Abstract
Extensive formation of nonfollicular sterile pustules on erythematous background combined with fever and peripheral blood leukocytosis are the characteristics of acute generalized exanthematous pustulosis. This uncommon eruption most often is an allergic reaction because of drugs such as aminopenicillins and sulfonamides inter alia. We recently demonstrated the important role of drug-specific T cells in the pathogenesis of this disease, showing that they produce high amounts of the neutrophil-attracting chemokine interleukin-8 and therefore stand out as a special subgroup of T cells, differing from the usual Th1 and Th2 subsets. In this study we use immunohistochemistry as well as cytotoxicity assays (4- and 18-hour assays) and fluorescence-activated cell-sorting analysis of drug-specific circulating T cells and of cells eluted from the skin of five patients with acute generalized exanthematous pustulosis, to analyze whether cytotoxic T-cell functions are important in the pathogenesis of this disease, in particular for the formation of vesicles. The data reveal that drug-specific CD4+ as well as CD8+ T cells both are activated and cytotoxic; perforin/granzyme B and to a variable degree the Fas/FasL-killing mechanism is involved in tissue destruction. These features allow the formation of vesicles. Additional secretion of interleukin-8 by T cells and keratinocytes attracts neutrophils that fill the vesicles and transform them into pustules.
Drug-induced hypersensitivity reactions can lead to many different clinical symptoms, which makes drug hypersensitivity reactions the great imitators of diseases. One peculiar form of drug hypersensitivity is acute generalized exanthematous pustulosis (AGEP), which is characterized by the rapid appearance of many pustules, which are sterile and located subcorneally in the epidermis; the patients have fever, leukocytosis, and sometimes also an eosinophilia. 1-3 This disease has some similarities to pustular psoriasis, but it has been shown that AGEP represents its own entity, clearly distinct from psoriasis. In >90% of the cases it is caused by drug intake, in particular aminopenicillins. 2 Lethality in AGEP is ∼1% for older patients. 3
The model character of drug hypersensitivity reactions for certain diseases makes them to an interesting topic of research. We recently analyzed patients with maculopapular drug eruptions (MPEs) and found that cytotoxic CD4+ T cells are involved, which are able to kill activated keratinocytes. 4 In AGEP, the formation of sterile pustules filled with polymorphonuclear neutrophils (PMNs) may be caused by drug-specific T cells, which are secreting high amounts of interleukin (IL)-8, now called CXCL8. 1,5 This chemokine can cause activation and recruitment of PMNs. However, it remained unclear how vesicles are formed and whether cytotoxic T cells are also involved in this form of drug allergy. Indeed, severe forms of AGEP are sometimes accompanied by skin detachment as well, making the disease somewhat similar to Stevens-Johnson syndrome or even toxic epidermal necrolysis. 2 The formation of bullae in the latter diseases has been associated with cytotoxic T lymphocytes, in particular CD8+ and natural killer T cells. 6,7
Here we analyze to what extent cytotoxic T cells can be detected in patients with AGEP, both in diseased skin lesions, in patch tests, and in the circulation. The data were obtained by performing activation analysis of drug-stimulated peripheral blood mononuclear cells (PBMCs) by fluorescence-activated cell sorting, cytotoxicity assays with drug-specific CD4+ and CD8+ T-cell clones, and immunohistochemical staining of cytospins, patch test lesions, and diseased skin lesions. As mouse models of the disease do not exist, patch tests are an important tool to analyze the pathogenesis of AGEP. They seem to mimic the original disease, reflecting early events of the reactions.
These data support the concept, that cytotoxic T cells, CD4+, and mainly CD8+, play an important role in AGEP as well. Different mechanisms of cytotoxicity, namely perforin/granzyme B- as well as FasL-mediated reactions, were found to be involved.
Materials and Methods
Patients
Patients AF, AP, JS, and EB have been described previously. 1 They were classified as having AGEP in accordance with the criteria of Roujeau 2 and with the AGEP EuroSCARE validation score. 8 An additional patient (CA, female, 64 years old) with the diagnosis of AGEP (fever >38.5°C and rapidly progressive generalized pustulosis) was included. Skin biopsy showed sterile pustulosis and abundant eosinophils. Her regular medication consisted of levodopa/benserazid, bromazepam, estrogen, calcium, and vitamin D; there was no history of intake of an additional drug. With oral retinoids and local antiseptic treatment the situation worsened with increasing fever, leukocytosis, and C-reactive protein as well as rapidly progressing detachment of the epidermis. She died in the intensive care unit a couple of days later.
Drugs and Reagents
The following drugs were used: amoxicillin (Sigma Chemical, St. Louis, MO), clavulanic acid (GlaxoSmithKline, Schönbühl, Switzerland), celecoxib (Pharmacia Corp., Stockholm, Sweden), and sulfamethoxazole (SMX; Hoffmann La-Roche, Basel, Switzerland). For patch tests drugs were prepared in phosphate-buffered saline (PBS): Augmentin (375 mg, half a pill/0.5 ml PBS), clamoxyl (375 mg/0.5 ml PBS), Celebrex (100 mg/0.5 ml PBS). For T-cell stimulation stock solutions of each drug were always freshly prepared in culture medium (CM) just before use. Celecoxib and SMX were dissolved in RPMI 1640 (Sigma Chemical) with 0.05 mol/L of NaOH.
Preparation and Staining of Skin Biopsy Specimens and Cytospins
Punch biopsy (5 mm) specimens were obtained from diseased skin lesions of patients AF and CA, fixed in 4% formalin, routinely processed, and paraffin-embedded. For standard histology they were stained with hematoxylin and eosin. In addition, epicutaneous testings (patch test) with corresponding drugs were performed on patients AP (amoxicillin), EB (celecoxib), and JS (amoxicillin) at least 2 months after recovery as described in detail previously. 1 The test reactions were read at 48 hours and scored as recommended by the International Contact Dermatitis Research Group. 9 No positive reactions to amoxicillin and celecoxib were found in 19 controls, confirming the specificity of the patch test (data not shown).
Immunohistochemistry was performed of skin biopsy specimens from the diseased skin lesions as well as from the positive epicutaneous test reactions. The biopsy specimens were snap-frozen in tissue-embedding medium using isopentane precooled in liquid nitrogen, and stored at −80°C. The following monoclonal antibodies (mAbs) were used in the study: anti-CD4, anti-CD8, 1 anti-granzyme B (clone HC4 at a concentration of 40 μg/ml; Hölzel Diagnostika GMBH, Köln, Germany), anti-perforin (clone δG9 at a concentration of 20 μg/ml, Hölzel Diagnostika GMBH), anti-Fas (clone APO-1-1 at a concentration of 1 μg/ml; Alexis Corp., San Diego, CA), anti-FasL (clone G 247-4 at a concentration of 70 μg/ml; PharMingen/Becton Dickinson, Mountain View, CA). Substitution of the primary antibody with isotype-matched IgG and omission of the primary antibody served as negative controls.
For immunostaining the avidin-biotin-complex/alkaline phosphatase method was used as described earlier. 1 Slides were fixed for 8 minutes with acetone (for CD4, CD8, and FasL) or 2% formaldehyde (Fas, granzyme B, and perforin) and incubated for 1 hour (for CD4, CD8) or 3 hours (other antibodies) with the primary antibody.
For preparation of cytospins drug-specific CD8+ T cells of patient AP were incubated with autologous B-lymphoblastoid cell line (B-LCL) as antigen-presenting cells (APCs) and 0.5 mg/ml of amoxicillin for 4 hours or 18 hours, respectively. Thereafter, cells were prepared in a cytospin centrifuge (cytocentrifuge II, Shandon, Ireland) and were stained either with anti-perforin mAb or with anti-FasL mAb, respectively. An additional negative control, autologous B-LCL without T cells was stained equally.
Culture Media
CM for PBMCs bulk cultures and CD4+ T-cell cultures consisted of RPMI 1640 supplemented with 10% pooled heat-inactivated human AB serum (Swiss Red Cross, Bern, Switzerland), 25 mmol/L HEPES buffer (no. L1613; Seromed, Switzerland), 2 mmol/L l-glutamine (no. 663.710; Biotest, Germany), 10 μg/ml streptomycin, and 100 U/ml penicillin (no. 4-01F00-H; Amimed, BioConcept, Allschwil, Switzerland). For propagation of CD4+ T-cell lines (TCLs) or clones (TCCs) CM was enriched with 40 U/ml of human recombinant IL-2.
CM for CD8+ T-cell cultures consisted of RPMI 1640 supplemented with 2 mmol/L l-glutamine, 50 U/ml penicillin, 50 μg/ml streptomycin, 5 mmol/L HEPES buffer, and 10% pooled heat-inactivated human AB serum. EBV-transformed B-LCLs were generated as described 10 and cultured in RPMI 1640 supplemented with 10% fetal calf serum, 25 mmol/L HEPES buffer, l-glutamine, and antibiotics.
Flow Cytometry Analysis
Aliquots containing 1 × 105 cells were stained with fluorochrome-conjugated mAb in 50 μl of buffer (PBS/1% fetal calf serum/0.02% NaN3) for 15 to 30 minutes at 4°C and subsequently fixed in buffer plus 0.5% paraformaldehyde. Analysis was done on a Coulter EPICS XL-MCL flow cytometer (Beckman Coulter, Hialeah, FL). Monoclonality of generated TCCs was shown by T-cell receptor Vβ-chain staining using fluorochrome-labeled anti-CD3 mAb (PharMingen/BD, Basel, Switzerland) and a panel of 22 mAbs recognizing different Vβ gene products (Coulter/Immunotech, Marseille, France) detecting ∼75% of all Vβ families as described. 11 Determination of activation markers on drug-induced PBMC bulk cultures and phenotypic characterization of TCLs and TCCs was done with fluorochrome-labeled anti-CD3, anti-CD4, anti-CD8, anti-CD25, and anti-HLA-DR mAb (all from PharMingen/BD). 11
Generation of Drug-Specific CD4+ and CD8+ T Cells
Generation of drug-specific CD4+ TCCs from peripheral blood and from positive epicutaneous test reactions of patients AP and JS was done as described previously. 1 CD8+ T cells were present in the observed lesions and additionally, we detected a drug-specific activation of CD8+ T cells in patients PBMCs. Because previous cloning procedures yielded a low frequency of CD8+ TCCs, we used a separate protocol to generate CD8+ T cells from patient AP. Briefly, freshly isolated PBMCs were depleted from CD4+ T cells using immunomagnetic-negative selection (Dynabeads, no. 111.08; Dynal SA, Oslo, Norway) and some 100 cells/well were seeded in 96-well plates in the presence of 5 × 105 irradiated (4500 rad) autologous PBMCs and 0.5 mg/ml of amoxicillin in CM for CD8+ cells. After the addition of 40 U/ml of IL-2 on day 3, drug-specific cultures were weekly selected for their cytotoxic activity using autologous B-LCL target cells at an effector to target (E:T) ratio of 20:1 in a standard 4-hour 51Cr-release assay as described below. Cultures with a specific lysis >20% were pooled and weekly restimulated as before. After several rounds, cultures with a drug-specific lysis >80% were further propagated using 106 cells/ml irradiated allogenic PBMCs, 105 cells/ml irradiated (6000 rad) autologous B-LCL, 40 U/ml IL-2, and amoxicillin.
Cytotoxicity Assays
Perforin/Granzyme-Mediated Killing
Cytotoxicity of the TCLs and TCCs was assessed in a standard 4-hour cytotoxicity assay in CM for B-LCL. Briefly, after 1 hour labeling of 1 × 106 autologous B-LCL as target cells with 50 μCi (51Cr-sodium chromate solution; Amersham Pharmacia Biotech, Cardiff, UK) at 37°C, duplicate cultures of 2.5 × 103 of targets were co-incubated in 96-well microtiter U-bottom plates with drug-specific T cells in presence or absence of soluble drug at mentioned E:T ratios at 37°C. After 4 hours, 100 μl of supernatant per well were collected and 51Cr-release was determined in a γ-counter (GAMMAmatic Basic; Kontron Analytical, Münchenstein, Switzerland).
Fas/FasL-Mediated Killing
To evaluate the cytotoxicity of FasL-mediated bystander killing by drug-specific T cells we used autologous B-LCL as APCs, 51Cr-labeled Fas-transfected L-1210 mouse lymphoblasts as target cells, and untransfected L-1210 cells as negative control. After labeling of mouse lymphoblasts for 90 minutes, some 2 × 103 target cells and nonlabeled B-LCL were co-incubated for 18 hours at 37°C in duplicate cultures with drug-specific cytotoxic T lymphocytes at mentioned E:T ratios in presence or absence of drug.
Specific lysis of target cells was proportional to the radioactivity released and was calculated as 100 × [(experimental release − spontaneous release)/(maximal release − spontaneous release)]. Maximum release was determined by lysis of targets with 2 mol/L of HCl, spontaneous release by lysis of targets in medium. Spontaneous release was always <20% of maximum release. Specific lysis was calculated as difference between lysis of targets with drug and targets without drug.
Results
Macroscopic Findings and Histology
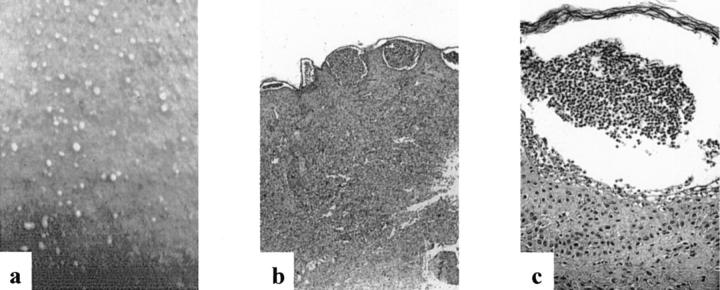
Figure 1a ▶ shows part of a skin lesion typically found in patients with AGEP: numerous pustules (<5 mm), mostly nonfollicular, arising on a widespread edematous and erythematous background. A representative histology of an AGEP skin lesion is shown in Figure 1, b and c ▶ (patient AF). The main histopathological findings were subcorneal pustules, a hyperplastic epidermis, papillary edema, and a subepidermal lymphohistiocytic perivascular infiltrate with some PMNs and eosinophils. Furthermore, hydropic degeneration of some keratinocytes, which most likely corresponds to cell death, was observed in the epidermis.
Figure 1.
a: Macroscopic finding of diseased skin. b and c: Representative histology of diseased skin of patient AF stained with H&E. Original magnifications: ×50 (b); ×250 (c).
CD25 and HLA-DR Expression after in Vitro Activation of PBMCs with Drugs
Previous in vitro studies revealed activation of both CD4+ and CD8+ T cells in penicillin- and SMX-sensitized patients. 6 Analysis of stimulated PBMCs of all AGEP patients with the corresponding drug in vitro revealed similar activation of PBMCs in all four patients (Table 1) ▶ : after 7 days of culture, CD4+ T cells showed a substantial elevation of activation markers. For CD4+ T cells the expression of CD25 increased to 8.3 to 42.2% and for HLA-DR to 4.9 to 16%. No, or only a marginal, increase of HLA-DR expression (AP, 7.1%; AF, 5.8%) and of CD25 expression (AP, 6%; AF, 0.9%; JS, 3.8%) was observed on CD8+ T cells. A further analysis after a cell culture period of 10 days confirmed these results (data not shown).
Table 1.
Drug-Specific Activation of Patients’ PBMCs—Analysis of Phenotype and Activation Marker as Percent on CD3-gated CD4+ and CD8+ T Cells (CD25 and HLA-DR) by Fluorescence-Activated Cell Analysis at Days 0 and 7 after Stimulation
| Patient | Phenotype | CD25 (%) | DR (%) | ||
|---|---|---|---|---|---|
| Day 0 | Day 7 | Day 0 | Day 7 | ||
| AP | CD4 | (6.3) | 42.2 | (3.0) | 11.8 |
| CD8 | (0.3) | 6.0 | (0.4) | 7.1 | |
| JS | CD4 | (2.7) | 21.0 | (2.6) | 16.0 |
| CD8 | (2.2) | 3.8 | (10.0) | 10.0 | |
| AF | CD4 | (4.7) | 23.8 | (2.8) | 4.9 |
| CD8 | (0.0) | 0.9 | (2.4) | 5.8 | |
| EB | CD4 | (5.7) | 8.3 | (4.5) | 5.2 |
| CD8 | (0.1) | 0.1 | (3.7) | 0.3 | |
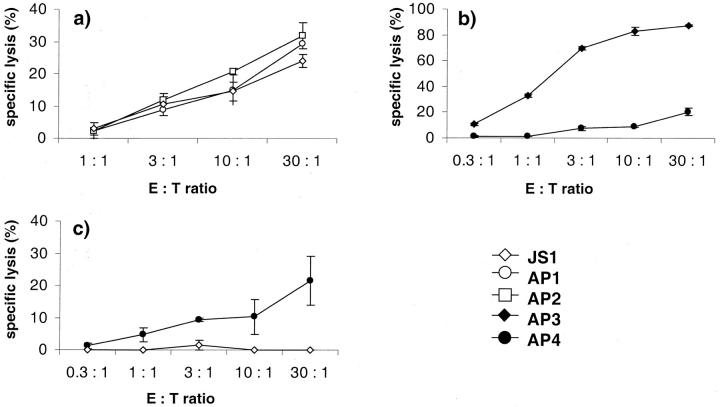
In Vitro Cytotoxicity of Drug-Specific T Cells
Incubation of autologous 51Cr-labeled B-LCL as target cells with CD4+ TCCs (patients AP and JS) as effector cells showed drug-specific cytotoxicity (Figure 2a) ▶ . Drug-specific cytotoxicity of CD8+ TCLs (patient AP) was shown in two different assays (Figure 2, b and c) ▶ . Using autologous 51Cr-labeled B-LCL as target cells incubated 4 hours with CD8+ TCLs we demonstrated a perforin/granzyme-mediated killing mechanism (Figure 2b) ▶ . To show Fas/FasL-mediated cytotoxicity, 51Cr-labeled Fas-transfected mouse lymphoblast L1210s as target cells were incubated for 18 hours with CD8+ TCLs (Figure 2c) ▶ . Specific stimulation of TCLs by drug-presenting B-LCL led to a moderate killing of Fas+ L1210, whereas Fas− L1210 were not killed (not shown).
Figure 2.
Perforin/granzyme-mediated (4-hour incubation with autologous B-LCL as target cells) and Fas/FasL-mediated cytotoxicity (18-hour incubation with Fas+ L-1210 mouse lymphoblasts as target cells) of drug-specific CD4+ TCCs (open symbols) or of drug-specific CD8+ TCLs (filled symbols) from patients AP and JS. Experiments were done in duplicate or triplicate cultures and SEM was calculated.
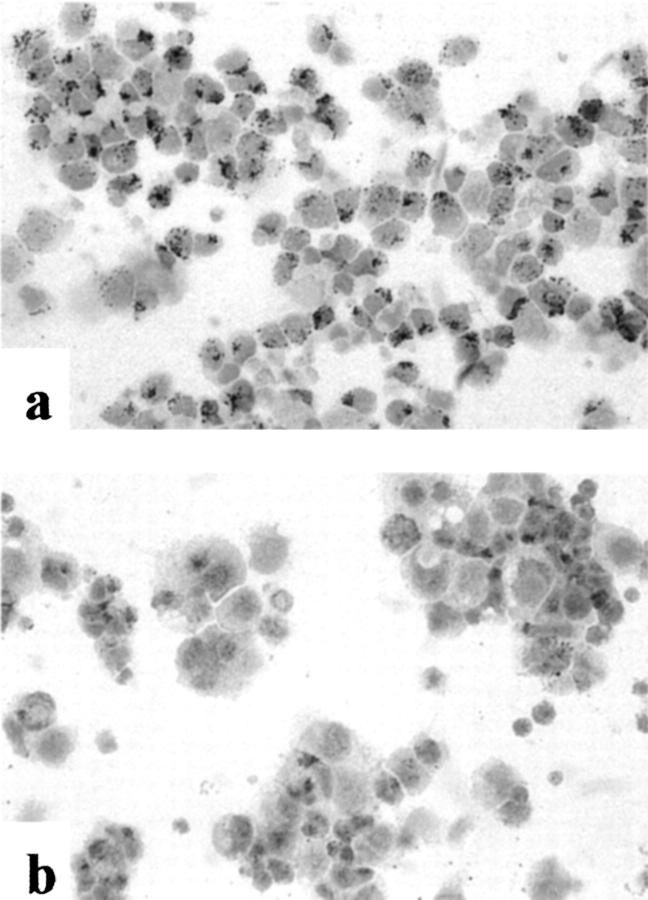
Staining of Drug-Specific CD8+ T Cells Prepared by Cytospin
Immunohistochemical staining of cytospin preparations confirmed a perforin or FasL positivity of the majority of the CD8+ T cells of patient AP, respectively (Figure 3, a and b) ▶ . There was no positive staining of the autologous B-LCL for either perforin or FasL (not shown).
Figure 3.
Cytospin preparations of drug-specific CD8+ TCLs of patient AP, immunohistochemical staining (ABC/AP method), with anti-perforin-mAb (a) and anti-FasL-mAb (b). Original magnifications, ×400.
Immunophenotyping of Patch Test Lesions
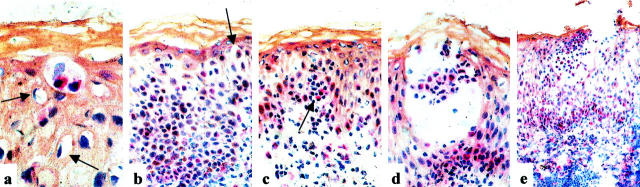
Patch tests are rather frequently positive in patients with AGEP 1,12 and even pustule formation can be observed after 96 hours. 13 Phenotypic and functional staining patterns of the inflammatory infiltrate in biopsies of patch test lesions at 48 hours (patients JS, AP, and EB) are shown in Figure 4 ▶ ; a to f. The infiltrate was still mainly mononuclear and located in the perivascular dermis, the epidermis and inside the subcorneal vesicles. Seventy to 80% of these cells were CD4+ and 20 to 30% were CD8+. About 10% of the infiltrating cells were perforin-positive and ∼20% of the cells were granzyme B-positive. Incubation with anti-Fas mAb showed positive staining of the epidermis (mainly of the basal layers) and of 70 to 80% of the mononuclear infiltrate. FasL-expression was observed in 5 to 10% of the infiltrating cells, mainly located in the vesicles or their surrounding, and to a lesser degree in the perivascular dermis. These FasL-positive cells were located in the immediate surrounding of the keratinocytes, which show hydropic cell degeneration (Figure 5a ▶ , patient JS) or which have lost the close cell-cell contact (Figure 5b ▶ , patient JS), and were part of infiltrating mononuclear cells forming conglomerates within the epidermis (Figure 5c ▶ , patient JS). In other locations of the epidermis, the FasL-positive cells were seen in subcorneal vesicles (Figure 5, d and e ▶ ; patients JS and EB).
Figure 4.
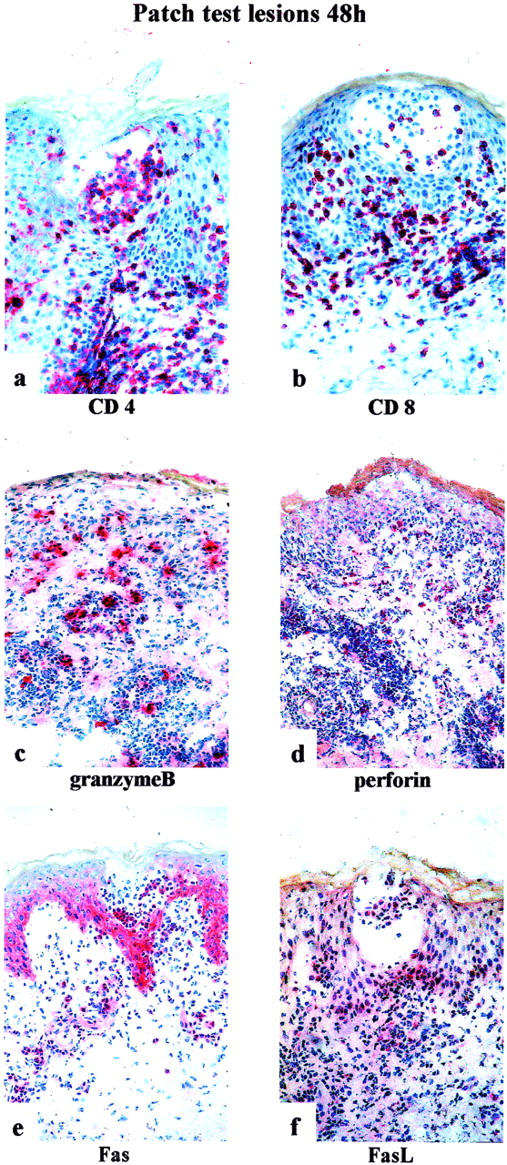
Immunohistochemistry of patch test lesions (48 hours) of patients JS, AP, and EB. For immunostaining (ABC/AP method), the following monoclonal antibodies were used: anti-CD4 (a), anti-CD8 (b), anti-granzyme B (c), anti-perforin (d), anti-Fas (e), and anti-FasL (f). Original magnifications, ×250.
Figure 5.
Immunohistochemical staining (ABC/AP method) of patch test lesions of patient JS (a–d) and patient EB (e) with anti-FasL-antibody. Original magnifications: ×1000 (a); ×400 (b–d); ×100 (e).
Immunophenotyping of Diseased Skin Lesions
Histology was obtained during the full blown disease, in which pustule formation had already occurred. Phenotypic and functional staining patterns of the inflammatory infiltrate in biopsies of diseased skin lesions (patients AF and CA) are shown in Figure 6; a to f ▶ . Of the infiltrating mononuclear cells, ∼90% were CD4+ and ∼10% were CD8+. In these diseased skin lesions CD4+ and CD8+ cells were observed in the perivascular dermis, but not in the epidermis or subcorneal pustules. Incubation with anti-perforin-mAb showed positive staining in <5% of the infiltrating cells and incubation with anti-granzyme B-mAb in ∼10% of the infiltrating cells. Fas-positivity was seen in the basal layers of the epidermis and in ∼50% of the infiltrating cells. Five to 20% of the mononuclear infiltrate in the dermis was FasL-positive, but we could not detect clearly FasL-expressing cells infiltrating the epidermis.
Figure 6.
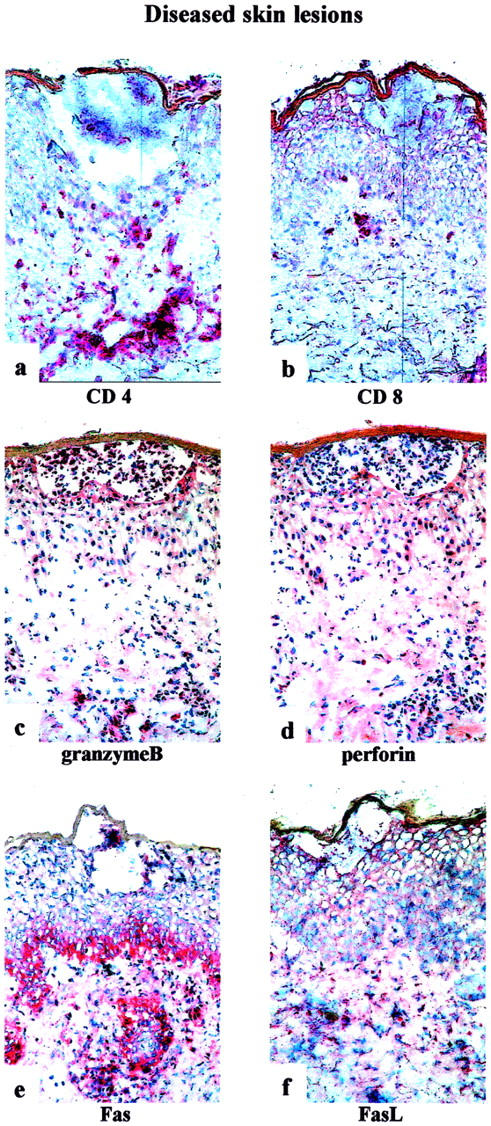
Immunohistochemistry of diseased skin lesions of patients AF and CA. For immunostaining (ABC/AP method) the following monoclonal antibodies were used: anti-CD4 (a), anti-CD8 (b), anti-granzyme B (c), anti-perforin (d), anti-Fas (e), and anti-FasL (f). Original magnifications, ×250.
Discussion
Drug hypersensitivity reactions are complex processes. In most reactions, drug-specific T cells are involved, either as regulators of the inflammatory process, or as effector cells with cytotoxic capacity. 14-16 The T cells thus fulfill distinct functions, and the process cannot be reduced to one cell function, ie, in MPEs, both cytotoxic and IL-5-secreting drug-specific CD4+ T cells can be found. They account for the major pathological changes, namely destruction of keratinocytes with hydropic degeneration and eosinophil infiltrations. 4,17
In previous studies, we and others have shown, that perforin-mediated killing may be involved in different forms of drug allergy such as maculopapular or bullous skin reactions. 18-20 Thereby, a high number of CD8+ cells in the epidermis may be associated with a more severe course, namely formation of bullae. 6 The presence of perforin in TCCs as well as in the skin lesions suggested a major role for this killing pathway. 17
Several studies in patients with AGEP have revealed a high rate of strongly positive patch tests to drugs compared to patients with other eruptions caused by allergic reactions to drugs. 1,12,13 Furthermore, these patch test lesions seem to mimic the original disease, because in our cases vesicles were formed after 48 hours of application, and another report on AGEP patients describes the formation of pustules after 96 hours of application. 13 Thus we believe that our biopsies taken 48 hours after patch testing might reflect early events of the reactions.
Comparing diseased and patch test lesions, we could see that in an earlier stage, which corresponds to patch test lesions, activated CD4+ and CD8+ T cells are infiltrating dermis and epidermis, subcorneal vesicles are formed and filled with (mainly CD4+) T cells. In a later stage (diseased skin lesions) CD4+ and CD8+ T cells are localized only in the dermis, while the vesicles are filled with neutrophils transforming them into pustules. Although in both stages most T cells are HLA-DR-positive, keratinocytes interestingly show no HLA-DR positivity. 1 There is a clearly higher number of perforin- and granzyme B-positive cells in early stages than in later stages. Activation and cytotoxicity of CD4+ and mainly CD8+ T cells was also shown by in vitro analysis. The strongest killing in the cytotoxicity assay was observed for perforin killing by CD8+ drug-specific T-cell clones. We assume that keratinocytes in AGEP may also work as APCs, because this was shown for keratinocytes in drug-induced MPEs. 4 However, in contrast to MPEs drug presentation by keratinocytes might be restricted to MHC class I molecules, activating cytotoxic CD8+ T cells, because the expression of MHC class II on keratinocytes in AGEP is low compared to immunohistochemistry of skin biopsies from patients with drug-induced MPEs. 18 This MHC class I presentation and CD8-mediated cytotoxicity might lead to vesicle formation.
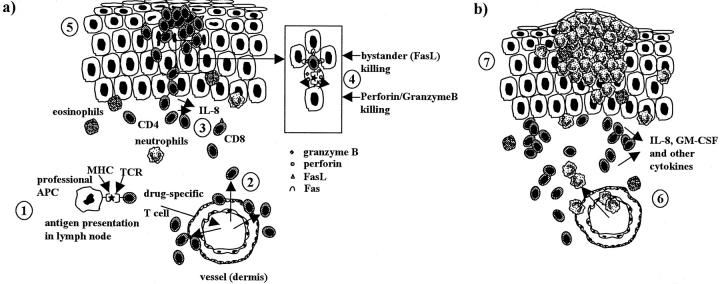
Because of the low MHC class II presentation on keratinocytes, infiltrating drug-specific CD4+ T cells might mainly be restimulated by resident APCs. They secrete high levels of IL-8, GM-CSF, and to a variable degree interferon-γ or IL-4/IL-5 and RANTES. 1 Taken together the in vitro data, the immunohistology of patch test, and of diseased skin lesions of AGEP, the following concept for pustule formation in AGEP can be outlined (Figure 7, a and b) ▶ :
Figure 7.
Concept for pustule formation in AGEP. a: After drug intake, professional APCs activate T cells by presentation of the drug in the lymph node (1); drug-specific T cells expand and migrate into the skin (2) where they are recruited to dermis and epidermis (3). They migrate to the epidermis, where keratinocytes are killed by CD4+ and mainly CD8+ cytotoxic drug-specific T cells. Perforin/granzyme B- and to a variable degree Fas/FasL-mediated mechanism is involved (4). This cell killing leads to tissue destruction and formation of subcorneal vesicles (5). Initially the vesicles are filled with T cells (mainly CD4+). b: The vesicles secrete IL-8, GM-CSF, and other cytokines and attract neutrophils and sometimes eosinophils through the dermis into the epidermis (6). The PMNs fill the vesicles and transform them to pustules (7).
The sensitization phase is not yet well understood: One assumes that after drug-intake, professional APCs activate T cells by presenting the drug in the lymph node; drug-specific T cells (CD4+ and CD8+) expand and migrate into the skin where they are recruited to dermis and epidermis. They migrate to the epidermis, where keratinocytes are killed mainly by CD8+ cytotoxic drug-specific T cells. A perforin/granzyme B- and to a variable degree Fas/FasL-mediated mechanism are involved. This cell killing leads to tissue destruction and formation of subcorneal vesicles. Initially these vesicles are filled with T cells (mainly CD4+) (Figure 7a) ▶ . They secrete IL-8, GM-CSF, and other cytokines and attract neutrophils and sometimes eosinophils through the dermis into the epidermis. The PMNs fill the vesicles and transform them into pustules (Figure 7b) ▶ .
This concept is still very crude and needs to be further refined, eg, the role of keratinocytes, which also secrete cytokines and chemokines (IL-8), needs to be investigated, and the puzzling differences to MPEs, in which CD4+ T-cell killing is so predominant, is not resolved. Nevertheless, AGEP as a drug-induced and T-cell-regulated disease might represent a model for T cell-PMN interaction, which is thought to be important in various other diseases such as pustular psoriasis, reactive arthritis, or Behçet’s disease, in which T cells orchestrate a PMN-rich inflammation. 21-25
Acknowledgments
We thank patients AP, JS, AF, and EB for helping to collaborate this study.
Footnotes
Address reprint requests to Werner J. Pichler, M.D., Clinic of Rheumatology and Clinical Immunology/Allergology, PKT2 D572, Inselspital, CH-3010 Bern, Switzerland. E-mail: werner.pichler@insel.ch.
Supported by the Swiss National Science Foundation (grant 31-61452.00 to W. J. P.).
References
- 1.Britschgi M, Steiner UC, Schmid S, Depta JP, Senti G, Bircher A, Burkhart C, Yawalkar N, Pichler WJ: T-cell involvement in drug-induced acute generalized exanthematous pustulosis. J Clin Invest 2001, 107:1433-1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roujeau JC: Neutrophilic drug eruptions. Clin Dermatol 2000, 18:331-337 [DOI] [PubMed] [Google Scholar]
- 3.Roujeau J, Bioulac-Sage P, Bourseau C: Acute generalized exanthematous pustulosis: analysis of 63 cases. Arch Dermatol 1991, 127:1333-1338 [PubMed] [Google Scholar]
- 4.Schnyder B, Frutig K, Mauri-Hellweg D, Limat A, Yawalkar N, Pichler WJ: T-cell-mediated cytotoxicity against keratinocytes in sulfamethoxazol-induced skin reaction. Clin Exp Allergy 1998, 28:1412-1417 [DOI] [PubMed] [Google Scholar]
- 5.Zlotnik A, Yoshie O: Chemokines: a new classification system and their role in immunity. Immunity 2000, 12:121-127 [DOI] [PubMed] [Google Scholar]
- 6.Hari Y, Frutig-Schnyder K, Hurni M, Yawalkar N, Zanni MP, Schnyder B, Kappeler A, Von Greyerz S, Braathen LR, Pichler WJ: T cell involvement in cutaneous drug eruptions. Clin Exp Allergy 2001, 31:1398-1408 [DOI] [PubMed] [Google Scholar]
- 7.Le Cleach L, Delaire S, Boumsell L, Bagot M, Bourgault-Villada I, Bensussan A, Roujeau JC: Blister fluid T lymphocytes during toxic epidermal necrolysis are functional cytotoxic cells which express human natural killer (NK) inhibitory receptors. Clin Exp Immunol 2000, 119:225-230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sidoroff A, Halevy S, Bavinck JN, Vaillant L, Roujeau JC: Acute generalized exanthematous pustulosis (AGEP)—a clinical reaction pattern. J Cutan Pathol 2001, 28:113-119 [DOI] [PubMed] [Google Scholar]
- 9.Wilkinson DS, Fregert S, Magnusson B, Bandmann HJ, Calnan CD, Cronin E, Hjorth N, Maibach HJ, Malalten KE, Meneghini CL, Pirila V: Terminology of contact dermatitis. Acta Derm Venereol 1970, 50:287-292 [PubMed] [Google Scholar]
- 10.Wyss-Coray T, Gallati H, Pracht I, Limat A, Mauri D, Frutig K, Pichler WJ: Antigen-presenting human T cells and antigen-presenting B cells induce a similar cytokine profile in specific T cell clones. Eur J Immunol 1993, 23:3350-3357 [DOI] [PubMed] [Google Scholar]
- 11.Zanni MP, Mauri-Hellweg D, Brander C, Wendland T, Schnyder B, Frei E, von Greyerz S, Bircher A, Pichler WJ: Characterization of lidocaine-specific T cells. J Immunol 1997, 158:1139-1148 [PubMed] [Google Scholar]
- 12.Wolkenstein P, Chosidow O, Fléchet ML, Robbiola O, Paul M, Dumé L, Revuz J, Roujeau JC: Patch-testing in severe cutaneous adverse drug reactions including Stevens-Johnson syndrome and toxic epidermal necrolysis. Contact Dermatitis 1996, 35:234-236 [DOI] [PubMed] [Google Scholar]
- 13.Jan V, Machet L, Gironet N, Martin L, Machet MC, Lorette G, Vaillant L: Acute generalized exanthematous pustulosis induced by dilitiazem: value of patch testing. Dermatology 1998, 197:274-275 [PubMed] [Google Scholar]
- 14.Pichler WJ, Yawalkar N, Britschgi M, Depta J, Strasser I, Schmid S, Kuechler P, Naisbitt D: Cellular and molecular pathophysiology of cutaneous drug reactions. Am J Clin Dermatol 2002, 3:229-238 [DOI] [PubMed] [Google Scholar]
- 15.Shiohara T, Kokaji T: Polysensitivity in fixed drug eruption. J Am Acad Dermatol 1997, 37:1017-1018 [DOI] [PubMed] [Google Scholar]
- 16.Shiohara T, Mizukawa Y, Teraki Y: Pathophysiology of fixed drug eruption: the role of skin-resident T cells. Curr Opin Allergy Clin Immunol 2002, 2:317-323 [DOI] [PubMed] [Google Scholar]
- 17.Yawalkar N, Pichler WJ: Pathogenesis of drug-induced exanthema. Int Arch Allergy Immunol 2001, 124:336-338 [DOI] [PubMed] [Google Scholar]
- 18.Yawalkar N, Egli F, Hari Y, Nievergelt H, Braathen LR, Pichler WJ: Infiltration of cytotoxic T cells in drug-induced cutaneous eruptions. Clin Exp Allergy 2000, 30:847-855 [DOI] [PubMed] [Google Scholar]
- 19.Yasukawa M, Ohminami H, Arai J, Kasahara Y, Ishida Y, Fujita S: Granule exocytosis, and not the Fas/Fas ligand system, is the main pathway of cytotoxicity mediated by alloantigen-specific CD4+ as well as CD8+ cytotoxic T lymphocytes in humans. Blood 2000, 95:2352-2355 [PubMed] [Google Scholar]
- 20.Posadas SJ, Padial A, Torres MJ, Mayorga C, Leyva L, Sanchez E, Alvarez J, Romano A, Juarez C, Blanca M: Delayed reactions to drugs show levels of perforin, granzyme B, and Fas-L to be related to disease severity. J Allergy Clin Immunol 2002, 109:155-161 [DOI] [PubMed] [Google Scholar]
- 21.Robert C, Kupper TS: Inflammatory skin diseases, T cells, and immune surveillance. N Engl J Med 1999, 341:1817-1828 [DOI] [PubMed] [Google Scholar]
- 22.Mochizuki M, Morita E, Yamamoto S, Yamana S: Characteristics of T cell lines established from skin lesions of Behcet’s disease. J Dermatol Sci 1997, 15:9-13 [DOI] [PubMed] [Google Scholar]
- 23.Terui T, Ozawa M, Tagami H: Role of neutrophils in induction of acute inflammation in T-cell-mediated immune dermatosis, psoriasis: a neutrophil-associated inflammation-boosting loop. Exp Dermatol 2000, 9:1-10 [DOI] [PubMed] [Google Scholar]
- 24.Mantas C, Direskeneli H, Oz D, Yavuz S, Akoglu T: IL-8 producing cells in patients with Behcet’s disease. Clin Exp Rheumatol 2000, 18:249-251 [PubMed] [Google Scholar]
- 25.Direskeneli H: Behcet’s disease: infectious aetiology, new autoantigens, and HLA-B51. Ann Rheum Dis 2001, 60:996-1002 [DOI] [PMC free article] [PubMed] [Google Scholar]