Abstract
Clinical disorders associated with increased serotonin [5-hydroxytryptamine (5-HT)] levels, such as carcinoid syndrome, and the use of serotonin agonists, such as fenfluoramine have been associated with a valvulopathy characterized by hyperplastic valvular and endocardial lesions with increased extracellular matrix. Furthermore, 5-HT has been demonstrated to up-regulate transforming growth factor (TGF)-β in mesangial cells via G-protein signal transduction. We investigated the hypothesis that increased exposure of heart valve interstitial cells to 5-HT may result in increased TGF-β1 expression and activity because of serotonin receptor-mediated signal transduction with activation of Gαq, and subsequently up-regulation of phospholipase C. Thus, in the present study we performed a clinical-pathological investigation of retrieved carcinoid and normal valve cusps using immunohistochemical techniques to detect the presence of TGF-β1 and other proteins associated with TGF-β expression, including TGF-β receptors I and II, latent TGF-β-associated peptide (LAP), and α-smooth muscle actin. Carcinoid valve cusps demonstrated the unusual finding of widespread smooth muscle actin involving the interstitial cells in the periphery of carcinoid nodules; these same cells were also positive for LAP. Normal valve cusps were only focally positive for smooth muscle actin and LAP. In sheep aortic valve interstitial cell cultures 5-HT induced TGF-β1 mRNA production and increased TGF-β1 activity. 5-HT also increased collagen biosynthesis at the dosages studied. Furthermore, TGF-β1 added to SAVIC cultures increased the production of sulfated glycan and hyaluronic acid. In addition, overexpression of Gαq using an adenoviral expression vector for a constitutively active Gαq mutant (Q209L-Gαq) resulted in increased phospholipase C activity as well as up-regulation of TGF-β expression and activity. These results strongly support the view that G-protein-related signal transduction is involved in 5-HT up-regulation of TGF-β1. In conclusion, 5-HT-associated valve disease may be, in part, because of TGF-β1 mechanisms.
Heart valve disease associated with increased serotonin [5 hydroxytryptamine (5-HT)] levels has been observed with carcinoid tumors, 1 and a 5-HT mechanism has been hypothesized to be responsible for the valvulopathy that has been described after prolonged administration of the diet drug combination of fenfluramine-phentermine. 2 Cuspal lesions associated with increased 5-HT levels have characteristically demonstrated subendothelial plaques with cuspal interstitial cell hyperplasia, and increased amounts of extracellular matrix constituents. 2 The presence of distinct subsets of 5-HT receptors in human heart valves 3,4 suggests that valvular interstitial cells have the potential to respond to serotonin.
A previous study of a clinical series of carcinoid valves removed at cardiac surgery investigated transforming growth factor (TGF)-β mechanisms and demonstrated that increased amounts of latent TGF-β-associated peptide and latent TGF-β-binding protein were present in the cuspal interstitial cells and extracellular matrix, respectively. 5 Furthermore, related research investigating mesangial cells has shown that 5-HT induces the up-regulation of TGF-β1 in this cell type. 6 Thus, serotonin could hypothetically mediate comparable effects by up-regulating TGF-β1 within heart valve interstitial cells.
Furthermore, because others using reverse transcriptase-polymerase chain reaction (RT-PCR) techniques have demonstrated the 5-HT type 2 receptor to be present in aortic valve interstitial cells (AVICs), 3 we further hypothesized that 5-HT control of TGF-β1 occurs through a G-protein signal transduction pathway. Thus, we hypothesized that 5-HT-associated heart valve disease is, in part, because of 5-HT-receptor-mediated up-regulation of TGF-β1 with resulting increased deposition of extracellular matrix components.
Materials and Methods
Human Pathology Specimens and Immunohistochemical Studies
Human aortic valves were obtained either at the time of cardiac surgery or at autopsy, under exemptions granted by the Institutional Review Boards of the Hospital of the University of Pennsylvania and the Children’s Hospital of Philadelphia. Carcinoid human tricuspid and pulmonary valve cusps were obtained from three female patients (age range, 47 to 75 years) with severe carcinoid heart disease undergoing valve removal and valve replacement. Normal human tricuspid valves were obtained at autopsy from human patients ranging in age from 65 to 74 years, including three males and two females. All valve specimens were fixed in neutral buffered formalin. Paraffin thin sections were prepared and routine microscopy hematoxylin and eosin staining was performed. Immunohistochemical studies were performed using an avidin-biotin complex technique (Vector Laboratories, Burlingame, CA), with immunoperoxidase methodology using diaminobenzidine tetrahydrochloride (Vector Laboratories) as a final chromogen. The primary antibodies, anti-TGF-β1 (1:400), anti-TGF-β RI (1:100), and anti-TGF-βRII (1:200), were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Antibody to human latent associated peptide-TGF-β1 (LAP-TGF-β1, AB-246-NA) was obtained from R&D Systems (Minneapolis, MN). Antigen was retrieved by heating the slides at 95°C in 10 mmol/L of sodium citrate (pH 6.0) for 20 minutes. Paraffin sections of human high-grade osteosarcoma and salivary gland were used as positive controls for TGF-β staining. 7,8 Slides incubated with nonspecific mouse or rabbit IgG (DAKO, Carpinteria, CA) were used as a negative control. Antibodies used for cell characterization studies included the following: a mouse monoclonal antibody against human (α/τ)-smooth muscle actin (SMA) was purchased from DAKO. A Serotonin 2A (5-HT2A) receptor polyclonal antibody was obtained from Oncogene Research Products (Boston, MA). No other anti-5-HT receptor antibodies were commercially or privately available for these studies. Other antibodies used in the study include: von Willebrand factor (DAKO), anti-desmin, anti-vimentin, and anti-calponin (all from Sigma, St. Louis, MO).
Cell Culture
Aortic valve leaflets were isolated from mature female sheep (Western Cross from Thomas Morris, Reisterstown, MD) as approved by the Institutional Animal Care and Use Committee (IACUC) of the Children’s Hospital of Philadelphia. Fresh excised cusps were rinsed in Medium 199 (Life Technologies, Inc., Gaithersburg, MD). Leaflets were then scraped lightly on both surfaces with a scalpel blade to remove endothelial cells. To obtain sheep aortic valve interstitial cells (SAVICs), 1- to 2-mm pieces of leaflets were cut by gentle rolling of the scalpel blade, and were cultivated in Medium 199 supplemented with 10% fetal bovine serum (FBS) (HyClone, Logan, UT) and penicillin/streptomycin (Life Technologies, Inc.) in a humidified atmosphere of 95% air-5% CO2 at 37°C. In most of the studies, cells were cultivated in six-well plates coated with bovine type I collagen (Cohesion Technologies, Inc., Palo Alto, CA) as previously described. 9 Cells from passages 3 to 10 were used in all experiments. Quiescence was induced by plating the cells in Medium 199 with 0.5% FBS and penicillin/streptomycin for 24 hours before the treatment with reagents of interest.
For cell characterization studies, SAVICs were plated on collagen gels at a density of 20 cells/mm2. After 24 to 72 hours of culture in Medium 199 (10% FBS), cells were fixed in 10% neutral buffered formalin. Cultures used for detection of 5-HT2A receptor were fixed in 4% paraformaldehyde for 30 minutes. Immunohistochemistry studies were performed with specific antibodies for α/β-SMA, the serotonin 5-HT2A receptor, von Willebrand factor, desmin, vimentin, and calponin. Immunostaining via immunoperoxidase used biotin-labeled peroxidase-conjugated secondary antibody incubations with diaminobenzidine tetrahydrochloride as a final chromogen as described above.
Transient Expression of Constitutively Active Mutant Gαq in SAVICs
A replication defective adenovirus (type V, E1, E3 deleted) containing the gene sequence for the constitutively active GTPase-deficient Gαq (Q209L) under the control of the human cytomegalovirus promoter (CMV) termed AdCMV-Gαq, was kindly provided by Dr. Morris Birnbaum (Department of Medicine, University of Pennsylvania, Philadelphia, PA). 10 SAVICs were plated on six-well plates overnight in Medium 199 containing 0.5% FBS. After washing, adenovirus suspensions were added at a titer of 10 7 or 10 8 plaque-forming units (PFU) per well (∼25 to 250 PFU/cell). A replication defective adenoviral vector encoding for the green fluorescent protein (AdCMV-GFP), a control vector, was obtained from the Institute for Human Gene Therapy of the University of Pennsylvania (Philadelphia, PA).
Real-Time RT-PCR of TGF-β1 Transcripts
Real-time RT-PCR was performed to measure TGF-β1 expression semiquantitatively as described. 11 Total RNAs were isolated (Trizol reagents, Life Technologies, Inc.) from SAVICs cultivated on bovine type I collagen stimulated with various concentrations of serotonin in Medium 199 with 0.5% fetal calf serum. Typically, 1 μg of total RNA was treated with amplification grade deoxyribonuclease I (Life Technologies, Inc.) and reverse-transcribed using the TaqMan reverse transcription kit (Perkin-Elmer, Foster City, CA). TGF-β1 primers and probe, based on the sheep TGF-β1 sequence, used for real time RT-PCR were: primers, TGTTCGTCAGCTCTACATTGACTTC and GGCCCCAGGCAGAAATTG; probe: TGGATTCACGAACCCAAGGGCTACC. The probe was labeled at the 5′ end with the reporter dye 6-FAM and on the 3′ end with the dye TAMRA (Perkin-Elmer, Foster City, CA). TaqMan ribosomal RNA was used as an internal control. TaqMan runs were performed in triplicate.
Phospholipase C (PLC) Assay
PLC activity was evaluated via the following procedures: 12 SAVICs cultivated on type I collagen-coated plates were labeled with [3H]myo-inositol (5 μCi/ml; NEN Life Science Products, Inc., Boston, MA) for 24 hours in Dulbecco’s modified Eagle’s medium (without inositol) (Life Technologies, Inc.) supplemented with 0.5% FBS. After washing, serotonin in different concentrations was diluted in 15 mmol/L of LiCl (Sigma) containing Dulbecco’s modified Eagle’s medium and incubated with [3H]inositol-labeled cells for various time points. Inositol-containing fractions were extracted from cells using chloroform/methanol/HCL (1:2:0.05). Inositol 1-phosphate (InsP1), inositol 1,4-bisphosphate [Ins(1,4)P2], and inositol 1,4,5-phosphate [Ins(1,4,5)P3] were eluted sequentially with 100 mmol/L formic acid and 200 mmol/L ammonium formate, 100 mmol/L formic acid and 600 mmol/L ammonium formate, and 100 mmol/L formic acid and 1 mol/L ammonium formate, respectively, on an anion-exchange columns (Agx8 resin, formate form). The columns were calibrated with each inositol phosphate standard to confirm complete separation of InsP1, Ins(1,4)P2, and Ins(1,4,5)P3.
Luciferase Assay for TGF-β Activity
Equal numbers of quiescent cells (∼4 × 105 cells) on collagen-coated six-well plates were treated with 10 μmol/L of 5-HT for 24 to 72 hours. After incubation, medium (test sample) was collected, centrifuged, and assayed for TGF-β1 activity by the plasminogen activator inhibitor I luciferase (PAI/L) assay, as described by Abe and colleagues. 13 Briefly, 1.6 × 104 mink lung epithelial cells, which were stably transfected with a portion of the plasminogen activator inhibitor 1 promoter (a generous gift from Dr. DB Rifkin, New York University Medical Center, New York, NY), were plated in 96-well tissue culture plates and allowed to attach for at least 3 hours at 37°C in a 5% CO2 incubator. The medium was replaced with the test sample for 14 hours at 37°C to assay the active TGF-β activity. For total TGF-β1 (active and latent), test samples were heated for 5 minutes at 80°C and diluted 4- to 10-fold before adding to mink lung epithelial cells. Cell extracts were prepared and assayed for luciferase activity using a luciferase assay kit (Tropix, Inc., Bedford, MA). TGF-β1-neutralizing antibody (AB-100-NA; R & D Systems, Minneapolis, MN) was also used in control studies to document the specificity of the assay.
Collagen and Total Protein Synthesis
Collagen synthesis was assessed by measuring the cellular uptake of 3H-proline as described by Hafizi and colleagues 14 with minor modifications. Briefly, SAVICs were seeded on type I collagen-coated 24-well plates at a density of 3 × 10 4 cells per well in growth medium overnight. To make the cells quiescent, cells were incubated with M199/0.5% FBS for at least 24 hours before adding various concentrations of 5-HT, TGF-β1, or anti-TGF-β1 antibody (R & D Systems). l-[2,3,4,5-3H]proline (NEN Life Science Products, Inc.) was added to each well at a final concentration of 10 μCi/ml and the cells were incubated for 48 hours before trichloroacetic acid precipitation. For total protein synthesis, cells were incubated with test samples for 24 hours and l-[3H]leucine (1 μCi/ml, NEN Life Science Products, Inc.) incorporation throughout the last 5 hours was measured. 15 The specificity of 5-HT stimulation of 3H-proline by a TGF-β1 mechanism was assessed by adding anti-TGF-β1 antibody (R & D Systems) to the 3H-proline samples just described.
Cell Proliferation Assay
A colorimetric assay was used according to manufacturer’s direction (Boehringer Mannheim, Indianapolis, IN) for the quantification of cell proliferation and cell viability, based on the cleavage of the tetrazolium salt WST-1 by mitochondrial dehydrogenases in viable cells. SAVICs were cultured on type I collagen-coated 96-well plates in a density of 1 × 104 cells/well. After 48 hours of incubation in reduced serum culture medium (M199/0.5% FBS), without or with test compounds, or a control positive stimulus (10% FBS), 10 μl of the cell proliferation reagent WST-1 was added to each well and incubated for 2 hours. The absorbance was measured using a microtiter plate reader at a wavelength of 418 nm.
Quantitative Assay for Hyaluronic Acid
Hyaluronic acid (HA) synthesis was measured by an enzyme-linked immunosorbent-like assay as described previously. 16 Conditioned medium and standards were first preincubated with protease (bacterial type XIV, Sigma) to remove interfering proteins, then preincubated with biotinylated HA-binding protein (bHABP; Seikagaku, Japan), and finally applied to microtiter plates coated with HA (ICN, Irvine, CA). The plates were washed and the bound bHABP was quantitated using an avidin-peroxidase system (Vectastain ABC kit, Vector Laboratories) with o-phenylenediamine (Sigma) and 0.015% hydrogen peroxide. The intensity of the color was thus inversely related to the amount of HA available for the bHABP.
Glycosaminoglycan Assay
Glycosaminoglycans in the conditioned medium were determined by the Blyscan assay (Accurate Chemical and Scientific Corporation, Westbury, NY) following the manufacturer’s instructions. Briefly, cells were cultured on bovine type I collagen (Vitrogen) as described above. Before adding 5-HT, cells were incubated in M199 containing 0.5% FBS for 48 hours to make the cells quiescent. Serum-free medium that did not contain phenol red was then used for the experiments. Conditioned media samples were collected at various time points, freeze dried, and kept at −70°C until the assays were performed.
Statistical Methods
All studies were performed in triplicate and data are the means of at least three independent experiments. Error bars indicate standard errors of the means. Differences between means were tested with one-way analysis of variance followed by Tukey-Kramer multiple comparisons post test (InStat; GraphPad Software, Inc., San Diego, CA). A value of P < 0.05 was considered statistically significant.
Results
Immunohistochemical Studies of Carcinoid Heart Valves and Autopsied Control Valves
We examined three tricuspid valve cusps and one pulmonary valve cusp taken from patients undergoing valve replacement surgery for severe carcinoid heart disease (Table 1) ▶ . Five clinically normal tricuspid valve cusps were included in the study as controls (Table 1) ▶ . Carcinoid valve cusps demonstrated numerous dense nodules, enriched in extracellular matrix, that were surrounded by hyperplasia of the valvular interstitial cells. These carcinoid perinodular interstitial cells showed a distinct spindle-like morphology (Figure 1A) ▶ . Immunostaining of the sectioned valves for SMA showed strong differences between the carcinoid valve cusps and control valve cusps (Figure 1 ▶ , Table 1 ▶ ). In carcinoid valve cusps, most of the interstitial cells surrounding the carcinoid nodules were immunopositive for SMA (Figure 1A) ▶ . In contrast, control valve cusps only showed focal subendothelial SMA staining (Figure 1B) ▶ . In one of the controls, a small focus of spindle-shaped cells was observed that was also immunopositive for SMA (data not shown). Nonspecific IgG exposure resulted in no apparent immunopositive staining (Figure 1E) ▶ .
Table 1.
Distribution of α/γ SMA, TGF-β1, LAP-TGF-β1, TGF-β Receptor (R)I, TGF-βRII on Normal Autopsied Tricuspid Valves and Valves Retrieved from Patients Undergoing Aortic Valve Replacement because of Carcinoid Valvular Disease
| SMA | TGF-β1 | LAP-TGF-β | TGF-β RI | TGF-β RII | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control tricuspid valves from autopsy | ||||||||||
| 1 | ++*† | − | + | + | − | |||||
| 2 | +*† | − | − | − | − | |||||
| 3 | ++* | + | − | ++ | + | |||||
| 4 | +* | +‡ | ++ | ++ | + | |||||
| 5 | +* | + | + | ++ | +++ | |||||
| Carcinoid valves from patients underwent valve replacement surgery | ||||||||||
| 6 | +++ | − | + | ++ | + | |||||
| 7 | +++ | − | ++ | − | ++ | |||||
| 8 | +++ | − | ++ | − | ++ | |||||
| 9 (pulmonary) | +++ | − | + | + | +++ | |||||
*Subendothelial staining.
†Focal spindle cells.
‡Endothelial staining.
Figure 1.
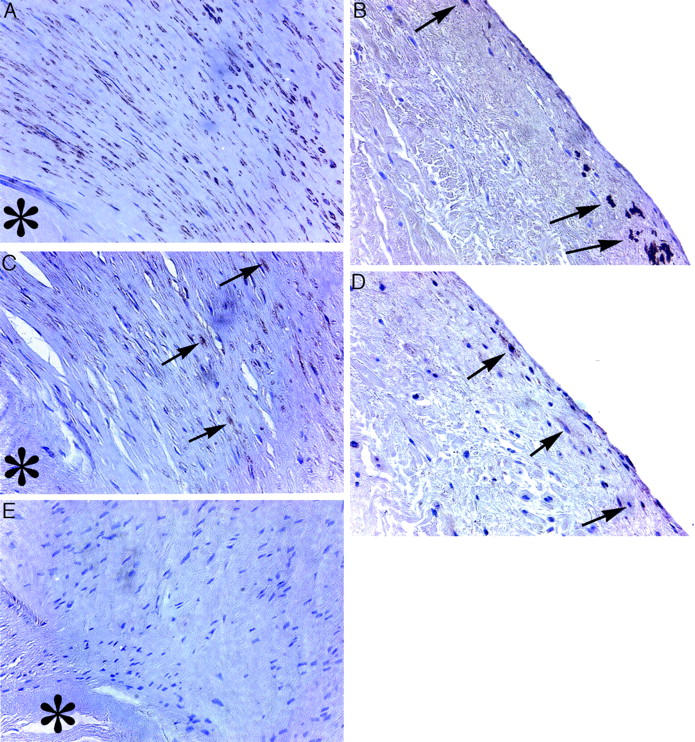
A: Representative micrographs of a carcinoid tricuspid valve cusp with prominent SMA staining by peroxidase immunochemistry (brown staining) with a hematoxylin counterstain. B: Normal control tricuspid valve cusp shows sparse subendothelial SMA staining (arrows indicate SMA-positive cells). C: A carcinoid valve cusp with strongly positive LAP-TGF-β1 immunostaining (arrows) localized in hyperplastic areas around carcinoid nodules. D: Focal subendothelial LAP-TGF-β1 immunostaining (arrows) in control valves. E: Negative IgG control shows an absence of peroxidase activity. Asterisk denotes carcinoid nodule. All micrographs were prepared with immunoperoxidase and hematoxylin staining techniques. Original magnifications, ×400.
We also performed immunostaining of the valve cusps for active TGF-β1, LAP-TGF-β1, and TGF-β receptors type I and II (Table 1) ▶ . Both receptors were present in carcinoid valve cusps and control valve cusps in variable amounts. Focal LAP-TGF-β1 staining was found in all carcinoid valve cusps and three of the control valves with important differences in immunostaining pattern. In carcinoid valve cusps, positive staining was observed mainly in the spindle-shaped cells around carcinoid nodules (Figure 1C) ▶ . In control cusps, positive immunostaining was found mainly in endothelial or subendothelial cells (Figure 1D) ▶ . However, TGF-β1 staining was not observed in carcinoid valve cusps and was detected in sparse amounts in normal cusps (Table 1) ▶ .
Characterization of SAVICs
Virtually all SAVICs in culture were immunopositive for the 5-HT2A receptor, compared to nonspecific IgG controls (Figure 2, A and B) ▶ . Unfortunately, no other antibodies were commercially available to screen for other 5-HT2 receptor subtypes. Other cell markers used in the characterization of SAVICs included von Willebrand factor, vimentin, desmin, and calponin. SAVICs were negative for vimentin and von Willebrand factor, and weakly positive for desmin and calponin (data not shown). When SAVICs were cultured in the presence of 10 ng/ml of TGF-β1, significantly more cells were immunopositive for α/τ-SMA than cells not exposed to TGF-β1 (Figure 2, C and D) ▶ . In control cultures (no added TGF-β1), 6.5 ± 1.2% of cells were α/τ-SMA-positive. However, after TGF-β1 treatment, 56.9 ± 10.7% of cells showed α/τ-SMA positivity (P < 0.05). Nonspecific IgG control showed no immunopositive staining (Figure 2E) ▶ .
Figure 2.
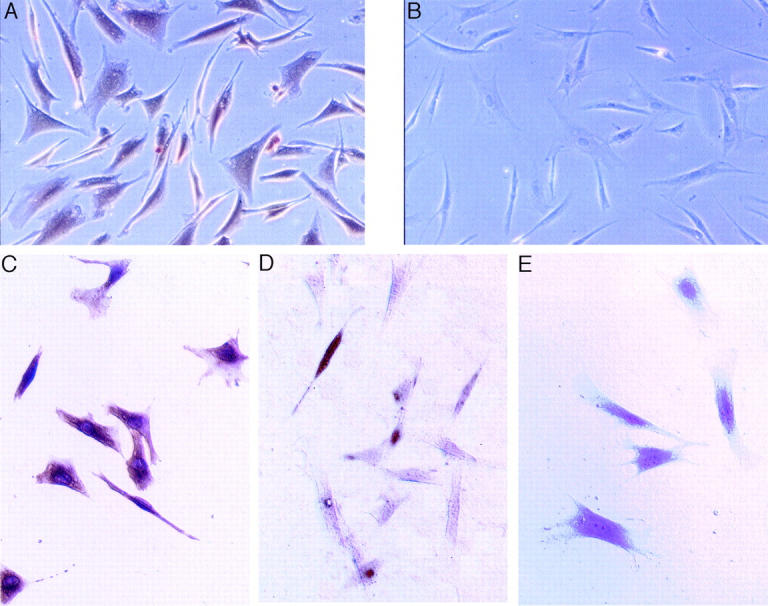
Immunostaining results demonstrating that SAVICs are immunopositive (brown) for the 5-HT2A receptor (A), compared to B, which demonstrates an absence of immunopositive cells using a nonspecific antibody. TGF-β1 exposure results in increased α/τ-SMA-immunopositive cells (brown) with an elongated cytoskeleton (C), compared to D. Cells not exposed to TGF-β1 are predominant by immunonegative for α/τ-SMA. Nonspecific IgG results were negative for α/τ-SMA (E). Peroxidase immunohistochemistry. Original magnifications: ×200 (A, B); ×400 (C–E).
TGF-β1 Is Up-Regulated by Serotonin (5-HT) in Aortic Valve Interstitial Cells
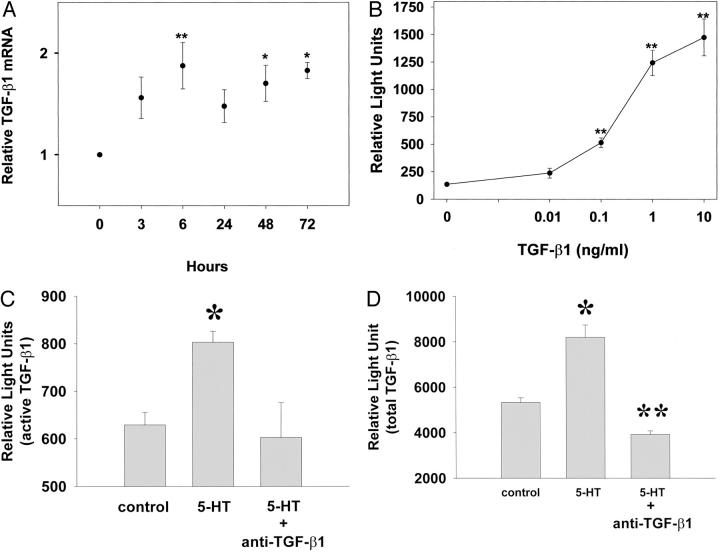
SAVICs were cultivated in the presence of 5-HT, and were monitored for TGF-β1 expression in terms of mRNA levels and TGF-β1 activity, using the PAI/luciferase assay. TGF-β1 mRNA, evaluated by real-time-RT-PCR, was increased significantly after 6 hours of 10 μmol/L 5-HT exposure and remained relatively constant up to 72 hours (Figure 3A ▶ , P < 0.05). For calibration, increasing concentrations of TGF-β1 were directly added to mink lung epithelial cells, and this resulted in a dose-dependent rise in luciferase activity (Figure 3B) ▶ . Furthermore, treatment of SAVICs with 10 μmol/L of 5-HT for 72 hours resulted in an increase in both direct and total TGF-β activity (Figure 3, C and D) ▶ . However, these increased luciferase activities were blocked completely by a TGF-β1 function-blocking antibody at a concentration of 10 μg/ml (Figure 3, C and D) ▶ . These studies conclusively demonstrate that 5-HT causes SAVICs to up-regulate TGF-β1, resulting in increased amounts of active (Figure 3C) ▶ and latent TGF-β1 (Figure 3D) ▶ .
Figure 3.
Serotonin up-regulates TGF-β1 expression and activity in AVICs. A: Time-dependent TGF-β1 mRNA induction by 10 μmol/L of 5-HT treatment evaluated by real-time RT-PCR. Results are expressed as mean ± SEM of four to six independent experiments (**, P < 0.01; *, P < 0.05; analysis of variance followed by Tukey-Kramer multiple comparisons post test). B: A standard calibration curve for the PAI/L construct with directly added TGF-β1 demonstrating a dose response. C: Active TGF-β stimulated by 10 μmol/L of 5-HT after 72 hours, per PAI/luciferase assays, and blocked by a TGF-β1 function-blocking antibody. D: Total TGF-β (active and latent), examined by PAI/luciferase assays, increased because of 72 hours exposure to 5-HT, and blocked by TGF-β1 function-blocking antibody. For B, C, and D, the results are expressed as mean ± SEM of triplicates, which are typical of four independent experiments (*, P < 0.05, significant from control; **, P < 0.01, significant from 5-HT; analysis of variance followed by Tukey-Kramer multiple comparisons post test).
5-HT and TGF-β1 Increase the Synthesis of Both Collagen and Glycosaminoglycans
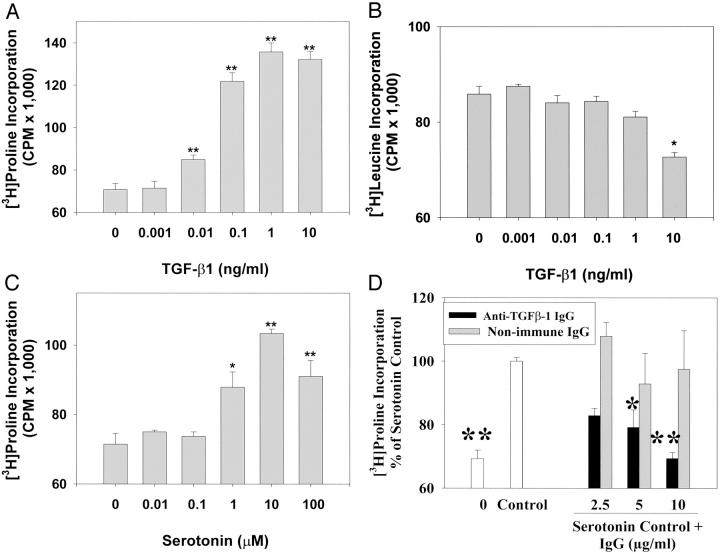
Both 5-HT and TGF-β1 (Figure 4) ▶ increased collagen synthesis by SAVICs, as measured by 3H-proline incorporation. The addition of TGF-β1 to SAVIC cultures resulted in significantly increased collagen synthesis at concentrations of 0.01 ng/ml to 10 ng/ml of TGF-β1 (Figure 4A) ▶ with results comparable to those seen with 5-HT. Total protein synthesis was assessed using a [3H]leucine incorporation assay. TGF-β1 has no effect on total protein synthesis at a concentration of 1 ng/ml or lower, but total protein synthesis was inhibited (P < 0.05) at 10 ng/ml of TGF-β1 (Figure 4B) ▶ . A stimulatory effect of 5-HT on collagen synthesis was observed at doses ranging from 1 μmol/L to 100 μmol/L of 5-HT (Figure 4C) ▶ . However, collagen synthesis stimulated by 5-HT was significantly reduced by an anti-TGF-β antibody (Figure 4D) ▶ , thus indicating the specificity of 5-HT stimulation of TGF-β1.
Figure 4.
Both 5-HT and TGF-β1 stimulate collagen synthesis by SAVICs. A: Stimulation of [3H]proline incorporation into cells by TGF-β1, significant stimulation was observed at 0.01 to 10 ng/ml of TGF-β1 (**, P < 0.01). B: The effects of TGF-β1 on total protein synthesis measured by [3H]leucine incorporation, demonstrating significant inhibition of protein synthesis at 10 ng/ml of TGF-β1 (*, P < 0.05). C: Stimulation of [3H]proline incorporation into cells by 5-HT, significant stimulation was observed from 1.0 to 100 μmol/L of 5-HT (*, P < 0.05; **, P < 0.01). D: Anti-TGF-β antibody blocked [3H]proline incorporation into cells stimulated by 5-HT. A significant blocking effect was observed at concentrations as low as 5 μg/ml of anti-TGF-β antibody (*, P < 0.05; **, P < 0.01). Results are expressed as mean ± SEM of four independent experiments (*, P < 0.05; **, P < 0.01, analysis of variance followed by Tukey-Kramer multiple comparisons post test).
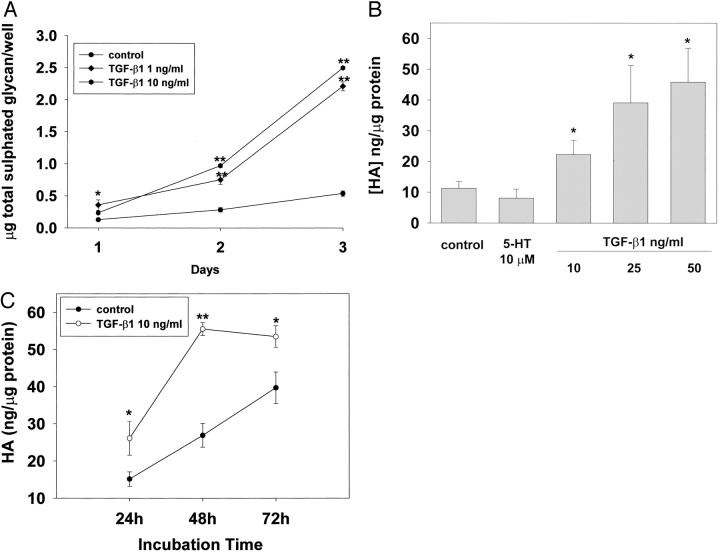
The secretion of glycosaminoglycan by SAVICs after 5-HT or TGF-β1 treatment was also investigated. The secretion of sulfated glycosaminoglycan was increased significantly after TGF-β1 treatment (Figure 5A) ▶ , but no significant increase was observed after 5-HT treatment (data not shown). Hyaluronic acid (HA), a nonsulfated glycosaminoglycan, was also studied. HA is the most abundant acidic glycosaminoglycan (AGAG) in aortic valve cusps, constituting almost 50% of the total AGAG. 17 It is hypothesized that the presence of HA is a marker for an initial phase of the extracellular matrix remodeling. 18 These experiments demonstrated that TGF-β1 increases SAVIC HA synthesis in a dose-dependent manner, and a significant cumulative effect was observed at a concentration of 10 ng/ml or greater of TGF-β1 after 24 hours of incubation (Figure 5B) ▶ . The effect of TGF-β1 on HA synthesis was also cumulative throughout time (Figure 5C) ▶ . We also examined the effect of direct addition of 5-HT on HA secretion. At a concentration of 10 μmol/L of 5-HT, no significant change in HA secretion was observed after 24 hours incubation (Figure 5B) ▶ . The effects of 5-HT and TGF-β1 on cell proliferation were also evaluated by a colorimetric assay 19 for the quantification of cell proliferation (WST-1, Boehringer Mannheim; see Materials and Methods). The concentration ranges of 5-HT and TGF-β1 used in these studies had little effect on proliferation: for serotonin, 0 to 10 μmol/L, cell counts ranged from 5.0 × 104 to 6.0 × 104; for TGF-β1, 0 to 10 ng/ml, cell counts ranged from 4.5 × 104 to 5.6 × 104, whereas a positive stimulus (10% FBS) produced a major increase (6 × 103 increasing to 3 × 104) in cell number.
Figure 5.
TGF-β1 stimulates both total glycosaminoglycan and HA production by SAVICs in a dose- and time-dependent manner. A: Time- and dose-dependent stimulation of total sulfated glycan by TGF-β1. B: Dose-dependent stimulation of HA production by TGF-β1, with a significant increase in HA production observed at 10 ng/ml or greater of TGF-β1 for 24 hours treatment. 5-HT at 10 μmol/L showed no significant effect on HA production. C: Time-dependent stimulation of HA production by TGF-β1, with the maximum HA production observed after 48 hours of incubation with TGF-β1. Results are expressed as mean ± SEM of triplicates, which are typical of three or four independent experiments (*, P < 0.05; **, P < 0.01, followed by Tukey-Kramer multiple comparisons post test).
Overexpression of Gαq via a Replication-Defective Adenovirus Increases PLC Activity, TGF-β1 Expression, and TGF-β1 Activity
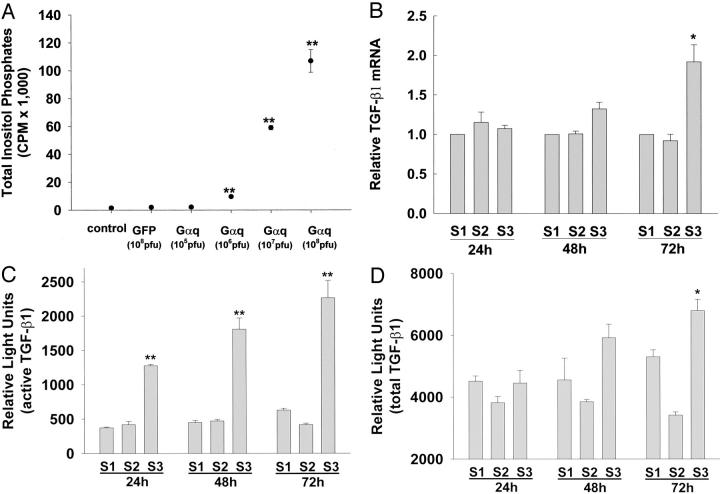
It was hypothesized that 5-HT-induced TGF-β1 production by SAVICs was because of receptor-mediated activation of the G-protein signal transduction pathway. Related research by our group established that 5-HT administration to SAVICs in the same dose range used in the present TGF-β1 studies, results in a dose-dependent increase in phospholipase C (PLC) activity. 20 Thus, using an adenoviral vector construct with constitutively active, GTPase-deficient mutant Gαq (AdCMV-G29), we investigated the effects of overexpression of Gαq on both PLC and TGF-β1 activity. Initial studies investigated the effects of increasing dosages of Gαq-adenovirus, compared to AdCMV-GFP on PLC activity. It was observed that a 40-fold (107 PFU Gαq) to 70-fold (108 PFU Gαq) increase in PLC activity occurred over the dosage range studied (Figure 6A) ▶ .
Figure 6.
Overexpression of Gαq via transduction of SAVICs with a replication defective adenovirus stimulates PLC activity and TGF-β1 expression, where S1 is the M199 medium (control); S2 is the AdCMV-GFP, 107 PFU; S3 is the AdCMV-Gαq, 107 PFU. Overexpression of Gαq significantly increases the accumulation of total inositol phosphates (A), stimulates TGF-β1 mRNA expression (B); increases the production of active TGF-β1 evaluated by PAI/L assay (C); and increases the production of total (active and latent) TGF-β1 evaluated by PAI/L assay (D). Results are expressed as mean ± SEM of triplicates, which are typical of three independent experiments (*, P < 0.05; **, P < 0.01, analysis of variance followed by Tukey-Kramer multiple comparisons post test).
The effects of Gαq on TGF-β1 mRNA level were not significantly increased until 72 hours (Figure 6B) ▶ . However, active TGF-β1 content, as assessed with the PAI/luciferase system using a dose of 107 PFU Gαq, was increased more than sixfold as early as 24 hours and remained constant for 72 hours (Figure 6C) ▶ . However, total TGF-β1 activity did not increase significantly in response to Gαq overexpression until 72 hours after the addition of adenoviral vector, in association with the increase of TGF-β1 mRNA levels (Figure 6B) ▶ . AdCMV-GFP had no effect on either PLC activity or on TGF-β expression and activity (Figure 6; A, C, and D) ▶ .
Discussion
The present studies have demonstrated that 5-HT-related heart valve disease may be, in part, because of TGF-β mechanisms. These results are in agreement with those reported previously 21-23 and provide insights concerning the mechanisms responsible for serotonin-mediated heart valve disease. Carcinoid heart valve cusps have been demonstrated to accumulate TGF-β latency associated peptide and latent binding protein. 5 Furthermore, serotonin has been demonstrated by our study and others to stimulate increased collagen synthesis by heart valve interstitial cells. 14 Serotonin up-regulation of TGF-β has also been observed in mesangial cells with associated increased PLC activity indicating G-protein signal transduction. 6 In addition, 5-HT2 receptors (A and B) have been demonstrated by RT-PCR studies to be present in human and porcine aortic valve cells. 3 We demonstrated 5-HT2A receptors with immunostaining in all SAVICs. Our results are consistent with these previous studies. Thus, the present results and related previous research are consistent with the view that heart valve interstitial cells are serotonin responsive, and thus it is likely that with an excessive serotonin exposure, as in the carcinoid syndrome, a TGF-β-related mechanism may explain at least in part the pathophysiological events.
Our clinical pathology study was comparable to the results of others 5 revealing relatively greater amounts of LAP in carcinoid cusps compared to normals, very likely reflecting up-regulation of TGF-β because of serotonin. However, relatively lower levels of the active form of TGF-β were detected compared to normal cusps. The mechanisms responsible for this observation are not completely understood, but may be, in part, because of rapid turnover of the active form of TGF-β with relatively little accumulation in the metabolically dynamic carcinoid cusps. Furthermore, hyperplastic interstitial cells within carcinoid cusps were for the most part positive for SMA, which was present to a markedly lesser extent in normal cusps. This may reflect up-regulation of SMA in cardiac valve interstitial cells because of TGF-β (Figure 2) ▶ . This observation is also comparable to the up-regulation of SMA by TGF-β in fibroblasts observed by others. 24 Thus, in carcinoid valves increased SMA could be hypothetically because of serotonin-mediated up-regulation of TGF-β.
Serotonin-related heart valve disease is a chronic process. Thus, the relatively rapid signaling events reported in this study can only explain the potential initiating mechanism that could be hypothetically associated with a cumulative effect throughout time, via changes in the extracellular matrix including accumulation of TGF-β. As discussed above, latent TGF-β-binding protein accumulates in the extracellular matrix, via covalent attachment, 25,26 and thus carcinoid heart valve disease provides an experiment in nature that supports our hypothesis. Furthermore, other studies from our group have shown that calcified human heart valves, but not normals, have high amounts of TGF-β accumulating in the extracellular matrix. 27 Although this observation is unlikely to be related to serotonin mechanisms, it nevertheless supports the view that chronic accumulation of TGF-β in human heart valve disease is a common pathological event that could have multiple pathophysiological impacts on heart valve disease in general. Therefore, the cell culture studies reported in this study with serotonin-induced TGF-β expression and activity, and increased biosynthesis of both collagen and glycosaminoglycans, may reflect incremental events of a chronic cumulative process.
The use of an adenoviral vector that overexpresses Gαq provided a unique opportunity to establish a strong association between G-protein signal transduction and TGF-β expression in heart valve interstitial cells. Our present studies demonstrate that there were significant increases in both PLC activity and TGF-β expression and activity because of overexpression of Gαq after adenoviral vector transduction. Interestingly, these results demonstrate that G-protein signal transduction appears to act at three levels to enable TGF-β1 expression and activity. As described above, an increase in the active TGF-β occurs after only 24 hours. Furthermore, TGF-β1 mRNA synthesis increases throughout a somewhat longer time course of 72 hours. In addition, 72 hours after Gαq exposure, both active and total TGF-β activity are significantly elevated (Figure 6) ▶ . Taken together, these data indicate a profound effect of G-protein signal transduction on TGF-β1 expression and processing in heart valve interstitial cells via Gαq, that may be related in part to the pathophysiological events in serotonin-associated heart valve disease.
The absence of any effect of 5-HT on glycosaminoglycan synthesis by SAVICs in culture in our studies (Figure 5) ▶ and a modest effect on proline incorporation (reflecting increased collagen synthesis) could be because of the fact that a far longer duration of exposure may be needed for a cumulative effect in a cell culture model system. Furthermore, our results also show that the TGF-β activity resulting from 5-HT stimulation is primarily in the latent form (thus requiring activation for physiological effects to ensue), in contrast to our studies involving direct addition of active TGF-β1 that had potent effects on both glycosaminoglycan and collagen production. These results concerning latent TGF-β are in agreement with our clinical pathology data. Because of the potential for serotonin exacerbation of heart valve disease, pharmaceuticals that have the capacity to increase serotonin levels could also contribute to the progression of heart valve disease in general. Thus, new and existing drugs could be screened in AVIC culture systems to determine whether the potential for TGF-β-related adverse effects exists. Further, it is conceivable that a novel therapeutic approach could arise based on the serotonin TGF-β pathway investigated in these studies. Numerous serotonin receptor antagonists have already been described, 28-30 and thus could be screened for their effects on cell cultures of AVICs. A similar approach could also be taken with the various PLC inhibitors that have been reported. 31,32 Therefore, because our results show that heart valve interstitial cells are unusually sensitive to serotonin, a serotonin-based therapeutic strategy is worthy of investigation.
Conclusions
Serotonin may contribute to the development and progression of cardiac valve disease through up-regulation of TGF-β1, and related increases in extracellular matrix components, including collagen and glycosaminoglycans. Serotonin signaling in heart valve interstitial cells leading to up-regulation of TGF-β1 likely takes place via a G-protein signal transduction mechanism, thus indicating potential therapeutic strategies that could be investigated.
Acknowledgments
We thank Ms. Xiumin Cui for her technical assistance, Ms. Jennifer LeBold for her assistance in preparing the manuscript, and Dr. D. B. Rifkin (New York University) for providing his mink lung epithelial cell line for TGF-β assays.
Footnotes
Address reprint requests to Robert J. Levy, M.D., Children’s Hospital of Philadelphia, Abramson Research Building, 3416 Civic Center Blvd., Philadelphia, PA 19104-4318. E-mail: levyr@email.chop.edu.
Supported in part by grants from the National Heart, Lung, and Blood Institute (RO1 HL38118, T32 HL07915 to R. J. L.; RO1 HL62868 and RO1 HL62472 to R. C. S.; and RO1 HL48225 to B. L.) and the William J. Rashkind Endowment of the Children’s Hospital of Philadelphia.
References
- 1.Robiolio PA, Rigolin VH, Wilson JS, Harrison JK, Sanders LL, Bashore TM, Feldman JM: Carcinoid heart disease: correlation of high serotonin levels with valvular abnormalities detected by cardiac catheterization and echocardiography. Circulation 1995, 92:790-795 [DOI] [PubMed] [Google Scholar]
- 2.Connolly HM, Crary JL, McGoon MD, Hensrud DD, Edwards BS, Edwards WD, Schaff HV: Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med 1997, 337:581-588 [DOI] [PubMed] [Google Scholar]
- 3.Fitzgerald LW, Burn TC, Brown BS, Patterson JP, Corjay MH, Valentine PA, Sun JH, Link JR, Abbaszade I, Hollis JM, Largent BL, Hartig PR, Hollis GF, Meunier PC, Robichaud AJ, Robertson DW: Possible role of valvular serotonin 5-HT(2B) receptors in the cardiopathy associated with fenfluramine. Mol Pharmacol 2000, 57:75-81 [PubMed] [Google Scholar]
- 4.Roy A, Brand NJ, Yacoub MH: Expression of 5-hydroxytryptamine receptor subtype messenger RNA in interstitial cells from human heart valves. J Heart Valve Dis 2000, 9:256-260 [PubMed] [Google Scholar]
- 5.Waltenberger J, Lundin L, Oberg K, Wilander E, Miyazono K, Heldin C-H, Funa K: Involvement of transforming growth factor-β in the formation of fibrotic lesions in carcinoid heart disease. Am J Pathol 1993, 142:71-78 [PMC free article] [PubMed] [Google Scholar]
- 6.Grewal JS, Mukhin YV, Garnovskaya MN, Raymond JR, Greene EL: Serotonin 5-HT2a receptor induces TGF-β1 expression in mesangial cells via ERK: proliferative and fibrotic signals. Am J Physiol 1999, 276:F922-F930 [DOI] [PubMed] [Google Scholar]
- 7.Franchi A, Arganini L, Baroni G, Calzolari A, Capanna R, Campanacci D, Caldora P, Masi L, Brandi M, Zampi G: Expression of transforming growth factor beta isoforms in osteosarcoma variants: association of TGF beta 1 with high-grade osteosarcomas. J Pathol 1998, 185:284-289 [DOI] [PubMed] [Google Scholar]
- 8.Mason GI, Hamburger J, Matthews JB: Mast cells, extracellular matrix components, TGFbeta isoforms and TGFbeta receptor expression in labial salivary glands in systemic sclerosis. Ann Rheum Dis 2000, 59:183-189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones P, Crack J, Rabinovitch M: Regulation of tenascin-C, a vascular smooth muscle cell survival factor that interacts with the alpha v beta 3 intergrin to promote epidermal growth factor receptor phosphorylation and growth. J Cell Biol 1997, 139:279-293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imamura T, Vollenweider P, Egawa K, Clodi M, Ishibashi K, Nakashima N, Ugi S, Adams JW, Brown JH, Olefsky JM: G alpha-q/11 protein plays a key role in insulin-induced glucose transport in 3T3–L1 adipocytes. Mol Cell Biol 1999, 19:6765-6774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibson UE, Heid CA, Williams PM: A novel method for real time quantitative RT-PCR. Genome Res 1996, 6:995-1001 [DOI] [PubMed] [Google Scholar]
- 12.Podrasky E, Xu D, Liang B: A novel phospholipase C- and cAMP-independent positive inotropic mechanism via a P2 purinoceptor. Am J Physiol 1997, 273:H2380-H2387 [DOI] [PubMed] [Google Scholar]
- 13.Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, Rifkin DB: An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem 1994, 216:276-284 [DOI] [PubMed] [Google Scholar]
- 14.Hafizi S, Taylor PM, Chester AH, Allen SP, Yacoub MH: Mitogenic and secretory responses of human valve interstitial cells to vasoactive agents. J Heart Valve Dis 2000, 9:454-458 [PubMed] [Google Scholar]
- 15.Dubey RK, Gillespie DG, Jackson EK: Adenosine inhibits collagen and total protein synthesis in vascular smooth muscle cells. Hypertension 1999, 33:190-194 [DOI] [PubMed] [Google Scholar]
- 16.Lokeshwar V, Obek C, Soloway M, Block N: Tumor-associated hyaluronic acid: a new sensitive and specific urine marker for bladder cancer. Cancer Res 1997, 57:773-777 [PubMed] [Google Scholar]
- 17.Murata K: Acidic glycosaminoglycans in human heart valves. J Mol Cell Cardiol 1981, 13:281-292 [DOI] [PubMed] [Google Scholar]
- 18.Riessen R, Wight TN, Pastore C, Henley C, Isner JM: Distribution of hyaluronan during extracellular matrix remodeling in human restenotic arteries and balloon-injured rat carotid arteries. Circulation 1996, 93:1141-1147 [DOI] [PubMed] [Google Scholar]
- 19.Bedard J, May S, Barbeau D, Yuen L, Rando R, Bowlin T: A high throughput colorimetric cell proliferation assay for the identification of human cytomegalovirus inhibitors. Antiviral Res 1999, 41:35-43 [DOI] [PubMed] [Google Scholar]
- 20.Xu J, Jian B, Chu R, Lu J, Li Q, Dunlop J, Rosenzweig-Lipson S, McGonigle P, Levy RJ, Liang B: Serotonin mechanisms in heart valve disease II: the 5-HT2A receptor and its signaling pathway in cultured aortic valve interstitial cells. Am J Pathol 2002, 161:2209-2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poncelet A-C, Schnaper HW: Regulation of human mesangial cell collagen expression by transforming growth factor-β1. Am J Physiol 1998, 275:F458-F466 [DOI] [PubMed] [Google Scholar]
- 22.Nishikawa H, Ueno A, Nishikawa S, Kido J, Ohishi M, Inoue H, Nagata T: Sulfated glycosaminoglycan synthesis and its regulation by transforming growth factor-beta in rat clonal dental pulp cells. J Endod 2000, 26:169-171 [DOI] [PubMed] [Google Scholar]
- 23.Dubaybo B, Thet L: Effect of transforming growth factor beta on synthesis of glycosaminoglycans by human lung fibroblasts. Exp Lung Res 1990, 16:389-403 [DOI] [PubMed] [Google Scholar]
- 24.Mattey DL, Dawes PT, Nixon NB, Slater H: Transforming growth factor beta 1 and interleukin 4 induced alpha smooth muscle actin expression and myofibroblast-like differentiation in human synovial fibroblasts in vitro: modulation by basic fibroblast growth factor. Ann Rheum Dis 1997, 56:426-431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonewald LF: Regulation and regulatory activities of transforming growth factor β. Crit Rev Eukaryot Gene Express 1999, 9:33-44 [PubMed] [Google Scholar]
- 26.Flanders K, Thompson N, Cissel D, Van Obberghen-Schilling E, Baker C, Kass M, Ellingsworth L, Roberts A, Sporn M: Transforming growth factor-beta 1: histochemical localization with antibodies to different epitopes. J Cell Biol 1989, 108:653-660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jian B, Mohler ER, Jones PL, Levy RJ: Transforming growth factor-beta promotes aortic valve interstitial cell calcification. Circulation 1999, 100(Suppl I):S706 (Abstract)
- 28.Uehara Y, Nagata T, Matsuoka H, Numabe A, Hirawa N, Takada S, Ishimitsu T, Yagi S, Sugimoto T: Antiproliferative effects of the serotonin type 2 receptor antagonist, ketanserin, on smooth muscle cell growth in rats. J Cardiovasc Pharmacol 1991, 17(Suppl 2):S154-S156 [DOI] [PubMed] [Google Scholar]
- 29.Pauwels PJ: 5-HT 1B/D receptor antagonists. Gen Pharmacol 1997, 29:293-303 [DOI] [PubMed] [Google Scholar]
- 30.Routledge C: Development of 5-HT1A receptor antagonists. Behav Brain Res 1996, 73:153-156 [DOI] [PubMed] [Google Scholar]
- 31.Lockhart LK, McNicol A: The phospholipase C inhibitor U73122 inhibits phorbol ester-induced platelet activation. J Pharmacol Exp Ther 1999, 289:721-728 [PubMed] [Google Scholar]
- 32.Bian JS, Zhang WM, Xia Q, Wong TM: Phospholipase C inhibitors attenuate arrhythmias induced by kappa-receptor stimulation in the isolated rat heart. J Mol Cell Cardiol 1998, 30:2103-2110 [DOI] [PubMed] [Google Scholar]