Abstract
Human prion diseases like Creutzfeldt-Jakob disease are infectious, inherited, or sporadic neurodegenerative disorders, characterized by the accumulationof an abnormal isoform of the host-encoded prion protein. This affects nervous tissue in sporadic Creutzfeldt-Jakob disease and, additionally, in lymphoid tissue in bovine spongiform encephalopathy-linked variant Creutzfeldt-Jakob disease. Experimental studies have established the involvement of cells of the lymphoid and peripheral nervous system in the transport of prions to their target central nervous system tissue. To evaluate the role of vessel wall-associated mobile cells, we obtained formalin-fixed tissue blocks from various brain regions and/or basal arteries from sporadic, variant and iatrogenic Creutzfeldt-Jakob disease, and unselected control cases. We demonstrate disease-associated prion protein deposits in intracranial vessel walls, in sporadic and variant Creutzfeldt-Jakob disease by performing immunohistochemical staining and paraffin-embedded tissue blotting. Using double immunofluorescence, these deposits co-localize with HLA-DR and S-100 immunoreactive cells in the intima, which are components of the vascular-associated dendritic cell network, as well as with HLA-DR and CD-68 immunopositive macrophages of the intima and media. We conclude that mobile cells in vessel walls like dendritic and monocyte/macrophage lineage cells may be involved in spread of disease-associated prion protein and possibly also of infectivity.
Prion diseases (PrD) are infectious, inherited, or sporadic neurodegenerative disorders. Beside the classical neuropathological triad (spongiform change, astrogliosis, and neuronal loss), PrD are characterized by the accumulation of an abnormal isoform of the host-encoded prion protein (PrP). 1 In human PrD, this affects nervous tissue in sporadic Creutzfeldt-Jakob disease (sCJD) 2 as well as in lymphoid tissue in bovine spongiform encephalopathy-linked variant Creutzfeldt-Jakob disease (vCJD). 3,4
Tissue-related diagnosis of PrD can be achieved by post-mortem examination of the brain 2 and also by detection of disease-associated prion protein (PrPSc) in lymphoid tissue biopsy in vCJD. 4 Detection of PrPSc in blood samples as a specific diagnosis for PrD would require its transport across vessel walls. As PrPSc is postulated as the infectious particle in PrD, 1 this transport can also be interpreted as spread of infectivity. Blood transfusion is not considered to carry a risk in sporadic cases, 5 although it may transmit disease in acquired PrD. 6 To evaluate this pathway we examined intracranial and extracranial vessels in different PrD subtypes, and we demonstrate the presence of PrPSc in vessel walls.
Materials and Methods
Case Selection
Formalin-fixed, paraffin-embedded tissue blocks from the cerebral cortex, basal ganglia, thalamus, brain stem, cerebellum and/or basal arteries (eg, basilar artery and/or branches of circulus Willisi) were obtained from 30 sCJD, 17 vCJD, 4 iatrogenic (growth hormone application) CJD (iCJD), and 30 unselected control cases with a variety of neuropathological diagnoses including brain tumors, inflammatory, neurodegenerative, and cerebrovascular diseases. In three sCJD, two vCJD, and two controls we investigated extracranial segments of carotid artery and/or ascending aorta as well.
Immunocytochemistry
For detection of PrPSc, we stained sections with 3F4 (monoclonal, 1:500, Senetek PLC, Napa, CA, USA) after a three-tiered tissue pretreatment, including 10 minutes of hydrated autoclaving at 121°C, 5 minutes of 96% formic acid, and 2 hours of 4 mol/L guanidine thiocyanate at 4°C. In cases with immunoreactivity (IR) in the vessel wall, we then obtained adjacent sections and used antibodies 6H4 (monoclonal, 1:500, Prionics, Zurich, Switzerland) and 12F10 (monoclonal, 1:1000, CEA, Fontenay-aux-Roses, France). To characterize labeled cells, we performed immunostaining for HLA-DR (CR3/43, monoclonal, 1:100, DAKO, Glostrup, Denmark), CD 68 (KP1, monoclonal, 1:100, DAKO), and S100 (polyclonal, 1:2000, DAKO). As a secondary system, we used the ChemMate detection kit (DAKO). Negative controls included omission or substitution of primary antibodies by nonspecific, isotype-matched antibodies.
Double Immunofluorescence
Double immunofluorescent labeling was evaluated by a Zeiss LSM 510 confocal laser microscope. The fluorescent-labeled secondary antibody for anti-PrP antibodies was Alexa Fluor 488 goat anti-mouse IgG (1:200 Molecular Probes, Eugene, OR, USA), for HLA-DR and CD68 we used Alexa Fluor 633 goat anti-mouse IgG (1:200, Molecular Probes), and for S-100 Alexa Fluor 633 goat anti-rabbit IgG (1:200, Molecular Probes) was utilized. We used argon 488-nm and helium/neon 633-nm lasers to elicit immunofluorescent staining. For nucleic acid counterstain we used SYTOX orange nucleic acid stain (1:1000, Molecular Probes) and a helium/neon 543-nm laser.
Paraffin-Embedded Tissue Blot
Paraffin-embedded tissue (PET) blot was performed on basal intracranial arteries from one sCJD and extracranial carotid and aorta from one vCJD case. Paraffin-embedded tissue sections were placed onto nitro-cellulose membrane for detection of PrPSc using the antibody 3F4 (monoclonal, 1:500, DAKO, Denmark) after proteinase K digestion. Labeling was carried out following the protocol of Schulz-Schaeffer et al, 7 with the alkaline phosphatase-coupled rabbit anti-mouse antibody replaced by an avidin-biotin system. A negative control, in which the primary antibody was omitted, was included for each section examined. Labeling was observed using a stereoscopic microscope.
Results
Using specific pretreatment and an immunostaining procedure for PrPSc, 8 we observe focal granular PrP IR in intracranial vessel walls of 6 of 30 sCJD, 3 of 17 vCJD, and none of the iCJD and control cases. It locates predominantly to the media of deep perforating and occasionally of meningeal and basal arteries (Figure 1a) ▶ as well as of small parenchymal vessels. Rarely do we observe it in the intima. We do not observe a correlation between PrP deposits in the brain parenchyma (eg, diffuse/synaptic, plaque) and presence of vessel wall PrP IR. In cases where we find PrP deposits in the vessel wall, it can be seen most often in more than one vessel and in different brain regions, whereas in other cases (iCJD), serial sections covering about 100 μm did not reveal PrP vessel wall deposits. In addition, perivascular PrP deposits are not only predominant in vCJD (Figure 1b) ▶ and iCJD, but also in sCJD cases. Nevertheless, as we rarely found more extensive PrP aggregates throughout the vessel wall (Figure 1c) ▶ , we excluded their amyloid nature by the absence of congophilia and birefringence.
Figure 1.
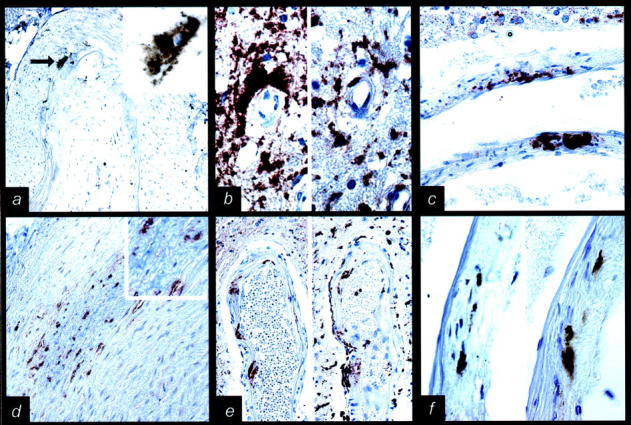
Disease-associated prion protein (PrP) deposits in vessel walls. a: PrP immunoreactivity (IR) (indicated by an arrow) in the media of basilar artery in sporadic Creutzfeldt-Jakob disease (sCJD) (3F4; magnification, ×100; enlarged in right upper corner, magnification, ×750). b: Perivascular PrP IR (left side of picture; 12F10; magnification, ×750) correlates with CD68 IR (right side of picture; magnification, ×750) in variant CJD (vCJD). c: Extensive PrP IR throughout an intracerebral (basal ganglia) vessel wall in vCJD (3F4; magnification, ×500). d: PrP IR in the outer part of the media in extracranial carotid artery in vCJD (6H4; magnification ×200; enlarged in right upper corner, magnification, ×750). e: PrP IR (left side of picture; 3F4; magnification, ×200) in a deep perforating artery in sCJD correlates with CD68 IR (right side of picture; magnification, ×200). f: PrP IR (left side of picture; 3F4; magnification, ×200) in a deep perforating artery in sCJD correlates with HLA-DR IR (right side of picture; magnification, ×200).
In extracranial carotid (Figure 1d) ▶ and ascending aorta we observe fine granular PrP IR in 2 of 2 vCJD, 0 of 3 of sCJD cases, and 0 of 2 controls. This is rather widespread and predominates in the outer part of the media and less in the intima and inner part of the media.
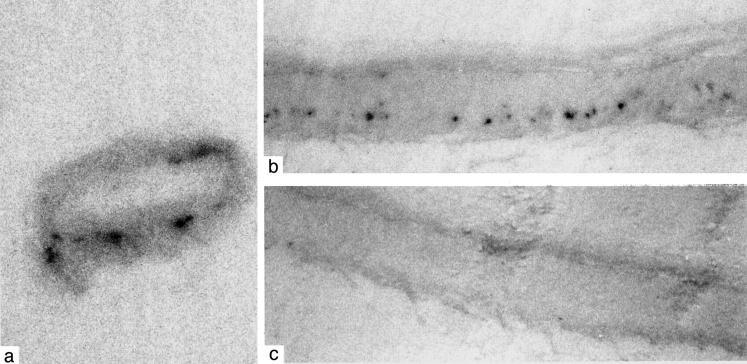
Using the PET blot, small, intense areas of PrP positivity are also observed within the intracranial vessels in one case of sCJD, selected from the six immunocytochemically positive sCJD cases. In many of the vessels, these intense clustered deposits appear to follow a linear pattern of labeling (Figure 2) ▶ . Extracranial carotid and aorta vessels in one vCJD case, selected from the two immunocytochemically positive vCJD cases, were found to be negative for PrP using the PET blot.
Figure 2.
Small, intense foci of disease-associated PrP positivity within the intracranial vessels in sporadic Creutzfeldt-Jakob disease visualized by the PET blot technique (a). These intense clustered deposits appear to follow a linear pattern of labeling (b). The control section for these in which the antibody was omitted is shown in (c). Magnification, ×60.
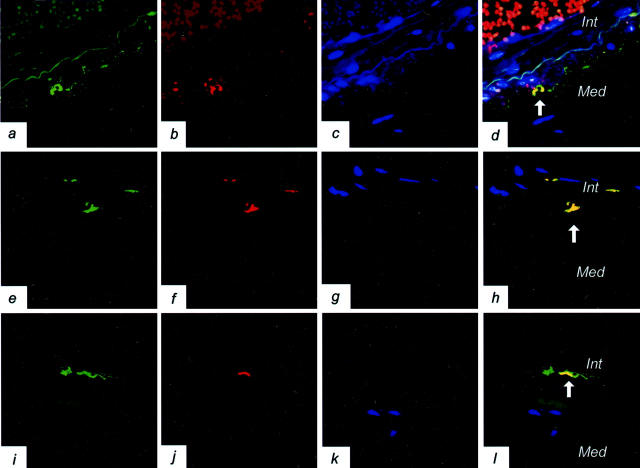
In adjacent sections of intracranial vessels and in double-immunofluorescent labeling, PrP deposits, in part, co-localize in the perivascular area and in the media of vessels with CD68 IR (Figure 1b) ▶ and HLA-DR IR (Figure 1f ▶ and Figure 3,a–d ▶ ) cells, and in the intima with HLA-DR IR and CD68 IR (Figure 1e ▶ and Figure 3, e–h ▶ ) or S-100 IR cells (Figure 3, i–l) ▶ .
Figure 3.
Disease-associated prion protein (PrP) co-localizes with immunocompetent cells in vessel walls. a–d: PrP co-localization (indicated by an arrow) with HLA-DR in the media of a leptomeningeal artery in sporadic Creutzfeldt-Jakob disease (sCJD) (3F4/HLA-DR double immunofluorescence; magnification, ×600; green color represents PrP, red HLA-DR represents immunopositivity, blue color indicates the nuclei; Int, intima; Med, media of vessel wall). All colors are combined in d. In the lumen of the artery red blood cells and between intima and media, the internal elastic layer shows autofluorescence. e–h: PrP co-localization (indicated by an arrow) with CD68 in the intima of a basal artery in sCJD (3F4/CD68 double immunofluorescence; magnification, ×1000; green color represents PrP, red CD68 indicates immunopositivity, blue color indicates the nuclei; Int, intima; Med, media of vessel wall). All colors are combined in h. i–l: PrP co-localization with S-100 in the intima of a basal artery in sCJD (3F4/CD68 double immunofluorescence; magnification, ×600; green color represents PrP, red S-100 represents immunopositivity, blue color indicates the nuclei; Int, intima; Med, media of vessel wall). All colors are combined in l. Between intima and media, the internal elastic layer (i and l) shows autofluorescence.
Discussion
Here we demonstrate the presence of PrP IR in vessel walls. We think that this represents predominantly PrPSc. We did not see similar IR in control cases, moreover, isotype antibody controls were negative. All three applied monoclonal anti-PrP antibodies, including 6H4 and 12F10 that we recently showed to be most reliable for PrPSc detection, 8 immunostained these deposits. Furthermore, PET blot studies fully support the immunocytochemical findings in the intracranial vessels, and confirm that protease-resistant PrP is present within the wall of the vessels in a focal distribution, consistent with the localization also demonstrated by double-immunofluorescence labeling. This type of PrP IR in vessel walls must be differentiated from that seen in stop mutation at codon 145 of the PRNP 9 since the presently described form lacks amyloid features.
The PET blot studies on two extracranial vessels of one vCJD case were unable to demonstrate protease-resistant PrP. The immunocytochemical findings in these vessels are therefore of uncertain significance at present. Possible explanations include up-regulation of the normal cellular isoform of the protein, or a conformational transition of PrP which is protease-sensitive but disease-associated, as has been recently described using conformation-dependent immunoassay in hamsters inoculated with different scrapie strains. 10 However, the most likely explanation might be that the very fine granular PrP deposits seen by immunocytochemistry are not visible in a “gross” method such as a PET blot. Further studies (including Western blot analysis and experimental transmission) are required to investigate these intriguing possibilities.
We did not detect any vessel wall PrP IR in the four iCJD cases. On one hand we examined only few cases, but, on the other hand, we performed serial sectioning covering about 100 μm without any result. In any case, we cannot exclude the possibility of missing focal PrP deposits in these vessels. The reason for not detecting PrP-IR in iCJD, if confirmed, remains obscure at present. Intracranial vessel wall PrP deposits may represent PrPSc derived from the periarterial interstitial fluid, which joins the cerebrospinal fluid that may harbor PrPSc 11,12 in some, but not all, CJD cases. 13 Our results resemble the findings in Alzheimer’s disease, where β-amyloid deposits are seen in intracranial but not in extracranial arteries. It is suggested that β-amyloid, often located perivascularly, follows interstitial fluid drainage along the periarterial space of intracerebral, leptomeningeal arteries, and intracranial carotid to join deep cervical lymph nodes. 14
The MHC class II antigen HLA-DR is expressed on numerous immunocompetent cells in the vasculature, including dendritic cells and macrophages. 15 We identified one PrPSc-associated cell type in the vessel wall and perivascular area as CD68-positive macrophages, which have a scavenger role in the central nervous system. 16,17 Macrophages are important in scrapie pathogenesis. They may take up PrPSc, sequester scrapie infectivity, and impair early scrapie agent replication, 18-20 or might be vehicles for exporting PrPSc from the brain to the periphery.
In a recent study, occasional perivascular macrophages surrounding cortical capillaries were PrP-immunoreactive in some non-PrD cases, 21 proposing that they express PrPC. The abundant aggregated PrP deposits in the perivascular area (Figure 1b) ▶ , some of which were related to CD68 IR cells, can be distinguished from these, as they represent disease-associated PrP deposits that are absent in non-PrD cases. Perivascular macrophages of the brain have a high rate of turnover by bone marrow-derived blood cells 22,23 and might represent a pathway for neuroinvasion of vCJD that is alternatively spread along peripheral nerves. 24
In addition to neural tissue, S-100 is expressed in vascular-associated dendritic cells (VADC). 25 Dendritic cells are potentially mobile, antigen processing, and presenting cells with endophagocytic and lysosomal activity, 26 and thus are ideal candidates for transport of the infectious agent. VADCs, a network of which is known to be present in arterial vessels of healthy individuals 25 are also related to atherosclerotic lesions with a possible role of T-cell activation. 27 Dendritic cells can migrate via the blood to the spleen or via lymph into lymph nodes where they are known as interdigitating cells. Close contact between VADCs and macrophages suggest a possible interaction between these cell types processing immunological information. VADCs may also migrate across vessel walls (to eg, lymphoid tissue). Thus, they might serve as common link between pathogenic events in the periphery as well as in neuroinvasion of prion diseases. 28
We widen the spectrum of tissues which contain PrPSc. Immunocompetent cells of vessel walls may participate in PrPSc production (analogously to follicular dendritic cells) and/or transport. As these cells might be in contact with both neural and extraneural tissue, our results raise the possibility of mobile cell-related spread of PrPSc and infectivity. Early detection of abnormal PrP in lymphoreticular tissues of gastrointestinal tract in mice after intracerebral inoculation of the CJD agent supports this theory. 29-31 In addition to established extraneural tissue infectivity in vCJD, 32 the occasional infectivity of extraneural tissues of sCJD patients 12 might result from this kind of PrPSc transport. Furthermore, our results suggest that PrPSc may be present in the blood, albeit in sCJD probably at very low levels. Moreover, this encourages the rationale for blood-based prion diagnostics using sensitive detection methods like protein misfolding cyclic amplification. 33
Acknowledgments
We thank Ms. Helga Flicker and Michaela Strohschneider for the excellent technical work.
Footnotes
Address reprint requests to Professor Herbert Budka, Institute of Neurology, AKH 4J, Waehringer Guertel 18–20, POB 48, A-1097 Vienna, Austria. E-mail: H.Budka@akh-wien.ac.at.
Supported by the Austrian Science Fund (Fonds zur Foerderung der Wissenschaftlichen Forschung No. P14584-PSY to H.B. and FWF No. 14741 to G.W.), and belongs to the framework of the European Union-funded project “Human TSEs: The Neuropathology Network (PRIONET).”
Oskar Koperek and Gábor G. Kovács contributed equally to this paper.
References
- 1.Prusiner SB: Prions. Proc Natl Acad Sci USA 1998, 95:13363-13383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Budka H: Histopathology and immunohistochemistry of human transmissible spongiform encephalopathies (TSEs). Arch Virol Suppl 2000, 16:135-142 [DOI] [PubMed] [Google Scholar]
- 3.Collinge J, Sidle KC, Meads J, Ironside J, Hill AF: Molecular analysis of prion strain variation and the aetiology of “new variant” CJD. Nature 1996, 383:685-690 [DOI] [PubMed] [Google Scholar]
- 4.Hill AF, Zeidler M, Ironside J, Collinge J: Diagnosis of new variant Creutzfeldt-Jakob disease by tonsil biopsy. Lancet 1997, 349:99-100 [DOI] [PubMed] [Google Scholar]
- 5.Budka H: Prions and transfusion medicine. Vox Sang 2000, 78:231-238 [PubMed] [Google Scholar]
- 6.Houston F, Foster JD, Chong A, Hunter N, Bostock CJ: Transmission of BSE by blood transfusion in sheep. Lancet 2000, 356:999-1000 [DOI] [PubMed] [Google Scholar]
- 7.Schulz-Schaeffer WJ, Tschoke S, Kranefuss N, Drose W, Hause-Reitner D, Giese A, Groschup MH, Kretzschmar HA: The paraffin-embedded tissue blot detects PrP(Sc) early in the incubation time in prion diseases. Am J Pathol 2000, 156:51-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kovacs GG, Head MW, Hegyi I, Bunn TJ, Flicker H, Hainfellner JA, McCardle L, Laszlo L, Jarius C, Ironside JW, Budka H: Immunohistochemistry for the prion protein: comparison of different monoclonal antibodies in human prion disease subtypes. Brain Pathol 2002, 12:1-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghetti B, Piccardo P, Spillantini MG, Ichimiya Y, Porro M, Perini F, Kitamoto T, Tateishi J, Seiler C, Frangione B, Bugiani O, Giaccone G, Prelli F, Goedert M, Dlouhy SR, Tagliavini F: Vascular variant of prion protein cerebral amyloidosis with τ-positive neurofibrillary tangles: the phenotype of the stop codon 145 mutation in PRNP. Proc Natl Acad Sci USA 1996, 93:744-748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Safar J, Wille H, Itri V, Groth D, Serban H, Torchia M, Cohen FE, Prusiner SB: Eight prion strains have PrP(Sc) molecules with different conformations. Nat Med 1998, 4:1157-1165 [DOI] [PubMed] [Google Scholar]
- 11.Bieschke J, Giese A, Schulz-Schaeffer W, Zerr I, Poser S, Eigen M, Kretzschmar H: Ultrasensitive detection of pathological prion protein aggregates by dual-color scanning for intensely fluorescent targets. Proc Natl Acad Sci USA 2000, 97:5468-5473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown P, Gibbs CJ, Jr, Rodgers-Johnson P, Asher DM, Sulima MP, Bacote A, Goldfarb LG, Gajdusek DC: Human spongiform encephalopathy : the National Institutes of Health series of 300 cases of experimentally transmitted disease. Ann Neurol 1994, 35:513-529 [DOI] [PubMed] [Google Scholar]
- 13.Wong BS, Green AJ, Li R, Xie Z, Pan T, Liu T, Chen SG, Gambetti P, Sy MS: Absence of protease-resistant prion protein in the cerebrospinal fluid of Creutzfeldt-Jakob disease. J Pathol 2001, 194:9-14 [DOI] [PubMed] [Google Scholar]
- 14.Weller RO, Massey A, Kuo YM, Roher AE: Cerebral amyloid angiopathy: accumulation of A β in interstitial fluid drainage pathways in Alzheimer’s disease. Ann NY Acad Sci 2000, 903:110-117 [DOI] [PubMed] [Google Scholar]
- 15.Wick G, Romen M, Amberger A, Metzler B, Mayr M, Falkensammer G, Xu Q: Atherosclerosis, autoimmunity, and vascular-associated lymphoid tissue. EMBO J 1997, 11:1199-1207 [DOI] [PubMed] [Google Scholar]
- 16.Mato M, Ookawara S, Sakamoto A, Aikawa E, Ogawa T, Mitsuhashi U, Masuzawa T, Suzuki H, Honda M, Yazaki Y, Watanabe E, Luoma J, Yla-Herttuala S, Fraser I, Gordon S, Kodama T: Involvement of specific macrophage-lineage cells surrounding arterioles in barrier and scavenger function in brain cortex. Proc Natl Acad Sci USA 1996, 93:3269-3274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamada Y, Doi T, Hamakubo T, Kodama T: Scavenger receptor family proteins: roles for atherosclerosis, host defence, and disorders of the central nervous system. Cell Mol Life Sci 1998, 54:628-640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carp RI, Callahan SM: Effect of mouse peritoneal macrophages on scrapie infectivity during extended in vitro incubation. Intervirology 1982, 17:201-207 [DOI] [PubMed] [Google Scholar]
- 19.Mabbott NA, Bruce ME: The immunobiology of TSE diseases. J Gen Virol 2001, 82:2307-2318 [DOI] [PubMed] [Google Scholar]
- 20.McHattie SJ, Brown DR, Bird MM: Cellular uptake of the prion protein fragment PrP106–126 in vitro. J Neurocytol 1999, 28:149-159 [DOI] [PubMed] [Google Scholar]
- 21.Esiri MM, Carter J, Ironside JW: Prion protein immunoreactivity in brain samples from an unselected autopsy population: findings in 200 consecutive cases. Neuropathol Appl Neurobiol 2000, 26:273-284 [DOI] [PubMed] [Google Scholar]
- 22.Santambrogio L, Belyanskaya SL, Fischer FR, Cipriani B, Brosnan CF, Ricciardi-Castagnoli P, Stern LJ, Strominger JL, Riese R: Developmental plasticity of CNS microglia. Proc Natl Acad Sci USA 2001, 98:6295-6300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams K, Alvarez X, Lackner AA: Central nervous system perivascular cells are immunoregulatory cells that connect the CNS with the peripheral immune system. Glia 2001, 36:156-164 [DOI] [PubMed] [Google Scholar]
- 24.Glatzel M, Aguzzi A: PrP(C) expression in the peripheral nervous system is a determinant of prion neuroinvasion. J Gen Virol 2000, 81:2813-2821 [DOI] [PubMed] [Google Scholar]
- 25.Millonig G, Niederegger H, Rabl W, Hochleitner BW, Hoefer D, Romani N, Wick G: Network of vascular-associated dendritic cells in intima of healthy young individuals. Arterioscler Thromb Vasc Biol 2001, 21:503-508 [DOI] [PubMed] [Google Scholar]
- 26.Mellman I, Steinman RM: Dendritic cells: specialized and regulated antigen processing machines. Cell 2001, 106:255-258 [DOI] [PubMed] [Google Scholar]
- 27.Bobryshev YV, Lord RS: Mapping of vascular dendritic cells in atherosclerotic arteries suggests their involvement in local immune-inflammatory reactions. Cardiovasc Res 1998, 37:799-810 [DOI] [PubMed] [Google Scholar]
- 28.Sozzani S, Allavena P, Vecchi A, Mantovani A: The role of chemokines in the regulation of dendritic cell trafficking. J Leukoc Biol 1999, 66:1-9 [DOI] [PubMed] [Google Scholar]
- 29.Radebold K, Chernyak M, Martin D, Manuelidis L: Blood-borne transit of CJD from brain to gut at early stages of infection. BMC Infect Dis 2001, 1:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bons N, Mestre-Frances N, Belli P, Cathala F, Gajdusek DC, Brown P: Natural and experimental oral infection of non-human primates by bovine spongiform encephalopathy agents. Proc Natl Acad Sci USA 1999, 96:4046-4051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lemaire-Vieille C, Schulze T, Podevin-Dimster V, Follet J, Bailly Y, Blanquet-Grossard F, Decavel JP, Heinen E, Cesbron JY: Epithelial and endothelial expression of the green fluorescent protein reporter gene under the control of bovine prion protein (PrP) gene regulatory sequences in transgenic mice. Proc Natl Acad Sci USA 2000, 97:5422-5427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bruce ME, McConnell I, Will RG, Ironside JW: Detection of variant Creutzfeldt-Jakob disease infectivity in extraneural tissues. Lancet 2001, 358:208-209 [DOI] [PubMed] [Google Scholar]
- 33.Saborio GP, Permanne B, Soto C: Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature 2001, 411:810-813 [DOI] [PubMed] [Google Scholar]