Abstract
Limited knowledge exists regarding the efficacy of insulin-like growth factor I (IGF-I) administration as a therapeutic intervention for muscular dystrophies, although findings from other muscle pathology models suggest clinical potential. The diaphragm muscles of mdx mice (a model for Duchenne muscular dystrophy) were examined after 8 weeks of IGF-I administration (1 mg/kg s.c.) to test the hypothesis that IGF-I would improve the functional properties of dystrophic skeletal muscles. Force per cross-sectional area was ∼49% greater in the muscles of treated mdx mice (149.6 ± 9.6 kN/m2) compared with untreated mice (100.1 ± 4.6 kN/m2, P < 0.05), and maintenance of force over repeated maximal contraction was enhanced ∼30% in muscles of treated mice (P < 0.05). Diaphragm muscles from treated mice comprised fibers with ∼36% elevated activity of the oxidative enzyme succinate dehydrogenase, and ∼23% reduction in the proportion of fast IId/x muscle fibers with concomitant increase in the proportion of type IIa fibers compared with untreated mice (P < 0.05). The data demonstrate that IGF-I administration can enhance the fatigue resistance of respiratory muscles in an animal model of dystrophin deficiency, in conjunction with enhancing energenic enzyme activity. As respiratory function is a mortality predictor in Duchenne muscular dystrophy patients, further evaluation of IGF-I intervention is recommended.
Patients with muscular dystrophies experience profound muscle wasting and functional impairment despite available therapies. Typically, the most severe neuromuscular dystrophies, such as Duchenne muscular dystrophy (DMD), are caused by genetic deficiencies of structural proteins, rendering muscle fibers more fragile and susceptible to injury during contraction. 1 As a consequence dystrophic muscle fibers continually undergo degeneration only to be replaced by regenerating fibers with the same genetic deficiency and susceptibility to injury. It is anticipated that genetic correction of the mutations responsible for the specific dystrophies will cure such conditions, but existing methodologies have yet to achieve optimum efficacy. 2 Until gene-based therapies are optimized, it is essential to continue evaluating nongenetic interventions to combat the symptoms of the muscular dystrophies to maximize a patient’s quality of life. Such therapies primarily focus on slowing the loss of muscle tissue to preserve muscle function.
Insulin-like growth factor-I (IGF-I) is an endogenous hormone produced by numerous tissues including skeletal muscle. Muscle IGF-I levels are elevated after injury and mechanical overload, when formation of new fibers or growth of existing fibers occurs. 3-5 IGF-I ligand-receptor interaction stimulates satellite cell proliferation and differentiation during muscle regeneration, as well as muscle fiber growth. 6 Experimental elevation of IGF-I levels has proven beneficial in various animal models of muscle wasting 7-9 and studies of age-related muscle deterioration. 10-12 Consequently, a basis exists for the administration of IGF-I to promote skeletal muscle hypertrophy in which loss of muscle mass and function occurs.
Recently published findings show a growing interest in the potential of IGF-I supplementation as an intervention for dystrophic muscle pathologies. Exciting work by Barton and colleagues 13 demonstrated that dystrophin-deficient mdx mice (which share the genetic aberration associated with DMD) cross bred with an IGF-I-overexpressing mouse strain produce dystrophic offspring with elevated intramuscular IGF-I levels and increased muscle fiber size and number. These important results highlight a link between up-regulation of endogenous IGF-I levels and amelioration of a dystrophin-deficient pathology. However, the aforementioned study did not examine the functional properties of the diaphragm muscle, which displays the progressive and continual deterioration most accurately modeling the decline in respiratory performance of DMD patients. 1,14-18 Additionally, the mode of intervention used is not clinically applicable. An immediate need for efficacious interventions for muscular dystrophies means that promising data must be derived using treatment methods that are clinically relevant.
Studies using the laminin-deficient 129P1 ReJ-Lama2dy mouse (a model of congenital muscular dystrophy) have noted enhanced limb muscle mass and function after systemic IGF-I administration, as well as attenuated muscle proteolysis and increased expression of muscle regulatory factors. 19-22 Using the mdx mouse, Granchelli and colleagues 23 observed enhanced hind limb volitional strength after 6 weeks of 5 mg/kg/day IGF-I administration. However, the aforementioned study did not examine the diaphragm muscle, and used an IGF-I dose exceeding that of most other studies, thus increasing the potential for undesirable IGF-I-related side effects with continued administration. 24 The data combined indicate that IGF-I supplementation can ameliorate some features of murine models of muscular dystrophy. Yet, no study has comprehensively evaluated the merits of a clinically achievable IGF-I intervention protocol on the functional properties of the murine model that currently best represents dystrophin-deficient dystrophies (dystrophinopathies); the diaphragm muscle of the mdx mouse.
The novelty of the present work lies in the strength of its clinical relevance based on the functional evaluation of the chosen model, and the mode of intervention used. For the purposes of evaluating the potential of IGF-I as a treatment for DMD-related muscle pathology, we examined the efficacy of a comparatively low systemic dose of IGF-I (1 mg/kg/day s.c. for 8 weeks) on benchmark indices of mdx mouse diaphragm muscle structure and function, to test the hypothesis that exogenous IGF-I administration ameliorates the structural and functional deterioration of dystrophinopathic skeletal muscles.
Materials and Methods
All procedures were approved by the Animal Experimentation Ethics Committee of The University of Melbourne and conformed to the Guidelines for the Care and Use of Experimental Animals as described by the National Health and Medical Research Council of Australia. Mice were housed in groups of four under an artificial light/dark cycle provided with light between 06:00 and 18:00 hours, and free access to drinking water and standard chow.
Male C57BL/10ScSn (wild-type control) and C57BL/10ScSn-mdx/J (dystrophic) mice (5 to 6 weeks old, 25 to 29 g body mass) were randomly assigned to either untreated or IGF-I-treated groups. Treated mice were administered recombinant human IGF-I (GroPep, Thebarton, SA, Australia) at a daily dose of 1 mg/kg body mass. This dose was chosen to elicit the beneficial functional effects of IGF-I from previous studies, 19,21-23 while minimizing the probability of deleterious side effects associated with elevated circulating IGF-I concentrations. 24
IGF-I dissolved in 10 mmol/L of HCl and sterile isotonic saline at a concentration of 8.3 mg/ml was loaded into miniosmotic pumps (model 1002; Alzet, Cupertino, CA) that had been partially dipped in paraffin wax to achieve a pumping rate of 0.125 μl/hour (3 μl/day) for 28 days. 25 Loaded pumps were primed in isotonic saline at 37°C for 24 hours before implantation. Mice assigned to receive IGF-I were anesthetized with pentobarbitone sodium (Nembutal, 40 mg/kg i.p.; Rhone Merieux, Pinkenba, QLD, Australia) such that they were unresponsive to tactile stimuli, whereupon an incision was made in the skin in the middle of the spinal curvature and a subcutaneous pocket created by way of blunt dissection with surgical scissors. Pumps were inserted into the subcutaneous space with the flow regulator cap directed distally and the incision was closed with Michel clips. At the completion of 28 days of continuous IGF-I administration, the pumps were replaced with freshly loaded and primed units to enable a further 28 days of treatment by the same procedure. On removal, pumps were aspirated to ensure that fouling had not occurred, and that the contents had been administered accordingly. The mode of IGF-I administration used in this study was chosen to enable continuous, systemic drug delivery as might be used for the treatment of a clinical condition affecting muscles throughout the body. IGF-I delivered according to this route exhibits sustained bioactivity throughout the treatment period. 26 Mice in untreated groups received no pump, because we have determined previously that there is no difference in the properties of skeletal muscles from unoperated mice and sham-operated mice receiving vehicle via osmotic pump. 22 Daily handling and examination of the animals for the duration of the study confirmed that no mice exhibited adverse effects to either the surgical procedures or the IGF-I treatments.
Contractile Properties
At the conclusion of 8 weeks of continuous treatment, the mice were anesthetized with pentobarbitone sodium (60 mg/kg i.p.) with supplemental doses administered to maintain a depth of anesthesia that prevented all responses to tactile stimuli. Once an appropriate depth of anesthesia was achieved, the entire diaphragm muscle and surrounding ribcage was rapidly excised to a dish containing oxygenated Krebs-Ringer solution (in mmol/L: NaCl, 137; NaHCO3,24; d-glucose, 11; KCl ,5; CaCl2, 2; NaH2PO4, 1; MgSO4, 0.487; d-tubocurarine chloride, 0.293) to microscopically isolate muscle portions for examination. Diaphragm strips composed of longitudinally arranged full-length muscle fibers were tied firmly with braided surgical silk (6/0; Pearsalls Sutures, Somerset, UK) at the central tendon, and sutured through a portion of rib bone affixed to the distal end of the strip. Mice were killed as a consequence of heart excision while still deeply anesthetized.
Isometric contractile properties of the diaphragm muscles were evaluated in vitro, using techniques described previously. 15,27 Each muscle was transferred to a custom-built Plexiglas bath filled with oxygenated Krebs-Ringer solution that was thermostatically maintained at 25°C for optimal oxygen diffusion. 28 The muscles were aligned horizontally and tied directly between a fixed pin and a dual-mode force transducer-servomotor (300B-LR; Aurora Scientific, Aurora, Ontario, Canada). Two platinum plate electrodes were positioned in the organ bath so as to flank the length of the muscle. Muscles were field stimulated by supramaximal square wave pulses (0.2-ms duration), that were amplified (EP500B; Audio Assemblers, Campbellfield, VIC Australia) to increase and sustain current intensity to a sufficient level to produce a maximum isometric tetanic contraction. 15
All stimulation parameters and contractile responses were controlled and measured using custom built applications (D.R. Stom Inc., Ann Arbor MI) of LabView software (National Instruments, Austin, TX) driving a personal computer with onboard controller (PCI-MIO-16XE-10, National Instruments) interfaced with the transducer-servomotor control/feedback hardware (Aurora Scientific). Optimum muscle length (Lo) and stimulation voltage were determined from micromanipulation of muscle length to produce maximum isometric twitch force (Pt). Optimum fiber length (Lf) was equal to Lo based on the alignment of the fibers in the preparation as determined previously. 15 Maximum isometric tetanic force (Po) was determined from the plateau of the frequency-force relationship after successive stimulations at 5 to 120 Hz for 450 ms, with 2 minutes of rest between stimuli.
After determination of isometric contractile properties, muscles were subjected to a 4-minute repeated stimulation protocol to induce muscle fatigue. Muscles at Lo were maximally stimulated for 450 ms once every 5 seconds for the duration of the fatigue protocol. Having completed 4 minutes of activity as described, electrical stimulation was ceased and the muscles remained inactive for 5 minutes. After the rest interval, the preparation was maximally stimulated once to determine the recovery of Po. After functional testing, the muscles were removed from the bath, trimmed of their tendons and any adhering nonmuscle tissue, blotted once on filter paper, and weighed on an analytical balance. Because the width of individual diaphragm preparations (and hence the total number of fibers tested) varies based on the discretion of the individual performing the dissections, comparisons of absolute Pt and Po values are not valid, and all data must be normalized with respect to the length and width (and therefore mass) of individual muscles. Muscle mass, Lf, and Po were used to calculate specific force [sPo, or force normalized per total muscle fiber cross-sectional area (CSA), kN/m2] as described elsewhere. 15,27 Calculation of muscle density (∼1.06 mg/mm3) based on muscle mass by the aforementioned methods is resistant to variation beyond the third decimal place (ie, < ±0.001 mg/mm3) from variations in connective tissue, fat, and water composition, thereby reinforcing the validity of the normalization technique. 15,29
Morphological and Histochemical Analyses
After weighing, each muscle was arranged at Lo on a small piece of aluminum foil, snap-frozen in thawing isopentane, and stored at −80°C. Muscles were cryosectioned transversely through the mid-length of the strip (CTI cryostat; IEC, Needham Heights, MA), and the sections placed on uncoated glass slides.
Serial 8-μm muscle sections were stained with hematoxylin and eosin for examination of tissue morphology, reacted for myosin ATPase enzymatic activity for determination of muscle fiber type proportions, and reacted for succinate dehydrogenase (SDH) activity as a marker of mitochondrial density and oxidative metabolic enzyme activity. Histochemical reaction for myosin ATPase activity used the method described by Hämäläinen and Pette 30 with serial sections preincubated at pH 4.3 and 4.55 for the determination of one slow and three fast muscle fiber types (types I, IIa, IIb, and IId/x). SDH activity was quantified according to the method of Blanco and colleagues. 31 As soon after sectioning as possible, sections were incubated in fresh reaction solution [in mmol/L: phosphate buffer, 100 (pH 7.4); succinic acid, 50; ethylenediaminetetraacetic acid, 5; nitro blue tetrazolium, 1.5; 1-methoxyphenzine methosulfate, 1; sodium azide, 0.75] at 25°C for exactly 3 minutes at which time the reaction was terminated with multiple rinses of distilled water. Reacted sections were air-dried, coverslipped, and stored in the dark for quantitative examination as described previously. 31
Digitized images (24 bit Red Green Blue (RGB)) of all muscle sections examined were acquired using an upright microscope (Axioplan; Zeiss, Oberkochen, Germany) with camera (Spot model 1.3.0; Diagnostic Instruments, Sterling Heights, MI), driven by Spot diagnostic software (v2.1, Diagnostic Instruments). Image files were analyzed in a double-blinded procedure using Image Pro software (v4.0; Media Cybernetics, Silver Spring, MD). The mean CSA of individual muscle fibers was calculated by interactive determination of the circumference of on average 240 adjacent fibers from every muscle examined. Muscle fibers in cross-section were traced on screen using a mouse-driven cursor, and the dimensions of highlighted fibers calculated within the software package based on a calibrated screen pixel-to-actual size ratio.
Quantitation of Muscle and Serum IGF-I Concentration
To verify exogenous administration of IGF-I, total (endogenous murine and recombinant human) IGF-I concentration was quantified in serum and diaphragm muscle samples of an extra group of C57BL/10ScSn mice after 2 weeks of treatment using established radioimmunoassay methods. 32 Blood was collected on cardiac excision, stored on ice for 30 minutes, and centrifuged at 3000 × g for 10 minutes (4°C) to obtain serum. Serum from each mouse was mixed 1:4 with extraction buffer (250 mmol/L HCl in ethanol) and incubated at room temperature for 30 minutes before centrifugation at 2000 × g for 10 minutes (4°C). The extracted supernatant was neutralized 2.5:1 with 1 mol/L of Tris base and subjected to a single freeze-thaw cycle and recentrifugation before analysis. IGF-I was extracted from muscle samples by homogenizing the samples in ice-cold 1 mol/L acetic acid (1:40 w:v) with a hand-held glass homogenizer, incubating the homogenates on ice for 2 hours, and centrifuging at 10,000 × g for 10 minutes (4°C) to obtain a supernatant. Volumes of sample supernatant were neutralized with 5 mol/L of NaOH (100 μl sample:12 μl NaOH), subjected to one freeze-thaw cycle, and centrifuged at 2000 × g for 10 minutes (4°C) to remove additional solid material before assay.
Statistical Analyses
Values in the text and tables are reported as mean ± SEM. Variables were compared between experimental groups using a general linear model, two factor analysis of variance for differences between strain (control or dystrophic) and treatment (control or treated) with Fisher’s least significant difference post hoc multiple comparison procedure to identify differences between specific groups. Differences between groups were considered significant when P was < 0.05.
Results
Control strain mice treated with IGF-I for 2 weeks exhibited a greater than fourfold elevation in serum total IGF-I concentration compared with untreated control mice (treated control versus untreated control: 2642 ± 141 ng/ml versus 622 ± 41 ng/ml, respectively; P < 0.05, n = 4 and 5). Total IGF-I concentrations of diaphragm muscle samples from treated (96.6 ± 5.4 ng/g, n = 4) and untreated mice (77.6 ± 18.4 ng/g, n = 5) were not different (P = 0.350). We have determined from pilot studies that the endogenous IGF-I concentration of the diaphragm muscle of untreated mdx mice at this age does not differ from that of control mice.
Morphometric and Functional Analyses
At the conclusion of the 8-week experimental intervention, untreated mdx mice exhibited a 14% greater body mass than untreated control mice. IGF-I administration had no effect on the body mass of either control or dystrophic mice.
When diaphragm muscles were tested for isometric contractile properties in vitro, preparations from untreated control mice recorded sPt and sPo values of ∼90 and ∼270 kN/m2, respectively (Table 1) ▶ . Muscles of untreated dystrophic mice demonstrated markedly diminished force-producing capacity with sPt and sPo values ∼69% and ∼63% lower than control values (Table 1) ▶ . Compared with untreated groups of respective strains, IGF-I administration reduced sPo ∼17% in control muscles, but increased sPt and sPo ∼70% and ∼49%, respectively, in mdx muscles. Despite the increased force-generating capacity of mdx muscles treated with IGF-I, both sPt and sPo remained substantially depressed compared with the muscles of untreated control mice (treated mdx versus untreated control, sPt and sPo; 52% and 55%, respectively; P < 0.05; Table 1 ▶ ).
Table 1.
Summary of Morphometric and Functional Data for Diaphragm Muscles Examined after 8 Weeks of Treatment from Treated and Untreated Control and mdx Mice
| Control | mdx | |||
|---|---|---|---|---|
| Untreated (n = 6) | Treated (n = 7) | Untreated (n = 7) | Treated (n = 7) | |
| BM (g) | 31.2 ± 1.6 | 30.3 ± 1.3 | 35.6 ± 1.4* | 35.8 ± 1.0 |
| TPT (ms) | 34.8 ± 2.3 | 37.0 ± 1.6 | 36.0 ± 1.3 | 39.3 ± 1.1 |
| dP/dt (mN/ms) | 4.4 ± 0.8 | 4.4 ± 0.3 | 3.7 ± 0.4 | 4.1 ± 0.5 |
| 1/2RT (ms) | 51.5 ± 4.6 | 51.6 ± 2.0 | 47.9 ± 3.7 | 56.0 ± 3.2 |
| sPt (kN/m2) | 89.9 ± 16.2 | 72.7 ± 6.1 | 27.5 ± 3.2* | 47.0 ± 5.3‡ |
| sPo (kN/m2) | 270.7 ± 10.7 | 225.6 ± 15.4† | 100.1 ± 4.6* | 149.6 ± 9.6‡ |
Values are means ± SEM. n, no. of mice; BM, body mass; TPT, time to peak twitch tension; dP/dt, peak rate of twitch force generation; 1/2RT, peak twitch half relaxation time; sPt, specific isometric twitch force; sPo, specific maximum isometric tetanic force.
*P < 0.05 versus untreated control.
†P < 0.05 for treated control versus untreated control.
‡P < 0.05 for treated mdx versus untreated mdx.
There were no differences observed in time to peak twitch tension (TPT), and for Pt to decrease by 50% on abolition of stimulation (half relaxation time, 1/2RT), between strains or treatment groups (treated mdx versus untreated mdx, TPT and 1/2RT; P = 0.088 and 0.125, respectively by Student’s t-test; Table 1 ▶ ). Similarly, there were no differences in peak rate of twitch force generation (dP/dt) between groups according to strain or treatment.
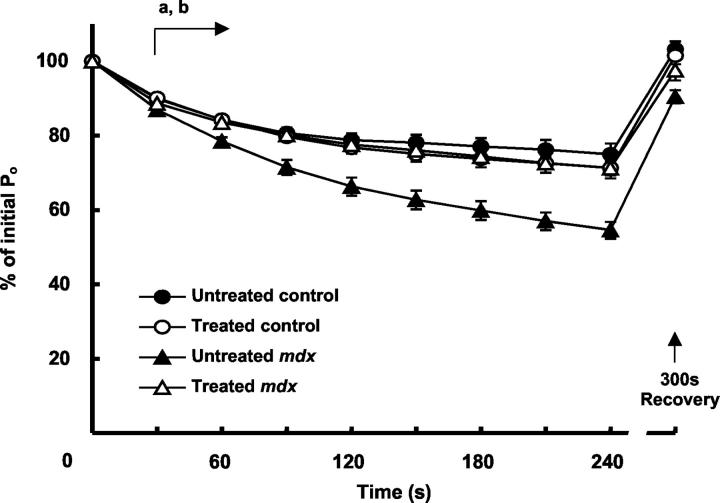
The 4-minute fatigue protocol of intermittent stimulation reduced the Po of muscle strips from control and mdx mice by ∼25% and ∼45% of initial values, respectively (Figure 1) ▶ . Peak force output as a percentage of initial Po was reduced in muscles of untreated mdx compared with untreated control mice within 30 seconds of starting the fatigue test, and the deficit between groups increased throughout the duration of the fatigue protocol (Figure 1) ▶ . In the diaphragm muscles of untreated control mice, cessation of stimulation for a period of 5 minutes immediately after the fatigue protocol restored Po to initial levels. After 5 minutes of recovery, Po of untreated mdx muscles was still depressed ∼10% relative to initial Po (recovery Po as a percentage of initial Po, untreated control versus untreated mdx, P < 0.05; Figure 1 ▶ ).
Figure 1.
Maximum force output during and 300 seconds after a repeated stimulation fatigue protocol for diaphragm muscle preparations from treated and untreated control and mdx mice. Note the comparatively greater reduction in force throughout time in the diaphragm muscles of untreated dystrophic mice compared with untreated control mice (a, P < 0.05), and the enhanced resistance of muscles from treated dystrophic mice compared with the untreated dystrophic muscles (b, P < 0.05) present from 30 seconds after commencement of stimulation, to the cessation of stimulation.
The fatigue response of muscles from treated and untreated control mice was not different. In contrast, diaphragm muscles of treated mdx mice showed an increased resistance to fatigue compared with muscles from untreated mdx mice throughout the 4-minute fatigue protocol (Figure 1 ▶ , P < 0.05). The difference in relative force output of muscles from treated relative to untreated mdx mice was significant after as little as 30 seconds of intermittent stimulation, and this greater relative force-producing capacity continued for the duration of the stimulation period (Figure 1) ▶ . Moreover, the diaphragm muscles of treated mdx mice maintained their force output (relative to initial Po) for the duration of the stimulation protocol similar to diaphragm muscles of control mice (Figure 1) ▶ . Recovery of force-producing capacity after the cessation of stimulation was greater in the muscles of treated compared with untreated dystrophic mice (percent of initial Po, 97.6 ± 2.7 versus 90.5 ± 1.8, respectively, P < 0.05; Figure 1 ▶ ). The recovery of force-producing capacity of muscles from treated dystrophic mice was not different from the muscles of control mice.
Structural and Histological Analyses
Cryosections of the diaphragm muscle preparations from untreated control mice that were tested in vitro were composed of fibers with a mean CSA of 709.2 ± 37.4 μm2. In comparison with control mice, sections of diaphragm muscles from untreated mdx mice were comprised of fibers that were on average ∼32% smaller (Table 2 ▶ , Figure 2C ▶ ). Histological examination of muscles from mdx mice identified widespread muscle degeneration and numerous small, regenerating muscle fibers that are characteristic of the dystrophic phenotype (Figure 2, A and B) ▶ . Population distribution of muscle fibers according to size demonstrated that the muscles of dystrophic mice contained fibers of varying CSA throughout a similar range of values as muscles of control mice (Figure 2C) ▶ . Thus, muscles of untreated dystrophic mice comprised a greater proportion of fibers with reduced CSA (in particular, fibers of less than 400 μm2), as opposed to a complete absence of fibers of greater CSA (ie, more than 1000 μm 2 in size, Figure 2C ▶ ). IGF-I treatment had no effect on mean muscle fiber CSA or on the distribution of muscle fiber proportions according to size in the diaphragm muscles of either control or mdx mice (Table 2 ▶ , Figure 2C ▶ ).
Table 2.
Summary of Morphological and Histochemical Data for Diaphragm Muscles Examined after 8 Weeks of Treatment from Treated and Untreated Control and mdx Mice
| Control | mdx | |||
|---|---|---|---|---|
| Untreated (n = 6) | Treated (n = 7) | Untreated (n = 7) | Treated (n = 7) | |
| Av CSA (μm2) | 709.2 ± 37.4 | 676.6 ± 62.9 | 483.1 ± 29.5* | 538.0 ± 58.8* |
| Type I (%) | 7.6 ± 0.9 | 7.6 ± 1.2 | 7.0 ± 2.4 | 11.9 ± 3.9 |
| Type IIa (%) | 33.7 ± 2.5 | 47.6 ± 4.7† | 30.6 ± 3.8 | 39.7 ± 6.2 |
| Type IId/x (%) | 58.6 ± 3.2 | 44.8 ± 5.6† | 62.4 ± 4.5 | 48.3 ± 4.4‡ |
| SDH (mmol/l/min) | 7.2 ± 0.7 | 7.8 ± 0.6 | 5.8 ± 0.4 | 7.9 ± 0.8‡ |
Values are means ± SEM. n, no. of mice; Av CSA, mean cross sectional area of all fibers counted; type I, IIa, IId/x, fiber types according to myosin ATPase activity; SDH, mean fiber succinate dehydrogenase activity as expressed in mmol of fumarate consumed/L tissue/minute.
*P < 0.05 versus untreated control.
†P < 0.05 for treated control versus untreated control.
‡P < 0.05 for treated mdx versus untreated mdx.
Figure 2.
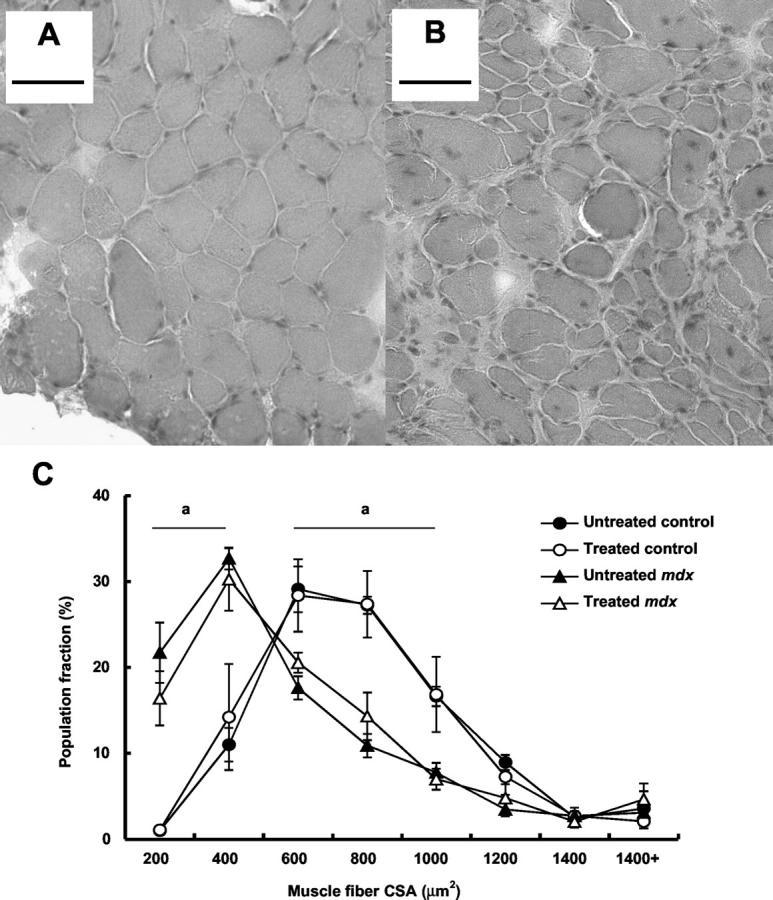
Photomicrographs of H&E-stained transverse sections of diaphragm muscles of untreated control mice (A) and untreated mdx mice (B), and population frequency distribution of muscle fibers from the diaphragm muscles of treated and untreated control and mdx mice according to cell CSA (C). Note the increased frequency of centrally nucleated muscle fibers, a characteristic of regenerating muscles, in the diaphragm samples of mdx mice (A) compared with control mice (B). The prevalence of numerous small regenerating fibers contributes to an increased proportion of fibers of small (ie, < 400 μm2) CSA in the muscles of mdx mice (C). Scale bars, 50 μm. a, P < 0.05, untreated control versus untreated mdx.
The muscles of the untreated control mice were composed of type I, IIa, and IId/x muscle fibers in the ratios of 1:4.4:7.7, respectively, and did not appear to comprise any type IIb fibers (Table 2) ▶ . There was no difference in the fiber type proportions within diaphragm muscles of mdx mice compared with control mice. Muscles from treated control mice comprised an ∼24% reduced proportion of IId/x fibers and an increased proportion of IIa fibers compared with untreated mice (Table 2) ▶ . Similarly, muscles from treated mdx mice comprised an ∼23% reduced proportion of IId/x fibers with concomitant increased distributions of type I and IIa fibers compared muscles from untreated mdx mice.
Quantitative histochemical determination of the SDH activity of individual muscle fibers was used to obtain a mean value equivalent to that capable of consuming 7.2 ± 0.7 mmol of fumarate/L of tissue/minute for the diaphragm muscles of untreated control mice. There was no difference in mean SDH activity between the diaphragm muscles of untreated control and mdx mice (P = 0.103, Table 2 ▶ ). No differences in SDH activity were evident between the diaphragm muscles of treated and untreated control mice. In contrast, the SDH activity of diaphragm muscles from treated mdx mice was ∼35% higher than that measured for muscles from untreated mdx mice (7.9 ± 0.8 versus 5.8 ± 0.4 mmol/L/minute, respectively; P < 0.05). Population distribution of muscle fibers according to SDH activity demonstrated that the muscles of untreated dystrophic mice contained a greater proportion of fibers with reduced SDH activity (<4 mmol/L/min) than muscles from control mice (Figure 3) ▶ . Sections of diaphragm muscle from treated mdx mice comprised a reduced population of fibers with <4 mmol/L/minute SDH activity such that the proportion of these fibers was not different from diaphragm muscles of untreated control mice (Figure 3C) ▶ .
Figure 3.
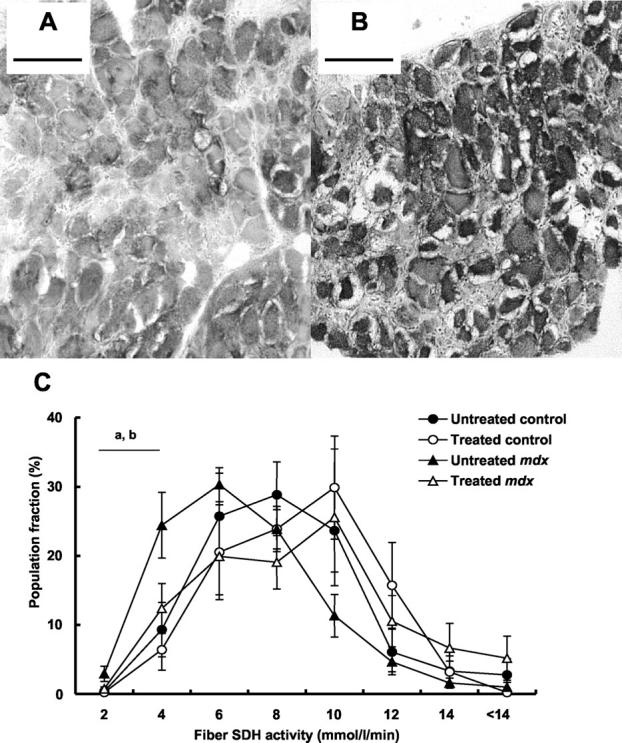
Photomicrographs of transverse sections of diaphragm muscles of untreated mdx mice (A) and treated mdx mice reacted for SDH activity (B), and population frequency distribution of muscle fibers from the diaphragm muscles of treated and untreated control and mdx mice according to cell SDH activity (C). Note the reduced frequency of muscle fibers with <4 mmol/L/minute SDH activity in mdx compared with control mice, and the reduced frequency of muscle fibers with <4 mmol/L/minute SDH activity in treated mdx compared to untreated mdx mice (C). The diminished prevalence of muscle fibers with low SDH activity contributes to an increased mean fiber SDH activity in the muscles of treated compared with untreated mdx mice. Scale bars, 100 μm. a, P < 0.05, untreated control versus untreated mdx; b, P < 0.05, untreated mdx versus treated mdx.
Discussion
The most important findings of this study were that IGF-I administration increased the specific force output and resistance to fatigue of diaphragm muscles from mdx dystrophic mice. These findings support our original hypothesis in part and indicate that exogenous administration of IGF-I can enhance the functional properties of skeletal muscles that exhibit pathology associated with a dystrophin deficiency.
Our reported deficits in force-producing capacity between the muscles of dystrophic and control mice agree with the literature. 15,17,18 The sPo values produced by the diaphragm muscles of treated dystrophic mice represent an improvement of ∼50% over those of untreated dystrophic mice. Despite this significant improvement in force-generating capacity, the values for sPo are still ∼55% of those for muscles from control mice. This finding illustrates that the IGF-I intervention alone was insufficient to surmount the deficiency in force-producing capacity as a consequence of the dystrophic pathology expressed in mdx mice. It should be noted, that unlike the host of genetic therapies under development that are concerned with stimulating endogenous production of the proteins deficient in various dystrophies, 2 the administration of IGF-I constitutes an intervention aimed at ameliorating the deleterious symptoms associated with the pathological phenotype. Therefore it is to be expected that the dystrophic mice would continue to exhibit some level of impairment of skeletal muscle structure and function despite the intervention.
Of clinical and functional significance was the fact that the muscles of treated dystrophic mice exhibited an enhanced resistance to muscle fatigue compared with untreated mice, similar to values for muscles from control mice. By virtue of their critical role in respiration, the nature of recruitment of the diaphragm muscles is highly regular, compared with the more sporadic recruitment of the majority of skeletal muscles. Furthermore, respiratory function impairment is recognized as a key predictor of mortality in DMD patients. 1,14 Therefore, the enhanced resistance to fatigue during regular activation of the diaphragm muscles of dystrophic mice treated with IGF-I represents a significant physiological benefit of this intervention with potential application to conditions such as DMD in which respiratory muscle function is compromised. 1,14 These data also indicate that some features of the muscle pathology of this model are affected by the intervention more than others. This idea is consistent with the concept that IGF-I can influence various features of skeletal muscle development and adaptation through several different mechanisms, some of which may modulate the structural and functional characteristics of dystrophic skeletal muscles more than others. 6
To explain the observed functional discrepancies between strains and groups, we also considered the histological and histochemical properties of the diaphragm muscles. Consistent with the findings of others, we noted widespread presence of immature, regenerating muscle fibers in the muscles of dystrophic mice, and extensive muscle degeneration. Although IGF-I influences numerous transduction pathways to exert seemingly contradictory effects during the life cycle of the skeletal muscle fiber, as a therapeutic intervention it is considered a potent stimulator of muscle anabolism. 6,7,12,22 In contrast to one aspect of our original hypothesis, and the findings of others, 13,22 we did not observe evidence of enhanced fiber size as a result of IGF administration in muscles of either dystrophic or control mice. As IGF-I is recognized to exert a variety of potential side effects subject to concentration, 24 we used a relatively low-dose protocol compared with previous studies examining skeletal muscle. 13,19,21-23 At the dose used, considerable increases in serum (and thus systemic) IGF-I concentration were noted after as little as 2 weeks of subcutaneous IGF-I administration, although muscle concentrations of IGF-I were not significantly different. Although uptake of IGF-I by skeletal muscles after systemic administration is modest compared with other tissues, 33 it is possible that intragroup variability and limited group size in this study reduced the probability of detecting a significant change in muscle IGF-I concentration. Although logistically difficult when using mice, it would be ideal to biopsy and test samples before and after IGF-I intervention in a repeated measures manner to accurately quantify changes in IGF-I concentrations in subjects. We propose that the IGF-I administration protocol used elicits beneficial effects in dystrophic skeletal muscles in the absence of altered IGF-I concentration of the magnitude required to induce muscle hypertrophy. 13
The data indicate that the underlying mechanism responsible for the enhanced fatigue resistance of the diaphragm muscles of treated mdx mice relates to IGF-I-induced alterations in muscle fiber type proportions. It should be noted that in the present study, the muscles of mdx mice comprised a considerable population of regenerating fibers with intermediate ATPase activity that may contribute to differences between the fiber type distributions reported in our study and that of Petrof and colleagues 34 who examined fiber proportions based on immunohistochemistry. However, the muscles of treated dystrophic mice comprised a reduced proportion of IId/x fibers with a concomitant increased distribution of fibers between the type I and type IIa classifications compared with the muscles of untreated mice. Furthermore, the muscles of treated dystrophic mice demonstrated ∼38% increased SDH activity relative to the untreated group. Both observations indicate an alteration in the properties of diaphragm muscle fibers in IGF-I treated dystrophic mice such that the muscle fibers become more highly oxidative and resistant to muscle fatigue. In rats it has been noted that stimulation of the IGF-I system via IGF-I/GH co-administration can increase muscle fiber SDH activity, 35 and in vitro there is evidence that IGF-I-mediated pathways can contribute to the regulation of the muscle fiber phenotype. 36 Therefore, IGF-I may be exerting effects on the mechanisms regulating fiber type in dystrophic muscles. Additional adaptations in fiber phenotype with IGF-I administration may extend to the various membrane-bound receptor/channel populations that regulate voltage- and ion-dependent calcium release within the muscle fibers. Down-regulation of the components of the intracellular calcium release/reuptake system has been implicated in the perturbation of muscle function with advancing age, and therefore it is logical to suggest that up-regulated expression of these structures (as observed with IGF-I administration in models of aging) might enhance the fatigue resistance of dystrophic skeletal muscles. 11,37
That alterations in fiber type proportions but not fiber SDH activity were recorded from the muscles of treated control mice suggests the two strains are not necessarily equally sensitive to the dose of IGF-I administered. Alternatively, it may be more accurate to suggest that the prevalence of large numbers of developing, regenerating muscle fibers in the dystrophic muscles constitutes a more sensitive and adaptive population of cells than the comparatively stable and mature fibers of nondystrophic muscles. 38 It is also unclear as to why the treatment protocol in question would result in a reduced sPo in control muscles, other than to suggest that the IGF-I system in the skeletal muscles of nonpathological animals probably operates near-optimally and does not always elicit equivalent responses compared with dystrophic muscles. 13 Clearly then, the present findings recommend further investigation into the precise actions of exogenous IGF-I on models of muscular dystrophy.
In the context of conditions afflicting skeletal muscle (such as the muscular dystrophies), the potential usefulness of an intervention is dependent on the extent to which it ameliorates the pathologically induced impairment of muscle function. The present findings demonstrate that the systemic administration of IGF-I meets this criterion in that it enhances the functional properties of muscles in an animal model of dystrophinopathy. An enhanced understanding of the workings of IGF-I is vital as an increasing number of clinically relevant trials demonstrates a growing interest in the use of therapeutic IGF-I interventions in medical practice. 39-42 However, we must define the full potential of such interventions, for the worth of all potential benefits is tempered by the recognized risks. 24 What must follow is an effort to elucidate the mechanisms by which IGF-I exerts its effects under these conditions, and the potential for benefit to be conferred to human neuromuscular disorders.
Footnotes
Address reprint requests to Gordon S. Lynch, Ph.D., Department of Physiology, The University of Melbourne, Victoria, Australia, 3010. E-mail: gsl@unimelb.edu.au.
Supported by the Muscular Dystrophy Association, USA.
References
- 1.Engel AG, Yamamoto M, Fischbeck KH: Dystrophinopathies. Engel AG Franzini-Armstrong C eds. Myology. 1994:pp 1133-1187 McGraw-Hill, New York
- 2.Hartigan-O’Connor D, Chamberlain JS: Developments in gene therapy for muscular dystrophy. Microsc Res Tech 2000, 48:223-238 [DOI] [PubMed] [Google Scholar]
- 3.Adams GR, Haddad F: The relationships among IGF-I, DNA content, and protein accumulation during skeletal muscle hypertrophy. J Appl Physiol 1996, 81:2509-2516 [DOI] [PubMed] [Google Scholar]
- 4.Marsh DR, Criswell DS, Hamilton MT, Booth FW: Association of insulin-like growth factor mRNA expressions with muscle regeneration in young, adult, and old rats. Am J Physiol 1997, 273:R353-R358 [DOI] [PubMed] [Google Scholar]
- 5.De Luca A, Pierno S, Camerino C, Cocchi D, Camerino DC: Higher content of insulin-like growth factor-I in dystrophic mdx mouse: potential role in the spontaneous regeneration through an electrophysiological investigation of muscle function. Neuromusc Disord 1999, 9:11-18 [DOI] [PubMed] [Google Scholar]
- 6.Singleton JR, Feldman EL: Insulin-like growth factor-I in muscle metabolism and myotherapies. Neurobiol Dis 2001, 8:541-554 [DOI] [PubMed] [Google Scholar]
- 7.Bark TH, McNurlan MA, Lang CH, Garlick PJ: Increased protein synthesis after acute IGF-I or insulin infusion is localized to muscle in mice. Am J Physiol 1998, 275:E118-E123 [DOI] [PubMed] [Google Scholar]
- 8.Fang CH, Li BG, Wang JJ, Fischer JE, Hasselgren PO: Treatment of burned rats with insulin-like growth factor I inhibits the catabolic response in skeletal muscle. Am J Physiol 1998, 275:R1091-R1098 [DOI] [PubMed] [Google Scholar]
- 9.Chrysis D, Underwood LE: Regulation of components of the ubiquitin system by insulin-like growth factor I and growth hormone in skeletal muscle of rats made catabolic with dexamethasone. Endocrinology 1999, 140:5635-5641 [DOI] [PubMed] [Google Scholar]
- 10.Chakravarthy MV, Davis BS, Booth FW: IGF-I restores satellite cell proliferative potential in immobilized old skeletal muscle. J Appl Physiol 2000, 89:1365-1379 [DOI] [PubMed] [Google Scholar]
- 11.Delbono O: Regulation of excitation contraction coupling by insulin-like growth factor-1 in aging skeletal muscle. J Nutr Health Aging 2000, 4:162-164 [PubMed] [Google Scholar]
- 12.Musaro A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, Barton ER, Sweeney HL, Rosenthal N: Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet 2001, 27:195-200 [DOI] [PubMed] [Google Scholar]
- 13.Barton ER, Morris L, Musaro A, Rosenthal N, Sweeney HL: Muscle-specific expression of insulin-like growth factor I counters muscle decline in mdx mice. J Cell Biol 2002, 157:137-148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matecki S, Topin N, Hayot M, Rivier F, Echenne B, Prefaut C, Ramonatxo M: A standardized method for the evaluation of respiratory muscle endurance in patients with Duchenne muscular dystrophy. Neuromusc Disord 2001, 11:171-177 [DOI] [PubMed] [Google Scholar]
- 15.Lynch GS, Hinkle RT, Faulkner JA: Force and power output of diaphragm muscle strips from mdx and control mice after clenbuterol treatment. Neuromusc Disord 2001, 11:192-196 [DOI] [PubMed] [Google Scholar]
- 16.Lynch GS, Hinkle RT, Chamberlain JS, Brooks SV, Faulkner JA: Force and power output of fast and slow skeletal muscles from mdx mice 6–28 months old. J Physiol 2001, 535:591-600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stedman HH, Sweeney HL, Shrager JB, Maguire HC, Panettieri RA, Petrof B, Narusawa M, Leferovich JM, Sladky JT, Kelly AM: The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature 1991, 352:536-539 [DOI] [PubMed] [Google Scholar]
- 18.Dupont-Versteegden EE, McCarter RJ: Differential expression of muscular dystrophy in diaphragm versus hindlimb muscles of mdx mice. Muscle Nerve 1992, 15:1105-1110 [DOI] [PubMed] [Google Scholar]
- 19.Zdanowicz MM, Moyse J, Wingertzahn MA, O’Connor M, Teichberg S, Slonim AE: Effect of insulin-like growth factor I in murine muscular dystrophy. Endocrinology 1995, 136:4880-4886 [DOI] [PubMed] [Google Scholar]
- 20.Hsu HH, Zdanowicz MM, Agarwal VR, Speiser PW: Expression of myogenic regulatory factors in normal and dystrophic mice: effects of IGF-1 treatment. Biochem Mol Med 1997, 60:142-148 [DOI] [PubMed] [Google Scholar]
- 21.Wingertzahn MA, Zdanowicz MM, Slonim AE: Insulin-like growth factor-I and high protein diet decrease calpain-mediated proteolysis in murine muscular dystrophy. Exp Biol Med 1998, 218:244-250 [DOI] [PubMed] [Google Scholar]
- 22.Lynch GS, Cuffe SA, Plant DR, Gregorevic P: IGF-I treatment improves the functional properties of fast- and slow-twitch skeletal muscles from dystrophic mice. Neuromusc Disord 2001, 11:260-268 [DOI] [PubMed] [Google Scholar]
- 23.Granchelli JA, Pollina C, Hudecki MS: Pre-clinical screening of drugs using the mdx mouse. Neuromusc Disord 2000, 10:235-239 [DOI] [PubMed] [Google Scholar]
- 24.Bach LA: The insulin-like growth factor system: basic and clinical aspects. Aust N Z J Med 1999, 29:355-361 [DOI] [PubMed] [Google Scholar]
- 25.Vahlsing HL, Varon S, Hagg T, Fass-Holmes B, Dekker A, Manley M, Manthorpe M: An improved device for continuous intraventricular infusions prevents the introduction of pump-derived toxins and increases the effectiveness of NGF treatments. Exp Neurol 1989, 105:233-243 [DOI] [PubMed] [Google Scholar]
- 26.Markowska AL, Mooney M, Sonntag WE: Insulin-like growth factor-I ameliorates age-related behavioral deficits. Neuroscience 1998, 87:559-569 [DOI] [PubMed] [Google Scholar]
- 27.Lynch GS, Rafael JA, Hinkle RT, Cole NM, Chamberlain JS, Faulkner JA: Contractile properties of diaphragm muscle segments from old mdx and old transgenic mdx mice. Am J Physiol 1997, 272:C2063-C2068 [DOI] [PubMed] [Google Scholar]
- 28.Segal SS, Faulkner JA: Temperature-dependent physiological stability of rat skeletal muscle in vitro. Am J Physiol 1985, 248:C265-C270 [DOI] [PubMed] [Google Scholar]
- 29.Mendez J, Keys A: Density and composition of mammalian muscle. Metabolism 1960, 9:184-199 [Google Scholar]
- 30.Hämäläinen N, Pette D: The histochemical profiles of fast fiber types IIB, IID and IIA in skeletal muscles of mouse, rat and rabbit. J Histochem Cytochem 1993, 41:733-743 [DOI] [PubMed] [Google Scholar]
- 31.Blanco CE, Sieck GC, Edgerton VR: Quantitative histochemical determination of succinic dehydrogenase activity in skeletal muscle fibres. Histochem J 1988, 20:230-243 [DOI] [PubMed] [Google Scholar]
- 32.Bach LA, Jerums G: Effect of puberty on initial kidney growth and rise in kidney IGF-I in diabetic rats. Diabetes 1990, 39:557-562 [DOI] [PubMed] [Google Scholar]
- 33.Ballard FJ, Knowles SJ, Walton PE, Edson K, Owens PC, Mohler MA, Ferraiolo BL: Plasma clearance and tissue distribution of labelled insulin-like growth factor-I (IGF-I), IGF-II and des(1-3)IGF-I in rats. J Endocrinol 1991, 128:197-204 [DOI] [PubMed] [Google Scholar]
- 34.Petrof BJ, Stedman HH, Shrager JB, Eby J: Sweeney HL, Kelly AM Adaptations in myosin heavy chain expression and contractile function in dystrophic mouse diaphragm. Am J Physiol 1993, 265:C834-C841 [DOI] [PubMed] [Google Scholar]
- 35.Lewis MI, LoRusso TJ, Fournier M: Anabolic influences of insulin-like growth factor I and/or growth hormone on the diaphragm of young rats. J Appl Physiol 1997, 82:1972-1978 [DOI] [PubMed] [Google Scholar]
- 36.Delling U, Tureckova J, Lim HW, De Windt LJ, Rotwein P, Molkentin JD: A calcineurin-NFATc3-dependent pathway regulates skeletal muscle differentiation and slow myosin heavy-chain expression. Mol Cell Biol 2000, 20:6600-6611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang ZM, Messi ML, Delbono O: Sustained overexpression of IGF-I prevents age-dependent decrease in charge movement and intracellular Ca2+ in mouse skeletal muscle. Biophys J 2002, 82:1338-1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Donovan CM, Faulkner JA: Plasticity of skeletal muscle: regenerating fibers adapt more rapidly than surviving fibers. J Appl Physiol 1987, 62:2507-2511 [DOI] [PubMed] [Google Scholar]
- 39.Vlachopapadopoulou E, Zachwieja JJ, Gertner JM, Manzione D, Bier DM, Matthews DE, Slonim AE: Metabolic and clinical response to recombinant human insulin-like growth factor I in myotonic dystrophy—a clinical research center study. J Clin Endocrinol Metab 1995, 80:3715-3723 [DOI] [PubMed] [Google Scholar]
- 40.Festoff BW: The preclinical rationale for the use of insulin-like growth factor-I in amyotrophic lateral sclerosis. Drugs Today 1998, 34:65-77 [DOI] [PubMed] [Google Scholar]
- 41.Herndon DN, Ramzy PI, Debroy MA, Zheng M, Ferrando AA, Chinkes DL, Barret JP, Wolfe RR, Wolf SE: Muscle protein catabolism after severe burn: effects of IGF-1/IGFBP-3 treatment. Ann Surg 1999, 229:713-722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laron Z: Somatomedin-1 (recombinant insulin-like growth factor-1)—clinical pharmacology and potential treatment of endocrine and metabolic disorders. Biodrugs 1999, 11:55-70 [DOI] [PubMed] [Google Scholar]