Abstract
Inflammatory bowel disease is associated with immune activation in Peyer’s patches and mucosal lymph nodes. The role of these organs in dextran sodium sulfate (DSS)-induced colitis was investigated. We used mice lacking Peyer’s patches and/or lymph nodes because of lymphotoxin-α gene deficiency or treatment in utero with lymphotoxin-β-receptor IgG and tumor necrosis factor-receptor-I (55)-IgG fusion proteins. Mice lacking Peyer’s patches and lymph nodes because of lymphotoxin-α deficiency or in utero fusion protein treatment developed more severe colitis than control mice as indicated by more severe intestinal shrinking, longer colonic ulcers, and higher histological disease scores. Oral DSS triggered the formation of colonic submucosal lymphoid patches in these mice and caused an increase in the number of submucosal lymphoid patches in mice treated in utero with the fusion proteins. Mice lacking Peyer’s patches only showed more submucosal lymphoid patches whereas intestinal length and histological disease score were similar to control mice. In conclusion, more severe DSS-induced colitis correlates with the loss of the mesenteric lymph nodes. However, neither the absence of Peyer’s patches nor the presence of colonic lymphoid patches were correlated with increased disease severity.
The physiological intestinal immune response toward intraluminal antigens include IgA secretion and the induction of systemic immune hyporesponsiveness oral tolerance. 1 Inflammatory bowel disease is associated with a breakdown of tolerance toward the resident intestinal flora 2,3 and immune activation in the gut-associated lymphatic tissue (GALT). The GALT consists of Peyer’s patches (PPs) and mesenteric lymph nodes (MLNs) as organized intestinal lymphoid follicles. PPs are lymphoid follicles in the intestinal wall and contain M cells that can uptake particulate intraluminal antigens. 4 Although the role of PPs and MLNs in the induction of intestinal immune responses 5-7 and of oral immune tolerance has recently been investigated, 8-10 little is known about their role in the induction of inflammatory bowel disease.
Lymphotoxin-α (LTα) and LTβ are members of the tumor necrosis factor (TNF) cytokine family. LTαβ is critical for the induction of secondary lymphoid organs and the development of the spleen. 11-14 LTα−/− mice do not develop PPs or LNs and have a disrupted splenic architecture. LTβ is required for the development of PPs but not of MLNs as LTβ (LTβ−/−) gene-deficient mice lack PPs but can develop at least some MLNs. 14,15 Gestational treatment of mice with lymphotoxin-β-receptor-IgG-fusion protein (LTβRIgG) or LTβRIgG and TNF-receptor-I(55)-IgG-fusion protein (TNFRIgG) inhibits the formation of PPs or of PPs and MLNs depending on the treatment regimen. 15,16
Little is known about the role of GALT organs in the induction and course of experimental colitis. We therefore used mice made deficient of either PPs or PPs and MLNs by fusion protein treatment (PP-null/LN+; PP/LN-null) or LTα deficiency (LTα−/−) to study the differential role of PPs and MLNs in the induction of colitis.
Materials and Methods
Mice
129xB6 wild-type (wt) and lymphotoxin-α gene-deficient (LTα−/−) mice were purchased from the Jackson Laboratories (Bar Harbor, ME). All mice were kept under sterile conditions in microisolator cages in the animal facilities of the Münster University Department of Dermatology with unlimited access to food and water according to federal animal protection regulations (permit G5/99 and G78/2000).
Abrogation of PPs or PP and MLN Development
Female 129xB6 mice were daily checked for vaginal plugs. To abrogate development of PPs alone, mice were intravenously injected with 200 μg of lymphotoxin-β-receptor IgG fusion protein (LTβRIgG; Biogen, Cambridge, MA) on days 16 and 18 after conception. Suppression of PP and MLN development was induced by intravenous injection of 100 μg of LTβRIgG and 100 μg of TNF-receptor-I(55kD)-IgG-fusion protein (TNFRIgG, Biogen) in pregnant mice on gestational days 11, 13, 15, and 17. Both regimens included treatment of the progeny with an intraperitoneal injection of 20 μg of LTβRIgG within 24 hours after birth. 16 As treatment with human IgG did not induce changes in lymphoid organ development 16,17 we used age- and sex-matched 129xB6 mice as controls to progeny of LTβRIgG-treated animals. Mice were individually checked for the absence of the respective lymphatic organs by gross examination (LNs) or soaking the intestines in 10% (v/v) acetic acid solution. Only mice devoid of PPs or PPs and LNs, respectively, were included in the analysis. Progeny of treated mice were 7 to 10 weeks of age at the time of the experiment.
Induction of Colitis
Colitis was induced in all groups by the addition of dextran sodium sulfate (DSS) [molecular weight, 40,000; 4% (w/v); ICN, Biochemicals, Eschwege, Germany] to the drinking water. The mean DSS/water consumption was recorded. Mice were treated for 7 days with DSS or normal drinking water (NDW). Body weight was assessed before and after 7 days of oral DSS.
MPO Assay
This assay was performed as previously described. 18
Assessment of Colitis
All mice were euthanized on day 7 after colitis induction. The entire colon was removed and the length was recorded.
Histology
To assess the distribution of inflammatory changes in the colon the organ was cut into three sections of one-third of the total colon length and attached to a cork board before fixation. Tissues were subsequently fixed in 2% (v/v) paraformaldehyde solution and embedded in paraffin and subsequently sectioned. Hematoxylin and eosin (H&E) staining was used for general assessment of intestinal inflammation. Gomori’s silver staining of reticular fibers was performed as described. 19
Immunohistochemistry
Tissues were snap-frozen in OCT compound (Miles, Elkhart, IL). Frozen serial sections were prepared and incubated as described previously. 17 Shortly, sections were fixed with acetone, washed, and preblocked with 5 μg/ml of anti-CD16/CD32 Fc block (Pharmingen, B&D, Heidelberg, Germany) in Tris-buffered saline with 0.25% bovine serum albumin, 0.05% Tween 20, and 10% heat-aggregated rabbit serum. Cells were stained with 5 μg/ml of biotinylated anti-B220 monoclonal antibody RA3–6B2 (Pharmingen) in the same buffer, followed by 10 μg/ml of fluorescein conjugated-neutralite (fluorescein isothiocyanate-avidin; Southern Biotechnology Associates, Birmingham, AL). Staining of CD4 and CD8 expression was performed in a similar manner using PE-labeled monoclonal antibody L3T4 (CD4) (Pharmingen) and biotin labeled antibody Ly-2 (CD8, clone 53-6.7). Staining of sections for MAdCAM-1 (Pharmingen) expression used monoclonal antibody MECA 367 (Pharmingen) followed by fluorescein isothiocyanate-conjugated goat anti-rat IgG (Pharmingen). Sections were visualized by a fluorescence microscope and photographed (Leica Microscan, Wetzlar, Germany) and photos were subsequently scanned for digital image processing.
Histological Scoring of Disease Severity
Tissue sections were examined by a gastrointestinal pathologist (HH) in a blinded manner. Each section was scored for severity and extent of ulceration, and the tissue thickness from the muscularis propria to the luminal border was determined. Lesion severity was graded using a modification of a previously defined scoring system 20 with a scale of 0 to 4: 0, normal; 1, minimal; 2, mild; 3, moderate; 4, severe. Minimal lesions contained small, focal, or widely dispersed areas of inflammation and/or fibrosis above the muscularis mucosae. Mild lesions were multifocal or locally extensive and contained inflammation or fibrosis extending into the submucosa. Moderate lesions consisted of multifocal lesions with ulcers consuming <10% of the assessed mucosal surface. Severe was defined similar to moderate lesions and/or ulcers consuming >10% of the assessed mucosal surface.
Percent length of colon ulcers was assessed as follows: the length of individual ulcers on longitudinal colon sections was measured, and the sum of all individual ulcers was multiplied × 100 and divided by the length of the colonic surface on the respective slide of the histological section.
Statistical Analysis
Differences between body weights, bowel lengths, and number of intestinal ulcers were compared using Mann-Whitney U statistics supported by InStat software for Macintosh computers.
Results
Colitis in LTα−/− Mice and in Mice Deficient of PPs and MLNs after Gestational Fusion Protein Treatment
We used LTα−/− mice, which are congenitally devoid of secondary lymphoid tissues, to study the role of PPs and MLNs in the course of DSS colitis. To further assess the role of PPs and PPs and MLNs in the regulation of experimental colitis in a manner separable from the LTα deficiency we also induced acute DSS colitis in mice made deficient of PPs and MLNs by treatment with LTβRIgG and TNFRIgG (PP/LN-null mice). Combined LTβRIgG and TNFRIgG treatment in mice from gestational day 11 through gestational day 17 inhibits PP and MLN formation without causing a life-long LTα and LTαβ cytokine defect.
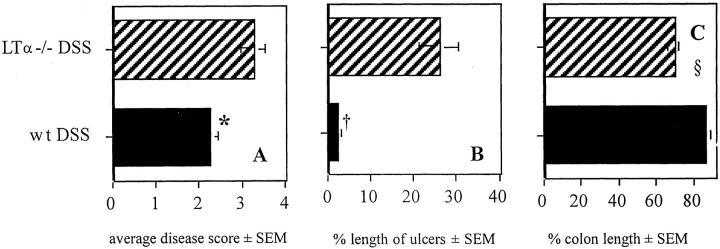
Acute colitis was more severe in LTα−/− mice than in wt mice. The average disease score was higher in mice deficient of the LTα gene and colonic ulcers covered <25% of the assessed mucosal surface (Figure 1, A and B) ▶ . There was more severe shrinking of the inflamed colon than in wt mice (Figure 1C) ▶ . To assess if the increased inflammatory activity was associated with an influx of neutrophil granulocytes, we also assessed colonic myeloperoxidase activity using the myeloperoxidase assay. However, myeloperoxidase activity was similar in diseased LTα−/− and wt mice (data not shown). In addition, body weight during the course of colitis did not differ between wt and LTα−/− mice.
Figure 1.
A and B: Average histological disease score (A) and length of ulcers as percentage of the total assessed colon length ± SEM (B) in wt and LTα−/− mice after a 7-day treatment with 4% DSS (n = 8 to 9/group). *, Disease score LTα−/− versus wt, P = 0.02; †, percent length of ulcers related to total colon length in wt versus LTα−/− mice, P < 0.001. Data are pooled from two individual experiments. C: Length of colon in wt mice and LTα−/− mice (n = 13/group) after a 7-day treatment with 4% DSS. Length is shown as relative length of untreated colon (in cm ± SD: wt, 8.25 ± 1.0; LTα−/−, 8.38 ± 1.3; n = 8/group). §, wt DSS versus LTα−/− DSS, P < 0.02; LTα−/− DSS versus LTα−/− NDW, P < 0.001; wt DSS versus wt NDW, P < 0.02.
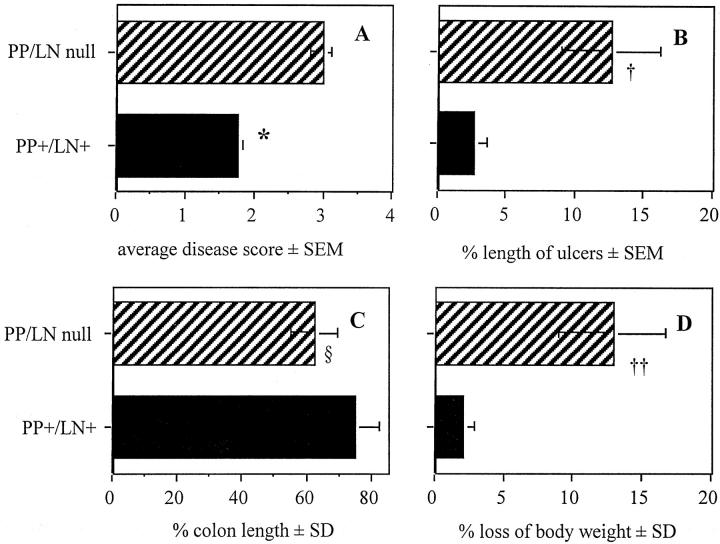
Colitis was also more severe in PP/LN-null mice than in wt mice, as indicated by a higher average disease score (Figure 2A) ▶ . Colonic ulcers covered up to 12% of the mucosal surface of PP/LN-null mice with colitis as compared to 3% in mice with PPs and MLNs (Figure 2B) ▶ . Colitis in the absence of PPs and LNs was associated with more severe shrinking of the inflamed colon (Figure 2C) ▶ and more weight loss (Figure 2D) ▶ . Leder staining of inflamed colon tissue from PP/LN-null mice for granulocytes did not show evidence of granulocyte infiltration in submucosal follicles (data not shown). In addition, MPO activity was similar in colonic tissue from PP/LN-null and wt mice undergoing colitis (data not shown).
Figure 2.
A and B: Average histological disease score (A) and length of mucosal ulcers (B) in mice with (PP+/LN+) and without (PP/LN-null) PPs and MLNs after a 7-day oral treatment with 4% DSS or NDW. *, †: PP/LN-null versus PP+/LN+, P < 0.05. Percentile length of colon (C) as related to colon length of untreated mice and loss of body weight (D) after oral administration of NDW or 4% DSS for 7 days in 129xB6 mice with (PP+/LN+) and without (PP/LN-null) PP and LN. Colon lengths were similar in NDW-treated mice with and without PPs and MLNs (PP+/LN+, 8.58 ± 0.78 cm; PP/LN-null, 8.6 ± 0.31 cm). The mean body weight changed from 20.3 ± 2.0 to 19.6 ± 1.5 (not significant) in PP+/LN+ mice and from 20.3 ± 2.1 g to 17.7 ± 1.7 g in PP/LN-null mice. §, PP+/LN+ DSS versus PP/LN-null NDW, P < 0.05. ††, PP/LN-null day 0 versus PP/LN-null day 7, P < 0.05. Colon length and body weight were assessed in 6 NDW and 11 DSS mice per group. Histology was assessed in at least five mice per group as the remaining intestines were used for MPO assay testing.
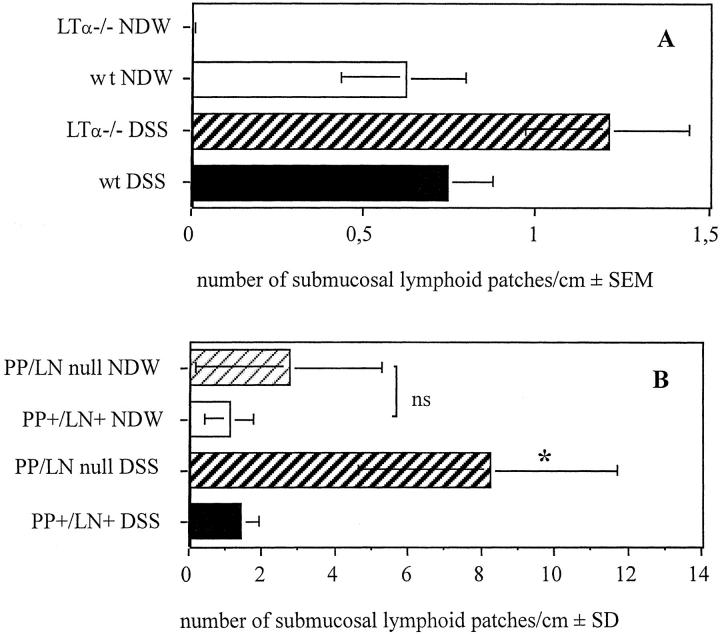
As shown in Figure 3A ▶ , we could not detect any lymphoid follicles in the colon of LTα−/− mice treated with NDW. Oral DSS induced the formation of lymphoid tissue in the absence of LTα (Figure 3A) ▶ . We found a higher number of lymphoid patches in the colon of LTα−/− mice than in diseased wt mice.
Figure 3.
A: Number of submucosal lymphoid patches/cm of colon length in wt and LTα−/− mice undergoing 7-day treatment with NDW or 4% DSS (n = 5 to 9/group). *, LTα−/− NDW versus LTα−/− DSS, P < 0.001. B: Number of submucosal lymphoid follicles/cm of colon length in mice with (PP+/LN+) and without (PP/LN-null) PPs and LNs (n = 5 to 6/group). *, PP/LN-null DSS versus PP/LN-null NDW, PP+/LN+ NDW and PP+/LN+ DSS, P < 0.008. PP/LN-null NDW versus PP+/LN+ NDW, not significant (ns); PP/LN-null NDW versus PP+/LN+ DSS, ns.
Fusion protein treatment in utero did not suppress the formation of lymphoid patches in the colon. There were similar numbers of submucosal lymphoid follicles in untreated control and PP-null/LN-null mice (Figure 3B) ▶ . As shown in Figure 3B ▶ , induction of colitis was associated with a dramatic increase of the number of submucosal lymphoid follicles in mice without GALT whereas the number of lymphoid follicles remained unchanged in control mice.
Figure 4 ▶ shows representative H&E stainings of colon sections from wt, LTα−/−, and PP/LN-null mice with and without colitis, respectively. Although the epithelial lining is intact in untreated wt and LTα−/− mice (Figure 4, A and C) ▶ , there are small epithelial defects in wt mice undergoing colitis (Figure 4B ▶ , arrows indicate limit of ulcers, disease severity score 3). There is also submucosal edema in wt mice after oral DSS. As shown in Figure 4D ▶ , epithelial ulcers in LTα−/− mice cover long colonic mucosal surface areas (disease severity score 4). Colonic submucosal edema in LTα−/− mice is more pronounced in areas without ulcerations (Figure 4E) ▶ . Notice the two lymphoid patches (Figure 4E ▶ , arrows) in the colon of a DSS-treated LTα−/− mouse. Colonic tissue of untreated PP/LN-null mice (Figure 4F) ▶ is similar to untreated wt mice (Figure 4A) ▶ . Colitis in PP/LN-null mice (Figure 4, G and H) ▶ is associated with submucosal edema (asterisk) and colonic epithelial ulceration (small arrows indicating areas of epithelial ulceration). Beside diffuse leukocyte infiltration there are multiple, confluent submucosal lymphoid follicles (large arrows) (Figure 4, G and H) ▶ .
Figure 4.
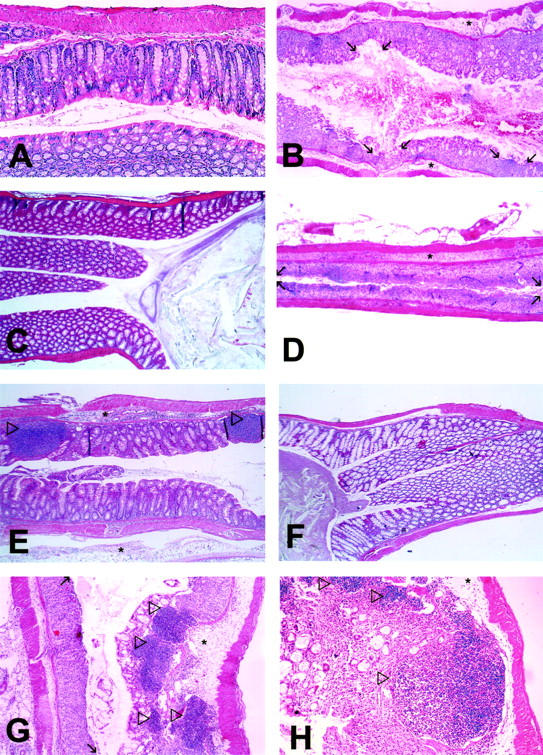
Microphotographs of representative sections of colon from wt (A, B), LTα−/− (C–E), and PP/LN-null (F–H) mice after oral treatment with NDW [wt (A), LTα−/− (C), PP/LN-null (F)] or 4% DSS in the drinking water for 7 days [wt (B), LTα−/− (D and E), PP/LN-null (G and H)]. Large arrows indicate lymphoid patches, small arrows indicate limits of epithelial ulcers. Areas of edema are indicated by asterisks. Original magnifications: ×5 (A–G); ×10 (H).
Immunohistochemical staining of colonic lymphoid follicles of untreated wt mice showed that these follicles predominantly consist of B220+ B cells and small distinct areas of CD8+ T cells (Figure 5, A and B) ▶ . Lymphoid follicles observed in untreated PP/LN-null mice (Figure 5, C and D) ▶ consisted of large B-cell areas and smaller T-cell areas. Inflammatory follicles observed in diseased PP/LN-null mice had even smaller and less sharp separated T-cell areas than wt and untreated PP/LN-null mice (Figure 5, E and F) ▶ . Intestinal inflammation in LTα−/− mice was associated with the formation of predominantly B-cell patches with scattered T-cell infiltrates that were not clearly distinct from B-cell areas of the patch (Figure 5, G and H) ▶ . We could detect only very few scattered CD4+ T cells in colonic follicles of untreated wt mice whereas CD4+ cell areas could be detected in cervical lymph nodes (CLNs) of wt mice (data not shown). We could also detect scattered CD4+ T cells in colonic lymphoid patches of diseased wt, LTα−/−, and PP/LN-null mice (not shown). We detected similar expression of the high endothelial venule marker MAdCAM-1, which is characteristic for mucosal lymph nodes, in lymphoid follicles of wt LTα−/− and PP/LN-null mice (not shown).
Figure 5.
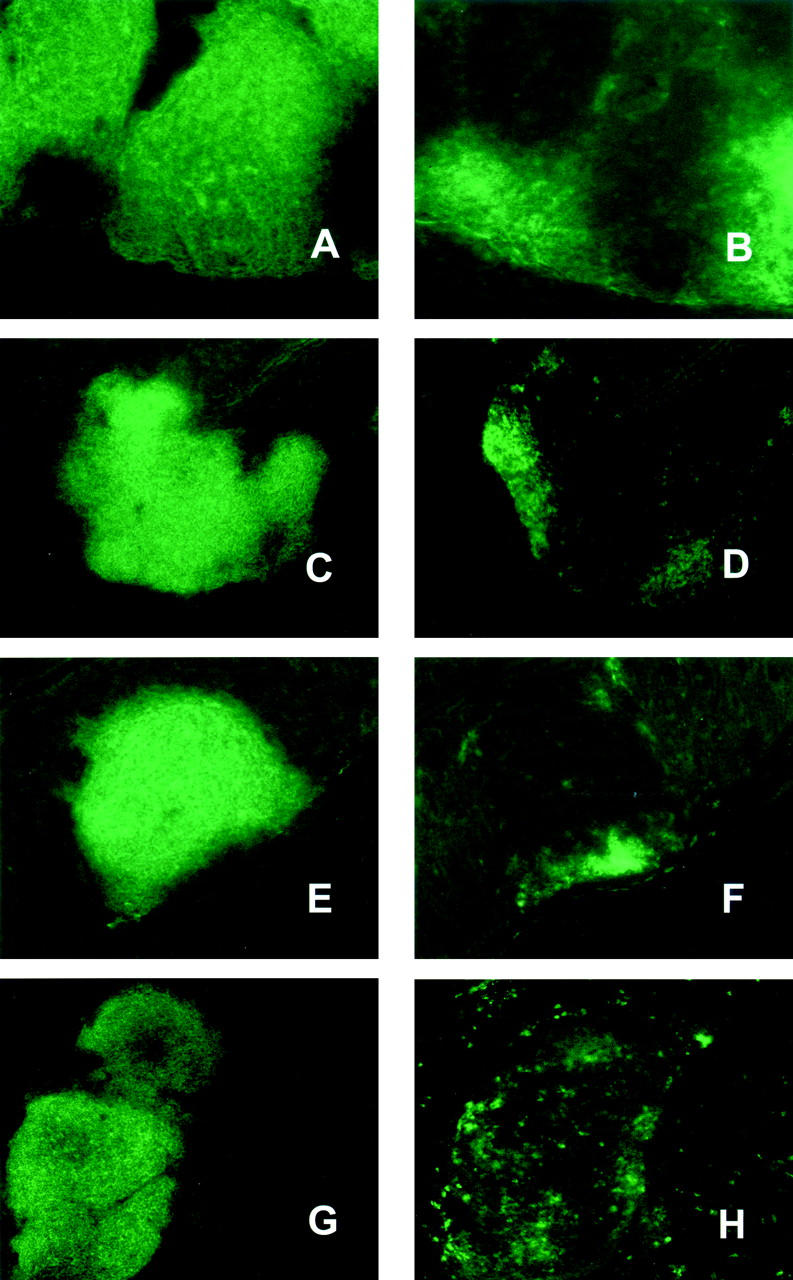
Immunohistochemical staining of colonic lymphoid aggregates from untreated wt mice (A and B), untreated PP/LN-null mice (C and D), PP/LN-null mice undergoing colitis (E and F), and LTα−/− mice undergoing colitis (G and H). Left: Staining for B220; right, staining for CD8. Original magnifications, ×10.
To further characterize the architecture of inflammatory colonic aggregates in wt, LTα−/−, and PP/LN-null mice, we performed silver staining of reticular fibers. Although organized lymphoid tissues contain a reticular fiber connective tissue structure, this is not expected in nonorganized leukocyte aggregates. As shown in Figure 6, A and B ▶ , there was similar lymphoid patch architecture in the colon of wt mice with colitis (Figure 6, A and B) ▶ , LTα−/− mice undergoing colitis (Figure 6, C and D) ▶ and diseased PP/LN-null mice was similar (Figure 6, E and F) ▶ .
Figure 6.
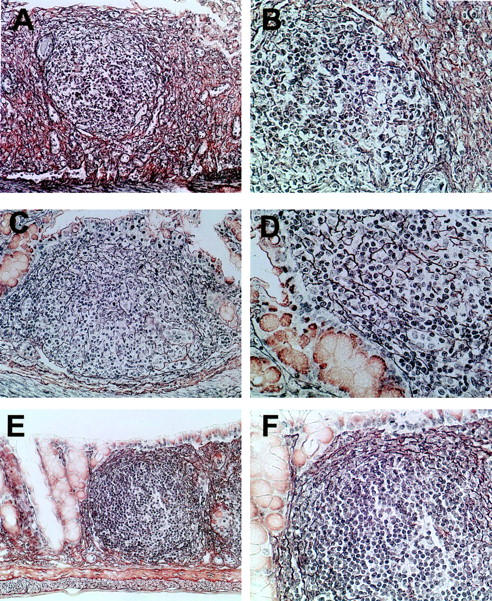
Gomori’s silver staining of colonic lymphoid patches of wt (A, B), LTα−/− (C, D), and PP/LN-null mice (E, F) undergoing colitis. Original magnifications: ×20 (A, C, E); ×40 (B, D, F).
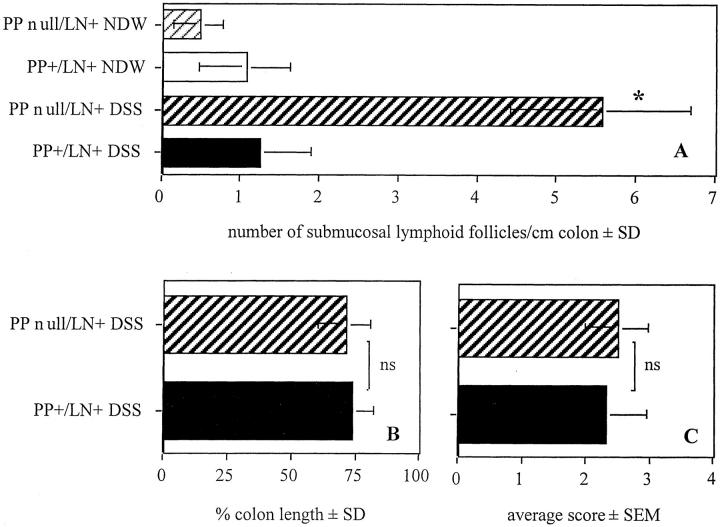
We next investigated the relative importance of PPs and MLNs in controlling the normal course of colitis by inducing DSS colitis in mice made deficient of PPs but not of MLNs by treatment with LTβRIgG in utero on gestational day 16 and gestational 18 (PP-null/LN+). As shown in Figure 7A ▶ , there were increased numbers of submucosal lymph patches in PP-null/LN+ mice undergoing colitis. As shown in Figure 7, B and C ▶ , intestinal shrinking and the average histological disease score were similar in wt and PP-null/LN+ mice with colitis. The length of colonic ulcers after oral DSS was similar in PP-null/LN+ and control mice (% length ± SEM: PP+/LN+, 5.3 ± 4.5; PP-null/LN+, 7.7 ± 3.6, not significant). There was no difference in weight loss between PP-null/LN+ mice and control mice undergoing colitis (data not shown).
Figure 7.
Number of submucosal lymphoid patches (A), percent colon length (B), and average disease score (C) in wt mice and mice without PPs but with LNs (PP-null/LN+ (n = 5/group). *, PP-null/LN+ DSS versus PP-null/LN+ NDW versus PP+/LN+ NDW versus PP+/LN+ DSS; P < 0.01; ns, not significant.
Discussion
We investigated the role of PPs and MLNs in the induction of acute DSS-induced colitis. We observed a more severe type of colitis with increased loss of body weight and more severe intestinal shrinking in mice without PPs and MLNs after oral DSS. Colitis in the absence of PPs and MLNs was associated with an increase in the number of colonic lymphoid patches. Although we could not detect extraintestinal lymphoid tissue or PPs in mice that had received LTβRIgG and TNFRIgG fusion protein treatment in utero, there were submucosally situated lymphoid follicles in the colon that predominantly consisted of B cells and relatively small T-cell areas. Although segregated T- and B-cell follicles could not be detected in the absence of LTα−/−, the formation of colonic B-cell patches was not suppressed by inhibition of LTαβ/LTβR interaction in utero. It is of interest, that although T cells were present in these patches, they did not appear in clearly segregated areas from the B cells. Colitis strongly induced additional formation of submucosal lymphoid aggregates. It remains unclear whether lymphatic cells migrating into de novo-formed colonic lymphoid tissue derive from the blood or expand from resident lamina propria cells. Colonic patches have been described in both mouse and human. 21,22 To distinguish the organized colonic patches from inflammatory leukocyte aggregates we also performed staining for reticular fibers. We found similar reticular staining in both colonic lymphoid follicles and patches of wt mice, PP/LN-null, and LTα−/− mice undergoing colitis suggesting that the inflammatory lymphoid patches observed in our study have similar connective tissue structures as organized lymphoid tissues. Recently, cryptopatches have been described in the murine intestine, which give rise to T-cell receptor (TCR) α/β+ and TCRγ/δ+ intraepithelial lymphocytes. 23,24
We obtained very similar data results from studies using LTα−/− mice, which also lack PPs and MLNs. Using LTα−/− mice we again observed significantly longer colonic ulcers as well as higher disease scores as compared to diseased wt mice. We can therefore conclude that the absence of organized intestinal lymphoid tissue at the onset of disease promotes the formation of colonic ulcers after oral DSS and the de novo formation of lymphoid patches triggered by inflammation. Thus the presence of organized intestinal lymphoid tissue at the time of disease induction attenuates the severity of colitis whereas de novo-formed lymphoid tissue is associated with colitis induction. It is of note that weight loss was more severe in PP-null/LN-null mice undergoing colitis than in diseased LTα−/− mice. This observation suggests that the proinflammatory cytokine LTα 25 might contribute to the wasting observed in DSS colitis.
Although we could not detect any lymphoid follicles in LTα−/− mice when treated with NDW, after oral DSS treatment we observed multiple colonic lymphoid patches in LTα−/− mice. It seems likely that the inability to detect the submucosal colonic lymphoid follicles in LTα−/− mice is because of a lack of a stimulus for cell migration to sites of lymph node development in the absence of LTα, which is partially reversed on inflammatory challenge.
This observation, and the observation that colonic follicles develop in wt mice treated with LTβR-IgG and TNFR55-IgG in utero, suggests that there is a lymphotoxin-independent pathway of secondary lymphoid tissue formation in the intestine. It is of note that this tissue consists primarily of B cells. Thus, B-cell follicle formation might be regulated in a LTα-LTβR-interaction-independent manner. Hamada and colleagues 26 have recently described follicle-like structures in the small intestine that mainly consist of B cells These lymphoid aggregates were named “isolated lymphoid follicles” (ILFs). The lymphoid patches observed in our study resemble ILFs and might be a counterpart to them in the large intestine. The presence of MLNs was initially reported in a small percentage of LTα−/− mice, 11,12 suggesting other signals could compensate for intestinal lymphoid tissue formation. Similar plasticity in mucosal LNs has been described in LTβ−/− mice. 13 In the example of mucosal LN formation the plasticity in reliance of LN development on LTβ was correlated with the immune status of the mother during gestation, that is, the LTβ-independent pathway of mucosal LN development was made manifest after a colony was established from sterilely rederived progeny. These observations on the development of various components of GALT suggest that a soluble factor produced by the mother can influence the development of lymphoid organs in the embryo. Although this novel observation makes evolutionary sense (the progeny are born equipped to deal with the immediate environment), and the influence of pathogens on GALT development is well appreciated 27 this finding is nonetheless unexpected and in need of further study. At this time the precise mechanism and source of newly detectable colonic patches in LTα−/− mice that were treated with DSS remains unclear.
Mice without PPs but with MLNs also showed an increased number of colonic lymphoid patches but no differences in the loss of body weight or severity of intestinal shrinking. Thus, abrogation of PP formation alone is sufficient to induce the migration of lymphoid cells into the inflamed intestine. Thus, induction of lymphoid aggregates in the inflamed intestine after oral DSS is not necessarily associated with more severe disease as indicated by longer intestinal ulcers and increased weight loss.
While our manuscript was in preparation, Dohi and colleagues 28 reported on the course of TNBS-induced colitis in mice made deficient of PPs by gestational fusion protein treatment. Using BALB/c mice, as compared to 129xB6 mice used in our study, they observed no organized lymphoid follicles in the colon after gestational fusion protein treatment. Furthermore, colitis induced by TNBS did not induce colonic lymphoid follicles. We suspect the differences in the mouse strains, disease models, or maternal environment are accountable for the differences observed in colonic lymphoid tissue and disease course.
In addition to LTβ LIGHT can also bind to the LTβR. Although the interaction between LIGHT and LTβR would be inhibited by continuous LTβRIgG treatment during disease, as administered in the study of Dohi and colleagues, 28 LIGHT could bind to the LTβR in our PP-null/LN-null and LTα−/− model as gestationally administered LTβRIgG is hardly detectable in the serum of treated progeny at the age of 6 to 8 weeks 17 when our mice were used for experiments. In addition, the ltα-gene deficiency does not interfere with LIGHT binding to the LTβR.
An important similarity also emerges from the study of Dohi and colleagues 28 and our studies. The disease that develops in response to oral DSS feeding or TNBS enema is much more severe in the absence of the normal array of mucosal secondary lymphoid organs such as PPs and draining mucosal LNs. It is therefore likely that populations of cells normally present in PPs and mucosal LNs play a role in modulating the response to intestinal inflammation. One important candidate cell to fulfill this role are dendritic cells, whose complexity and function in PPs and LNs are only recently becoming well understood. 29 For example, such dendritic cells have recently been shown to be sources of interleukin-10, a cytokine known to influence the outcome of gut inflammation. 30
Despite the acute course that DSS-induced colitis takes in mice there is good evidence in the literature that the adaptive immune system can indeed regulate the disease. DSS-pulsed macrophages stimulate T cells from colitis mice for secretion of T helper 1 cytokines 31 in vitro. Inflammatory infiltrates consist of plasma cells, neutrophils, and mononuclear cells. 32 Severity of DSS-induced colitis can be modulated by cyclosporin-induced suppression of T-cell activation 33 and intravenous immunoglobulin. 34
There is evidence for preferential immune activation in regional lymphoid follicles in animal models of inflammatory bowel disease. In indomethacin-induced colitis, there is proliferation and apoptosis of M cells in the inflamed intestine. 35 In TCR-α−/− mice, there was more proliferation and a higher level of autoantibody production in appendix lymphoid follicle tissue than in PP tissue during colitis. 36 In a Th-2 model of inflammatory bowel disease induced in interferon-γ−/− mice, colitis is associated with colonic patch hypertrophy. 21 Lymphoid hyperplasia has been reported as a feature of chronic DSS-induced colitis. 37 Secondary lymphoid follicles are also observed in patients with Crohn’s disease. Our results suggest an important role of the GALT in maintenance of mucosal integrity and control of mucosal inflammation. Indeed, development of colonic follicles during colitis may be an attempt to control intestinal inflammation.
It is reasonable to speculate that the severe colitis that develops in the absence of PPs and MLNs may be in part related to a failure of tolerance induction because of the loss of normally present regulatory cells in these organs such as dendritic cell populations mentioned above. Oral feeding of sonicates from anaerobic intestinal bacteria and of haptenized colonic protein down-modulates colitis in mice. 38,39 Thus intraluminal and intestinal wall antigens have the capacity to induce tolerance toward inflammatory intestinal immune responses. Our previous study and those of other groups have implicated both PPs and MLNs in the development of intestinal oral tolerance 8,9 with MLNs playing a pivotal role. There is evidence showing that lymph nodes draining mucosal sites are critical for the induction of immune tolerance. We have recently demonstrated that induction of MLN formation in the LTα−/− model restores the capacity to induce tolerance to oral antigen in the absence of PPs. 10 Wolvers and colleagues 40 have shown that CLNs are critical for the induction of nasal tolerance. Surgical removal of CLNs abrogated the capacity to induce tolerance by the nasal route and transplantation of CLNs but not of other peripheral LNs to mice without CLNs restored the capacity to induce nasal tolerance.
In this study we observed an increase in the disease score in mice deficient of PPs and MLNs whereas disease activity in mice lacking PPs only was comparable to control mice. We therefore assume that the lack of the capacity to induce oral tolerance in the absence of MLNs 8 may be associated with an exacerbation of intestinal inflammation.
Recently, it has been reported that treatment of adult mice with LTβRIgG could attenuate experimental colitis in mice. 41 This observation is not in contradiction to our study as we used LTβRIgG fusion protein for gestational inhibition of lymphotoxin-receptor interaction to ablate the formation of secondary lymphoid organs, rather than treating them once formed. The LTαβ/LTβR pathway has varied and distinct biological roles to play during development and in the normal physiology of the adult. 42 Because LTβR-IgG treatment of adult wt mice seems to influence the development of a variety of immune diseases, including inflammatory bowel disease, it might be anticipated that treatment of adult wt mice would have efficacy in the DSS model as well.
In summary we report more severe intestinal inflammation in the absence of MLNs whereas the absence of PPs alone did not affect disease severity. These observations suggest a regulatory function for MLNs but not for PPs in intestinal inflammation.
Acknowledgments
We thank Mrs. Jansen and Mrs. Krull for the dedicated and skillful tissue preparations and stainings.
Footnotes
Address reprint requests to Thomas W. Spahn, M.D., Department of Medicine B, Albert Schweitzer-Str. 33, D-48129 Münster, Germany. E-mail: spahnth@t-online.de.
Supported by funds from the Innovative Medizinische Forschung of the Westfälische Wilhelms-Universität, Münster, Germany (SP 119914).
References
- 1.Spahn T, Weiner HL: The nature of oral gastrointestinal tolerance. Kirsner J eds. Inflammatory Bowel Disease. 2000:pp 240-250 W. B. Saunders Company, Philadelphia
- 2.Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, Meyer zum Buschenfelde KH: Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD). Clin Exp Immunol 1995, 102:448-455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duchmann R, Schmitt E, Knolle P, Meyer zum Buschenfelde KH, Neurath M: Tolerance towards resident intestinal flora in mice is abrogated in experimental colitis and restored by treatment with interleukin-10 or antibodies to interleukin-12. Eur J Immunol 1996, 26:934-938 [DOI] [PubMed] [Google Scholar]
- 4.Neutra MR: Current concepts in mucosal immunity. V. Role of M cells in transepithelial transport of antigens and pathogens to the mucosal immune system. Am J Physiol 1998, 274:G785-G791 [DOI] [PubMed] [Google Scholar]
- 5.Kawanishi H, Saltzman LE, Strober W: Characteristics and regulatory function of murine con A-induced, cloned T cells obtained from Peyer’s patches and spleen: mechanisms regulating isotype-specific immunoglobulin production by Peyer’s patch B cells. J Immunol 1982, 129:475-483 [PubMed] [Google Scholar]
- 6.Kawanishi H, Saltzman LE, Strober W: Mechanisms regulating IgA class-specific immunoglobulin production in murine gut-associated lymphoid tissues. I. T cells derived from Peyer’s patches that switch sIgM B cells to sIgA B cells in vitro. J Exp Med 1983, 157:433-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamoto M, Rennert P, McGhee JR, Kweon MN, Yamamoto S, Dohi T, Otake S, Bluethmann H, Fujihashi K, Kiyono H: Alternate mucosal immune system: organized Peyer’s patches are not required for IgA responses in the gastrointestinal tract. J Immunol 2000, 164:5184-5191 [DOI] [PubMed] [Google Scholar]
- 8.Spahn TW, Fontana A, Faria AM, Slavin AJ, Eugster HP, Zhang X, Koni PA, Ruddle NH, Flavell RA, Rennert PD, Weiner HL: Induction of oral tolerance to cellular immune responses in the absence of Peyer’s patches. Eur J Immunol 2001, 31:1278-1287 [DOI] [PubMed] [Google Scholar]
- 9.Fujihashi K, Dohi T, Rennert PD, Yamamoto M, Koga T, Kiyono H, McGhee JR: Peyer’s patches are required for oral tolerance to proteins. Proc Natl Acad Sci USA 2001, 98:3310-3315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spahn TW, Weiner HL, Rennert PD, Lugering N, Fontana A, Domschke W, Kucharzik T: Mesenteric lymph nodes are critical for the induction of high-dose oral tolerance in the absence of Peyer’s patches. Eur J Immunol 2002, 32:1109-1113 [DOI] [PubMed] [Google Scholar]
- 11.De Togni P, Goellner J, Ruddle NH, Streeter PR, Fick A, Mariathasan S, Smith SC, Carlson R, Shornick LP, Strauss-Schoenberger J, et al: Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science 1994, 264:703-707 [DOI] [PubMed] [Google Scholar]
- 12.Banks TA, Rouse BT, Kerley MK, Blair PJ, Godfrey VL, Kuklin NA, Bouley DM, Thomas J, Kanangat S, Mucenski ML: Lymphotoxin-alpha-deficient mice. Effects on secondary lymphoid organ development and humoral immune responsiveness. J Immunol 1995, 155:1685-16937636227 [Google Scholar]
- 13.Koni PA, Sacca R, Lawton P, Browning JL, Ruddle NH, Flavell RA: Distinct roles in lymphoid organogenesis for lymphotoxins alpha and beta revealed in lymphotoxin beta-deficient mice. Immunity 1997, 6:491-500 [DOI] [PubMed] [Google Scholar]
- 14.Rennert PD, Hochman PS, Flavell RA, Chaplin DD, Jayaraman S, Browning JL, Fu YX: Essential role of lymph nodes in contact hypersensitivity revealed in lymphotoxin-alpha-deficient mice. J Exp Med 2001, 193:1227-1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rennert PD, James D, Mackay F, Browning JL, Hochman PS: Lymph node genesis is induced by signaling through the lymphotoxin beta receptor. Immunity 1998, 9:71-79 [DOI] [PubMed] [Google Scholar]
- 16.Rennert PD, Browning JL, Mebius R, Mackay F, Hochman PS: Surface lymphotoxin alpha/beta complex is required for the development of peripheral lymphoid organs. J Exp Med 1996, 184:1999-2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rennert PD, Browning JL, Hochman PS: Selective disruption of lymphotoxin ligands reveals a novel set of mucosal lymph nodes and unique effects on lymph node cellular organization. Int Immunol 1997, 9:1627-1639 [DOI] [PubMed] [Google Scholar]
- 18.Krawisz JE, Sharon P, Stenson WF: Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology 1984, 87:1344-1350 [PubMed] [Google Scholar]
- 19.Gomori G: Silver impregnation of reticulum in paraffin sections. Am J Pathol 1937, 13 [PMC free article] [PubMed]
- 20.Andres PG, Beck PL, Mizoguchi E, Mizoguchi A, Bhan AK, Dawson T, Kuziel WA, Maeda N, MacDermott RP, Podolsky DK, Reinecker HC: Mice with a selective deletion of the CC chemokine receptors 5 or 2 are protected from dextran sodium sulfate-mediated colitis: lack of CC chemokine receptor 5 expression results in a NK1.1+ lymphocyte-associated Th2-type immune response in the intestine. J Immunol 2000, 164:6303-6312 [DOI] [PubMed] [Google Scholar]
- 21.Dohi T, Fujihashi K, Rennert PD, Iwatani K, Kiyono H, McGhee JR: Hapten-induced colitis is associated with colonic patch hypertrophy and T helper cell 2-type responses. J Exp Med 1999, 189:1169-1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Leary AD, Sweeney EC: Lymphoglandular complexes of the colon: structure and distribution. Histopathology 1986, 10:267-283 [DOI] [PubMed] [Google Scholar]
- 23.Ishikawa H, Saito H, Suzuki K, Oida T, Kanamori Y: New gut associated lymphoid tissue “cryptopatches” breed murine intestinal intraepithelial T cell precursors. Immunol Res 1999, 20:243-250 [DOI] [PubMed] [Google Scholar]
- 24.Suzuki K, Oida T, Hamada H, Hitotsumatsu O, Watanabe M, Hibi T, Yamamoto H, Kubota E, Kaminogawa S, Ishikawa H: Gut cryptopatches: direct evidence of extrathymic anatomical sites for intestinal T lymphopoiesis. Immunity 2000, 13:691-702 [DOI] [PubMed] [Google Scholar]
- 25.Sacca R, Cuff CA, Ruddle NH: Mediators of inflammation. Curr Opin Immunol 1997, 9:851-857 [DOI] [PubMed] [Google Scholar]
- 26.Hamada H, Hiroi T, Nishiyama Y, Takahashi H, Masunaga Y, Hachimura S, Kaminogawa S, Takahashi-Iwanaga H, Iwanaga T, Kiyono H, Yamamoto H, Ishikawa H: Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J Immunol 2002, 168:57-64 [DOI] [PubMed] [Google Scholar]
- 27.Cebra JJ, Periwal SB, Lee G, Lee F, Shroff KE: Development and maintenance of the gut-associated lymphoid tissue (GALT): the roles of enteric bacteria and viruses. Dev Immunol 1998, 6:13-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dohi T, Rennert PD, Fujihashi K, Kiyono H, Shirai Y, Kawamura YI, Browning JL, McGhee JR: Elimination of colonic patches with lymphotoxin beta receptor-Ig prevents Th2 cell-type colitis. J Immunol 2001, 167:2781-2790 [DOI] [PubMed] [Google Scholar]
- 29.Anjuere F, Martin P, Ferrero I, Fraga ML, del Hoyo GM, Wright N, Ardavin C: Definition of dendritic cell subpopulations present in the spleen, Peyer’s patches, lymph nodes, and skin of the mouse. Blood 1999, 93:590-598 [PubMed] [Google Scholar]
- 30.Iwasaki A, Kelsall BL: Freshly isolated Peyer’s patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J Exp Med 1999, 190:229-239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shintani N, Nakajima T, Okamoto T, Kondo T, Nakamura N, Mayumi T: Involvement of CD4+ T cells in the development of dextran sulfate sodium-induced experimental colitis and suppressive effect of IgG on their action. Gen Pharmacol 1998, 31:477-481 [DOI] [PubMed] [Google Scholar]
- 32.Fedorak R, Madsen K: Naturally occurring and experimental models of inflammatory bowel disease. Kirsner J eds. Inflammatory Bowel Disease. 2000:pp 113-143 J. B. Saunders Company, Philadelphia
- 33.Murthy SN, Cooper HS, Shim H, Shah RS, Ibrahim SA, Sedergran DJ: Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Dig Dis Sci 1993, 38:1722-1734 [DOI] [PubMed] [Google Scholar]
- 34.Shintani N, Nakajima T, Nakakubo H, Nagai H, Kagitani Y, Takizawa H, Asakura H: Intravenous immunoglobulin (IVIG) treatment of experimental colitis induced by dextran sulfate sodium in rats. Clin Exp Immunol 1997, 108:340-345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kucharzik T, Lugering A, Lugering N, Rautenberg K, Linnepe M, Cichon C, Reichelt R, Stoll R, Schmidt MA, Domschke W: Characterization of M cell development during indomethacin-induced ileitis in rats. Aliment Pharmacol Ther 2000, 14:247-256 [DOI] [PubMed] [Google Scholar]
- 36.Mizoguchi A, Mizoguchi E, Chiba C, Bhan AK: Role of appendix in the development of inflammatory bowel disease in TCR-alpha mutant mice. J Exp Med 1996, 184:707-715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R: A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 1990, 98:694-702 [DOI] [PubMed] [Google Scholar]
- 38.Verdu EF, Bercik P, Cukrowska B, Farre-Castany MA, Bouzourene H, Saraga E, Blum AL, Corthesy-Theulaz I, Tlaskalova-Hogenova H, Michetti P: Oral administration of antigens from intestinal flora anaerobic bacteria reduces the severity of experimental acute colitis in BALB/c mice. Clin Exp Immunol 2000, 120:46-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neurath MF, Fuss I, Kelsall BL, Presky DH, Waegell W, Strober W: Experimental granulomatous colitis in mice is abrogated by induction of TGF-beta-mediated oral tolerance. J Exp Med 1996, 183:2605-2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolvers DA, Coenen-de Roo CJ, Mebius RE, van der Cammen MJ, Tirion F, Miltenburg AM, Kraal G: Intranasally induced immunological tolerance is determined by characteristics of the draining lymph nodes: studies with OVA and human cartilage gp-39. J Immunol 1999, 162:1994-1998 [PubMed] [Google Scholar]
- 41.Mackay F, Browning JL, Lawton P, Shah SA, Comiskey M, Bhan AK, Mizoguchi E, Terhorst C, Simpson SJ: Both the lymphotoxin and tumor necrosis factor pathways are involved in experimental murine models of colitis. Gastroenterology 1998, 115:1464-1475 [DOI] [PubMed] [Google Scholar]
- 42.Fu YX, Chaplin DD: Development and maturation of secondary lymphoid tissues. Annu Rev Immunol 1999, 17:399-433 [DOI] [PubMed] [Google Scholar]