Abstract
Glial Müller cells are known to undergo functional and morphological changes during retinal proliferative disorders, but very little is known of the contribution of these cells to extracellular matrix deposition during retinal wound healing and gliosis. This study constitutes the first demonstration that retinal Müller cells express two major matrix metalloproteinases (MMPs), gelatinase A (MMP-2) and gelatinase B (MMP-9), implicated in cell migration and matrix degradation. Although mRNA and gelatinolytic activity of MMP-2 remained unchanged in cultured Müller cells, basal levels of MMP-9 mRNA observed after subculture at 24 hours, markedly declined after 48 or 72 hours. This correlated with the expression of MMP-9 gelatinolytic activity that peaked at 24 hours, but gradually decreased at 48 and 72 hours. Tumor necrosis factor-α, in both a soluble form or bound to collagen and fibronectin, increased MMP-9 mRNA and gelatinolytic activity, but not MMP-2 expression, and its effect could be blocked by anti-tumor necrosis factor-α antibodies. The results suggest that Müller cells may aid in the local control of extracellular matrix deposition during retinal proliferative disorders, and that interaction of these cells with matrix-bound cytokine may influence their pathological behavior. Control of Müller cell production of MMP-9 may constitute an important target for the design of new therapeutic approaches to treat and prevent retinal proliferative disease.
Müller cells constitute the main glial cells of the retina. They stabilize the complex retinal architecture, provide structural support to retinal neurons and blood vessels, and prevent aberrant photoreceptor migration into the subretinal space. 1 It is well recognized that Müller cells undergo functional and phenotypic changes in retinal pathological conditions such as proliferative vitreoretinopathy (PVR), 2 proliferative diabetic retinopathy, 3 age-related macular degeneration, 4 and inherited macular dystrophies. 5 Early events leading to the development of proliferative retinopathy involve local cell migration and proliferation followed by extracellular matrix (ECM) accumulation. 6,7 This results in the formation of retinal membranes and resembles physiological processes of wound healing and fibrosis. 6,7 Similar changes occur in animal models of retinal proliferation in which intravitreal injections of inflammatory mediators cause rapid Müller cell migration and proliferation, followed by formation of gliotic adhesions. 8,9 Migration as well as matrix deposition are controlled by degradation of the ECM by proteolytic enzymes known as matrix metalloproteinases (MMPs). 10 These constitute a family of zinc-binding, calcium-dependent molecules, whose activity is in turn regulated by natural inhibitors, known as tissue inhibitors of metalloproteinases (TIMPs). 11 Two major matrix-degrading enzymes, known as MMP-2 (collagenase A) and MMP-9 (collagenase B), which have been implicated in cell migration and proliferation, 12-15 may be found in vitreous and retinal membranes from eyes with retinal proliferative disorders. 16-19 The main source of these MMPs has been thought to be the retinal pigment epithelial cells, which are well known to produce these molecules. 20-22 Although there is no evidence that Müller cells produce these MMPs, it is possible that they produce these enzymes while undergoing phenotypic and metabolic changes during retinal proliferative disease. This is suggested by evidence that glial cells, such as rat astrocytes produce MMP-2 and MMP-9 23 and that the membrane-type (MT)-1, -2, and -3 MMPs are expressed by astrocytes of glioblastoma tissues. 24
During proliferative retinopathy, retinal Müller cells encounter different ECM proteins, cytokines, and growth factors, because these are often present in vitreous and retinal tissues from affected eyes. 25-28 Therefore, Müller cell functions, including production of matrix-degrading enzymes, may be influenced by cytokines and growth factors present in the retinal microenvironment during the course of various pathological processes. A cytokine, known as tumor necrosis factor-α (TNF-α), is known to regulate expression and production of MMPs by various cells, 29,30 and is found in vitreous 25,26 and ECM of retinal membranes from eyes with PVR and proliferative diabetic retinopathy. 27,28 It is well recognized that TNF-α binds to ECM proteins, including collagen, fibronectin, and laminin, 31-33 which constitute the main matrix components of epiretinal membranes. 34 In addition, TNF-α bound to ECM exhibits biological activity, as demonstrated by findings that when bound to fibronectin, this cytokine modifies β1-integrin-mediated adhesion of T lymphocytes, 31 provides a stop signal for migration of T cells, 32 and stimulates MMP-9 expression by monocytes. 35 On this basis, it is likely that TNF-α bound to ECM during processes of retinal fibrosis and neovascularization may affect Müller cell function, and hence influence the development of retinal proliferative disease.
Given the functional importance of Müller cells in the maintenance of retinal integrity, the role of MMPs in the promotion of cell migration and matrix degradation, and the activity of TNF-α in promoting cell activation and MMP production, we investigated the expression and production of gelatinases A (MMP-2) and B (MMP-9) by Müller cells, as well as the ability of TNF-α in soluble and ECM-bound forms to modulate the production of these MMPs by retinal Müller cells in culture.
Materials and Methods
Müller Cell Culture
Müller cells were isolated from donor eyes consented for research use, from Moorfields Hospital Eye Bank, by a modification of a established method. 36 Briefly, the retina was homogenized by vigorous pipetting, followed by incubation with Trypsin-EDTA (5% trypsin, 2% ethylenediaminetetraacetic acid; Gibco BRL, UK) for 20 minutes at 37°C, and filtration through a stainless steel sieve. Cells were washed and cultured to confluence in Dulbecco’s modified Eagle’s medium containing L-glutamax I (Gibco BRL) and 10% fetal calf serum (Gibco BRL). Two primary cultures of Müller cells identified by the donor numbers 5418 and 5427 were derived from a 58-year-old female and a 44-year-old male, respectively. One of the preparations (5418 cells) became spontaneously immortalized after subsequent passages. 37 However, cells used in the study were obtained from passages 3 to 6 of both preparations and were identified by their characteristic morphology (Figure 1A) ▶ and by their expression of Müller cell markers. 36,38 These included cellular retinaldehyde-binding protein (>98%, Figure 1B ▶ ), epidermal growth factor receptor (>70%), vimentin (>95%), glutamine synthetase (>90%), α-smooth muscle cell actin (100%), and glial fibrillary acidic protein (<10%).
Figure 1.
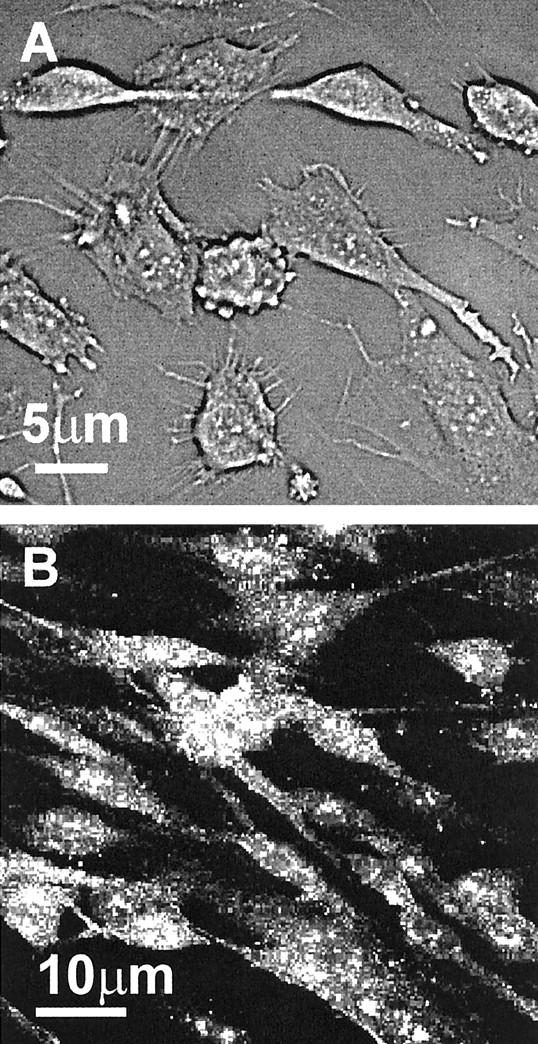
Characteristic morphology of Müller cells in culture. A: Subconfluent monolayer of Müller cells in culture seen under phase contrast microscope displaying long branching and microvilli processes. Intracellular granular appearance was often observed in primary cultures. B: Confocal image of Müller cells stained for CRALBP. The majority of cells (>98%) stained for this molecule with a characteristic granular pattern.
To determine production of MMPs, Müller cells grown to confluence were detached by incubation with Trypsin-EDTA acid (5.0 g/L trypsin, 2.0 g/L EDTA) (GIBCO, BRL) for 2 minutes at 37°C. Cells were then suspended at a concentration of 2.5 × 104/ml in Dulbecco’s modified Eagle’s medium containing 10% of gelatinase-depleted fetal calf serum, and 0.5-ml aliquots of this suspension were laced in each of 24-well tissue culture plates (Nunc, UK). After incubation at 37°C for various periods of time, supernatants were harvested, centrifuged, and kept at −70°C until use. Gelatinase-depleted serum was obtained by incubation of 18 ml of heat-inactivated fetal calf serum with 3 ml of gelatin-Sepharose 4B (Pharmacia Biotech, Sweden) for 2 hours at room temperature on a shaker, followed by centrifugation and filtration.
Analysis of MMP-2 and MMP-9 Production by Gelatin Zymography
Aliquots of Müller cell supernatants were mixed with an equal volume of dissociating buffer [63 mmol/L Tris-HCl,pH 6.8, containing 10% glycerol, 2% sodium dodecyl sulfate, and 0.0025% bromophenol blue (Novex; Invitrogen, The Netherlands)] for 5 minutes at room temperature. Using zymogram gels containing 0.1% gelatin (Novex), samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis for 90 minutes at 125 V in Tris-glycine running buffer (25 mmol/L Tris, 192 mmol/L glycine, 0.1% sodium dodecyl sulfate, pH 8.3; Novex). Gels were then renatured in 2.7% Triton X-100 (Novex) for 30 minutes at room temperature, followed by a further 30-minute incubation in developing buffer (50 mmol/L Tris, 200 mmol/L NaCl, 5 mmol/L CaCl2, 0.2% Brij35; Novex). Gels were then incubated in fresh developing buffer at 37°C for 20 hours and finally stained with 0.5% Coomassie blue (Bio-Rad) dissolved in 45% methanol, 10% acetic acid, and 45% distilled water for 2 hours. To determine the degree of gelatinolytic activity, as judged by the presence of clear bands, gels were photographed using a digital camera (model DC120; Kodak, UK), and the images analyzed using the computer software program KDS1D, version 2.0 (Kodak).
RNA Isolation and Reverse Transcriptase-Polymerase Chain Reaction (PCR) Analysis of MMP-2 and MMP-9
After trypsinization of Müller cell monolayers, total cellular RNA was isolated from cell pellets using the RNeasy system (Qiagen GmbH, Hilden, Germany) according to the manufacturer’s instructions. For the reaction, 1 μg of total RNA was reverse-transcribed in 20-μl reactions consisting of 5 mmol/L MgCl2, 1 mmol/L dNTP, 1 U/μl RNase inhibitor, 0.75 U/μl AMV reverse transcriptase (Promega, UK), and 25 ng/μl oligo dT-15 primers (Pharmacia, UK) in 10 mmol/L of Tris/HCl buffer containing 50 mmol/L KCl, for 40 minutes at 42°C, and 5 minutes at 95°C in a thermal cycler (PCR Express; Hybaid, UK). PCR amplification was then performed by published methods 39 using specific primers for MMP-2 (forward primer 5′-CCTGTTTGTGCTGAAGGACA-3′, reverse primer 5′-GTACTTGCCATCCTTCTCAA-3′) and MMP-9 (forward primer 5′-AAACCGGTCGTCGGTGTCGT-3′, reverse primer 5′-GTCGAAATCTCTGGGGCCTG-3′) derived from the human sequence (GenBank). The amplification was performed in a final volume of 50 μl by addition of 1.5 mmol/L MgCl2, 0.2 mmol/L dNTP, 2 U Taq DNA polymerase (Promega), 0.5 μmol/L primers in 50 mmol/L KCl, 10 mmol/L Tris/HCl, pH 8.0. The mixture was initially incubated at 94°C for 5 minutes, followed by 27 cycles at the following conditions: 94°C for 1 minute, 58°C for 1 minute, 72°C for 1 minute, and 1 cycle of 72°C for 5 minutes. Kinetic analysis of amplified products was applied to all samples for each gene to ensure that signals derive only from the exponential phase of amplification. PCR products were then analyzed by agarose gel electrophoresis (2%) containing 25 ng/ml of ethidium bromide.
Optimization of TNF-α Binding to Collagen and Fibronectin for Müller Cell Activation
To investigate the effect of matrix-bound TNF-α on the modulation of MMP production by Müller cells, a modified enzyme-linked immunosorbent assay was used to assess the optimal binding of TNF-α to collagen type IV and fibronectin. The assay was based on conventional capture enzyme-linked immunosorbent assay protocol as follows: flat-bottom 96-well plates (Maxisorb Immunoplates; Nunc, UK) were coated with 500 μl of a 10-μg/ml solution of either collagen type IV or fibronectin (Sigma) in coating buffer (15 mmol/L Na2CO3, 35 mmol/L NaHCO3, pH 9.6), and incubated overnight at 4°C. Unbound protein was removed by four washes with phosphate-buffered saline (PBS) (Oxoid, UK) containing 0.05% Tween-20 (PBS/Tween). Aliquots of 100 μl of recombinant TNF-α (R&D Systems, UK) ranging from 0 to 100 ng/ml in PBS were then added to the wells of protein-treated plates, followed by incubations for 1 hour at 37°C and 1 hour at 4°C. Adhesion of TNF-α to nonprotein-coated plates or plates coated with bovine serum albumin (BSA) (10 μg/ml, Sigma) was also performed to establish the specificity of adhesion of this cytokine to ECM proteins. After washing unbound TNF-α with PBS/Tween buffer, 500-μl volumes of anti-TNF-α antibodies were added to the wells at a concentration of 1 μg/ml in PBS/Tween-20. To further confirm the binding of this cytokine to the ECM, three different anti-TNF-α antibodies [4H31-PG2 and HTNF10 (clones provided by Dr. W. A. Buurman and antibodies produced by Celltech, UK) and B-C7 (Serotec, UK)] were tested for the recognition of the bound cytokine. Plates were then incubated for 1 hour at room temperature, washed with the same buffer, and incubated for a further 1 hour at room temperature with 100 μl of a 1:1000 dilution (in PBS/Tween) of biotin-labeled goat anti-mouse IgG-biotin conjugate (DAKO, UK). After a final washing step, 200 μl of horseradish peroxidase-avidin D conjugate (Vector Laboratories, UK) was added to each well and the plates were incubated for 20 minutes at room temperature. The reaction was then stopped by the addition of 50 μl of 12.5% sulfuric acid. Optical density was measured at 450 nm using an enzyme-linked immunosorbent assay plate reader (model MR 5000; Dynatech Labs, UK).
Examination of the Effect of ECM-Bound TNF-α on the Production of MMP-2 and MMP-9 by Müller Cells
To determine the effect of ECM-bound TNF-α on the production of MMP-2 and MMP-9 by Müller cells, 24-well tissue-culture plates were coated with 500 μl of a 10-μg/ml solution of collagen type IV or fibronectin (Sigma), according to the optimal conditions established by the above experiments. After removal of unbound ECM protein, recombinant TNF-α (R&D Systems) was added to the wells (500 μl of a 100-ng/ml solution) and the plates were incubated for 1 hour at 37°C, followed by 1 hour at 4°C. Unbound TNF-α was removed by four washes with Dulbecco’s modified Eagle’s medium. Cells were then added to the wells (500 μl of a 2.5 × 104 cells/ml suspension in Dulbecco’s modified Eagle’s medium containing 10% gelatinase-depleted fetal calf serum), and the plates incubated for various periods of time at 37°C. Controls for these experiments constituted collagen type IV or fibronectin-coated plates without TNF-α and TNF-α bound to nonprotein-coated plates. After various periods of culture, supernatants were harvested, centrifuged, and kept at −70°C until use. Further confirmation of the effect of TNF-α on MMP production was achieved by inhibiting the effect of matrix-bound TNF-α with the anti-TNF-α antibody 4H31-PG2 (10 μg/ml) added at the beginning of the culture.
Results
Optimization of TNF-α Binding to Collagen and Fibronectin
As shown in Figure 2, A and B ▶ , recombinant TNF-α bound in a dose-response manner to immobilized fibronectin and collagen type IV when used at concentrations ranging from 0 to 100 ng/ml. The maximum binding of this cytokine to both fibronectin and collagen type IV was observed at the highest concentration used (100 ng/ml), and was detected with three different recombinant anti-TNF-α antibodies (4H31-PG2, HTNF10, and B-C7). Binding of TNF-α to culture plates without ECM also occurred (Figure 2C) ▶ , and only a residual binding of this cytokine to bovine serum albumin was observed when TNF-α was used at the highest concentration (100 ng/ml). However, this binding of TNF-α to bovine serum albumin was significantly lower than that observed when this cytokine bound to collagen type IV and fibronectin (Figure 2, A and B) ▶ . These findings support previous observations that TNF-α associates to the ECM, and indicates that this cytokine is antigenically accessible when bound to extracellular matrices.
Figure 2.
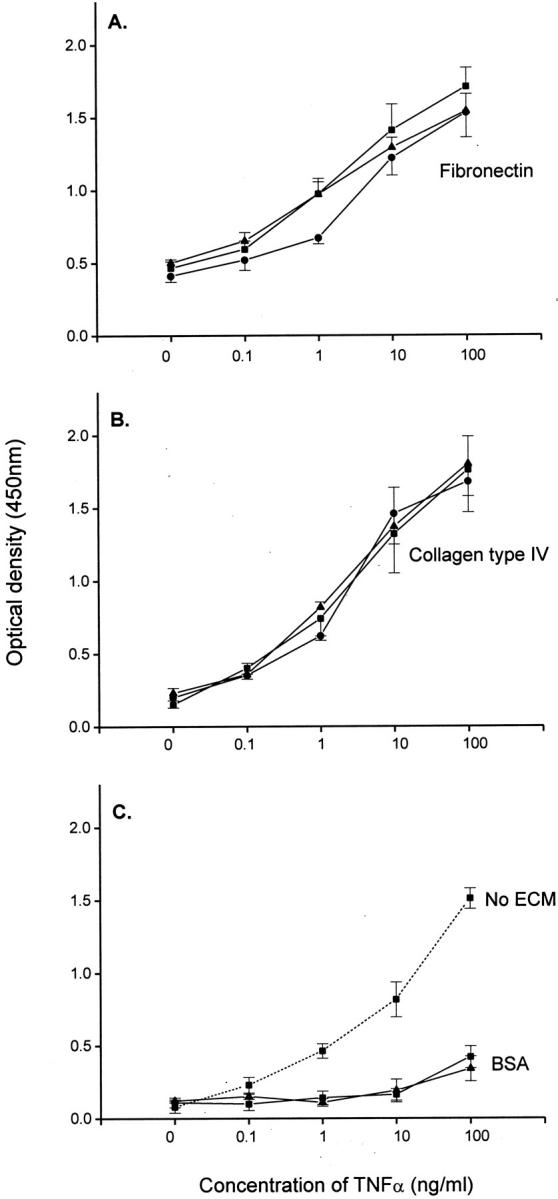
Binding of TNF-α to fibronectin-, collagen type IV-, bovine serum albumin-, and nonprotein-coated culture plates. The linear plots illustrate the binding to fibronectin, collagen type IV, bovine serum albumin, and noncoated plastic of various concentrations of recombinant TNF-α, as detected with the monoclonal antibodies 4H31-PG2 (▪), HTNF10 (▴), and B-C7 (•) used at a concentration of 1 μg/ml. Each point represents the mean ± SEM of three separate experiments.
Expression of MMP-2 by Retinal Müller Cells in Culture
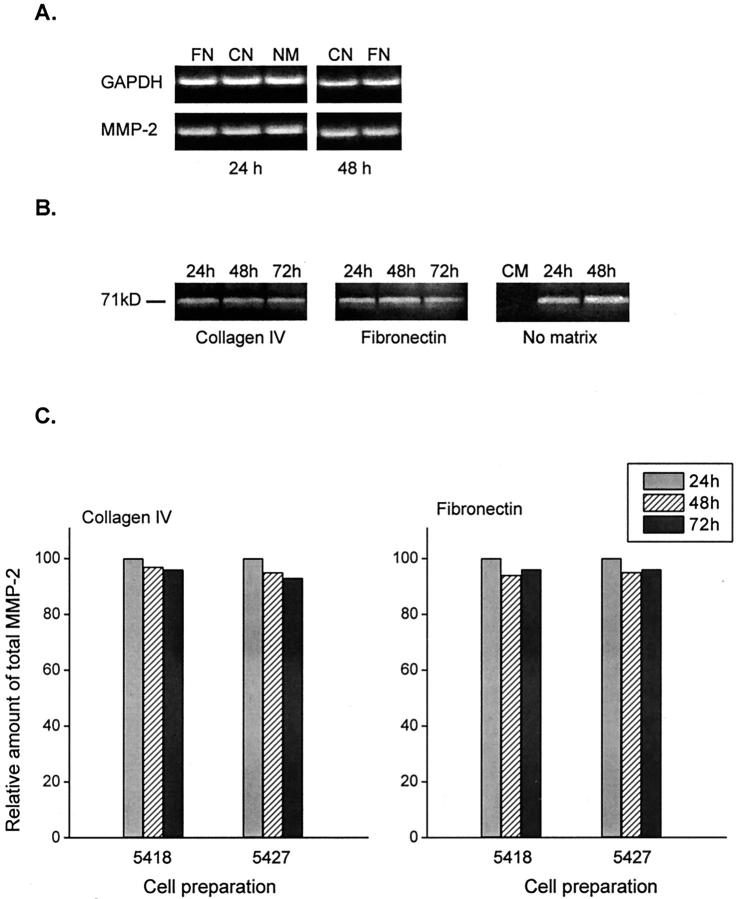
As observed in Figure 3A ▶ , constant levels of MMP-2 mRNA were detected in retinal Müller cells after 24 and 48 hours of culture. Levels of MMP-2 mRNA were not modified by culturing the cells on either fibronectin- or collagen-coated plates, or in the absence of ECM. As judged by gelatin zymography, MMP-2 was not detected in culture medium used to culture the cells, but after 24 hours it was present in culture supernatants as an inactive zymogen, corresponding to the 71-kd molecular weight gelatinase. Levels of MMP-2 enzymatic activity in culture medium were not modified after 24, 48, or 72 hours of culture of Müller cells on collagen- or fibronectin-coated plates, or on plates not coated with ECM (Figure 3B) ▶ , and a similar pattern of MMP-2 production was observed when two different Müller cell preparations were used in the study (Figure 3C) ▶ .
Figure 3.
mRNA expression and production of MMP-2 by retinal Muller cells in culture. A: Reverse transcriptase-PCR showing the expression of MMP-2 mRNA after 24 hours of culture in the presence of fibronectin (FN) and collagen type IV (CN), or in the absence of ECM (NM), and after 48 hours of culture in the presence of CN or FN. B: Gelatin zymography showing the gelatinolytic activity of MMP-2 (molecular weight, ∼72 kd) in control culture medium (CM) and in supernatants of Müller cells cultured on FN or CN matrices or in the absence of ECM for 24 to 72 hours C: Relative amount of MMP-2 gelatinolytic activity in culture supernatants calculated in relation to the levels of MMP-2 at 24 hours (100% increase over control culture medium at 0 hours). The histogram shows the production of this enzyme by two different individual preparations (5418 and 5427) cultured on CN or FN matrices.
Expression of MMP-9 by Retinal Müller Cells in Culture
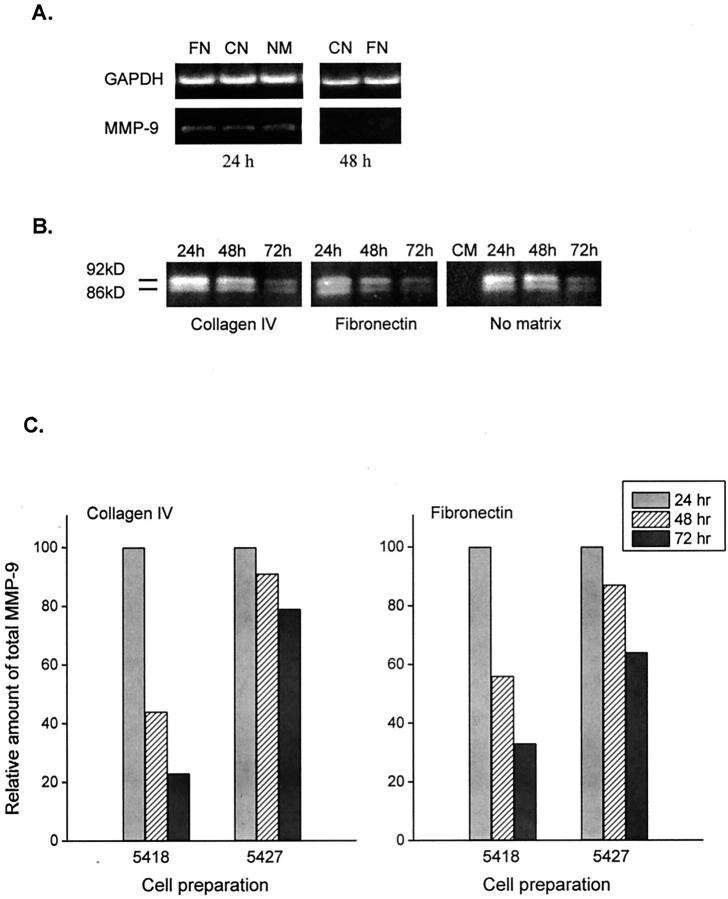
Figure 4A ▶ shows that mRNA coding for MMP-9 may be observed in retinal Müller cells after 24 hours of culture on plates coated with collagen type IV or fibronectin, or in the absence of ECM. After 48 hours, MMP-9 mRNA was not detected under the same culture conditions. Similarly, levels of MMP-9 enzymatic activity, corresponding to the latent (92 kd) and active (86 kd) forms of this gelatinase, were observed in 24-hour culture supernatants and progressively decreased after 48 and 72 hours of culture (Figure 4B) ▶ . This enzyme was not detected in culture medium (Figure 4B) ▶ . As observed with MMP-2, levels of MMP-9 gelatinolytic activity were not modified by culturing the cells on collagen type IV or fibronectin substrates, or in the absence of ECM. A similar pattern of MMP-9 production was also observed when different Müller cell preparations were used in the study (Figure 4C) ▶ .
Figure 4.
mRNA expression and production of MMP-9 by retinal Muller cells in culture. A: Reverse transcriptase-PCR showing the expression of MMP-9 mRNA after 24 hours of culture in the presence of fibronectin (FN) and collagen type IV (CN), or in the absence of ECM (NM), and after 48 hours of culture in the presence of CN or FN. B: Gelatin zymography showing the gelatinolytic activity of MMP-9 (approximate molecular weights of 92 kd and 86 kd, corresponding to the proactive and active forms) in control culture medium (CM) and in supernatants of Müller cells cultured for 24, 48, and 72 hours on CN or FN matrices. C: Percentage of increase in MMP-9 gelatinolytic activity in culture medium after 24, 48, and 72 hours of culture, when compared with control culture medium at time 0. The histogram shows the production of this enzyme by two individual cell preparations (5418 and 5427) cultured on CN or FN matrices.
Effect of ECM-Bound TNF-α on the Production of MMP-2 and MMP-9 by Retinal Müller Cells
Figure 5A ▶ shows that TNF-α in the absence of ECM proteins did not modify MMP-2 mRNA expression by Muller cells after 24 hours of culture. Similarly, TNF-α bound to collagen type IV or fibronectin did not modify MMP-2 mRNA expression by Müller cells after 24 and 48 hours of culture. In contrast, cells cultured for 24 hours on plates coated with TNF-α in the absence of ECM exhibited an increase in MMP-9 mRNA expression. Müller cells cultured in the presence of TNF-α bound to collagen type IV or fibronectin also showed an increase in MMP-9 mRNA expression after 24 and 48 hours of culture. As judged by gelatin zymography, levels of enzymatic activity of MMP-2 and MMP-9 correlated well with mRNA expression. In resemblance to the unchanged expression of MMP-2 mRNA, the enzymatic activity of this MMP was not modified after 24, 48, or 72 hours of culture of Müller cells in the presence of TNF-α adhered to culture plates, or collagen- and fibronectin-bound TNF-α (Figure 5B) ▶ . An increase in MMP-9 mRNA was accompanied by an increase in MMP-9 gelatinolytic activity when Müller cells were cultured for 24, 48, and 72 hours in the presence of TNF-α bound to collagen and fibronectin (Figure 5B) ▶ . Although there was a decrease in the gelatinolytic activity of MMP-9 in 48- and 72-hour culture supernatants when compared with 24 hours, a significant increase in MMP-9 activity was observed at all times when cells were cultured in the presence of collagen- or fibronectin-bound TNF-α. This effect was observed with different cell preparations (Figure 5C) ▶ .
Figure 5.
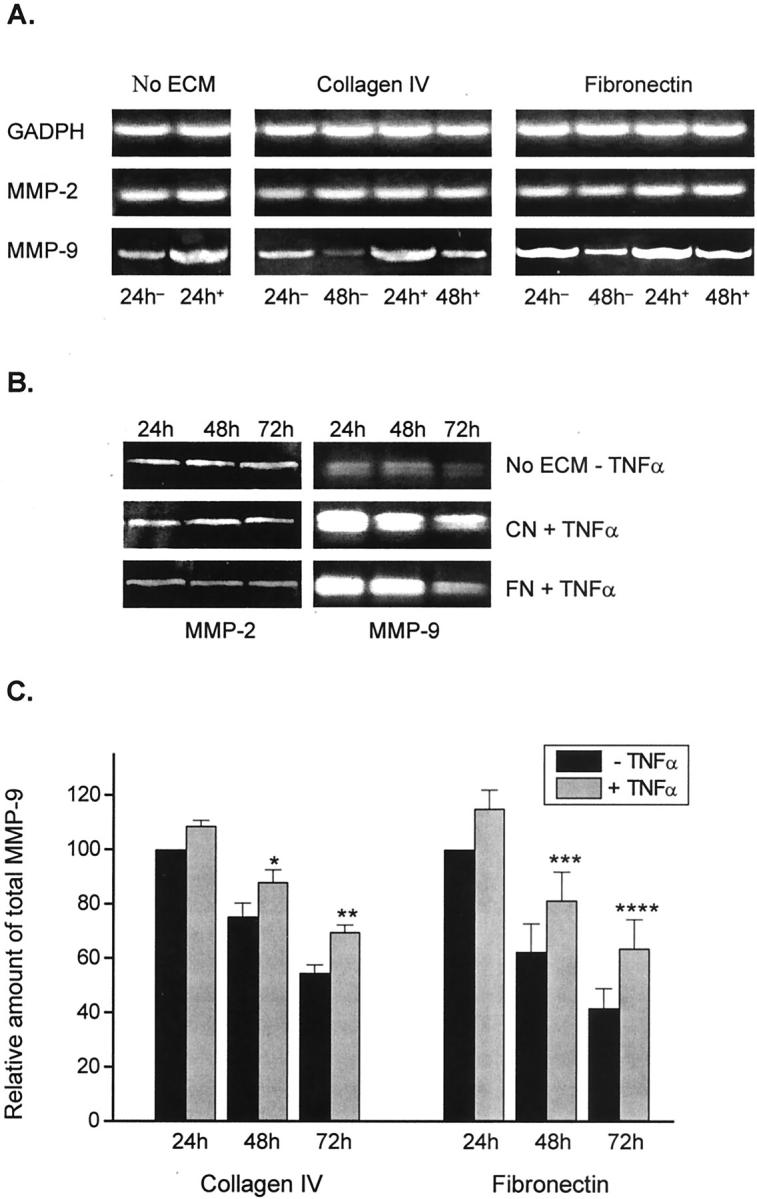
Effect of TNF-α on the mRNA expression and production of MMP-2 and MMP-9 by Müller cells in culture. A: Reverse transcriptase-PCR showing the expression of MMP-2 and MMP-9 mRNA after 24 hours of culture in the absence of ECM and in the presence (+) or absence (−) of plate-bound TNF-α, and 24 and 48 hours of culture on fibronectin (FN) or collagen type IV (CN) matrices associated (+) or nonassociated (−) with TNF-α. Results shown in the three columns are not comparable as they were derived from different experiments B: Gelatin zymography showing the gelatinolytic activity of MMP-2 and MMP-9 in supernatants of Muller cells cultured for 24, 48, and 72 hours on non-ECM-coated plates in the absence of TNF-α, and on CN or FN matrices associated (+) with TNF-α. C: Percentage of increase in MMP-9 gelatinolytic activity of supernatants from Müller cells cultured for 24, 48, and 72 hours on CN and FN matrices associated with TNF-α, when compared with control culture medium at time 0. Comparison with supernatants from cells cultured on CN and FN matrices but in the absence of TNF-α for the same periods of time. Each point represents the mean ± SEM of three separate experiments. *, P = 0.03 versus −TNF-α at 48 hours; **, P = 0.025 versus −TNF-α at 72 hours; ***, P = 0.0006 versus −TNF-α at 48 hours; ****, P = 0.001 versus −TNF-α at 72 hours.
Effect of Soluble TNF-α on the Production of MMP-9 by Müller Cells and Inhibition of ECM-Bound TNF-α Activity by Anti-TNF-α Antibodies
Addition to Müller cells cultured for 24 hours with increasing concentrations of soluble TNF-α within levels comparable to those bound to collagen and fibronectin in previous experiments (0 to 10 ng/ml), induced an increase in the production of MMP-9 by these cells in a dose-response manner (Figure 6A) ▶ . Addition of anti-TNF-α antibody 4H31-PG2 (10 μg/ml) at the time of plating Müller cells onto collagen type I- and fibronectin-bound TNF-α, resulted in inhibition of TNF-α activity (Figure 6B) ▶ , as judged by the lower levels of gelatinolytic activity of MMP-9 observed in culture supernatants, when compared with cultures to which antibody was not added.
Figure 6.
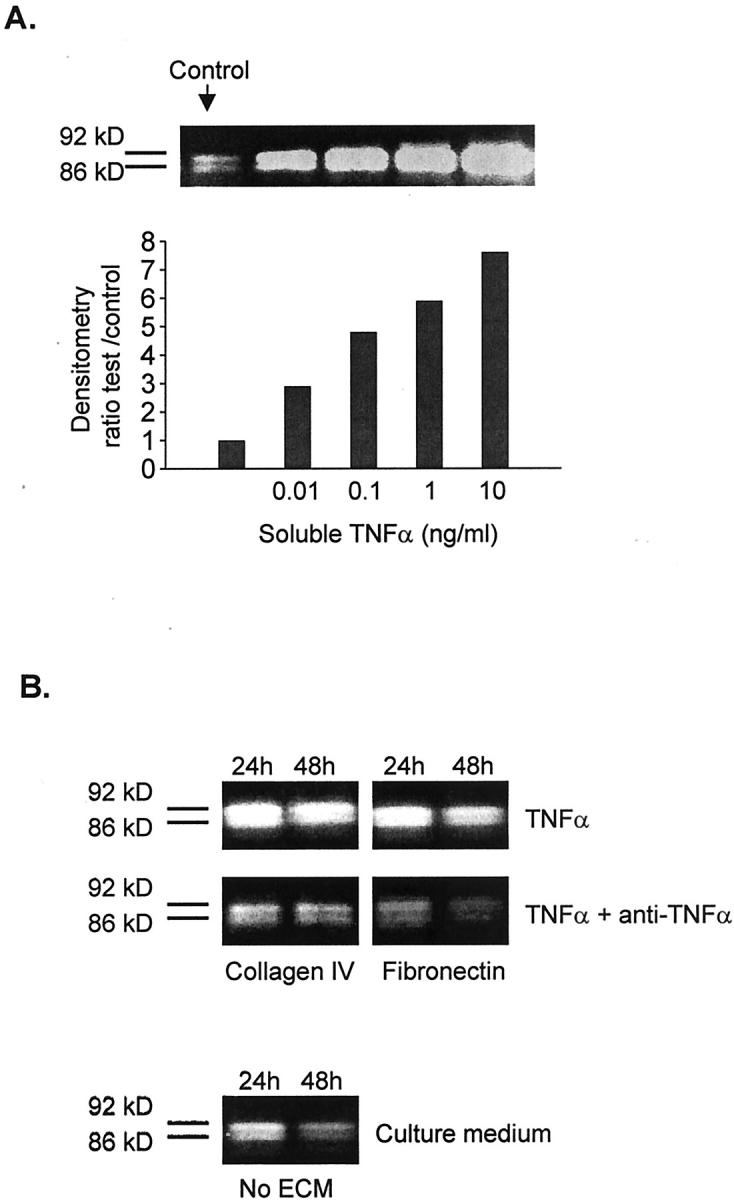
Effect of soluble TNF-α on MMP-9 production by Müller cells. Inhibition of ECM-bound TNF-α by anti-TNF-α antibodies. A: Gelatin zymography showing the MMP-9 gelatinolytic activity of Müller cell supernatants after 24 hours of culture with various concentrations of soluble TNF-α. B: Gelatin zymography of supernatants from Müller cells cultured on collagen type IV and fibronectin-bound TNF-α in the presence or absence of the anti-TNF-α antibody 4H31-PG2 (10 μg/ml). Comparison with supernatants from cells cultured on non-ECM-coated plates.
Discussion
The present study shows that MMP-2 and MMP-9, known as gelatinases A and B, respectively, are expressed by retinal Müller cells in culture. Significant levels of mRNA and gelatinolytic activity of MMP-2 were observed 24 hours after subculture and these remained unchanged after 48 and 72 hours. In contrast, basal levels of MMP-9 mRNA observed at 24 hours, were hardly detectable after 48 or 72 hours of culture. This correlated with the expression of MMP-9 gelatinolytic activity, which peaked at 24 hours, but gradually declined at 48 and 72 hours of culture. Interestingly, although mRNA and enzymatic activity of MMP-2 were not modified by soluble TNF-α or TNF-α bound to collagen type IV or fibronectin, mRNA and gelatinolytic activity of MMP-9 were up-regulated by this cytokine in both soluble and immobilized forms.
The main features that characterize the development of retinal proliferative disease are cell migration and proliferation, followed by local synthesis and deposition of ECM. 6,7 Because MMPs have been associated with these processes, 16-19 it may be possible that MMPs released by Müller cells may facilitate the migration of other cells onto the retina and into the vitreous cavity, including retinal pigment epithelial (RPE) cells, fibroblasts, and inflammatory leukocytes. This is supported by observations that MMP-2 is actively involved in keratinocyte 40 and glioma cell migration, 12,41 and that MMP-9 overexpression enhances smooth muscle cell migration 13 and accumulates at the advancing lamellipodia of migrating cells. 42 The finding that MMP-2 is constitutively expressed by Müller cells suggests that this molecule may play a physiological role in the maintenance of retinal integrity, whereas the observation that MMP-2 production is not modified by TNF-α indicates that the signaling pathway of activation of this molecule differs from that of MMP-9. Because MMP-2 is secreted in pro-active form and only activated by interaction with membrane-type MMPs (MT-MMPs) associated to the cell surface, 43,44 it is conceivable that complex Müller cell functions are required to selectively trigger this activation. It is also possible that soluble pro-MMP-2 released by Müller cells is converted to its active form by binding to MT-MMPs expressed by other cells of the retinal microenvironment, such as RPE cells, and this constitutes a subject of further investigation. That mRNA expression and enzymatic activity of MMP-9 decline with time in culture, suggests that this MMP may be induced in Müller cells by conformational membrane changes resulting from trypsinization and reattachment in vitro. These changes may reflect in vivo processes of cell activation and suggest that production of MMP-9 by Müller cells may constitute a first line of reactivity during pathological processes of retinal proliferation.
It has been suggested that retinal pigment epithelial cells are a main source of MMPs involved in the pathology of retinal proliferative disorders and macular degeneration, because of their ability to produce MMP-2 and tissue inhibitor of metalloproteinases 1 and 3. 20-22,45 In addition, optic nerve head astrocytes have been shown to produce MMP-2, MT1-MMP, TIMP-1, and TIMP-2, but not MMP-9, 46 for which it has been thought that these cells may aid in the remodeling of the ECM in glaucoma. Because bovine retinal endothelial cells have been shown to produce MMP-9 on interaction with glial cells and in response to transforming growth factor-β, 47 it is possible that this MMP is locally produced by vascular endothelium and Müller cells during retinal processes of inflammation, neovascularization, and proliferation.
Integrity of the ECM is critical for the maintenance of function of normal tissues, and changes in this integrity because of enzymatic degradation or association to growth factors and cytokines are known to modify cell functions. 48 It is therefore of interest that Müller cells cultured on collagen or fibronectin substrates did not exhibit differences in their ability to produce MMP-2 and MMP-9 when compared with cells cultured in the absence of ECM. This contrasts with previous observations that transient adherence of T lymphocytes to fibronectin, induces production of activated forms of MMP-2 and MMP-9, 49 and that fibronectin-mediated cell adhesion is required for induction of MMP-9. 50 The findings that MMP-9 but not MMP-2 expression was up-regulated by soluble and ECM-bound TNF-α, are in accordance with other reports that MMP-9 but not MMP-2 expression is increased by TNF-α in mucosal keratinocytes, 51 cervical smooth muscle cells, 52 and endometrial fibroblasts. 53 The importance of TNF-α in the modulation of MMP-9 production by Müller cells lies in that this cytokine predominates in the ECM of PVR 18 and proliferative diabetic retinopathy 19 epiretinal membranes, and that it is often found in vitreous from eyes with these retinal complications. 16,17 In addition, MMP-2, which is found in normal human vitreous, 54 is increased in vitreous from eyes with proliferative retinopathy, together with MMP-9, 16,17 and both MMP-2 and MMP-9 are often present in epiretinal membranes of PVR and proliferative diabetic retinopathy, 18,19 and in retinectomy specimens from eyes with anterior PVR 55 (Sethi et al, manuscript in preparation). However, from the present observations it is possible to suggest that Müller cells may constitute an important source of MMP-9 found in vitreous and retinal tissues during pathological processes, and that these cells may play a more important role in the control of matrix deposition during retinal proliferative disease than previously thought.
To our knowledge, this is the first demonstration that MMP-2 and MMP-9 are expressed by retinal Müller cells, and our findings that TNF-α bound to ECM proteins up-regulates expression of MMP-9 by Müller cells are supported by a recent report that fibronectin-bound TNF-α stimulates monocyte expression of MMP-9. 35 Because Müller cells constitute the main macroglial cells of the retina, these observations have important implications in the understanding of the cellular and molecular mechanisms underlying not only retinal proliferative disorders, but pathological processes of the brain characterized by glial cell activation and proliferation. In this context, it is of significance that TNF-α has been implicated in the pathology associated with neuroinflammatory diseases such as multiple sclerosis, 56 Alzheimer’s disease, 57 AIDS-dementia complex, 58 and cerebral ischemia, 59 and that MMPs are increasingly thought to play an important role in the pathogenesis of all of these conditions. 60 On this basis, a better understanding of the mechanisms that control MMP production resulting from glial cell interaction with matrix-bound cytokines, may pave the way for the design of therapeutic approaches to treat and prevent aberrant retinal and brain response to injury.
Acknowledgments
We thank Prof. Gill Murphy, University of East Anglia, for her invaluable advice and for reviewing the manuscript; and I. Blood, F. Sethna, and B. Ellis for their technical support with preliminary research leading to this study.
Footnotes
Address reprint requests to Dr. G. A. Limb, Division of Cell Biology, Institute of Ophthalmology, 11 Bath St., London, United Kingdom EC1V 9EL. E-mail: g.limb@ucl.ac.uk.
Supported by the Wellcome Trust (grant no. 062290/Z/00/Z/JHW); the Henry Smith Charity, the Guide Dogs for the Blind Association (GBDA), and the Royal National Institute for the Blind (RNIB), United Kingdom.
References
- 1.Newman E, Reichenbach A: The Müller cell: a functional element of the retina. Trends Neurosci 1996, 19:307-312 [DOI] [PubMed] [Google Scholar]
- 2.Okada M, Matsumura M, Ogino N, Honda Y: Müller cells in detached human retina express glial fibrillary acidic protein and vimentin. Graefe’s Arch Clin Exp Ophthalmol 1990, 228:467-474 [DOI] [PubMed] [Google Scholar]
- 3.Mizutani M, Gerhardinger C, Lorenzi M: Müller cell changes in human diabetic retinopathy. Diabetes 1998, 47:445-449 [DOI] [PubMed] [Google Scholar]
- 4.Madigan MC, Penfold PL, Provis JM, Balind TK, Billson FA: Intermediate filament expression in human retinal macroglia: histopathologic changes associated with age-related macular degeneration. Retina 1994, 14:65-74 [PubMed] [Google Scholar]
- 5.Birnbach CD, Jarvelainen M, Possin DE, Milam AH: Histopathology and immunohistochemistry of the neurosensory retina in fundus flavimaculatus. Ophthalmology 1994, 101:1211-1219 [DOI] [PubMed] [Google Scholar]
- 6.Hiscott P, Sheridan C, Magee TM, Grierson I: Matrix and the retinal pigment epithelium in proliferative retinal disease. Prog Retin Eye Res 1999, 18:167-190 [DOI] [PubMed] [Google Scholar]
- 7.Casaroli-Marano RP, Preissner KT, Vilaro S: Fibronectin, laminin, vitronectin and their receptors at newly-formed capillaries in proliferative diabetic retinopathy. Exp Eye Res 1995, 60:5-17 [DOI] [PubMed] [Google Scholar]
- 8.Lewis G, Mervin K, Valter K, Maslim J, Kappel PJ, Stone J, Fisher S: Limiting the proliferation and reactivity of retinal Müller cells during experimental retinal detachment: the value of oxygen supplementation. Am J Ophthalmol 1999, 128:165-172 [DOI] [PubMed] [Google Scholar]
- 9.Kimura H, Spee C, Sakamoto T, Hinton DR, Ogura Y, Tabata Y, Ikada Y, Ryan SJ: Cellular response in subretinal neovascularization induced by bFGF-impregnated microspheres. Invest Ophthalmol Vis Sci 1999, 40:524-528 [PubMed] [Google Scholar]
- 10.Seiki M: Membrane-type matrix metalloproteinases. APMIS 1999, 107:137-143 [DOI] [PubMed] [Google Scholar]
- 11.Henriet P, Blavier L, Declerck YA: Tissue inhibitors of metalloproteinases (TIMP) in invasion and proliferation. APMIS 1999, 107:111-119 [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa T, Kubota T, Kabuto M, Fujimoto N, Okada Y: Secretion of matrix metalloproteinase-2 (72 kD gelatinase/type IV collagenase= gelatinase A) by malignant glioma cell lines: implications for the growth and cellular invasion of the extracellular matrix. J Neurooncol 1996, 28:13-24 [DOI] [PubMed] [Google Scholar]
- 13.Mason DP, Kenagy RD, Hasenstab D, Bowen-Pope DF, Seifert RA, Coats S, Hawkins SM, Clowes AW: Matrix metalloproteinase-9 overexpression enhances smooth muscle cell migration and alters remodelling in the injured rat carotid artery. Circ Res 1999, 85:1179-1185 [DOI] [PubMed] [Google Scholar]
- 14.Turck J, Pollock AS, Lee LK, Marti H-P, Lovett DH: Matrix metalloproteinase 2 (gelatinase A) regulates glomerular mesangial cell proliferation and differentiation. J Biol Chem 1996, 271:15074-15083 [DOI] [PubMed] [Google Scholar]
- 15.Johnson S, Knox A: Autocrine production of matrix metalloproteinase-2 is required for human airway smooth muscle proliferation. Am J Physiol 1999, 277:L1109-L1117 [DOI] [PubMed] [Google Scholar]
- 16.Kon CH, Occleston NL, Charteris D, Daniels J, Aylward GW, Khaw PT: A prospective study of matrix metalloproteinases in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci 1998, 39:1524-1529 [PubMed] [Google Scholar]
- 17.De La Paz MA, Itoh Y, Toth CA, Nagase H: Matrix metalloproteinases and their inhibitors in human vitreous. Invest Ophthalmol Vis Sci 1998, 39:1256-1260 [PubMed] [Google Scholar]
- 18.Webster L, Chignell AH, Limb GA: Predominance of MMP-1 and MMP-2 in epiretinal membranes of proliferative vitreoretinopathy. Exp Eye Res 1999, 68:91-98 [DOI] [PubMed] [Google Scholar]
- 19.Salzmann J, Limb GA, Khaw PT, Gregor ZJ, Webster L, Chignell AH, Charteris DG: Matrix metalloproteinases and their natural inhibitors in fibrovascular membranes of proliferative diabetic retinopathy (PDR). Br J Ophthalmol 2000, 84:1091-1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Shabrawi Y, Eckhardt M, Berghold A, Faulborn J, Auboeck L, Mangge H, Ardjomand N: Synthesis pattern of matrix metalloproteinases (MMPs) and inhibitors (TIMPs) in human explant organ cultures after treatment with latanoprost and dexamethasone. Eye 2000, 14:375-383 [DOI] [PubMed] [Google Scholar]
- 21.Padgett LC, Lui G-M, Werb Z, Lavail MM: Matrix-metalloproteinase-2 and tissue inhibitors of metalloproteinase-1 in the retinal pigment epithelium and interphotoreceptor matrix: vectorial secretion and regulation. Exp Eye Res 1997, 64:927-938 [DOI] [PubMed] [Google Scholar]
- 22.Alexander JP, Bradley JMB, Gabourel JD, Acott TS: Expression of matrix metalloproteinases and inhibitor by human retinal pigment epithelium. Invest Ophthalmol Vis Sci 1990, 31:2520-2528 [PubMed] [Google Scholar]
- 23.Gottschall PE, Yu X: Cytokines regulate gelatinase A and B (matrix metalloproteinase 2 and 9) activity in cultured rat astrocytes. J Neurochem 1995, 64:1513-1520 [DOI] [PubMed] [Google Scholar]
- 24.Nakada M, Nakamura H, Ikeda E, Fujimoto N, Yamashita J, Sato H, Seiki M, Okada Y: Expression and tissue localization of membrane-type 1, 2 and 3 matrix metalloproteinases in human astrocytic tumors. Am J Pathol 1999, 154:417-428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Limb GA, Little BC, Meager A, Ogilvie JA, Wolstencroft RA, Franks WA, Chignell AH, Dumonde DC: Cytokines in proliferative vitreoretinopathy. Eye 1991, 5:686-693 [DOI] [PubMed] [Google Scholar]
- 26.Franks WA, Limb GA, Stanford MR, Ogilvie J, Wolstencroft RA, Chig-nell AH, Dumonde DC: Cytokines in human intra-ocular inflammation. Curr Eye Res 1992, 11:187-191 [DOI] [PubMed] [Google Scholar]
- 27.Limb GA, Alam E, Earley O, Chignell AH, Dumonde DC: Distribution of cytokine proteins within epiretinal membranes in proliferative vitreoretinopathy. Curr Eye Res 1994, 13:791-798 [DOI] [PubMed] [Google Scholar]
- 28.Limb GA, Chignell AH, Green W, LeRoy F, Dumonde DC: Distribution of TNFα and vascular adhesion molecules in fibrocellular membranes of proliferative diabetic retinopathy. Br J Ophthalmol 1996, 80:168-173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, McCluskey K, Fujii K, Wahl LM: Differential regulation of monocyte matrix metalloproteinase and TIMP-1 production by TNF-alpha, granulocyte-macrophage CSF and IL-1 beta through prostaglandin-dependent and independent mechanisms. J Immunol 1998, 161:3071-3076 [PubMed] [Google Scholar]
- 30.Migita K, Eguchi K, Kawabe Y, Ichinose Y, Tsukada T, Aoyagi T, Nakamura H, Nagataki S: TNF-alpha-mediated expression of membrane-type matrix metalloproteinase in rheumatoid synovial fibroblasts. Immunology 1996, 89:553-557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alon R, Cahalon L, Hershkoviz R, Elbaz D, Reizis B, Wallach D, Akiyama SK, Yamada KM, Lider O: TNF-α binds to the N-terminal domain of fibronectin and augments the β1-integrin-mediated adhesion of CD4+ T lymphocytes to the glycoprotein. J Immunol 1994, 152:1304-1313 [PubMed] [Google Scholar]
- 32.Hershkoviz R, Goldkorn I, Lider O: Tumour necrosis factor-alpha interacts with laminin and functions as a pro-adhesive cytokine. Immunology 1995, 85:125-130 [PMC free article] [PubMed] [Google Scholar]
- 33.Franitza S, Hershkoviz R, Kam N, Lichtenstein N, Vaday GG, Alon R, Lider O: TNF-alpha associated with extracellular matrix fibronectin provides a stop signal for chemotactically migrating T cells. J Immunol 2000, 165:2738-2747 [DOI] [PubMed] [Google Scholar]
- 34.Scheiffarth OF, Kampik A, Günther H, von der Mark K: Proteins of the extracellular matrix in vitreoretinal membranes. Graefe’s Arch Clin Exp Ophthalmol 1988, 226:357-361 [DOI] [PubMed] [Google Scholar]
- 35.Vaday GG, Hershkoviz R, Rahat MA, Lahat N, Cahalon L, Lider O: Fibronectin-bound TNF-alpha stimulates monocyte matrix metalloproteinase-9 expression and regulates chemotaxis. J Leukoc Biol 2000, 68:737-747 [PubMed] [Google Scholar]
- 36.Sarthy VP, Brodjian SJ, Dutt K, Kennedy BN, French RP, Crabb JW: Establishment and characterization of a retinal Muller cell line. Invest Ophthalmol Vis Sci 1998, 39:212-216 [PubMed] [Google Scholar]
- 37.Limb GA, Salt TE, Munro PMG, Moss SE, Khaw PT: Spontaneously immortalized human Müller cell line (MIO-M1) retains the characteristics of primary cells in vitro. Invest Ophthalmol Vis Sci 2002, 43:864-869 [PubMed] [Google Scholar]
- 38.Roque RS, Caldwell RB, Behzadian MA: Cultured Muller cells have high levels of epidermal growth factor receptors. Invest Ophthal Vis Sci 1992, 33:2587-2595 [PubMed] [Google Scholar]
- 39.Yokoi H, Natsuyama S, Iwai M, Noda Y, Mori T, Mori KJ, Fujita K, Nakayama H, Fujita J: Non-radioisotopic quantitative RT-PCR to detect changes in mRNA levels during early mouse embryo development. Biochem Biophys Res Commun 1993, 195:769-775 [DOI] [PubMed] [Google Scholar]
- 40.Makela M, Larjava H, Pirila E, Maisi P, Salo T, Sorsa T, Uitto V-J: Matrix metalloproteinase 2 (gelatinase A) is related to migration of keratinocytes. Exp Cell Res 1999, 251:67-78 [DOI] [PubMed] [Google Scholar]
- 41.Deryugina EI, Bourdon MA, Luo G-X, Reisgeld RA, Strongin A: Matrix metalloproteinase 2 activation modulates glioma cell migration. J Cell Sci 1997, 110:2473-2482 [DOI] [PubMed] [Google Scholar]
- 42.Legrand C, Gilles C, Zahm JM, Polette M, Buisson AC, Kaplan H, Birembaut P, Tournier JM: Airway epithelial cell migration dynamics. MMP-9 role in cell-extracellular matrix remodelling. J Cell Biol 1999, 146:517-529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D’Ortho MP, Will H, Atkinson S, Butler G, Messent A, Gavrilovic J, Smith B, Timpl R, Zardi L, Murphy G: Membrane-type matrix metalloproteinases 1 and 2 exhibit broad-spectrum proteolytic capacities comparable to many matrix metalloproteinases. Eur J Biol Chem 1997, 250:751-757 [DOI] [PubMed] [Google Scholar]
- 44.Murphy G, Stanton H, Cowell S, Butler G, Knauper V, Atkinson S, Gavrilovic J: Mechanisms for pro matrix metalloproteinase activation. APMIS 1999, 107:38-44 [DOI] [PubMed] [Google Scholar]
- 45.Della NG, Campochiaro PA, Zack DJ: Localization of TIMP-3 mRNA expression to the retinal pigment epithelium. Invest Ophthalmol Vis Sci 1996, 37:1921-1924 [PubMed] [Google Scholar]
- 46.Agapova OA, Ricard CS, Salvador-Silva M, Hernadez MR: Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human optic nerve head astrocytes. Glia 2001, 33:205-216 [DOI] [PubMed] [Google Scholar]
- 47.Behzadian MA, Wang X-L, Windsor LJ, Ghaly N, Caldwell RB: TGB-β increases retinal endothelial cell permeability by increasing MMP-9: possible role of glial cells in endothelial barrier function. Invest Ophthalmol Vis Sci 2001, 42:853-859 [PubMed] [Google Scholar]
- 48.Streuli C: Extracellular matrix remodelling and cellular differentiation. Curr Opin Cell Biol 1999, 11:634-640 [DOI] [PubMed] [Google Scholar]
- 49.Esparza J, Vilardell C, Clavo J, Juan M, Vives J, Urbano-Marquez A, Yague J, Cid MM: Fibronectin upregulates gelatinase B (MMP-9) and induces coordinated expression of gelatinase A (MMP-2) and its activator MT1-MMP (MMP-14) by human T lymphocyte cell lines. A process repressed through RAS/MAP kinase signalling pathways. Blood 1999, 94:2754-2766 [PubMed] [Google Scholar]
- 50.Xie B, Laouar A, Huberman E: Fibronectin-cell mediated cell adhesion is required for induction of 92-kDa type IV collagenase/gelatinase (MMP-9) gene expression during macrophage differentiation. J Biol Chem 1998, 273:11576-11582 [DOI] [PubMed] [Google Scholar]
- 51.Makela M, Salo T, Larjava H: MMP-9 from TNFα-stimulated keratinocytes binds to cell membranes and type I collagen: a cause for extended matrix degradation in inflammation? Biochem Biophys Res Commun 1998, 253:325-335 [DOI] [PubMed] [Google Scholar]
- 52.Watari M, Watari H, DiSanto ME, Chacko S, Shi PG, Strauss JF, III: Pro-inflammatory cytokines induce expression of matrix-metabolizing enzymes in human cervical smooth muscle cells. Am J Pathol 1999, 154:1755-1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singer CF, Marbaix E, Lemoine P, Courtoy PJ, Eeckhout Y: Local cytokines induce differential expression of matrix metalloproteinases but not their tissue inhibitors in human endometrial fibroblasts. Eur J Biochem 1999, 259:40-45 [DOI] [PubMed] [Google Scholar]
- 54.Brown D, Hamdi H, Bahri S, Kenney MC: Characterization of an endogenous metalloproteinase in human vitreous. Curr Eye Res 1994, 13:639-647 [DOI] [PubMed] [Google Scholar]
- 55.Sethi CJ, Limb GA, Charteris DG, Luthert PJ: Differential expression of matrix metalloproteinases in normal and chronically detached retina. Invest Ophthalmol Vis Sci 1999, 40:S950(Abstract) [Google Scholar]
- 56.Bitsch A, Kuhlmann T, Da-Costa C, Bunkowski S, Polak T, Bruck W: Tumour necrosis factor alpha mRNA expression in early multiple sclerosis lesions: correlations with demyelinating activity and oligodendrocyte pathology. Glia 2000, 29:366-375 [DOI] [PubMed] [Google Scholar]
- 57.Garlind A, Brauner A, Hojeberg B, Basun H, Schultzberg M: Soluble interleukin-1 receptor type II levels are elevated in cerebrospinal fluid in Alzheimer’s disease patients. Brain Res 1999, 826:112-116 [DOI] [PubMed] [Google Scholar]
- 58.Nuovo GJ, Alfieri ML: AIDS dementia is associated with massive, activated HIV-1 infection and concomitant expression of several cytokines. Mol Med 1996, 2:358-366 [PMC free article] [PubMed] [Google Scholar]
- 59.Botchkina GI, Meistrell ME, III, Botchkina IL, Tracey KJ: Expression of TNF and TNF receptors (p55 and p75) in the rat brain after focal cerebral ischemia. Mol Med 1997, 3:765-781 [PMC free article] [PubMed] [Google Scholar]
- 60.Yong VW, Krekoski CA, Forsyth PA, Bell R, Edwards DR: Matrix metalloproteinases and diseases of the CNS. Trends Neurosci 1998, 21:75-80 [DOI] [PubMed] [Google Scholar]