Abstract
We recently found that human small cell lung carcinomas (SCLCs) express, in addition to other neuroendocrine markers, vesicular monoamine transporters. Our present results indicate that SCLCs are histaminergic. We detected the biosynthetic enzyme histidine decarboxylase by immunohistochemistry in paraffin sections of 12 biopsies of SCLC tumors. This finding was supported by immunoblotting and reverse transcription-polymerase chain reaction experiments using established SCLC cell lines, frozen and paraffin-embedded SCLC tumors. Moreover, we found histamine to be synthesized, stored, and released by cultured SCLC cells. Our novel observations may be useful for developing new diagnostic tools for this frequent and highly malignant tumor.
Catecholamines, serotonin, and histamine, synthesized in the cytoplasm of aminergic cells, are taken up into intracellular storage vesicles by proton-driven vesicular monoamine transporters. 1 We recently detected both types of known vesicular monoamine transporters (VMAT1 and VMAT2) in human small cell carcinomas (SCLCs), whereas non-SCLC tumors such as large cell carcinomas, adenocarcinomas, and squamous cell carcinomas did not express VMATs. 2 In the present study we attempted to identify the nature of the amine stored by the high-grade malignant SCLCs. 3 The enzymes involved in the biosynthesis of biogenic amines which are synthesized from amino acid precursors are well known. Tyrosine hydroxylase (TH) is the key enzyme in the synthesis of catecholamines, tryptophan hydroxylase (TPH) in that of serotonin, and histidine decarboxylase (HDC) in that of histamine. Thus, we first determined whether the biosynthetic enzymes are present in SCLCs. Subsequently, we examined the amine suggested by the enzymatic makeup by established human SCLC cell lines. Our results show that histamine is a major secretory product of human SCLCs.
Materials and Methods
Immunohistochemistry and Immunoblotting
Twelve bronchoscopic biopsies of human SCLC tumors, a snap-frozen tumor, and two established SCLC cell lines (see below) shown previously to express SNAREs (SNAP receptors) and VMATs were investigated. Immunohistochemistry and immunoblotting were conducted as described. 2 The following primary antibodies were used for immunohistochemistry: polyclonal anti-HDC (1:10000, Euro-Diagnostica AB, Malmö, Sweden), monoclonal anti-TH (1:40, Loxo GmbH, Dossenheim, Germany), monoclonal anti-TPH (1:1000, Sigma-Aldrich, Deisenhofen, Germany) and monoclonal anti-tryptase (1:100; DAKO, Hamburg, Germany). For control purposes the first antibody was replaced by nonimmune mouse or rabbit serum in concentrations matching the immunoglobulin concentrations of the specific antibodies used. One such control is shown in Figure 1 ▶ G. Paraffin sections of human adrenals, duodenum, stomach, and testis served as positive controls. For immunoblotting, the anti-HDC antibody was diluted 1:8000.
Figure 1.
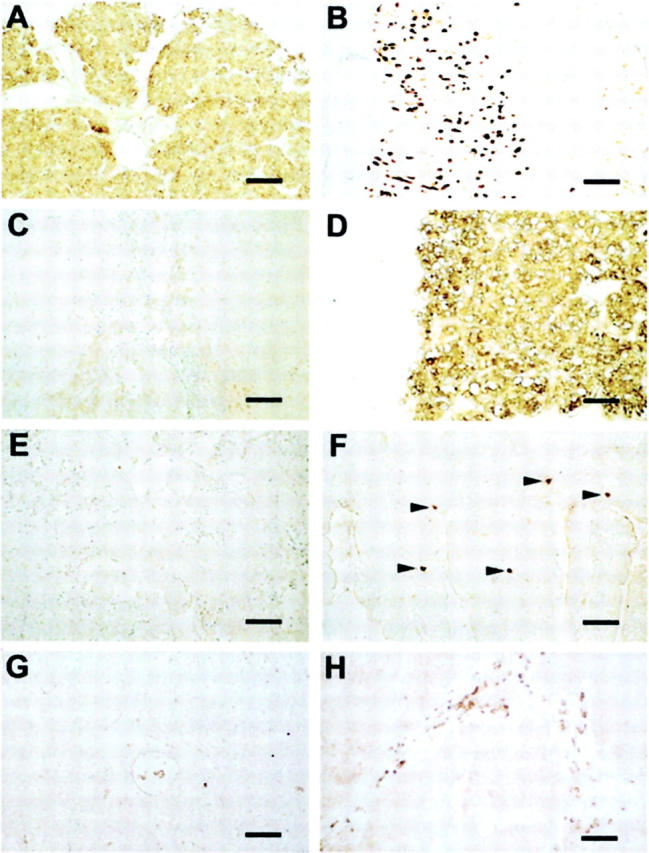
Presence of HDC and absence of TH and TPH in SCLC tumors. Immunohistochemistry of a SCLC tumor (case 11 in Table 1 ▶ ), stomach, adrenal, and duodenum is shown. HDC immunostaining was found in the tumor (A) and in the ECL cells of the human stomach mucosa (B). No staining was observed in the SCLC tumor using antibodies directed against TH (C) and TPH (E), but strong immunostaining is apparent in adrenal medullary chromaffin cells (D) and in enterochromaffin cells (arrowheads) of the duodenum mucosa (F). Nonimmune rabbit serum (1:10,000) is shown as a negative control (G). The location of mast cells is shown by immunostaining with an antibody directed against tryptase. Groups of mast cells present in the stroma of the tumor are shown between the clusters of unstained SCLC cells (H). The size of the bars (equivalent to 100 μm) is given on the right.
Reverse Trancription-Polymerase Chain Reaction Analysis
RNA from cultured SCLC cell lines (see below) was prepared using the RNeasy kit (Qiagen, Hilden, Germany). RNA (500 ng) from cultured cells was used for reverse transcription as described previously. 2 The following primers designed to span exons 4 and 5 of HDC (GenBank accession no. M60445) were used: for the first PCR 5′-GAA CGA ATC ATC ATG CCT and 3′-TTC CAC AGA GGA GTG AGC and the primers for nested PCR were 5′-CTA CTA CCC AGC CCT CAC C and 3′-AGG CAG GAC TCA TCA GCA. PCR conditions were as follows: 2 minutes of initial denaturation at 94°C and 34 cycles of 30 seconds, annealing at 56°C, with a 1-minute extension at 72°C. Reverse transcription-polymerase chain reaction (RT-PCR) analysis of paraffin-embedded SCLC tumors was performed as previously described. 4 In brief, deparaffinized 5-μm sections of SCLC tumors were scratched from the slides, and RNA was extracted using the Purescript kit (Biozym, Hessisch Oldenburg, Germany) followed by PCR amplification with the primers described above. PCR products were subcloned into the pGEMT vector (Promega, Mannheim, Germany) and sequenced using a fluorescence-based dideoxy sequencing reaction (ABI model 377 DNA sequencer; Perkin Elmer, Ueberlingen, Germany).
Histamine Production by SCLC Cell Lines
The human SCLC cell lines SCLC-24H 5 and NCI-H69 6 , for simplicity termed H24 and H69 in this contribution, were cultivated at a density of 1 × 106 cells/ml in 100-ml flasks (NUNC GmbH, Wiesbaden, Germany) in 4 ml of RPMI-1640 (Sigma-Aldrich, Deisenhofen, Germany) supplemented with 5% fetal calf serum (FCS) or without serum in F12-Dulbecco’s modified Eagle’s medium (DMEM) (Biochrom, Berlin, Germany) supplemented with 0.5 mg/ml bovine serum albumin, 15 mM HEPES, 50 μmol/L ethanolamin, 10 μg/ml insulin, 10 μg/ml transferrin, and 10 μg/ml selenit (Sigma) as described. 5;7 Additional L-histidine (100 μmol/L; 250 μmol/L; 500 μmol/L) was added to the medium to study the influence of the substrate concentration on histamine synthesis. Supernatants and cells were separated by centrifugation at 8000 rpm for 4 minutes. Cells were washed twice with NPE (150 mmol/L NaCl, 10 mmol/L PIPES, 1 mmol/L EDTA) (pH 7.4) and sonified. The samples were stored at −20°C in the dark until measurement of histamine using a commercial radioimmunoassay kit (Immunotech, Marseille, France) with a detection limit of 0.2 nmol/L. The within-assay variability was less than 1%, the between-assay variability less than 5%.
Results
Identification of HDC in Human SCLC
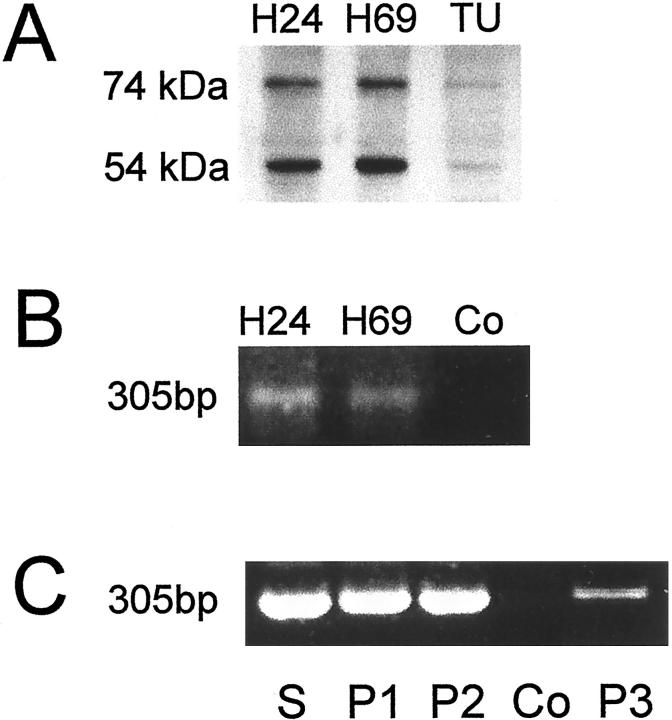
To examine the presence of the key enzymes of amine biosynthesis we performed immunohistochemistry on biopsies (n = 12) of SCLCs shown previously to exhibit specific immunoreactivity for VMAT1 and VMAT2 as well as SNAP-25 and syntaxin1. 2 While the presence of VMATs indicates the possibility of amine storage by the tumor cells, the expression of the SNAREs, SNAP-25 and syntaxin1, is in accordance with the known neuroendocrine nature of SCLCs. Immunostainings of paraffin sections with antibodies directed against HDC, TH, and TPH are shown in Figure 1 ▶ . In all SCLC tumors HDC was detected (Figure 1A ▶ and Table 1 ▶ ). As a positive control we show the presence of HDC in histaminergic enterochromaffin-like (ECL) cells (Figure 1B) ▶ of the human stomach. 8 In contrast, TH and TPH were absent in the tumor tissue (Figure 1, C and E) ▶ , although both enzymes were readily detected in tissues as positive controls, ie, in human adrenal medullary cells (Figure 1D) ▶ and enterochromaffin cells of the human duodenum (Figure 1F) ▶ . HDC is known to be present also in human mast cells which are, however, distinct from tumor cells. Thus, mast cell location within the tumor tissue is clearly shown by immunostaining with an antibody directed against the mast cell protein tryptase. 9 In Figure 1H ▶ groups of mast cells present in the stroma of an SCLC tumor are shown between the clusters of unstained SCLC cells. Our immunohistochemical data are supported by Western blot analysis of SCLC cell lines and a snap-frozen SCLC tumor. We detected two immunoreactive bands of 54-kd and 74-kd in SCLC cell lines H24 and H69 as well as in the SCLC tumor (Figure 2A) ▶ . Thus, both functional HDC types with different subcellular localization 10 are present in both samples. Thus HDC in the cell lines can be clearly assigned to SCLC cells, but it cannot be ruled out that mast cells present in the stoma (see above) may have contributed to HDC-immunoreactive bands in tumor biopsies.
Table 1.
Immunohistological Analysis of Paraffin Sections
| Section | No. | Age/Sex | HDC | TH | TPH |
|---|---|---|---|---|---|
| SCLC biopsies | |||||
| 1 | 56/m | + | − | − | |
| 2 | 75/f | + | − | − | |
| 3 | 79/f | + | − | − | |
| 4 | 95/m | + | − | − | |
| 5 | 67/m | + | − | − | |
| 6 | 51/m | + | − | − | |
| 7 | 64/m | + | − | − | |
| 8 | 49/f | + | − | − | |
| 9 | 43/m | ++ | − | − | |
| 10 | 70/m | + | − | − | |
| 11 | 51/m | ++ | − | − | |
| 12 | 55/f | + | − | − | |
| Adrenals | |||||
| 13 | 43/m | − | ++ | − | |
| 14 | 75/f | − | ++ | − | |
| Stomach | |||||
| 15 | ++ | − | − | ||
| Duodenum | |||||
| 16 | − | − | ++ |
++, strong immunostaining; +, moderate immunostaining; −, no staining.
HDC, histidine decarboxylase; TH, tyrosine hydroxylase; TPH, tryptophan hydroxylase.
Figure 2.
Identification of HDC in SCLC cell lines and SCLC tumors by immunoblotting and RT-PCR. A Western blot of protein extracts of the SCLC cell lines H24 and H69 and a snap-frozen tumor (TU, case 13 in Table 2 of reference 2 is shown in A). We observed two bands with the expected molecular weight indicating the presence of both functional forms of HDC. The molecular weight of the bands is indicated on the left. We performed nested RT-PCR with mRNA extracted from the SCLC cell lines H24 and H69 (B) and of paraffin sections of resected SCLC tumors (C) (P1 and P2, cases 2 and 12 in Table 2 of reference 2 ) and of a biopsy (P3, case 9 in Table 1 ▶ ). We obtained a band of the expected size indicating the presence of RNA coding for HDC. PCR reaction without input cDNA served as negative controls (Co) and stomach (S) as a positive control. The identity of the PCR products was confirmed by sequencing.
To extend the results obtained at the protein level we examined the presence of mRNA coding for HDC by RT-PCR in the SCLC cell lines (H24 and H69) (Figure 2B) ▶ . Using the primers outlined above, we obtained bands of 305-bp indicating the presence of HDC RNA in the tumor cells. The PCR products were identical with the published data (GenBank accession no. M60445) as determined by sequencing. Moreover, RT-PCR analysis and sequencing with RNA extracted from paraffin-embedded sections of SCLC tumors (Figure 2C) ▶ , indicated HDC gene expression in SCLC tumors as well as in the mucosa of the stomach which served as a control. As mentioned for the HDC Western blot, in tissue samples, a contribution by tissue mast cells cannot be ruled out.
We conclude from our data that SCLC tumors like the established SCLC cell lines H24 and H69 express HDC. This finding suggests biosynthesis of histamine which can be stored via vesicular monoamine transporters in the storage organelles within the tumor cells. 2
Biosynthesis, Storage, and Release of Histamine by SCLC Cell Lines
Direct evidence for the biosynthesis of histamine by SCLC cells was obtained by determination of the histamine content in the cells and in the cellular supernatant with time in culture. In RPMI-1640 medium containing 5% FCS (see Materials and Methods), histamine was continuously released from the cells into the supernatants (Figure 3A) ▶ while the amount of histamine within the cells remained roughly constant. However, increasing the levels of l-histidine in the medium, led to an enhanced storage and release of histamine by the cells (Figure 3B) ▶ . We also determined histamine storage and release in cells cultivated in the absence of serum. In this way, possible effects of factors present in FCS and of serotonin (about 300 nmol/L, released from thrombocytes during the production of the serum) on histamine production can be excluded. Also, in the absence of serum, storage and release of histamine was substantial and of a similar order of magnitude when compared with the values observed in the presence of serum (compare Figure 3A ▶ to Figure 3C ▶ ). Moreover, addition of 500 μmol/L l-histidine caused an increase of histamine in the cells and in the supernatants with and without serum present (compare Figure 3B ▶ to Figure 3C ▶ ). These observations indicate that synthesis, storage, or release of histamine by SCLC cells are not greatly affected by factors present in FCS and serotonin.
Figure 3.
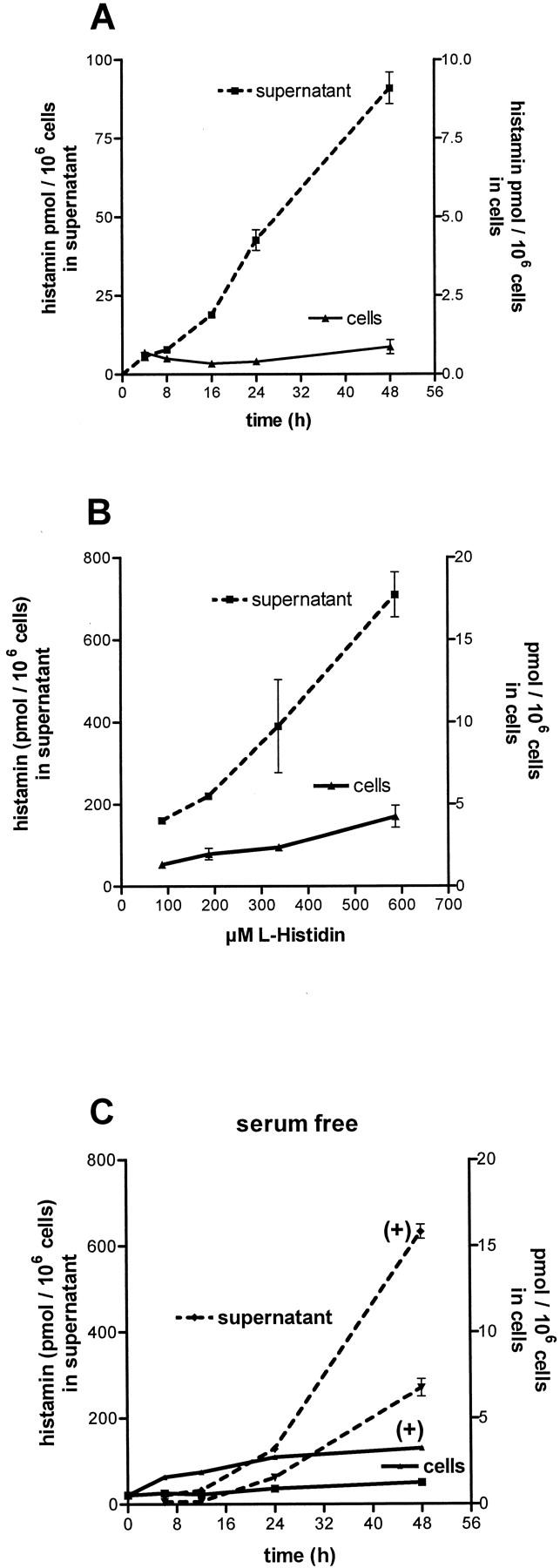
Histamine production by SCLC cell lines. A: Histamine release by SCLC cells (H69) with time in culture in RPMI-1640 containing 97 μmol/L histidine. B: Cell contents and histamine in supernatants after 48 hours in culture were enhanced by increasing the substrate concentration. C: Histamine in cells and supernatants was also determined in cells cultivated without serum. Experiments in F12-DMEM medium containing 194 μmol/L histidine and after further addition of 500 μmol/L histidine (+), are shown. Under both conditions histamine in cells and supernatants increased. Values are means of four experiments ± SD.
Discussion
Neuroepithelial bodies are airway oxygen sensors in the lung, which, like their vascular counterparts in the carotid body, release neurotransmitter during hypoxia. SCLC cells share with sensor cells of neuroepithelial bodies some neuroendocrine properties 11 and oxygen-sensitive potassium channels. 12 Neuroepithelial bodies are commonly identified by positive immunostaining for serotonin. 13 Serotonin was also believed to be a major secretory product of SCLC cells but it is unclear whether serotonin is taken up or synthesized by the tumor cells. Serotonin biosynthesis requires two enzymes, TPH and aromatic amino acid decarboxylase. While the decarboxylase is frequently observed in SCLC tumors, 11,14 the presence of TPH mRNA 15 but not of TPH protein and its enzymatic activity has been documented in SCLC. Production of histamine by SCLC cell lines as well as presence of HDC in SCLC cell lines and SCLC tumors shown in this study is a novel observation with important implications.
First, the properties of SCLC elucidated in this study may allow definition of the origin of this tumor. Other than mast cells, which are not neuroendocrine in nature, no other histamine producing cell types have yet been detected in the lung. At present it is unclear whether SCLCs are derived from a neuroendocrine precursor cell or from an undifferentiated epithelial cell. Studies on the expression of HDC and of neuoendocrine markers 2,7,16,17 may be useful in addressing this question.
Second, new markers may be suitable to distinguish SCLC tumors from other lung tumors and to identify primary tumors and metastases of SCLCs in patients. The SNAREs, syntaxin 1 and SNAP-25, and the vesicular monoamine transporters, VMAT1 and VMAT2, 2 add to other proteins such as synaptophysin, NCAM, chromogranins, and neuron-specific enolase 7,16,17 which are often variably or inconsistently expressed when investigated in paraffin sections of SCLC tumors. Thus, the World Health Organization defines the diagnosis of SCLC by light microscopic criteria 18 . However, newly established markers have been used in related neuroendocrine tumors such as ECL tumors which can be readily identified with a VMAT2 antibody. 19,20 It is interesting to note that very recently another highly malignant tumor type, human melanoma, has been observed to express HDC. 21
Substrates of cellular enzymes may serve as tools to examine tumors noninvasively by PET. For example, carcinoids take up 11C-labeled 5-hydroxytryptophane, which allows localization of these tumors in patients. 22 Our findings on the presence of HDC (this study) and VMATs 2 in human SCLC indicate that histidine/histamine-derived tracers may well be used in similar attempts to follow and characterize SCLC tumors and occult metastases in patients.
Acknowledgments
We thank Martina Haasemann for critical reading of the manuscript and Marlies Rauchfuβ, Barbara Zschiesche, and Andrea Thalhammer for excellent technical assistance. The human tissues and tumor samples used in this study were a kind gift from Drs. H. Höfler and M. Bauer, Institut für Allgemeine Pathologie und Pathologische Anatomie, Technische Universität München, München, Germany.
Footnotes
Address reprint requests to Prof. Dr. Manfred Gratzl, Anatomisches Institut, Universität München, Biedersteiner Str. 29, 80802 München, Germany. E-mail: gratzl@lrz.uni-muenchen.de.
Supported by Deutsche Forschungsgemeinschaft (Graduiertenkolleg 333: “Biology of Human Diseases”), Fonds der Chemischen Industrie, and by Wilhelm Sander-Stiftung.
C. Prinz is a Heisenberg fellow of the DFG (Pr 411/7–1).
References
- 1.Eiden LE: The vesicular neurotransmitter transporters: current perspectives and future prospects. FASEB J 2000, 14:2396-2400 [DOI] [PubMed] [Google Scholar]
- 2.Graff L, Castrop F, Bauer M, Höfler H, Gratzl M: Expression of vesicular monoamine transporters, synaptosomal-associated protein 25, and syntaxin 1: a signature of human small cell lung carcinoma. Cancer Res 2001, 61:2138-2144 [PubMed] [Google Scholar]
- 3.Wingo PA, Ries LA, Giovino GA, Miller DS, Rosenberg HM, Shopland DR, Thun MJ, Edwards BK: Annual report to the nation on the status of cancer, 1973–1996, with a special section on lung cancer and tobacco smoking. J Natl Cancer Inst 1999, 91:675-690 [DOI] [PubMed] [Google Scholar]
- 4.Mayerhofer A, Frungieri MB, Fritz S, Bulling A, Jessberger B, Vogt HJ: Evidence for catecholaminergic, neuron-like cells in the adult human testis: changes associated with testicular pathologies. J Androl 1999, 20:341-347 [PubMed] [Google Scholar]
- 5.Bepler G, Jaques G, Neumann K, Aumüller G, Gropp C, Havemann K: Establishment, growth properties, and morphological characteristics of permanent human small cell lung cancer cell lines. J Cancer Res Clin Oncol 1987, 113:31-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gazdar AF, Carney DN, Russell EK, Sims HL, Baylin SB, Bunn PA, Jr, Guccion JG, Minna JD: Establishment of continuous, clonable cultures of small-cell carcinoma of the lung which have amine precursor uptake and decarboxylation cell properties. Cancer Res 1980, 40:3502-3507 [PubMed] [Google Scholar]
- 7.Aletsee UM, Langley K, Rotsch M, Havemann K, Gratzl M: NCAM: a surface marker for human small cell lung cancer cells. FEBS Lett 1990, 267:295-300 [DOI] [PubMed] [Google Scholar]
- 8.Prinz C, Zanner R, Gerhard M, Mahr S, Neumayer N, Hohne-Zell B, Gratzl M: The mechanism of histamine secretion from gastric enterochromaffin-like (ECL) cells. Am J Physiol 1999, 277:C845-C855 [DOI] [PubMed] [Google Scholar]
- 9.Meineke V, Frungieri MB, Jessberger B, Vogt H, Mayerhofer A: Human testicular mast cells contain tryptase: increased mast cell number and altered distribution in the testes of infertile men. Fertil Steril 2000, 74:239-244 [DOI] [PubMed] [Google Scholar]
- 10.Yatsunami K, Tsuchikawa M, Kamada M, Hori K, Higuchi T: Comparative studies of human recombinant 74- and 54-kDa L-histidine decarboxylases. J Biol Chem 1995, 270:30813-30817 [DOI] [PubMed] [Google Scholar]
- 11.Gazdar AF, Helman LJ, Israel MA, Russell EK, Linnoila RI, Mulshine JL, Schuller HM, Park J-G: Expression of neuroendocrine cell markers L-dopa decarboxylase, chromogranin A, and dense core granules in human tumors of endocrine and nonendocrine origin. Cancer Res 1988, 48:4078-4082 [PubMed] [Google Scholar]
- 12.O’Kelly I, Stephens RH, Peers C, Kemp PJ: Potential identification of the O2-sensitive K+ current in a human neuroepithelial body-derived cell line. Am J Physiol 1999, 276:L96-L104 [DOI] [PubMed] [Google Scholar]
- 13.Lauweryns JM, van Ranst L, Verhofstad AAJ: Ultrastructure localization of serotonin in the intrapulmonary neuroepithelial bodies of neonatal rabbits by use of immuno-electron microscopy. Cell Tissue Res 1986, 243:455-459 [DOI] [PubMed] [Google Scholar]
- 14.Nakajima T, Shimosato Y, Morinaga S, Terasaki T, Tsumuraya M, Yamaguchi K, Ichinose H, Nagatsu T, Kato K, Nakazato Y: Immunohistochemical study of small cell lung carcinoma; with special reference to the neuroendocrine markers aromatic L-amino acid decarboxylase and gastrin-releasing peptide. Jpn J Clin Oncol 1986, 16:223-233 [PubMed] [Google Scholar]
- 15.Newman C, Wang D, Cutz E: Serotonin (5-hydroxytryptamine) expression in pulmonary neuroendocrine cells (NE) and a NE tumor cell line. Adv Exp Med Biol 1993, 337:73-78 [DOI] [PubMed] [Google Scholar]
- 16.Capella C, Heitz PU, Hofler H, Solcia E, Kloppel G: Revised classification of neuroendocrine tumours of the lung, pancreas, and gut. Virchows Arch 1995, 425:547-560 [DOI] [PubMed] [Google Scholar]
- 17.Kibbelaar RE, Moolenaar CECK, Michalides RJAM, Bitter-Suermann D, Addis BJ, Mooi WJ: Expression of the embryonal neural cell adhesion molecule N-CAM in lung carcinoma: diagnostic usefulness of monoclonal antibody 735 for the distinction between small cell lung cancer and non-small cell lung cancer. J Pathol 1989, 159:23-28 [DOI] [PubMed] [Google Scholar]
- 18.Travis WD, Colby TV, Corrin B, Shimosato Y, Brambilla E, : Collaborators from 14 countries: Histological typing of lung and pleural tumors. 1999. Springer Berlin
- 19.Eissele R, Anlauf M, Sch, Eiden LE, Arnold R, Weihe E: Expression of vesicular monoamine transporters in endocrine hyperplasia and endocrine tumors of the oxyntic stomach. Digestion 1999, 60:428-439 [DOI] [PubMed] [Google Scholar]
- 20.Rindi G, Paolotti D, Fiocca R, Wiedenmann B, Henry JP, Solcia E: Vesicular monoamine transporter 2 as a marker of gastric enterochromaffin-like cell tumors. Virchows Arch 2000, 436:217-223 [DOI] [PubMed] [Google Scholar]
- 21.Haak-Frendscho M, Darvas Z, Hegyesi H, Karpati S, Hoffman RL, Laszlo V, Bencsath M, Szalai C, Furesz J, Timar J, Bata-Csorgo Z, Szabad G, Pivarcsi A, Pallinger E, Kemeny L, Horvath A, Dobozy A, Falus A: Histidine decarboxylase expression in human melanoma. J Invest Dermatol 2000, 115:345-352 [DOI] [PubMed] [Google Scholar]
- 22.Orlefors H, Sundin A, Ahlstrom H, Bjurling P, Bergstrom M, Lilja A, Langstrom B, Oberg K, Eriksson B: Positron emission tomography with 5-hydroxytryprophan in neuroendocrine tumors. J Clin Oncol 1998, 16:2534-2541 [DOI] [PubMed] [Google Scholar]