Abstract
CD44 is a major cell-surface receptor for hyaluronic acid (HA), a glycosaminoglycan component of extracellular matrix. HA-CD44 interactions have been implicated in leukocyte extravasation into an inflammatory site. This study examined the role of CD44 in acute inflammatory responses during pneumonias induced by Escherichia coli and Streptococcus pneumoniae using CD44-deficient mice. In E. coli-induced pneumonia, neutrophil accumulation in the lungs and edema formation was increased by 84% and 88%, respectively, in CD44-deficient mice compared to wild-type mice. In contrast, no difference was observed between these genotypes in S. pneumoniae-induced pneumonia, and the HA content in the lungs decreased after instillation of S. pneumoniae, but not E. coli, in both genotypes. Studies to determine the mechanisms for this enhanced response showed that: 1) neutrophil apoptosis was not different between these two genotypes in either type of pneumonia; 2) CD44 deficiency resulted in enhanced mRNA expression of several inflammatory genes; and 3) CD44-deficient neutrophils migrated through Matrigel in response to chemoattractants faster and in greater numbers than wild-type neutrophils in vitro and this increase was in part dependent on HA content in the Matrigel. These data demonstrate that CD44 deficiency results in enhanced inflammation in E. coli but not S. pneumoniae-induced pneumonia, suggesting a previously unrecognized role for CD44 in limiting the inflammatory response to E. coli.
Neutrophil accumulation at inflammatory sites is an important feature of acute inflammatory responses. During pulmonary inflammation, circulating neutrophils become sequestered within pulmonary capillaries, a process that does not require rolling. Neutrophils then migrate across the endothelium and through the pulmonary interstitium, which contains fibroblasts and extracellular matrix proteins such as collagens and proteoglycans. 1-4 Finally, neutrophils migrate between alveolar epithelial cells, often between a type II and a type I pneumocyte, into the airspace. 1,5
CD44 is a type I transmembrane glycoprotein that is expressed by most cell types, including leukocytes, and is the major cell surface receptor for hyaluronan (HA). HA is a nonsulfated glycosaminoglycan component of the extracellular matrix and plays a major role in maintenance of tissue integrity. 6-8 CD44 has 10 different splice variants and neutrophils express the standard CD44 isoform, CD44s. 9 CD44 may modulate immunological and inflammatory responses through at least two mechanisms. First, CD44 may play important roles in modulating leukocyte extravasation. Interaction between CD44 and HA is implicated in lymphocyte rolling and extravasation, and optimal binding of these two molecules is modulated by proinflammatory cytokines such as tumor necrosis factor-α. 10-14 The role of CD44 in mediating neutrophil emigration during acute inflammatory responses is less well understood. Second, CD44 is capable of inducing signal transduction pathways and cell activation. Ligation of CD44 triggers lytic function of cytotoxic T cells and enhances natural killer cell activity. 15,16 In addition, ligation of CD44 by HA fragments results in induction of inflammatory genes in alveolar macrophages. 17-19 Moreover, a recent study by Sconocchia and colleagues 20 demonstrated that ligation of CD44 on neutrophils by antibodies or HA induces production of interleukin (IL)-6. Proteolysis of CD44 seems to play important roles in triggering the signaling events induced by CD44. 21 A CD44 intracellular domain fragment, released by proteolysis, acts as a signaling molecule and activates transcription of several genes, including possibly CD44 itself. 21 Thus, CD44 may function as a signaling molecule to modulate the activation of leukocytes and alveolar macrophages during inflammatory responses.
CD44 binding to HA is regulated by inflammatory mediators through, at least in part, posttranslational modification. CD44 is present on the surface of lymphocytes and monocytes in an inactive state that does not bind HA. When monocytes undergo differentiation in vitro into macrophages in the presence of serum, HA binding activity is acquired. 22,23 On activation by antigens or cytokines, CD44 can be converted, through glycosylation and addition of glycosaminoglycans, to an active state that binds HA. 24-27 In addition, on stimulation by tumor necrosis factor-α, but not by interferon-γ, CD44 can be induced to bind HA through the sulfation of CD44, 14 and this posttranslational modification is required for CD44-mediated binding of a leukemic cell line to the extracellular matrix and to vascular endothelial cells. 14 These studies suggest that inflammatory mediators modulate the interactions between CD44 on leukocytes and HA, which may, in turn, regulate leukocyte extravasation.
This study examined the role of CD44 in inflammatory responses during acute bacterial pneumonia induced by either E. coli or S. pneumoniae. These two organisms were chosen for the following reasons: 1) E. coli is a gram-negative organism, whereas S. pneumoniae is a gram-positive organism; 2) S. pneumoniae expresses hyaluronidase, an enzyme that cleaves HA, a major ligand for CD44, 28,29 whereas E. coli does not; 3) E. coli elicits CD18-dependent neutrophil emigration, whereas S. pneumoniae induces CD18-independent neutrophil emigration. 4,30
The role of CD44 was examined by comparing CD44-deficient mice to wild-type mice. CD44-deficient mice lack all known isoforms of CD44 and exhibit no obvious developmental deficits. 31 These mice do have hematological impairments as evidenced by altered distribution of myeloid progenitors in bone marrow and spleen, and they exhibit exaggerated responses to granuloma-inducing bacteria. 31 The present study demonstrates that CD44 deficiency resulted in more pronounced inflammatory responses, including enhanced neutrophil accumulation and edema, in E. coli- but not S. pneumoniae-induced pneumonia, suggesting a role for CD44 in limiting the inflammatory response to E. coli.
Materials and Methods
Animals
CD44-deficient mice backcrossed at least six generations to C57BL/6J mice were generously given by Dr. Tak Mak, University of Toronto, Toronto, Canada. Generation and characterization of the mutant mice have been described. 31 Age-matched C57BL/6J mice were used as controls. All animals in this study were 6 to 8 weeks old. All experiments received institutional approval.
Bacterial Pneumonia
Clinical isolates of E. coli and S. pneumoniae were used in this study. Stock cultures were maintained on trypticase soy agar (TSA 2; Becton Dickinson, Cockeysville, MD) for 18 hours before the study. Bacterial suspensions were prepared at a concentration of 0.5 to 1 × 108 organisms/ml phosphate-buffered saline (PBS) containing 5% colloidal carbon. These doses of organisms were chosen because they induced similar numbers of neutrophil emigration into the lungs and similar amounts of edema accumulation. 32,33 Concentrations were determined by optical density. In addition, serial dilutions of the instillates were prepared to determine the colony-forming units (CFUs) of the instillates.
Pneumonias were induced in mice as previously described. 32,33 The animals were studied 6 hours after instillation of the organisms because this is the earliest time point when consistent quantifiable neutrophil emigration is occurring. Mice were anesthetized by intramuscular injection of ketamine hydrochloride (100 mg/kg), ace-promazine maleate (5 mg/kg), and atropine (0.1 mg/kg). In experiments measuring the accumulation of extravascular albumin (EVA) as a measure of edema formation, 125I-albumin (0.1 μCi/mouse) was injected intravenously 15 minutes before instilling the bacteria into the lungs. The tracheas were exposed, and bacterial suspensions were instilled in the left lung at a dose of 50 μl/22 g body weight. 51Cr-labeled red blood cells (0.30 μCi/mouse) were injected intravenously 2 minutes before the end of 6 hours of pneumonia. At 6 hours after instillation, the mice received an overdose of inhaled halothane. The thoracic cavity was opened, and the base of the heart was tied to prevent blood loss from the pulmonary circulation. Peripheral blood samples were drawn from the inferior vena cava and a blood and plasma sample were weighed. The lungs were removed and fixed by intratracheal instillation of 6% buffered glutaraldehyde at a pressure of 22 cm H2O. The radioisotopes in blood, plasma, and the pneumonic lungs were quantitated in a gamma counter (Cobra Quantum; Packard Instrument Company, Meriden, CT). The EVA was calculated according to the following formula: EVA (μl) = (total 125I-albumin volume in lung) − (intravascular 125I-albumin volume), where total 125I-albumin volume in lung = (125I-albumin in lung)/(125I-albumin/g plasma), and intravascular 125I-albumin volume = (51Cr-RBC in lung)/(51Cr-RBC/g blood) × (1-hematocrit), assuming the density of plasma is 1.
Morphometric Quantification of Neutrophil Accumulation in the Lungs
Histological slides were prepared from the pneumonic region by sectioning the blackened area of the lung, embedding in paraffin, and staining 5-μm sections with hematoxylin and eosin. The neutrophils that accumulated in the lungs were counted at ×200 magnifications by point-counting. 34 A grid containing 100 points was reflected onto the view of field and the number of points that landed on neutrophils was counted. For each slide, six randomly selected fields of distal lung tissue were counted and averaged. Neutrophils accumulated in the lungs were quantified as the percentage of the distal lung volume that was occupied by neutrophils.
Bacterial Counts
Bacterial pneumonia was induced in mice as described above. After 6 hours, the mice received an overdose of inhaled halothane. The lungs were removed from the thoracic cavity and homogenized in sterile saline. Appropriate serial dilutions of the homogenates and of the instillate were then plated onto trypticase soy agar plates, and the number of recovered CFUs was determined.
Evaluation of Apoptotic Neutrophils Recovered in the Bronchoalveolar Lavage (BAL)
Bacterial pneumonia was induced in mice as described above. After 6 hours, the mice received an overdose of inhaled halothane. The diaphragm was cut to release the negative pleural pressure. The mouse lung was filled with 0.9 ml of ice-cold PBS containing 0.6 mmol/L of ethylenediaminetetraacetic acid. Thirty seconds later, the BAL fluid was slowly withdrawn while massaging the chest cavity. This procedure was repeated three more times. The total number of cells and cell differentials in the BAL were measured. The cells were stained immediately with fluorescein isothiocyanate-conjugated annexin V using an apoptosis detection kit, according to the manufacturer’s protocols (R&D Systems, Minneapolis, MN). To identify neutrophils in the BAL, 2 μg/ml of phycoerythrin-conjugated anti-mouse Ly-6G (Gr-1) antibody (PharMingen, San Diego, CA) was also included in the staining solution. For each sample, a negative staining control was prepared using a solution that did not contain annexin V and anti-Gr-1 antibody to determine background fluorescence. After staining, the cells were washed and immediately analyzed by flow cytometry. The percentage of apoptotic neutrophils was considered to be the percentage of Gr-1-positive cells that were annexin V-positive. In all of the samples examined, the annexin V staining intensity of the annexin V-positive neutrophils was barely above the background level. In addition, examination of cytospins prepared from BAL fluid showed no visible morphological changes of neutrophils characteristic of apoptosis, such as nuclear condensation or blebbing.
Measurement of the Distance that Neutrophils Migrated into Matrigel
Neutrophil migration through Matrigel in response to a chemoattractant was assessed using a modified Boyden chamber assay as previously described. 35 Hanks’ balanced salt solution (HBSS) (100 μl) containing 500 μg of Matrigel (Becton Dickinson, Bedford, MA) was added to the top chamber of each transwell insert (pore size, 8 μm; diameter, 6.5 mm) and allowed to gel at 37°C for 1 hour. Leukocytes were isolated from venous blood by hypotonically lysing the red blood cells. Leukocytes were incubated with 5 μg/ml of calcein AM (Molecular Probes, Eugene, OR) in the dark at room temperature for 15 minutes and washed once. The number of leukocytes and neutrophils was counted. The leukocyte suspension containing 2.8 × 103 neutrophils in 200 μl was added to each insert, and allowed to settle for 15 minutes at 37°C. HBSS or 100 ng/ml of recombinant mouse MIP-2 (R&D Systems, Inc.) was added to the bottom chamber. Because MIP-2 induces chemotaxis of neutrophils but not monocytes, 36 only neutrophils will migrate into the Matrigel. After incubation at 37°C for 30 minutes, the Matrigel was fixed with 3.7% paraformaldehyde and examined under a fluorescent microscope. The number of neutrophils that migrated into the gels toward the chemoattractant was counted from the top of the gels at increments of 50 μm. For each experiment, the number of neutrophils in five fields (×200) was counted and averaged at each incremental level.
Measurement of the Number of Neutrophils that Migrated across Matrigel
The number of neutrophils that migrated across Matrigel was assessed using a modified Boyden chamber assay as described above. Matrigel and leukocytes were prepared as described above, and the number of leukocytes and neutrophils was counted. Approximately 106 leukocytes were added to each insert, and HBSS or 10−7 mol/L of fMLP was added to the bottom chamber. After incubation at 37°C for 6 hours, the number of neutrophils in the bottom chamber was counted and the percentage of neutrophils that migrated was calculated.
To examine the role of hyaluronic acid in mediating neutrophil migration across Matrigel, the Matrigel was pretreated with Streptomyces hyaluronidase (Calbiochem, San Diego, CA), which breaks down only HA. Matrigel at two concentrations (1.78 mg/ml or 1.25 mg/ml) was incubated with 10 U/ml hyaluronidase or control vehicle for 8 hours at 4°C. The Matrigel (100 μl) was then added to the top chamber of each transwell insert and allowed to incubate at 37°C overnight to ensure complete digestion of HA by hyaluronidase. The number of neutrophils that migrated across the transwells in response to fMLP after 6 hours was measured as described above.
Measurement of HA Content in Lung Tissue
HA content was measured using a colorimetric enzyme-linked immunosorbent -like assay as previously described, based on competitive binding of biotinylated HA binding protein (bHABP) to HA in samples and to microtiter plate-bound HA. 37,38 Briefly, dehydrated lungs were incubated with Pronase protease (Calbiochem, La Jolla, CA) at 5 U/10 mg of tissue in pronase buffer (0.05 mol/L Tris, 0.01 mol/L CaCl2, pH 7.2) for 16 hours at 55°C with occasional vortexing, followed by incubation at 100°C for 10 minutes. The samples were centrifuged briefly at 10,000 rpm and the supernatants were collected. Ninety-six-well microtiter plates (Covalink; Nalge Nunc International, Rochester, NY) were coated with HA by incubating with 100 μl of 100 μg/ml high-molecular weight rooster comb HA (Sigma, St. Louis, MO), 50 μl of 200 μmol/L 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (Pierce, Rockford, IL) and 50 μl of 0.4 mmol/L HCl for 2 hours. Samples (100 μl) and HA standards were incubated with 100 μl of 4 μg/ml of bHABP (generous gift from Dr. C. B. Underhill, Georgetown Medical Center, Washington, DC) for 1 hour, and added to HA-coated wells at 50 μl/well in duplicates. The plate was agitated on a rocking platform at 200 rpm for 1 hour and washed three times with PBS containing 2 mol/L NaCl and 40 μmol/L MgSO4 and twice with PBS. The wells were incubated with 100 μl of 1 μg/ml horseradish peroxidase streptavidin (Vector Laboratories, Burlingame, CA) for 20 minutes and washed five times with H2O. Peroxidase substrate was added to each well according to manufacturer’s instructions (ABTS Kit, Vector Laboratories) and absorbance at 405 nm was measured. The HA concentration in the samples was calculated from a standard curve between 0 to 2000 ng/ml.
Northern Blots
RNA was extracted from lungs using Trizol reagent according to the manufacturer’s protocols (Life Technologies, Inc., Grand Island, NY). Equal amounts of RNA were electrophoresed on a formaldehyde agarose gel, transferred to a nylon membrane, and crosslinked with ultraviolet radiation. 32P-Labeled DNA probes were generated using a NEBlot Kit (New England Biolabs, Beverly, MA). Hybridization of the membrane, washes, and autoradiography were performed as previously described. 16 Aldolase was used as a housekeeping gene to verify RNA loading.
Statistical Analysis
Data comparing two groups were analyzed using the Student’s t-test for unpaired data. One-way analysis of variance and posthoc comparison were used when more than two groups were compared. A P value less than 0.05 using a two-tailed test was considered significant. The data were expressed as the mean value ± SEM.
Results
Neutrophil Accumulation and Edema Formation in the Lungs of CD44-Deficient Mice and Wild-Type Mice after 6 Hours of Bacterial Pneumonia
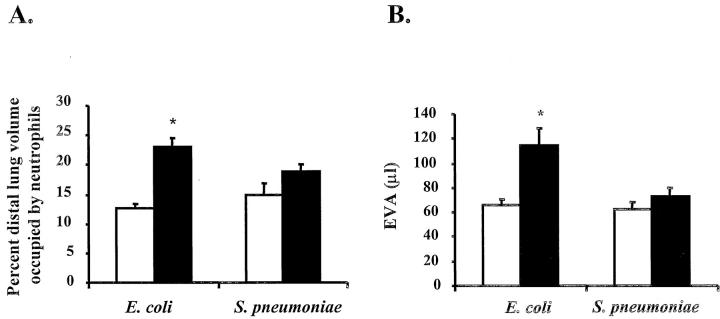
Histological examination showed more severe capillary disruption and more neutrophil accumulation in the lungs of CD44-deficient mice 6 hours after instillation of E. coli (Figures 1 and 2) ▶ ▶ . Neutrophil accumulation in the lungs was quantified by point counting. As shown in Figure 2A ▶ , the percentage of the distal lung volume occupied by neutrophils was significantly higher in CD44-deficient mice compared to wild-type mice after 6 hours of E. coli pneumonia (23.0 ± 1.7% versus 12.7 ± 0.7%, n = 6 and 7, P < 0.05). In contrast, neutrophil accumulation 6 hours after instillation of S. pneumoniae was not different in CD44-deficient mice and wild-type mice (18.9 ± 1.2% and 14.7 ± 2.0% neutrophils, respectively, n = 6). Circulating leukocyte counts in untreated mice or after 6 hours of pneumonia were not different in mice of either genotype (Table 1) ▶ . In addition, examination of lung histology of untreated mice showed no differences between wild-type and CD44-deficient mice (data not shown).
Figure 1.
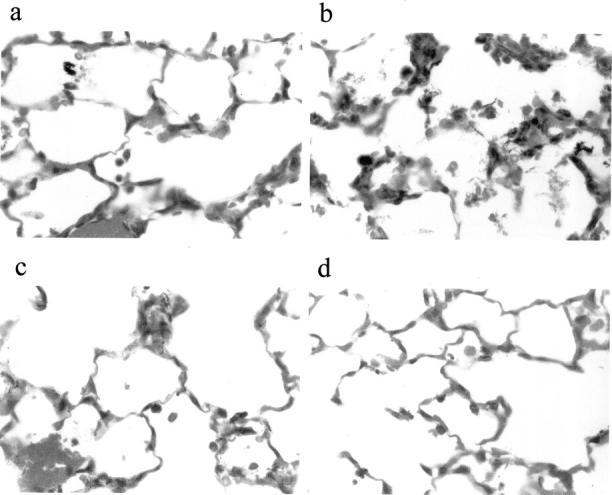
Histological sections of lungs of wild-type (a, c) and CD44-deficient mice (b, d) after 6 hours of pneumonia induced by E. coli (a, b) or S. pneumoniae (c, d). Original magnification, ×600.
Figure 2.
Neutrophil accumulation (A) and edema formation (B) in the lungs of CD44-deficient mice and wild-type mice after 6 hours of pneumonia induced by E. coli or S. pneumoniae. A: The neutrophil accumulation was quantified by point counting as described in Material and Methods. The data are presented as percent distal lung volume occupied by neutrophils. B: Edema formation was evaluated by measuring the accumulation of EVA as described in Material and Methods. Open bars, wild-type mice; filled bars, CD44-deficient mice. All of the data are presented as mean ± SEM (n ≥ 5 in each group). *, P < 0.05 when compared to wild-type mice.
Table 1.
Circulating Leukocyte or Neutrophil Counts Were Not Different in CD44-Deficient Mice and Wild-Type Mice without Bacteria Instillation or after 6 Hours of Pneumonia
| Wild-type mice | CD44-deficient mice | |
|---|---|---|
| Circulating leukocyte counts | ||
| No instillation | 6.83 ± 0.36 | 7.10 ± 0.14 |
| E. coli pneumonia | 1.71 ± 0.09 | 1.92 ± 0.15 |
| S. pneumoniae pneumonia | 1.84 ± 0.24 | 2.41 ± 0.48 |
| Circulating neutrophil counts | ||
| No instillation | 0.65 ± 0.07 | 0.64 ± 0.09 |
| E. coli pneumonia | 0.77 ± 0.16 | 0.81 ± 0.08 |
| S. pneumoniae pneumonia | 0.56 ± 0.06 | 0.67 ± 0.09 |
Data are presented as means ± SEM × 106/ml blood (n ≥ 5 in each group).
In addition to enhanced neutrophil emigration in CD44-deficient mice after E. coli pneumonia, edema accumulation was greater in these mice compared to wild-type mice. The EVA measured 114.5 ± 14.1 μl (n = 6) and 66.1 ± 4.8 μl (n = 7) in CD44-deficient mice and wild-type mice, respectively (P < 0.05, Figure 2B ▶ ). In contrast, edema induced by S. pneumoniae was similar with the two genotypes, because the EVA measured 73.2 ± 6.6 μl (n = 5) and 62.5 ± 5.7 μl (n = 6) in CD44-deficient mice and wild-type mice, respectively (Figure 2B) ▶ . In the absence of any inflammatory stimulus, the EVA was similar in CD44-deficient mice and wild-type mice [25.1 ± 1.5 and 21.2 ± 1.8 μl (n = 5), respectively].
Bacterial Counts in the Lungs of CD44-Deficient Mice and Wild-Type Mice after 6 Hours of Bacterial Pneumonia
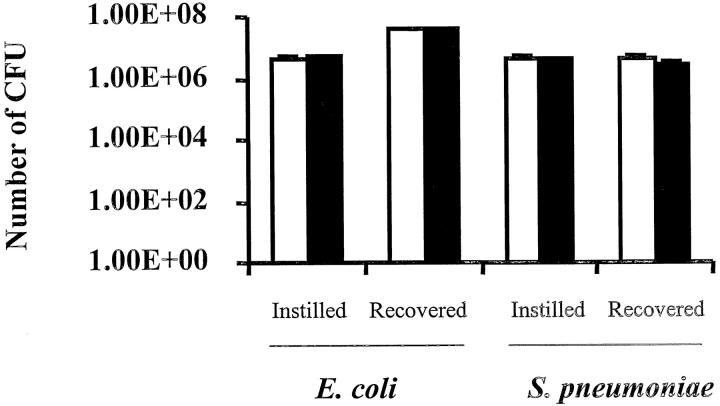
To determine whether the increased edema formation and neutrophil accumulation in the lungs of CD44-deficient mice after 6 hours of E. coli pneumonia were because of defective clearance of instilled bacteria, the number of bacteria remaining in the lungs 6 hours after instillation of organisms was determined. In CD44-deficient mice and wild-type mice, the number of CFUs of E. coli instilled was 5.27 ± 0.13 × 106 and 4.21 ± 0.09 × 106, respectively, and the number of CFUs of E. coli recovered was 4.30 ± 0.54 × 107 and 3.75 ± 0.82 × 107, respectively (n = 5 in each group, Figure 3 ▶ ). In S. pneumoniae pneumonia, 4.05 ± 0.2 × 106 and 3.98 ± 0.19 × 106 CFUs were instilled in CD44-deficient mice and wild-type mice, and 3.10 ± 0.66 × 106 and 4.60 ± 1.40 × 106 CFUs were recovered, respectively (Figure 3) ▶ . These data indicate that there was no difference in the clearance of either organism in CD44-deficient compared with wild-type mice.
Figure 3.
Bacteria recovered after 6 hours of pneumonia induced by E. coli or S. pneumoniae in the lungs of CD44-deficient mice and wild-type mice. The number of CFU of either bacterium instilled was determined. After 6 hours of pneumonia, the lungs were homogenized, and the number of CFU of either bacterium in the lung homogenate was measured. Open bars, wild-type mice; filled bars, CD44-deficient mice. The data are presented as number of CFU of bacteria instilled or recovered (n = 5 in each group).
Evaluation of Apoptotic Neutrophils Recovered in BAL
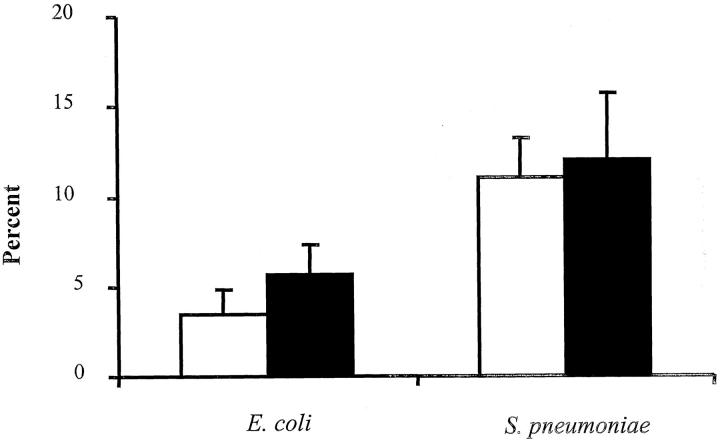
Because CD44 has been implicated in the clearance of apoptotic neutrophils during inflammatory responses, 39,40 studies were performed to determine whether the observed increased in neutrophil accumulation in CD44-deficient mice after instillation of E. coli was because of accumulation of apoptotic neutrophils in the lung. Six hours after bacterial instillation, the lungs were lavaged, and apoptosis of neutrophils recovered in the BAL fluid was evaluated by staining with annexin V, which detects changes in cell surface that occur early during apoptosis. Only a small percentage of neutrophils recovered in the BAL fluid were apoptotic, and there was no significant difference in response to either E. coli or S. pneumoniae between wild-type and CD44-deficient mice (Figure 4) ▶ . These data suggest that the increased neutrophil accumulation in CD44-deficient compared to wild-type mice during E. coli pneumonia was not because of differences in neutrophil apoptosis at this early time point.
Figure 4.
Percentage of apoptotic neutrophils recovered in the BAL fluid of wild-type or CD44-deficient mice 6 hours after instillation of E. coli or S. pneumoniae. Apoptotic neutrophils in the BAL fluid were evaluated by annexin V and Gr-1 staining and quantified by flow cytometry as described in Material and Methods. The data are expressed as the percentage of neutrophils that are annexin V-positive and presented as mean ± SEM (n = 5 or 6 in each group). Open bars, wild-type mice; filled bars, CD44-deficient mice.
Neutrophil Chemotaxis through Matrigel in Vitro
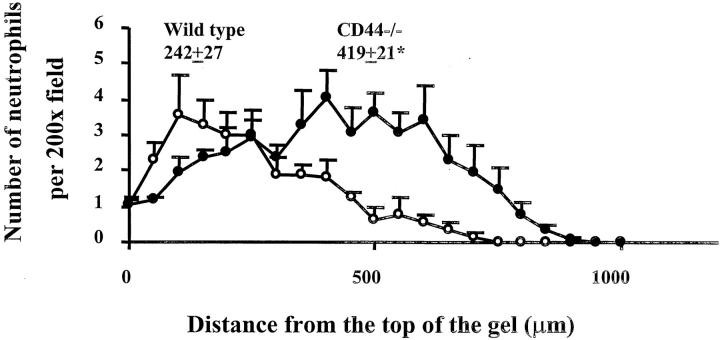
To determine whether CD44 deficiency altered the ability of neutrophils to migrate through extracellular matrix induced by a chemoattractant, neutrophil chemotaxis assays through Matrigel, a matrix containing HA, were performed in vitro. The distance that neutrophils migrated into Matrigel within 30 minutes of exposure to MIP-2 was first determined. In the absence of MIP-2, few neutrophils migrated into the Matrigel. MIP-2 caused both wild-type and CD44-deficient neutrophils to migrate into the Matrigel, but CD44-deficient neutrophils migrated further into the Matrigel than wild-type neutrophils (Figure 5) ▶ . On average, CD44-deficient neutrophils moved 419 ± 21 μm toward MIP-2, whereas wild-type neutrophils moved only 242 ± 27 μm (n = 5 or 6, P < 0.05). Thus, CD44-deficient neutrophils (14.0 ± 0.7 μm/minute) migrated faster than wild-type neutrophils (8.0 ± 0.9 μm/minute) into Matrigel in response to MIP-2.
Figure 5.
The distance that neutrophils migrated into Matrigel after 30 minutes. CD44-deficient and wild-type leukocytes isolated from venous blood were labeled with calcein AM and added to transwell filters coated with Matrigel. The number of neutrophils that migrated into the gels was counted from the top of the gels toward the chemoattractant at increments of 50 μm. For each experiment, the number of neutrophils in five fields (×200) was counted and averaged at each incremental level. Data are presented as mean ± SEM from five or six separate experiments. Open symbols, wild-type neutrophils; filled symbols, CD44-deficient neutrophils. The average distance that neutrophils migrated into Matrigel was calculated and included in the graph. *, P < 0.05 when compared with wild-type neutrophils.
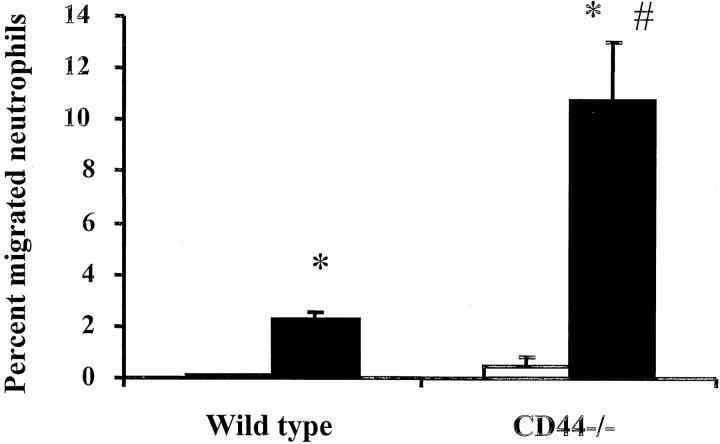
The number of neutrophils that migrated across Matrigel and accumulated in the bottom chamber after 6 hours was also measured. As shown in Figure 6 ▶ , in the absence of a chemoattractant, few neutrophils migrated into the bottom chamber. fMLP induced both CD44-deficient and wild-type neutrophils to migrate through Matrigel. However, significantly more CD44-deficient neutrophils migrated than wild-type neutrophils, and the percentage of neutrophils that migrated within 6 hours measured 10.8 ± 2.2% and 2.2 ± 0.3%, respectively (P < 0.05, n ≥12). In the absence of Matrigel, the ability of CD44-deficient and wild-type neutrophils to migrate across filters in response to fMLP was similar, and the percentage of neutrophils that migrated in 1 hour measured 8.8 ± 0.5% and 7.1 ± 0.5%, respectively (n = 5). Taken together, these data suggested that CD44 deficiency resulted in enhanced neutrophil migration through Matrigel in the presence of a chemoattractant.
Figure 6.
The number of neutrophils that migrated across Matrigel after 6 hours. CD44-deficient and wild-type leukocytes were isolated from venous blood and added to transwell filters coated with Matrigel. The percentage of neutrophils that migrated through the transwells in response to either buffer or fMLP placed in the bottom chambers was calculated. Open bars, buffer in the lower chamber; filled bars, 10−7 mol/L fMLP in the lower chamber. The data were presented as percent neutrophils migrated and was presented as means ± SEM (n ≥ 8 in each group). *, P < 0.05 when compared to buffer; #, P < 0.05 when compared to wild-type neutrophils.
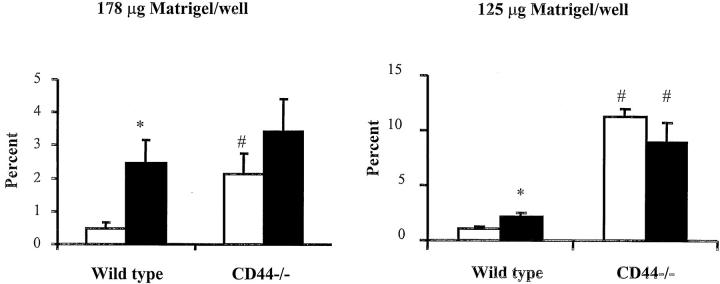
To determine whether CD44 interacts with HA in Matrigel to modulate neutrophil migration, Matrigel was pretreated with hyaluronidase to degrade HA. At the higher concentration of Matrigel, hyaluronidase increased the number of wild-type neutrophils that migrated across Matrigel to that observed for CD44-deficient neutrophils, whereas the migration of CD44-deficient neutrophils was unchanged by hyaluronidase treatment (Figure 7) ▶ . At the lower concentration of Matrigel, hyaluronidase also increased the number of wild-type neutrophils that migrated across Matrigel, but not to that attained by CD44-deficient neutrophils (Figure 7) ▶ . These data show that hyaluronidase enhances wild-type but not CD44-deficient neutrophil migration, suggesting that HA contributes to the observed differences between these genotypes, and that other ligands of CD44 in the Matrigel may also play a role depending on the conditions of the Matrigel.
Figure 7.
The effect of treatment of Matrigel with hyaluronidase on the migration of wild-type and CD44-deficient neutrophils. Matrigel at a higher concentration (178 μg/well) or a lower concentration (125 μg/well) was treated with 10 U/ml hyaluronidase or control vehicle as described in Material and Methods. The number of neutrophils that migrated across Matrigel in response to fMLP after 6 hours was measured as described above. Open bars, treatment of Matrigel with control vehicle; filled bars, treatment of Matrigel with hyaluronidase. The data were presented as percent neutrophils migrated and was presented as means ± SEM (n ≥ 5 in each group). *, P < 0.05 when compared to treatment with control vehicle; #, P < 0.05 when compared to wild-type neutrophils.
HA Content in the Lungs of CD44-Deficient Mice and Wild-Type Mice after 6 Hours of Bacterial Pneumonia
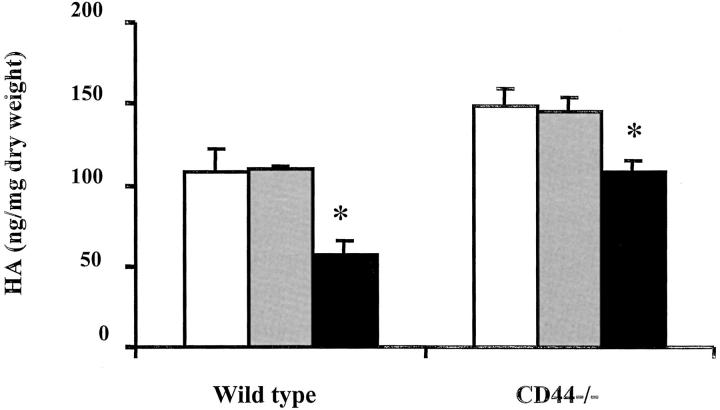
To determine whether the lack of difference in inflammatory responses between wild-type and CD44-deficient mice in S. pneumoniae pneumonia was associated with a difference in HA concentration, HA content in lungs of wild-type and CD44-deficient mice after instillation of saline, E. coli, or S. pneumoniae was compared. After 6 hours of E. coli pneumonia, the HA concentration remained unchanged in both wild-type and CD44-deficient mice when compared to their respective saline-treated control animals (Figure 8) ▶ . In contrast, in S. pneumoniae pneumonia, a significant decrease in HA concentration occurred in both wild-type (57 ± 9 versus 108 ± 14 ng/mg dry weight, P = 0.025, n = 3) and CD44-deficient mice (108 ± 7 versus 148 ± 11 ng/mg dry weight, P = 0.047, n = 3) when compared to their saline-treated controls (Figure 8) ▶ . These data suggest that the decrease in HA content in S. pneumoniae pneumonia may possibly contribute to the lack of differences in inflammatory responses observed between wild-type and CD44-deficient mice.
Figure 8.
HA content in the lungs of CD44-deficient mice and wild-type mice after instillation of saline, E. coli, or S. pneumoniae. HA content was measured as described in Materials and Methods. White bars, saline-treated mice; gray bars, E. coli-treated mice; black bars, S. pneumoniae-treated mice. The data are means ± SEM (n = 3). *, P < 0.05 when compared to their respective saline-treated control mice.
Inflammatory Gene Expression in the Lungs of CD44-Deficient Mice and Wild-Type Mice after 6 Hours of Bacterial Pneumonia
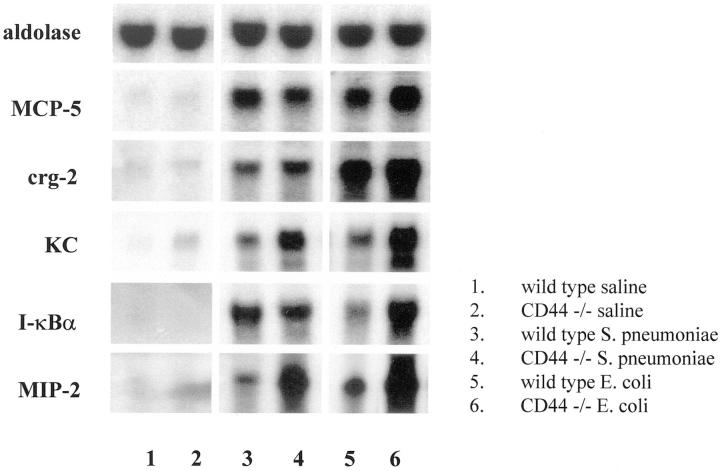
To determine whether the enhanced inflammatory responses observed in CD44-deficient mice during E. coli pneumonia was associated with altered inflammatory gene expression, Northern blot analysis was performed. The CD44-deficient mice expressed more mRNA for I-κBα than wild-type mice in E. coli, but not S. pneumoniae pneumonia (Figure 9) ▶ . The mRNA expression of KC and MIP-2, two neutrophil chemoattractants, was significantly higher in CD44-deficient mice than wild-type mice in both types of pneumonia (Figure 9) ▶ . In contrast, there was no difference between wild-type and CD44-deficient mice in the expression of MCP-5 or crg-2 (IP-10), chemokines that recruit other leukocytes. These data suggest that CD44 deficiency resulted in increased mRNA expression of several inflammatory genes.
Figure 9.
Inflammatory gene expression in the pneumonic lungs of CD44-deficient mice and wild-type mice after 6 hours of pneumonia induced by E. coli or S. pneumoniae. Expression of mRNAs from whole lungs was detected in Northern blots as described in Materials and Methods. Aldolase was used as a loading control. Lanes 1 to 6: mRNA in lungs of wild-type and CD44-deficient mice after saline (lanes 1 and 2), S. pneumoniae (lanes 3 and 4), and E. coli (lanes 5 and 6), respectively. Data shown is representative of three independent experiments.
Discussion
Bacteria within the airspace of the lungs induce edema formation and neutrophil emigration from blood into the airspaces. In E. coli-induced pneumonia, the number of neutrophils accumulating in the lungs was increased by 84% in CD44-deficient mice, and edema formation was 88% greater in CD44-deficient mice compared to wild-type mice. This difference was not observed in S. pneumoniae pneumonia. In addition, CD44-deficient neutrophils migrated through Matrigel more efficiently in response to chemoattractants than wild-type neutrophils in vitro that was in part dependent on HA in the Matrigel. Moreover, CD44 deficiency resulted in increased mRNA expression of several inflammatory genes. Together, these data demonstrate that CD44 deficiency results in enhanced inflammation and lung injury in E. coli- but not S. pneumoniae-induced pneumonia, suggesting a previously unrecognized role for CD44 in limiting the inflammatory response to E. coli.
CD44 has been implicated in acute and chronic immunological responses, as demonstrated using several models of chronic inflammatory responses. Administration of blocking antibodies against CD44 prevents cutaneous delayed-type hypersensitivity responses, 41 attenuates the responses of collagen II arthritis, 42 and prevents tissue edema formation and leukocyte infiltration in murine arthritis. 43 In addition, CD44-deficient mice exhibit a markedly reduced vascular leak syndrome in the lungs and liver induced by IL-2, and this reduction in endothelial cell injury was associated with a marked decrease in IL-2-induced lymphokine-activated killer cell activity. 44 A recent study by Teder and colleagues 40 demonstrated that CD44-deficient mice exhibit increased mortality from lung injury induced by bleomycin, implicating a role for CD44 in the resolution of pulmonary inflammation. This present study suggests that during acute bacterial pneumonia induced by E. coli, CD44 acts to limit neutrophil emigration and edema formation.
The increased neutrophil accumulation in the lungs of CD44-deficient mice during E. coli pneumonia was not because of a defect in clearing instilled bacteria or a difference in neutrophil apoptosis. Several studies have implicated a role for CD44 in clearing apoptotic neutrophils both in vitro and in vivo during chronic inflammatory responses. 39,40 This present study demonstrates that during acute bacterial pneumonia, the percentage of emigrated neutrophils in the alveolar space that were apoptotic was small, and there was no difference in response to either E. coli or S. pneumoniae between wild-type and CD44-deficient mice. In addition, examination of the emigrated neutrophils in all samples demonstrates no morphological alterations of neutrophils characteristic of apoptosis and no phagocytosis of neutrophils by macrophages. These data suggest that the increased neutrophil accumulation in the lungs of CD44-deficient mice during early E. coli pneumonia is not because of accumulation of apoptotic neutrophils in the alveolar space.
This increased neutrophil accumulation in the lungs of CD44-deficient mice during E. coli pneumonia may occur because of enhanced neutrophil migration as a result of absent interactions between CD44 and its ligands. HA, the major ligand for CD44, is expressed in the interstitium of the lungs, including within the alveolar walls. 45,46 During migration through the interstitium, neutrophils are in close contact with fibroblasts and extracellular matrix components, 1 and these macromolecules may play important roles in mediating neutrophil adhesion and migration. 47 A recent study by Si-Tahar and colleagues 9 demonstrates that ligation of neutrophil CD44 by a specific activating antibody (clone IM7) or HA inhibits neutrophil migration across cultured epithelium or microfilters in response to fMLP in vitro without reducing neutrophil adhesion. Incorporation of HA into collagen matrices decreases neutrophil migration. 48,49 Our studies demonstrate that CD44 deficiency results in enhanced neutrophil migration through Matrigel in response to chemoattractants in vitro that is in part dependent on HA in the Matrigel. CD44-deficient neutrophils migrate faster through Matrigel than wild-type neutrophils, and more CD44-deficient neutrophils accumulate into the bottom chamber. Moreover, degradation of HA in the Matrigel by hyaluronidase results in an increase in migration of wild-type neutrophils but not CD44-deficient neutrophils. These data suggest that HA in the matrix and CD44 expressed by the neutrophils serve to limit neutrophil migration across matrix proteins. Interestingly, the ability of hyaluronidase to increase the migration of wild-type neutrophils to a similar level as the CD44-deficient neutrophils depends on the concentration of Matrigel. These results suggest that CD44 may slow neutrophil migration through extracellular matrix through a mechanism that is at least in part dependent on HA, and that other CD44 ligands in the matrix may also play a role. These roles of CD44 in limiting neutrophil migration may be because of CD44-induced intracellular signaling events in neutrophils on binding by HA or other ligands. This enhanced migration observed with CD44-deficient neutrophils may contribute to the observed increase in neutrophil accumulation in E. coli pneumonia. Taken together, these studies demonstrate a role for CD44 in slowing neutrophil migration through extracellular matrix, suggesting that CD44 interactions with HA or other matrix components may limit neutrophil migration across the interstitium in vivo.
CD44 deficiency also resulted in increased mRNA expression of several inflammatory genes. The CD44-deficient mice expressed more mRNA for I-κBα than wild-type mice in response to E. coli, but not S. pneumoniae. The mRNA expression of KC and MIP-2, two neutrophil chemoattractants, was significantly higher in CD44-deficient mice than wild-type mice in both types of pneumonia. In contrast, the expression of MCP-5 or crg-2 (IP-10), chemokines that recruit other leukocytes was not different between these two genotypes, suggesting that CD44 deficiency does not result in an overall increase in inflammatory gene expression. This increase is not solely because of the increased neutrophil emigration because the expression of KC and MIP-2 was higher in CD44-deficient mice than wild-type mice in both types of pneumonias while neutrophil emigration was only higher in CD44-deficient mice in response to E. coli. The cellular sources of this increased gene expression remain to be determined and alveolar macrophages, epithelium, endothelium, and neutrophils are all likely to contribute. Interestingly, a recent study by Sconocchia and colleagues 20 demonstrated that ligation of CD44 by antibodies or HA on neutrophils induces IL-6 expression. IL-6, a cytokine with anti-inflammatory properties, down-regulates the synthesis of IL-1, tumor necrosis factor-α, and MIP-2. 50,51 Thus, this increase in mRNA expression of these genes may reflect a dysregulated response of several cell types to instilled bacteria in the absence of CD44.
Interestingly, CD44 deficiency results in enhanced inflammatory responses only in E. coli-, and not in S. pneumoniae-induced pneumonia. If this effect of CD44 is mediated through CD44/HA interactions, the difference between these two pneumonias may be because of differences in HA content and/or in the regulation of CD44/HA interactions by inflammatory cytokines. After instillation of S. pneumoniae but not E. coli, there was a significant decrease in HA content in the lungs at 6 hours. This may be because of the fact that S. pneumoniae expresses hyaluronidase, which is localized to the capsule and can be secreted into the local environment, 28,29 whereas E. coli does not. Because there were similar mRNA levels of chemokines that recruit neutrophils in both types of pneumonia, these results suggest that a decrease in HA content may minimize the role of CD44 and result in the lack of enhanced inflammatory responses in CD44-deficient mice with S. pneumoniae pneumonia. Our in vitro studies indeed demonstrate that the increased migration of CD44-deficient neutrophils compared with wild-type neutrophils depends on HA in the matrix. In addition, the regulation of CD44/HA interaction by cytokines may account for differences in CD44’s role in S. pneumoniae compared to E. coli pneumonia. These two organisms induce differential activation of transcription factors and production of inflammatory cytokines, as well as differential utilization of adhesion molecules. 52-54 CD44/HA interactions may be enhanced only by the cytokine cascades induced by E. coli.
In summary, this study demonstrated that CD44 deficiency resulted in an enhanced inflammatory response during E. coli pneumonia, but not S. pneumoniae pneumonia, suggesting that CD44 may serve to down-regulate this response. Evidence is provided for both enhancement of chemokine production and accelerated crawling of neutrophils as mechanisms through which CD44 may dampen the acute response.
Acknowledgments
We thank Dr. Jindrich Soltys for his help in flow cytometry studies.
Footnotes
Address reprint requests to Claire M. Doerschuk, M.D., Room 787, 11100 Euclid Ave., RB&C, Cleveland, OH 44106. E-mail: cmd22@po.cwru.edu.
Supported by National Institutes of Health grants HL52466 and HL48160 (to C. M. D.); the Burroughs Wellcome Fund (Clinical Scientist Award in Translational Research to C. M. D.), the American Lung Association (to Q. W.), the Parker B. Francis Fellowship from the Francis Families Foundation (to Q. W.), and National Institutes of Health grants HL60539 and HL10177-02 (to P. W. N.).
References
- 1.Behzad AR, Chu F, Walker DC: Fibroblasts are in a position to provide directional information to migrating neutrophils during pneumonia in rabbit lungs. Microvasc Res 1996, 51:303-316 [DOI] [PubMed] [Google Scholar]
- 2.Hogg JC, Doerschuk CM: Leukocyte traffic in the lung. Annu Rev Physiol 1995, 57:97-114 [DOI] [PubMed] [Google Scholar]
- 3.Gebb SA, Graham JA, Hanger CC, Godbey PS, Capen RL, Doerschuk CM, Wagner WW: Sites of leukocyte sequestration in the pulmonary microcirculation. J Appl Physiol 1995, 79:493-497 [DOI] [PubMed] [Google Scholar]
- 4.Doerschuk CM, Winn RK, Coxson HO, Harlan JM: CD18-dependent and -independent mechanisms of neutrophil emigration in the pulmonary and systemic microcirculation of rabbits. J Immunol 1990, 144:2327-2333 [PubMed] [Google Scholar]
- 5.Walker DC, Behzad AR, Chu F: Neutrophil migration through preexisting holes in the basal laminae of alveolar capillaries and epithelium during streptococcal pneumonia. Microvasc Res 1995, 50:397-416 [DOI] [PubMed] [Google Scholar]
- 6.Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B: CD44 is the principal cell surface receptor for hyaluronate. Cell 1990, 61:1303-1313 [DOI] [PubMed] [Google Scholar]
- 7.Laurent TC, Fraser JR: Hyaluronan. EMBO J 1992, 6:2397-2404 [PubMed] [Google Scholar]
- 8.Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A, Jr, Kubalak S, Klewer SE, McDonald JA: Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest 2000, 106:349-360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Si-Tahar M, Sitaraman S, Shibahara T, Madara JL: Negative regulation of epithelium-neutrophil interactions via activation of CD44. Am J Physiol 2001, 280:C423-C432 [DOI] [PubMed] [Google Scholar]
- 10.Jalkanen S, Saari S, Kalimo H, Lammintausta K, Nainio E, Leino R, Duijvestijin AM, Kalimo K: Lymphocyte migration into the skin: the role of lymphocyte homing receptor (CD44) and endothelial cell antigen (HECA-452). J Invest Dermatol 1990, 94:786-792 [DOI] [PubMed] [Google Scholar]
- 11.Picker LJ, Terstappen LW, Rott LS, Streeter PR, Stein H, Butcher EC: Differential expression of homing-associated adhesion molecules by T cell subsets in man. J Immunol 1990, 145:3247-3255 [PubMed] [Google Scholar]
- 12.Mohamadzadeh M, DeGrendele H, Arizpe H, Estess P, Siegelman M: Proinflammatory stimuli regulate endothelial hyaluronan expression and CD44/HA-dependent primary adhesion. J Clin Invest 1998, 101:97-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeGrendele HC, Estess P, Siegelman MH: Requirement for CD44 in activated T cell extravasation into an inflammatory site. Science 1997, 278:672-675 [DOI] [PubMed] [Google Scholar]
- 14.Maiti A, Maki G, Johnson P: TNF-α induction of CD44-mediated leukocyte adhesion by sulfation. Science 1998, 282:941-943 [DOI] [PubMed] [Google Scholar]
- 15.Seth A, Gote L, Nagarkatti M, Nagarkatti PS: T-cell-receptor-independent activation of cytolytic activity of cytotoxic T lymphocytes mediated through CD44 and gp90MEL-14. Proc Natl Acad Sci USA 1991, 88:7877-7881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galandrini R, Albi N, Tripodi G, Zarcone D, Terenzin A, Moretta A, Grossi CE, Velardi A: Antibodies to CD44 trigger effector functions of human T cell clones. J Immunol 1993, 150:4225-4235 [PubMed] [Google Scholar]
- 17.McKee C, Margaret BP, Cowman M, Burdick M, Strieter RM, Bao C, Noble PW: Hyaluronan (HA) fragment induced chemokine gene expression in alveolar macrophages: the role of HA size and CD44. J Clin Invest 1996, 98:2403-2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKee C, Lowenstein C, Horton M, Wu J, Bao C, Chin B, Choi A, Noble P: Hyaluronan fragments induce nitric oxide in murine macrophages through NF-κB-dependent mechanism. J Biol Chem 1997, 272:8013-8020 [DOI] [PubMed] [Google Scholar]
- 19.Noble P, Lake F, Henson P, Riches D: Hyaluronate activation of CD44 induces insulin-like growth factor-1 expression by a tumor necrosis factor-a-dependent mechanism in murine macrophages. J Clin Invest 1993, 91:2368-2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sconocchia G, Campagnano L, Adorno D, Iacona A, Cococcetta NY, Boffo V, Amadori S, Casciani CU: CD44 ligation on peripheral blood polymorphonuclear cells induces interleukin-6 production. Blood 2001, 97:3621-3627 [DOI] [PubMed] [Google Scholar]
- 21.Okamoto I, Kawano Y, Murakami D, Sasayama T, Araki N, Miki T, Wong AJ, Saya H: Proteolytic release of CD44 intracellular domain and its role in the CD44 signaling pathway. J Cell Biol 2001, 155:755-762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levesque MC, Haynes BF: In vitro culture of human peripheral blood monocytes induces hyaluronan binding and up-regulates monocyte variant CD44 isoform expression. J Immunol 1996, 156:1557-1565 [PubMed] [Google Scholar]
- 23.Culty M, O’Mara TE, Underhill CB, Yeager H, Jr, Swartz RP: Hyaluronan receptor (CD44) expression and function in human peripheral blood monocytes and alveolar macrophages. J Leukoc Biol 1994, 56:605-611 [DOI] [PubMed] [Google Scholar]
- 24.Lesley J, English N, Perschl A, Gregoroff J, Hyman R: Variant cell lines selected for alterations in the functions of the hyaluronan receptor CD44 show differences in glycosylation. J Exp Med 1995, 182:431-437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lesley J, Hyman R, Kincade PW: CD44 and its interaction with extracellular matrix. Adv Immunol 1993, 54:271-335 [DOI] [PubMed] [Google Scholar]
- 26.Katoh S, Enf Z, Oritani K, Shiozato T, Kincade PW: Glycosylation of CD44 negatively regulates its recognition of hyaluronan. J Exp Med 1995, 182:419-429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skelton TP, Zeng C, Nocks A, Stamenkovic I: Glycosylation provides both stimulatory and inhibitory effects on cell surface and soluble CD44 binding to hyaluronan. J Cell Biol 1998, 140:431-446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrieri P: GBS enzymes, hemolysin, toxins and other products. Antibiot Chemother 1985, 35:57-70 [DOI] [PubMed] [Google Scholar]
- 29.Berry AM, Lock RA, Thomas SM, Rajan DP, Hansman D, Paton JC: Cloning and nucleotide sequence of the Streptococcus pneumoniae hyaluronidase gene and purification of the enzyme from recombinant Escherichia coli. Infect Immun 1994, 62:1101-1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doerschuk CM: Mechanisms of leukocyte sequestration in inflamed lungs. Microcirculation 2001, 8:71-88 [PubMed] [Google Scholar]
- 31.Schmits R, Filmus J, Gerwin N, Senaldi G, Kiefer F, Kundig T, Wakeham A, Shahinian A, Catzavelos C, Rak J, Furlonger C, Zakarian A, Simard JJ, Ohashi PA, Paige CJ, Gutierrez-Ramos JC, Mak TW: CD44 regulates hematopoietic progenitor distribution, granuloma formation, and tumorigenicity. Blood 1997, 90:2217-2233 [PubMed] [Google Scholar]
- 32.Qin L, Quinlan WM, Doyle NA, Graham L, Sligh JE, Takei F, Beaudet AL, Doerschuk CM: The roles of CD11/CD18 and ICAM-1 in acute Pseudomonas aeruginosa-induced pneumonia in mice. J Immunol 1996, 157:5016-5021 [PubMed] [Google Scholar]
- 33.Mizgerd JP, Meek BB, Kutkoshi GJ, Bullard DC, Beaudet AL, Doerschuk CM: Selectins and neutrophil traffic: margination and Streptococcus pneumoniae-induced emigration in murine lungs. J Exp Med 1996, 184:639-645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weibel ER: Morphometry: stereological theory and practical methods. Gill J eds. Models of Lung Disease: Microscopy and Structural Methods, 1990, vol 47.:pp 199-252 Marcel Dekker, Inc., New York [Google Scholar]
- 35.Loike JD, Cao L, Budhu S, Marcantonio EE, Khoury J, Hoffman S, Yednock TA, Silverstein SC: Differential regulation of β1 integrins by chemoattractants regulates neutrophil migration through fibrin. J Cell Biol 1999, 144:1047-1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolpe SC, Cerami A: Macrophage inflammatory proteins 1 and 2: members of a novel superfamily of cytokines. FASEB J 1989, 3:2565-2573 [DOI] [PubMed] [Google Scholar]
- 37.Underhill CB, Nguyen HA, Shizari M, Culty M: CD44 positive macrophages take up hyaluronan during lung development. Dev Biol 1993, 155:324-336 [DOI] [PubMed] [Google Scholar]
- 38.Guo MM, Hildreth JE: Assessment of cell binding to hyaluronic acid in a solid-phase assay. Anal Biochem 1996, 233:216-220 [DOI] [PubMed] [Google Scholar]
- 39.Hart SP, Dougherty GJ, Haslett C, Dransfield I: CD44 regulates phagocytosis of apoptotic neutrophil granulocytes, but not apoptotic lymphocytes, by human macrophages. J Immunol 1997, 159:919-925 [PubMed] [Google Scholar]
- 40.Teder P, Vandivier RW, Jiang D, Liang J, Cohn L, Pure E, Henson PM, Noble PW: Resolution of lung inflammation by CD44. Science 2002, 296:155-158 [DOI] [PubMed] [Google Scholar]
- 41.Camp RL, Scheynius A, Johansson C, Pure E: CD44 is necessary for optimal contact allergic responses but not required for normal leukocyte extravasation. J Exp Med 1993, 178:497-507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verdrengh M, Holmdahl R, Tarkowski A: Administration of antibodies to hyaluronan receptor (CD44) delays the start and ameliorates the severity of collagen II arthritis. Scand J Immunol 1995, 42:353-358 [DOI] [PubMed] [Google Scholar]
- 43.Mikecz K, Brennan FR, Kim JH, Glant TT: Anti-CD44 treatment abrogates tissue oedema and leukocyte infiltration in murine arthritis. Nat Med 1995, 1:558-563 [DOI] [PubMed] [Google Scholar]
- 44.Rafi-Janajreh AQ, Chen D, Schmits R, Mak TW, Grayson RL, Sponenberg DP, Nagarkatti M, Nagarkatti PS: Evidence for the involvement of CD44 in endothelial cell injury and induction of vascular leak syndrome by IL-2. J Immunol 1999, 163:1619-1627 [PubMed] [Google Scholar]
- 45.Allen SJ, Sedin EG, Johzon A, Wells AF, Laurent TC: Lung hyaluronan during development: a quantitative and morphological study. Am J Physiol 1991, 260:H1449-H1454 [DOI] [PubMed] [Google Scholar]
- 46.Kobayashi H, Sun GW, Terao T: Immunolocalization of hyaluronic acid and inter-alpha-trypsin inhibitor in mice. Cell Tissue Res 1999, 296:587-597 [DOI] [PubMed] [Google Scholar]
- 47.Pakianathan DR: Extracellular matrix proteins and leukocyte functions. J Leukocyte Biol 1995, 57:699-702 [DOI] [PubMed] [Google Scholar]
- 48.Reid GG, Lackie JM, Gorham SD: The behaviour of BHK cells and neutrophil leukocytes on collagen gels of defined mechanical strength. Cell Biol Int Rep 1990, 14:1033-1045 [DOI] [PubMed] [Google Scholar]
- 49.Reid GG, Newman I: Human leukocyte migration through collagen matrices containing other extracellular matrix components. Cell Biol Int Rep 1991, 15:711-720 [DOI] [PubMed] [Google Scholar]
- 50.Barton BE: IL-6: insights into novel biological activities. Clin Immunol Immunopathol 1997, 85:16-20 [DOI] [PubMed] [Google Scholar]
- 51.Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, Achong MK: IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest 1998, 101:311-320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kubo H, Bell FP, Manning AM, Doerschuk CM: NF-κB translocation in CD18-dependent and -independent neutrophil emigration. Am J Respir Crit Care Med 1997, 155:A611 [Google Scholar]
- 53.Burns AR, Takei F, Doerschuk CM: Quantitation of ICAM-1 expression in mouse lung during pneumonia. J Immunol 1994, 153:3189-3198 [PubMed] [Google Scholar]
- 54.Kumasaka T, Doyle NA, Doerschuk CM: Inflammatory cytokines and soluble ICAM-1 in pneumonias induced by E. coli endotoxin or S. pneumoniae. Am J Respir Crit Care Med 1995, 151:A343 [Google Scholar]