Abstract
To establish a model for adoptive transfer of endothelial cells, we transferred human umbilical vein endothelial cells (HUVECs) to immunodeficient mice (Rag 2−/−). HUVECs were suspended as single cells in Matrigel and injected subcutaneously in the abdominal midline. Within 10 days after injection, HUVECs expressed pseudopod-like extensions and began to accumulate in arrays. By day 20, we observed human vessels that contained erythrocytes, and on day 30 we observed perivascular cells that expressed smooth muscle actin, thus resembling mature vessels. Throughout the experimental period, HUVECs bound Ulex europaeus lectin and expressed CD31, VE-cadherin, von Willebrand factor, as well as ICAM-2. A fraction of the cells also expressed the proliferation marker Ki67. Moreover, the sialomucin CD34, which is rapidly down-regulated in cultured HUVECs, was reinduced in vivo. However, we found no reinduction of CD34 in cells cultured inside or on top of Matrigel in vitro. We also injected cells suspended in Matrigel around the catheter tip of implanted osmotic pumps. Delivery of recombinant human interferon-γ by this route led to strong induction of MHC class II and ICAM-1 on the human vessels. In conclusion, isolated human endothelial cells can integrate with the murine vascular system to form functional capillaries and regain in vivo properties.
Vascular endothelial cells (ECs) are adapted to the needs of the surrounding tissue and exhibit a remarkable heterogeneity at the functional and structural level. The best-characterized examples of vascular differentiation are found in secondary lymphoid organs and in the brain. In the former, ECs of postcapillary, high endothelial venules support the migration of lymphocytes by means of adhesion molecules that bind lymphocyte homing receptors. 1,2 On the other hand, the ECs that line the small blood vessels of the brain possess a unique expression pattern of cell surface receptors, transporters, and intracellular enzymes that serve to tightly regulate the exchange of solutes between blood and brain parenchyma. 3,4 Distinct EC phenotypes have likewise been documented in other organs such as the liver, kidney, intestine, and lung. 5,6 In addition, ECs vary in their response to pathophysiological stimuli. For instance, Escherichia coli sepsis in baboons selectively induces tissue factor in a subpopulation of ECs within the marginal zone of splenic follicles. 7 Furthermore, systemic delivery of lipopolysaccharide to mice specifically up-regulates the acute-phase protein pentraxin-3 within the vascular beds of striated muscles. 8
Beyond a large descriptive catalogue of EC phenotypes, surprisingly little is known about the molecular basis of vascular diversity. One of the most fundamental questions in vascular biology is whether phenotypic patterns are genetically inherited from distinct sublineages or instead governed by signals generated within the microenvironment. In vitro investigations with embryonic stem cell cultures suggest that EC differentiation and early vasculogenesis are genetically predetermined. 9 Furthermore, retroviral cell-tagging studies in chicken embryos has revealed different clonal origins for endocardial versus coronary artery ECs. 10 On the other hand, in vivo transplant studies with avian embryos have pointed to the critical role of environmental cues in establishing blood vessel patterning during development. 11 Unfortunately, these experimental approaches are difficult to adapt to the mammalian system, because of poor accessibility of embryos and the lack of appropriate cell markers. Nevertheless, there is evidence that regional specialization of the endothelium in mammals may be conditioned by exogenous factors. Perhaps the best examples are studies of the blood-brain barrier, in which both cell culture and in vivo transfer have documented the ability of astrocytes to induce and maintain the appropriate EC phenotype. 12-15 Furthermore, a direct influence of extracellular signals has also been demonstrated in other EC types. Thus, preproendothelin-1 is up-regulated in rat cardiac microvascular ECs when co-cultured with ventricular myocytes. 16 Moreover, shear stress modulates the transcription of a number of EC genes. 17 Although some phenotypic changes are observed by changing the microenvironment, other EC characteristics seem well imprinted into the cell’s genetic program. Thus, several constitutive EC markers, eg, von Willebrand factor (vWF), CD31 (PECAM-1), and VE-cadherin (CD144) remain stably expressed long after explantation and adaptation to the in vitro environment. 18,19 Moreover, several phenotypic and functional differences between microvascular and large vessel-derived ECs are retained even when cultured under identical conditions throughout a period of several weeks. 20-22 There is also evidence to suggest that the malleability of the EC phenotype may change during development, as the high endothelial venule phenotype of lymph nodes transplanted to other regions of lymphatic drainage is influenced by donor age. 23
The development of transgenic animals that express reporter genes (eg, LacZ) under the control of EC-relevant promoter regions, has been an important step toward understanding the role of the microenvironment in EC phenotype regulation. 24 However, such experimental models cannot serve to dissect the individual role of particular non-EC types or their products. Although co-culture systems using ECs and other cell types have identified relevant exogenous components, ECs adapted to the in vitro environment may no longer possess important properties present in intact vessels in vivo.
Here we describe a method for adoptive transfer of human umbilical vein endothelial cells (HUVECs) to immunodeficient (Rag 2−/− knockout) mice. HUVECs cast in subcutaneously injected Matrigel assembled to form functional, mature vessels that contained murine erythrocytes, and were surrounded by smooth muscle α-actin-expressing cells. Furthermore, the sialomucin CD34 was rapidly reinduced in cells that lacked CD34 before transfer, reaching expression levels resembling those observed in human microvessels. We also demonstrate how to modulate the phenotype of the transferred endothelium with controlled, local delivery of cytokines by osmotic pumps.
Materials and Methods
Chemicals, Growth Factors, and Other Reagents
Acridine orange, ethidium bromide, hydrocortisone, recombinant human (rh) epidermal growth factor, rh basic fibroblast growth factor (bFGF), MCDB 131, and Dulbecco’s phosphate-buffered saline (PBS) were purchased from Sigma Chemical Co. (St. Louis, MO). Fetal calf serum, gentamicin, and amphotericin B were obtained from Life Technologies (Paisley, Scotland) and trypsin/ethylenediaminetetraacetic acid solution from BioWhittaker (Walkersville, MD), bovine dermal type I collagen (Vitrogen 100) from Celtrix Laboratories (Palo Alto, CA) and dried dimethyl sulfoxide from Merck (Darmstadt, Germany). Collagenase A and DNase-1 were from Boehringer Mannheim (Mannheim, Germany). Paramagnetic monodisperse beads (4.5 μm) coated with sheep anti-mouse IgG were obtained from Dynal (Oslo, Norway). OCT compound was from Tissue Tec, Miles Laboratories (Elkhart, IN). Matrigel basement-membrane matrix was from Becton Dickinson Labware (Bedford, MA), rh interferon-γ from R&D Systems Europe Ltd. (Abingdon, UK), Luconyl Blue 9600 from BASF Corporation (Ludvigshafen, Germany), the Ridascreen mycoplasma immunofluorescence assay from R-Biopharm (Darmstadt, Germany), chamber slides from Nunc (Roskilde, Denmark), Alzet osmotic pumps and catheters from Durect Corp. (Cupertino, CA), and 6.5-mm transwells from Costar (Cambridge, MA). Hoechst nuclear dye was from Molecular Probes (Eugene, OR).
Primary Antibodies and Secondary Reagents
The primary antibodies (Abs) used in this study are listed in Table 1 ▶ . Rhodamine- and fluorescein isothiocyanate (FITC)-conjugated Ulex europaeus Lectin I, biotinylated horse anti-mouse IgG, and aminomethyl coumarin acetic acid-conjugated goat anti-rabbit IgG (H+L) were from Vector Laboratories (Burlingame, CA). FITC-conjugated donkey anti-rabbit IgG, Texas Red-conjugated donkey anti-mouse IgG (H+L), and FITC-conjugated donkey anti-rat IgG were from Jackson ImmunoResearch Laboratories (West Grove, PA), Alexa 594-conjugated donkey anti-rat IgG was from Molecular Probes and streptavidin-Texas Red was from Life Technologies.
Table 1.
Primary Antibodies Used for Immunoselection and Immunostaining
| Antibody/clone | Specificity | Working concentration | Specifications | Source |
|---|---|---|---|---|
| hec 7 | hCD31 | 0.4 μg/ml | Mouse IgG2a | W.A. Muller, New York, NY |
| 561 | hCD34 class III | 10 μg/ml | Mouse IgG2a | G. Gaudernack, Oslo, Norway |
| H18/7 | hE-Selectin | 10 μg/ml | Mouse IgG1 | Becton-Dickinson, Bedford, MA |
| NaM185-2C3 | hDARC | 1/10 | Mouse IgG1 | Y. Colin, Paris, France |
| RRI/1.1.1 | hICAM-1 | 10 μg/ml | Mouse IgG1 | R. Rothlein, Ridgefield, CT |
| CBR-C2/2 | hICAM-2 | 1 μg/ml | Mouse IgG2a | Bender Med Systems, Vienna, Austria |
| 4B9 | hVCAM-1 | 3.5 μg/ml | Mouse IgG1 | J. Harlan, Seattle, WA |
| OKM5 | hCD 36 | 1/200 | Mouse IgG1 | Ortho Systems Inc., Raritan, NJ |
| 4.8.G | hVE-cadherin | 1/1000 | Mouse IgG1 | O. Bakke, Oslo |
| IV.3 | hCD 32 | 1/100 | Mouse IgG1 | L. Bjoerge, Bergen, Norway |
| B8.11 | HLA-DR | 1.3 μg/ml | Mouse IgG2b | B. Malissen, Marseille, France |
| MEC 13.3 | mCD31 | 1/100 | Rat IgG2a | A. Vecchi, Milano, Italy |
| YN 1.1.7.4 | mICAM-1 | 10 μg/ml | Rat Ig | F. Takei, Vancouver, Canada |
| MK 27 | mVCAM-1 | 10 μg/ml | Rat Ig | P. Kincade, Oklahoma City, OK |
| von Willebrand factor | vWf | 4.1 μg/ml | Rabbit IgG | DAKO, Glostrup, Denmark |
| 42/2 | Potato virus | 10 μg/ml | Mouse IgG2a | R. Burns, Edinburgh UK |
| 60/3.4 | Tomato nepovirus | Corresponding to relevant Ab | Mouse IgG1 | R. Burns |
| F4/80 | Monocytes/ macrophages | 10 μg/ml | Rat IgG2b | S. Gordon, Oxford, UK |
| MIB 1 | Ki67 | 6.7 μg/ml | Mouse IgG1 | Dianova, Hamburg, Germany |
| M 0851 | Smooth muscle actin | 10 μg/ml | Mouse IgG2a | DAKO |
Cell Cultures
HUVECs obtained from the Dept. of Gynecology and Obstetrics at Rikshospitalet were isolated as described by Jaffe and colleagues 25 and cultured in MCDB 131 containing 7.5% fetal calf serum, 10 ng/ml rh epidermal growth factor, 1 ng/ml rhbFGF, 1 μg/ml hydrocortisone, 50 μg/ml gentamicin, and 0.25 μg/ml amphotericin B. The cells were maintained at 37°C in a humid 95% air/5% CO2 atmosphere and split at ratios of 1:2 to1:3. All cultures were tested for infection with Mycoplasma by means of the Ridascreen immunoassay according to instructions of the manufacturer (R-Biopharm).
Cultures depleted of CD34+ cells were generated by means of immunomagnetic beads. To this end, cells were incubated with monoclonal antibody (mAb) to human CD34 (10 μg/ml) (see Table 1 ▶ for details) for 15 minutes on ice, then washed by centrifugation before addition of paramagnetic monodisperse beads coated with goat anti-murine IgG. The proper number of beads was enumerated by mixing a small fraction of the mAb-coated cells with beads in excess followed by centrifugation (1000 × g, 1 minute, 4°C); the percentage of rosetted cells was then determined by light microscopy. On the basis of this preliminary test, the cell suspensions were rosetted at a bead:target cell ratio of 20:1 (45 minutes at 4°C on a rock’n roller). Rosetted CD34+ cells were removed with two rounds of extraction on a magnetic particle concentrator (Dynal), and the depletion of CD34+ cells was verified by immunocytochemistry on chamber slides (see below).
In Vivo Experiments
Animal experiments were performed in accordance with institutional guidelines and national legislation. Specific pathogen-free mice of the Rag 2−/− B6.SJL-Ptprca strain (age, 6 to 8 weeks; weight, 20 to 22 g) were purchased from M&B (Ry, Denmark), and housed in air-flow racks on a restricted access area. Mice were fed BK001 pelleted rat and mouse diet (Grimston, Aldbruugh, Hull) and tap water ad libitum.
Anesthesia was induced by intraperitoneal injection of a combination of Hypnorm (fentanyl plus fluanisone) plus Dormicum (midazolam) solutions. HUVECs (3 × 105) suspended in 200 μl of Matrigel were injected subcutaneously in the midline of the abdomen, carefully positioning the needle between the epidermis and the muscle layer. All batches of Matrigel were adjusted to 9.3 mg/ml by addition of PBS before aliquoting. On termination of the experiments, some mice were reanesthetized and given an intracardial injection of Luconyl Blue (200 μl, 50% solution over PBS) 5 minutes before sacrifice by cervical dislocation. In experiments designed to modulate the endothelial phenotype, Alzet osmotic pumps (model 2004; 0.25 μl/hour, 28 days) were filled with 200 μl of rh interferon-γ (4000 UI/μl) and implanted subcutaneously on the back of anesthetized mice for cytokine delivery. To enable local cytokine delivery to the Matrigel plug, a catheter tube was attached to the opening of the pump and the other end cannulated subcutaneously to the center of the abdomi-nal wall. Matrigel with ECs was subsequently injected around the tip of the catheter tube, thus allowing local delivery to the center of the Matrigel plug.
The Matrigel plugs were removed by a wide excision of the abdominal wall, including the skin and all muscle layers, and transferred to PBS on ice. Each specimen was then divided into two pieces, one being immediately snap-frozen (liquid N2) in OCT compound for storage at −70°C until use, the other being fixed for 24 hours in methanol (−20°C) and further processed as follows: 96% ethanol (24 hours at 4°C), absolute ethanol (2 hours at 4°C) twice, xylene twice (20 hours at 4°C and 30 minutes at 22°C) before paraffin embedding.
In Vitro Experiments
CD34-depleted and nondepleted cells were suspended in Matrigel on ice, cast on top of the membrane in Transwell cell culture inserts (3 × 105 cells, 200 μl of Matrigel, 6.5-mm wells) by incubating for 1 hour at 37°C, before adding serum-free culture medium. After 3, 10, and 12 days, membrane inserts were fixed in methanol (24 hours at −20°C) and paraffin-embedded. In other inserts, the Matrigel was dissolved by incubation with a collagenase/dispase solution (1.5 hours at 37°C). Cells thus obtained were washed and subsequently centrifuged onto glass slides with a cytospin centrifuge. The cells were then air-dried (24 hours), fixed with methanol (15 minutes), and air-dried for another 30 minutes. Cells (104) were also cultured in chamber slides precoated with Matrigel (100 μl per well, 1 hour at 37°C), and incubated for 24 hours and 48 hours (37°C). The chamber slides were then immersed in methanol (15 minutes) and air-dried for 30 minutes.
Immunostaining Protocols
All of the following steps were performed at 22°C unless otherwise noted. Frozen tissue sections (8 μm) were air-dried overnight and subsequently acetone-fixed (10 minutes). Paraffin sections (4 μm) were dried for 24 hours at 4°C, dewaxed with xylene (10 minutes), and taken through 96% ethanol (1 minute) and PBS (3 minutes) before staining.
Tissue sections were in one protocol first incubated with mouse anti-human CD31 in combination with a rat anti-mouse CD31, next with a combination of Texas Red-conjugated donkey anti-mouse IgG (final concentration, 13 μg/ml) and FITC-conjugated donkey anti-rat IgG (15 μg/ml). In some experiments, a rabbit Ab that detects both human and murine vWF was added in the first step and an aminomethyl coumarin acetic acid-conjugated goat anti-rabbit IgG (18.8 μg/ml) was added to the mixture of secondary reagents. Cryosections were incubated with the primary and secondary reagents for 1 hour each, whereas paraffin sections were incubated with primary Abs for 20 hours and secondary reagents for 3 hours, respectively.
In another protocol, tissue sections, chamber slides, and cytospins were immunostained with a mixture of primary mouse mAbs (see Table 1 ▶ for details) and rabbit anti-vWF, next with a mixture of biotinylated horse anti-mouse IgG (6 μg/ml) and FITC-conjugated donkey anti-rabbit IgG (3.8 μg/ml), and finally with streptavidin-Texas Red (2.5 μg/ml, 60 minutes). Alternatively, we detected the binding of rat mAbs by means of Alexa 594-conjugated donkey anti-rat IgG (10 μg/ml) and human ECs by means of FITC-conjugated Ulex lectin (10 μg/ml). In some experiments we also added Hoechst blue nuclear stain (50 ng/ml) to the final volume of washing buffer after the last incubation.
Negative controls were tissue sections incubated with primary irrelevant isotype- and concentration-matched mAbs, whereas tissue sections from human tonsils served as positive controls. Microscopy was performed with a Nikon E-800 fluorescence microscope (Nikon Corp., Tokyo, Japan) equipped with a charge-coupled device camera (Hamamatsu Photonics, Hamamatsu City, Japan).
Results
Development of Functional Human Vessels in Immunodeficient Mice
Injected Matrigel formed a solid tumorous mass on the abdominal wall and retained its shape during the experimental period. Tissue sections of abdominal wall samples revealed the Matrigel plugs in the subcutaneous connective tissue (Figure 1a) ▶ . To follow the fate of injected HUVECs and relate them to pre-existing murine vessels, we stained tissue sections with anti-human CD31 (red) in combination with anti-mouse CD31 (green), in both species a pan-EC marker. Thus specimens excised 1 or 3 days after Matrigel injection revealed red single HUVECs uniformly distributed in the gel (data not shown and Figure 1b ▶ ). However, after 10 days we observed cells with elongated cytoplasmic protrusions resembling pseudopods (Figure 1c) ▶ . These cells appeared to accumulate and line up in arrays in areas of the gel surrounding murine vessels at the edges of the plug (Figure 1d) ▶ . By day 20 we observed vessel-like structures of accumulated HUVECs in all areas of the gels (Figure 1e) ▶ and in some mice a few of these structures contained erythrocytes, indicative of functional vessels (data not shown). At day 30 several human vessels that contained erythrocytes were found in all mice and in particular when close to murine vessels (Figure 1g) ▶ . On day 40 nearly all human vessels contained erythrocytes and to a greater extent than on day 30 (Figure 1j) ▶ . After day 30, the majority of all vessels were of human origin whereas the fraction of murine and hybrid vessels amounted to less than 10% and 3%, respectively. The fraction of single HUVECs was gradually reduced during the experimental period and amounted to less than 10% of all HUVEC-derived elements at day 30 and 40. Luconyl Blue, a water-insoluble dye that remains in the vascular compartment after intracardial injection, was found inside some of the human vessels (in addition to almost all murine vessels), thus providing further evidence of functional vessels at the time point of sacrifice (Figure 1i) ▶ . Furthermore, after 30 and 40 days we observed areas of markedly increased cellular density. These hotspots contained high numbers of human and murine ECs that were mostly assembled to erythrocyte-containing vessels (Figure 1h) ▶ . However, these areas also contained high numbers of extravascular erythrocytes perhaps indicating vascular leakage at points of xenogeneic contact.
Figure 1.
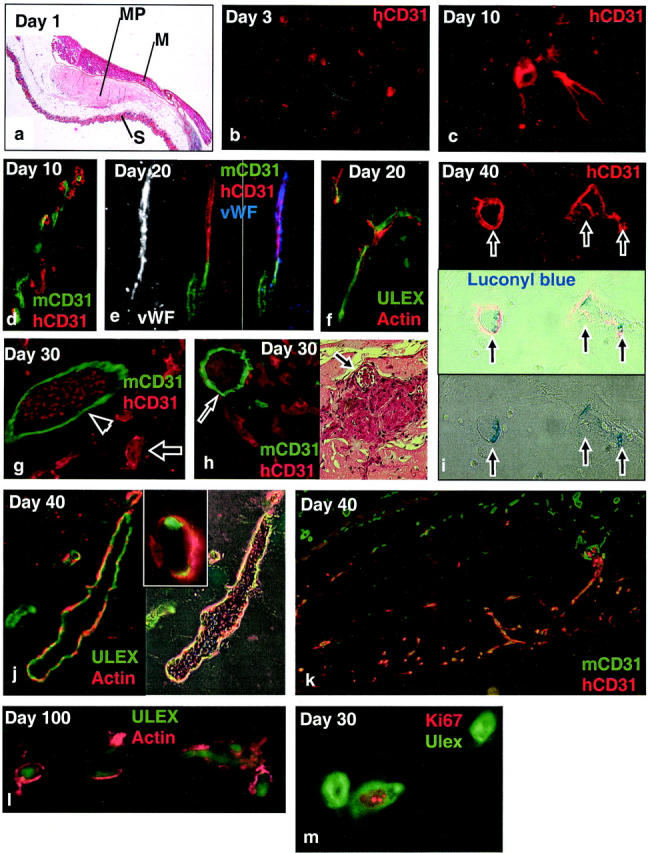
Development of functional human vessels in Matrigel. a: H&E staining of Matrigel plug (MP) positioned between the skin (S) and abdominal muscle layer (M). b–m: Immunofluorescence staining of human and murine endothelium in the Matrigel with markers as indicated in the panels. Note close association between human and murine ECs in d, as well as apparent junction between human and murine vessels in e. Note also erythrocytes inside human (arrow) and murine (arrowhead) vessels in g, as well as Luconyl Blue (arrows) in human vessels in i (phase contrast and immunofluorescence image of identical fields). Methanol-fixed, paraffin-embedded samples were cut at 4 μm. Original magnifications: ×40 (a); ×200 [b and h (right)]; ×100 (d, e, and k); ×600 (c and m); ×400 [f, g, h (left), i, j, and l].
To investigate whether the novel human vessels could recruit other cellular components of mature vessels such as pericytes and vascular smooth muscle cells, we co-stained sections with an antibody to smooth muscle α-actin and Ulex lectin. Smooth muscle actin is expressed by smooth muscle cells and most pericytes, 26 whereas Ulex lectin labels human but not murine ECs. The temporal appearance of complex vessels that were associated with α-actin-expressing cells was somewhat variable. Thus, at day 20 we observed scattered complex vessels in one of four mice (Figure 1f) ▶ , whereas at day 30 approximately two-thirds of all human vessels were associated with α-actin-positive cells. By day 40 and day 100 almost all of the human vessels were associated with actin-positive cells, forming a distinct layer outside the Ulex-positive ECs (Figure 1 ▶ ; j, k, and l).
We next wished to evaluate the proliferative potential of adoptively transferred HUVECs. To this end, tissue sections were stained for the proliferation marker Ki67 in combination with Ulex lectin. Ki67 is a nuclear antigen expressed by proliferating cells but down-regulated in cells re-entering the G0 phase. 27 We found a fraction of all HUVECs to express Ki67 throughout the whole experimental period, thus indicating stable proliferative activity in this cell population (Figure 1m) ▶ .
Phenotype of Adoptively Transferred Endothelium
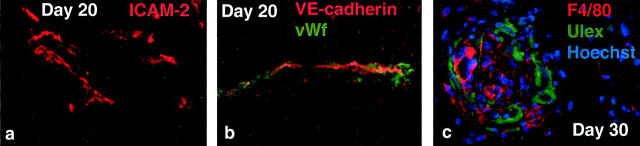
To begin characterizing the phenotype of HUVECs in their new environment, we stained tissue sections with Abs to well-known EC markers, showing that the human microvessels expressed vWF (Figure 3 ▶ ; a to d), ICAM-2 (Figure 2a) ▶ , and VE-cadherin (Figure 2b) ▶ throughout the whole experiment. On the other hand, HUVECs failed to stain with Abs to ICAM-1, VCAM-1, E-selectin, DARC (Duffy antigen/receptor of chemokines), CD36, CD32, and MHC class ΙΙ (HLA-DR) at all time points, despite strong staining of control tissue sections that contained relevant target cells (data not shown). Murine vessels expressed CD31 (Figure 1) ▶ and vWF (Figure 1e) ▶ , but failed to bind Ulex (relevant double staining not shown).
Figure 3.
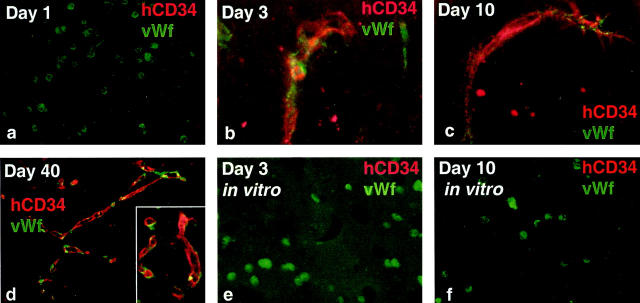
Reinduction of CD34 expression. Immunofluorescence staining of Matrigel in vivo (a–d) and in vitro (e and f) with markers as indicated (all paraffin sections). Original magnifications: ×100 (a); ×400 (b); ×600 (c); ×200 (d–f).
Figure 2.
Phenotypic analysis of human ECs and murine macrophages. Immunofluorescence staining of frozen (b) or paraffin-embedded (a and c) sections from the Matrigel plug with markers as indicated. Original magnifications, ×200.
Phagocytic cells observed by electron microscopy (T. Yamanaka, unpublished data) also prompted us to analyze the gels with mAb F4/80, a pan marker for murine macrophages. 28 Co-staining with Ulex revealed large amounts of F4/80+ cells in close association with human vessels (Figure 2c) ▶ . The F4/80+ macrophages were also present in areas of increased cellular density (see above) as seen in the center of Figure 2c ▶ . These areas corresponded to the hotspots observed in hematoxylin- and eosin-stained sections that contained large accumulations of extravascular erythrocytes as well as murine and human ECs (Figure 1h) ▶ .
Reinduction of the Sialomucin CD34
When phenotyping day 10 tissue sections containing second passage HUVECs, we surprisingly observed that virtually all HUVECs expressed CD34 (data not shown). This finding was in strong contrast to published data on cultured HUVECs, according to which CD34 is rapidly down-regulated on passage of the cells. 29 We confirmed these findings by observing that the number of CD34+ cells in HUVEC cultures was gradually reduced from ∼10 to 20% (at passage levels 1 and 2) to being undetectable at passage levels 3 to 5. Wishing to determine the fate of both high- and low-passage CD34− HUVECs, we depleted first passage cultures of CD34+ cells by means of immunomagnetic beads, plated the remaining cells in chamber slides, and found the fraction of CD34+ cells reduced to less than 1% by means of immunocytochemical staining. Cells from depleted cultures or high passage CD34− HUVECs were subsequently injected in mice. One day after injection, HUVECs in Matrigel were CD34− and vWF+ (Figure 3a) ▶ , but from day 3 and onwards we found virtually all vessels to express CD34, regardless of passage level (Figure 3, b to d ▶ , and data not shown).
To exclude the possibility that the Matrigel itself might reinduce CD34 expression, we immunostained depleted and nondepleted second passage HUVECs cultured on the top or cast in Matrigel, after 3, 10, and 12 days. However, all in vitro cultures failed to express CD34 despite strong expression of vWF (Figure 3, e and f ▶ , and data not shown).
Modulation of the EC Phenotype by Osmotic Pumps
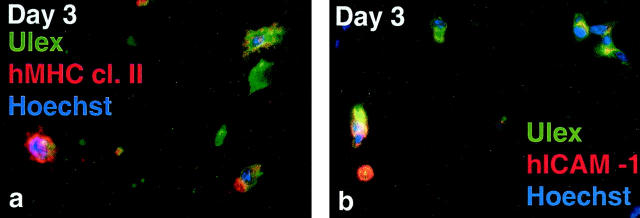
To investigate whether osmotic pumps could be used for controlled delivery of mediators to human ECs in Matrigel, we exposed gels to locally delivered rh interferon-γ for 3 days and observed gross induction (∼90% of the population) of hMHC class II (Figure 4a) ▶ and hICAM-1 (Figure 4b) ▶ , whereas no signal for hVCAM-1 or hE-selectin could be detected (data not shown). Furthermore, we observed induction of mICAM-1 on non-ECs (vWF-negative) both inside and outside the gel as well as induction of mVCAM-1 on non-ECs outside the gel (data not shown).
Figure 4.
Modulation of the EC phenotype. Immunofluorescence staining of paraffin-embedded interferon-γ-stimulated day 3 samples with markers as indicated. Original magnifications, ×600.
Discussion
This study describes a simple and reproducible method for adoptive transfer of ECs. HUVECs assembled to form capillaries that contained murine red blood cells within 4 weeks after subcutaneous injection in Matrigel, thus mimicking the later steps of sprouting angiogenesis in which ECs terminate migration and proliferation, and begin accumulating and remodeling into new blood vessels. 30,31 The finding of Luconyl Blue inside only some of the human vessels indicated that newly formed vessels remain in a plastic state of remodeling, perhaps depending on the proper combination of stimulating survival signals such as vascular endothelial growth factor or Angiopoetin-1 for further maturation. 32,33 Thus, this system may be suitable for analyses of vascular remodeling and evaluation of factors that affect the later steps of angiogenesis.
The factors that enable the capillary morphogenesis and the successful anastomosis with the host vasculature in our model are currently unknown. Nevertheless, it is highly likely that both the murine vessels surrounding the gel and the non-ECs that invade it play a role. Indeed, HUVECs tended to accumulate close to murine vessels, possibly getting additional morphogenic signals from their murine counterparts. It is also reasonable to assume that the pericytes found in close contact with capillaries can influence vascular differentiation and stabilization. Indeed, pericyte-derived factors may affect the phenotype of nearby vessels. 34,35 It is also possible that gel-invading macrophages may influence the survival and phenotype of the HUVECs. Given that these macrophages are derived from peripheral blood monocytes, future studies might estimate the role of macrophages in vascular differentiation by means of mice depleted of monocytes. 36
A model for adoptive transfer of ECs also lends itself to studies of their phenotype. We initially assumed that the transfer of ECs to an in vivo environment (and subsequent capillary morphogenesis) would induce vascular changes that might enable studies of the phenotype not permitted in vitro. Although several markers that are predominantly expressed by microvessels or segments thereof (CD32, CD36, DARC, or HLA-DR 20,21,37,38 ) were not induced in our model, our assumption was confirmed in observing reinduction of an EC marker that in HUVECs is rapidly lost in vitro. The sialomucin CD34, a putative L-selectin ligand in the high endothelium of lymphoid organs, but of unknown function in flat-walled vessels, 39 was absent at day 1 but reinduced within 3 days in vivo. However, this reinduction was not mediated by Matrigel itself, because CD34− HUVEC cultures failed to express CD34 when cultured in or on Matrigel. Instead, CD34 expression may have been stimulated by the altered microenvironment in the Matrigel, perhaps provided by one or several of the cellular components discussed above. There is also a small possibility that the CD34+ cells found in the Matrigel at day 3 were not CD34− at the time of transfer but rather generated from the small number of cells (<1%) having escaped our immunomagnetic depletion. However, the high numbers of positive cells at day 3 (∼70%) are unlikely to have risen from at the most four population doublings of contaminating CD34+ cells.
Our model may prove particularly useful to assess the effect of phenotypic stimuli other than those recruited from the host. To this end, the usefulness of osmotic pumps for soluble substance delivery and their effect on vascular phenotype regulation were clearly demonstrated by the de novo induction of cytokine-responsive EC markers. It may also be possible to transfer growth-arrested, stably transfected cell lines that would produce soluble or cell-bound mediators, preferably under the control of an in vivo-inducible promoter. Furthermore, the influence of non-ECs from organs with EC phenotypes of interest could be estimated by including them in the Matrigel. Finally, cultured microvascular ECs from several organs have a phenotype different from HUVECs and may therefore enable the generation of data not obtainable by means of the latter.
The present model of adoptive transfer was partly chosen because of technical simplicity. Alternatively, it would be interesting to transfer ECs directly to the tissue without Matrigel. To this end, it remains unknown whether human ECs would be targets for natural killer cells or granulocytes once they were transferred directly to the immunodeficient mice used in this study, but in the absence of Matrigel. It is possible that the Matrigel provides immunoisolation, although HUVECs sometimes observed outside the gel appeared healthy. Nevertheless, our model nicely proves a principle that can be adopted to other settings. Thus, congenic transfer of murine ECs might provide another modality useful for studies in immunocompetent animals.
The technique described here has advantages over two alternative, recently published models. 40,41 First, the injection of cells in liquid gel is efficient and less traumatizing to the surrounding tissue than surgical implantation of solidified gels 40 or porous polymer matrices. 41 In fact, the vessels that developed in the latter transiently expressed ICAM-1 and VCAM-1, indicating proinflammatory activation of the microenvironment and contrasting the complete lack of such induction (including E-selectin) in our model. Second and more importantly, HUVECs in our system developed into complex microvessels, which were stable up to 100 days (instead of regressing within day 28), without genetic modification designed to suppress apoptosis. Such manipulation may interfere with the ability to understand physiological human EC behavior. Although Matrigel is an incompletely defined substance of animal origin that would carry potential xenogeneic hazards, it is reasonable to assume that one or several of its relevant components would support the survival of native, nontransduced endothelium. In fact, it is tempting to speculate that improved vascular perfusion in clinical settings might be achieved by simple injection of healthy ECs in a completely defined substitute for Matrigel.
In conclusion, we have developed a system for adoptive transfer of ECs. This system may be useful to study vascular remodeling and regulation of the EC phenotype. Such EC transfer also holds promise for enhanced vascularization in clinical settings.
Acknowledgments
We would like to thank the technical staff at Institute of Pathology for expert technical assistance.
Footnotes
Address reprint requests to Dag K. Skovseth, LIIPAT, Rikshospitalet, N-0027 Oslo, Norway. E-mail: d.k.skovseth@farmasi.uio.no.
Supported by National Institutes of Health Grant GM37734 (to E.C.B.) and by grants from the Norwegian Cancer Society (B88136), the Research Council of Norway [13546/310 and 13932/300], the Anders Jahre’s Fund, and the Independent Order of Odd Fellows.
References
- 1.Kraal G, Mebius RE: High endothelial venules: lymphocyte traffic control and controlled traffic. Adv Immunol 1997, 65:347-395 [PubMed] [Google Scholar]
- 2.Girard JP, Springer TA: High endothelial venules (HEVs): specialized endothelium for lymphocyte migration. Immunol Today 1995, 16:449-457 [DOI] [PubMed] [Google Scholar]
- 3.Bradbury MW: The blood-brain barrier. Exp Physiol 1993, 78:453-472 [DOI] [PubMed] [Google Scholar]
- 4.Schlosshauer B: The blood-brain barrier: morphology, molecules, and neurothelin. Bioessays 1993, 15:341-346 [DOI] [PubMed] [Google Scholar]
- 5.DeFouw DO: Structural heterogeneity within the pulmonary microcirculation of the normal rat. Anat Rec 1988, 221:645-654 [DOI] [PubMed] [Google Scholar]
- 6.Fleming S, Jones DB: Antigenic heterogeneity of renal endothelium. J Pathol 1989, 158:319-323 [DOI] [PubMed] [Google Scholar]
- 7.Drake TA, Cheng J, Chang A, Taylor FB, Jr: Expression of tissue factor, thrombomodulin, and E-selectin in baboons with lethal Escherichia coli sepsis. Am J Pathol 1993, 142:1458-1470 [PMC free article] [PubMed] [Google Scholar]
- 8.Introna M, Alles VV, Castellano M, Picardi G, De Gioia L, Bottazzai B, Peri G, Breviario F, Salmona M, De Gregorio L, Dragani TA, Srinivasan N, Blundell TL, Hamilton TA, Mantovani A: Cloning of mouse ptx3, a new member of the pentraxin gene family expressed at extrahepatic sites. Blood 1996, 87:1862-1872 [PubMed] [Google Scholar]
- 9.Wang R, Clark R, Bautch VL: Embryonic stem cell-derived cystic embryoid bodies form vascular channels: an in vitro model of blood vessel development. Development 1992, 114:303-316 [DOI] [PubMed] [Google Scholar]
- 10.Mikawa T, Fischman DA: Retroviral analysis of cardiac morphogenesis: discontinuous formation of coronary vessels. Proc Natl Acad Sci USA 1992, 89:9504-9508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noden DM: Origins and assembly of avian embryonic blood vessels. Ann N Y Acad Sci 1990, 588:236-249 [DOI] [PubMed] [Google Scholar]
- 12.Janzer RC, Raff MC: Astrocytes induce blood-brain barrier properties in endothelial cells. Nature 1987, 325:253-257 [DOI] [PubMed] [Google Scholar]
- 13.Maxwell K, Berliner JA, Cancilla PA: Induction of gamma-glutamyl transpeptidase in cultured cerebral endothelial cells by a product released by astrocytes. Brain Res 1987, 410:309-314 [DOI] [PubMed] [Google Scholar]
- 14.Tao-Cheng JH, Nagy Z, Brightman MW: Tight junctions of brain endothelium in vitro are enhanced by astroglia. J Neurosci 1987, 7:3293-3299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lobrinus JA, Juillerat-Jeanneret L, Darekar P, Schlosshauer B, Janzer RC: Induction of the blood-brain barrier specific HT7 and neurothelin epitopes in endothelial cells of the chick chorioallantoic vessels by a soluble factor derived from astrocytes. Brain Res Dev Brain Res 1992, 70:207-211 [DOI] [PubMed] [Google Scholar]
- 16.Nishida M, Springhorn JP, Kelly RA, Smith TW: Cell-cell signaling between adult rat ventricular myocytes and cardiac microvascular endothelial cells in heterotypic primary culture. J Clin Invest 1993, 91:1934-1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Resnick N, Gimbrone MA, Jr: Hemodynamic forces are complex regulators of endothelial gene expression. FASEB J 1995, 9:874-882 [DOI] [PubMed] [Google Scholar]
- 18.Muller WA, Ratti CM, McDonnell SL, Cohn ZA: A human endothelial cell-restricted, externally disposed plasmalemmal protein enriched in intercellular junctions. J Exp Med 1989, 170:399-414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lampugnani MG, Resnati M, Raiteri M, Pigott R, Pisacane A, Houen G, Ruco LP, Dejana E: A novel endothelial-specific membrane protein is a marker of cell-cell contacts. J Cell Biol 1992, 118:1511-1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groger M, Sarmay G, Fiebiger E, Wolff K, Petzelbauer P: Dermal microvascular endothelial cells express CD32 receptors in vivo and in vitro. J Immunol 1996, 156:1549-1556 [PubMed] [Google Scholar]
- 21.Swerlick RA, Lee KH, Wick TM, Lawley TJ: Human dermal microvascular endothelial but not human umbilical vein endothelial cells express CD36 in vivo and in vitro. J Immunol 1992, 148:78-83 [PubMed] [Google Scholar]
- 22.Utgaard JO, Jahnsen FL, Bakka A, Brandtzaeg P, Haraldsen G: Rapid secretion of prestored interleukin 8 from Weibel-Palade bodies of microvascular endothelial cells. J Exp Med 1998, 188:1751-1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mebius RE, Breve J, Kraal G, Streeter PR: Developmental regulation of vascular addressin expression: a possible role for site-associated environments. Int Immunol 1993, 5:443-449 [DOI] [PubMed] [Google Scholar]
- 24.Aird WC, Edelberg JM, Weiler-Guettler H, Simmons WW, Smith TW, Rosenberg RD: Vascular bed-specific expression of an endothelial cell gene is programmed by the tissue microenvironment. J Cell Biol 1997, 138:1117-1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaffe EA, Nachman RL, Becker CG, Minick CR: Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest 1973, 52:2745-2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C: Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 1999, 126:3047-3055 [DOI] [PubMed] [Google Scholar]
- 27.Scholzen T, Gerdes J: The Ki-67 protein: from the known and the unknown. J Cell Physiol 2000, 182:311-322 [DOI] [PubMed] [Google Scholar]
- 28.Hume DA, Robinson AP, MacPherson GG, Gordon S: The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80. Relationship between macrophages, Langerhans cells, reticular cells, and dendritic cells in lymphoid and hematopoietic organs. J Exp Med 1983, 158:1522-1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fina L, Molgaard HV, Robertson D, Bradley NJ, Monaghan P, Delia D, Sutherland DR, Baker MA, Greaves MF: Expression of the CD34 gene in vascular endothelial cells. Blood 1990, 75:2417-2426 [PubMed] [Google Scholar]
- 30.Folkman J, D’Amore PA: Blood vessel formation: what is its molecular basis? Cell 1996, 87:1153-1155 [DOI] [PubMed] [Google Scholar]
- 31.Risau W: Mechanisms of angiogenesis. Nature 1997, 386:671-674 [DOI] [PubMed] [Google Scholar]
- 32.Ferrara N: Molecular and biological properties of vascular endothelial growth factor. J Mol Med 1999, 77:527-543 [DOI] [PubMed] [Google Scholar]
- 33.Papapetropoulos A, Garcia-Cardena G, Dengler TJ, Maisonpierre PC, Yancopoulos GD, Sessa WC: Direct actions of angiopoietin-1 on human endothelium: evidence for network stabilization, cell survival, and interaction with other angiogenic growth factors. Lab Invest 1999, 79:213-223 [PubMed] [Google Scholar]
- 34.Orlidge A, D’Amore PA: Inhibition of capillary endothelial cell growth by pericytes and smooth muscle cells. J Cell Biol 1987, 105:1455-1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, Betsholtz C: Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol 2001, 153:543-553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evans R: Effect of X-irradiation on host-cell infiltration and growth of a murine fibrosarcoma. Br J Cancer 1977, 35:557-566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hadley TJ, Lu ZH, Wasniowska K, Martin AW, Peiper SC, Hesselgesser J, Horuk R: Postcapillary venule endothelial cells in kidney express a multispecific chemokine receptor that is structurally and functionally identical to the erythroid isoform, which is the Duffy blood group antigen. J Clin Invest 1994, 94:985-991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daar AS, Fuggle SV, Fabre JW, Ting A, Morris PJ: The detailed distribution of MHC Class II antigens in normal human organs. Transplantation 1984, 38:293-298 [DOI] [PubMed] [Google Scholar]
- 39.Baumheter S, Singer MS, Henzel W, Hemmerich S, Renz M, Rosen SD, Lasky LA: Binding of L-selectin to the vascular sialomucin CD34. Science 1993, 262:436-438 [DOI] [PubMed] [Google Scholar]
- 40.Schechner JS, Nath AK, Zheng L, Kluger MS, Hughes CC, Sierra-Honigmann MR, Lorber MI, Tellides G, Kashgarian M, Bothwell AL, Pober J: In vivo formation of complex microvessels lined by human endothelial cells in an immunodeficient mouse. Proc Natl Acad Sci USA 2000, 97:9191-9196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nor JE, Peters MC, Christensen JB, Sutorik MM, Linn S, Khan MK, Addison CL, Mooney DJ, Polverini P: Engineering and characterization of functional human microvessels in immunodeficient mice. Lab Invest 2001, 81:453-463 [DOI] [PubMed] [Google Scholar]