Abstract
Purpose
Physicians often order periodic bone scans (BS) to check for metastases in patients with an increasing prostate-specific antigen (PSA; biochemical recurrence [BCR]) after radical prostatectomy (RP), but most scans are negative. We studied patient characteristics to build a predictive model for a positive scan.
Patients and Methods
From our prostate cancer database we identified all patients with detectable PSA after RP. We analyzed the following features at the time of each bone scan for association with a positive BS: preoperative PSA, time to BCR, pathologic findings of the RP, PSA before the BS (trigger PSA), PSA kinetics (PSA doubling time, PSA slope, and PSA velocity), and time from BCR to BS. The results were incorporated into a predictive model.
Results
There were 414 BS performed in 239 patients with BCR and no history of androgen deprivation therapy. Only 60 (14.5%) were positive for metastases. In univariate analysis, preoperative PSA (P = .04), seminal vesicle invasion (P = .02), PSA velocity (P < .001), and trigger PSA (P < .001) predicted a positive BS. In multivariate analysis, only PSA slope (odds ratio [OR], 2.71; P = .03), PSA velocity (OR, 0.93; P = .003), and trigger PSA (OR, 1.022; P < .001) predicted a positive BS. A nomogram for predicting the bone scan result was constructed with an overfit-corrected concordance index of 0.93.
Conclusion
Trigger PSA, PSA velocity, and slope were associated with a positive BS. A highly discriminating nomogram can be used to select patients according to their risk for a positive scan. Omitting scans in low-risk patients could reduce substantially the number of scans ordered.
INTRODUCTION
Radical prostatectomy for clinically localized prostate cancer provides excellent cancer control and was recently shown to improve disease-specific survival when compared with surveillance.1-3 Nevertheless, biochemical progression, manifested by increasing prostate-specific antigen (PSA), occurs in 15% to 40% of patients within 10 years after radical prostatectomy.4-6 Distant clinical progression after radical prostatectomy almost never develops without increasing PSA levels.7 The optimal means of evaluating patients with increasing serum PSA after radical prostatectomy has not been determined. However, it is clear that imaging should not be used for patients without elevated PSA levels after radical prostatectomy.8,9
For patients with biochemical failure after radical prostatectomy, the ability to differentiate between local and distant recurrence is critical for choosing appropriate treatment. Although predictors for metastases after radical prostatectomy include rapid PSA doubling time (PSADT), short time from surgery to biochemical recurrence (BCR), and pathology Gleason sum of 8 to 10,7 conclusive evidence for metastatic disease is usually a positive imaging test. The organ most frequently involved in distant progression is bone, and the imaging of choice is bone scan with technetium-99m (99mTc) methylene diphosphonate.9 More than a decade ago, Terris et al10 suggested that patients with elevated PSA after radical prostatectomy should be evaluated by bone scan because 47% of those patients had positive bone scans.
There is evidence that a bone scan could be omitted for patients with newly diagnosed prostate cancer with PSA of less than 20 ng/mL, given that less than 1% of these have a positive bone scan.11,12 Regarding patients with increasing PSA after radical prostatectomy, Lee et al13 recommended omitting bone scans for patients with PSA of less than 2 ng/mL. More recently, it was shown that a higher threshold of PSA can be used to decrease the number of bone scans in patients who develop BCR after radical prostatectomy.14,15 In both studies, the only predictor for a positive bone scan according to multivariate analysis was PSA level before bone scan. However, simply using a single variable for determining whether a bone scan should be performed likely predicts suboptimally because of the heterogeneity of the disease. For this reason, models that include multiple variables (eg, nomograms) were developed and validated for different disease states in prostate cancer.16 These models have been shown to be more accurate, measured by concordance index, than risk group assignment. We evaluated pre and postoperative variables in a large cohort of patients with elevated serum PSA after radical prostatectomy for association with a positive bone scan. The main goals of our study were to identify predictors for a positive bone scan and to construct a nomogram for predicting the bone scan result.
PATIENTS AND METHODS
From January 1985 to March 2003, 4,823 patients underwent retropubic radical prostatectomy at Memorial Sloan-Kettering Cancer Center (New York, NY) or by a single surgeon at Baylor College of Medicine (P.T.S.; Houston, TX). Data were collected in the Specialized Program of Research Excellence (SPORE) prostate cancer database. The study was approved by the Institutional Review Board committee of Memorial Sloan-Kettering Cancer Center. We excluded 181 patients (3.8%) because of preoperative radiation therapy, chemotherapy (n = 178), or adjuvant hormonal therapy before the biochemical failure event (n = 3). Patients were staged according to the 1992 American Joint Committee on Cancer.17 Pathologic assessment of the specimen was performed according to previous descriptions,18 and histologic grading was based on the Gleason grading system. The patients were observed either at the above-mentioned institutions or by the referring physician. The recommendation for surveillance after the operation was as follows: every 3 months for the first year, semiannually between years 1 to 3, and annually thereafter. Patients who developed biochemical failure were observed with medical history, physical examination, PSA test, and imaging tests including bone scan, at the discretion of the treating physician. Because androgen deprivation treatment (ADT) leads to significant changes in serum PSA, pathologic assessment of the radical prostatectomy specimen and bone scan results, we omitted from the analysis all bone scans performed after treatment with ADT (including neoadjuvant hormonal treatment).
We identified bone scans of patients with biochemical failure after radical prostatectomy, and their pre- and postoperative characteristics are summarized in Table 1. The PSA threshold for defining biochemical failure was 0.4 ng/mL. Patients with increasing PSA treated by secondary treatment (radiation or hormones) before they reached the PSA threshold of 0.4 ng/mL were considered to have biochemical failure as well. Bone scans were performed after intravenous administration of 25 mCi 99mTc methylene diphosphonate. Total body images were obtained at 2 to 3 hours after injection and supplemented by spot views of spine, pelvis, or skull, if indicated. All bone scans were interpreted by nuclear radiologists. Bone scan results (positive v negative) and the nature of positive bone scans (malignant v nonmalignant) were recorded. Equivocal scans were considered to be negative for our analysis unless additional imaging demonstrated metastases. The trigger PSA (tPSA) was defined as the PSA level before the bone scan (within 1 month before the bone scan). The PSA slope, velocity, and PSADT were calculated by using three PSA values, at least 30 days apart, before bone scan, of which the third value was the tPSA. PSA velocity was calculated as the change in PSA over time (in months). PSA slope was calculated as the change in PSA over time divided by the time interval (in months). PSADT was calculated as the natural log of 2 divided by the PSA slope (http://www.nomograms.org).
Table 1.
Pre- and Postoperative Characteristics of 239 Patients
| Variable | No. of Patients | % |
|---|---|---|
| Preoperative PSA, ng/mL | ||
| 0-4 | 14 | 6.2 |
| 4.01-10 | 97 | 42.9 |
| 10.01-20 | 68 | 30 |
| 20.01-50 | 40 | 17.7 |
| > 50 | 7 | 3.1 |
| Positive surgical margin | 108 | 45.2 |
| Extracapsular extension | 133 | 55.6 |
| Seminal vesicle involvement | 73 | 30.5 |
| Lymph node involvement | 30 | 12.5 |
| Pathology Gleason score | ||
| 2-6 | 57 | 23.8 |
| 7 | 110 | 46.0 |
| 8-10 | 72 | 30.1 |
Abbreviation: PSA, prostate-specific antigen.
The following parameters were evaluated for predicting the probability of a positive bone scan: serum PSA before surgery, pathology Gleason sum, presence of a positive surgical margin, extracapsular extension, seminal vesicle invasion, lymph node involvement, PSA slope, PSA velocity, PSADT, and tPSA.
For univariate and multivariate analysis, we used a mixed model, with the patient as a random effect because some of the patients had more than one observation. SAS software was used (Version 8.2; SAS Institute, Cary, NC) for the mixed model analysis. The nomogram was constructed based on a logistic regression model using the design library of S-plus software (Insightful Corp, Seattle, WA). The nomogram was evaluated for its ability to discriminate among patients’ risk of recurrence. Discrimination was measured as a concordance index, which represents the probability that when two patients are randomly selected— one with positive bone scan and one with negative bone scan—the patient with the positive scan had a higher predicted probability of a positive bone scan. Calibration was assessed by applying a nonparametric smoothing algorithm (lowess) to the jackknife-predicted probabilities. All P values resulted from use of two-sided statistical tests.
RESULTS
Patient Characteristics
We identified 927 bone scans performed in 330 patients with increasing serum PSA after radical prostatectomy. We excluded 195 scans in patients with a history of neoadjuvant hormonal therapy, and an additional 318 scans performed after the administration of ADT. These exclusions left 414 scans performed in 239 patients for analysis. There were 155 patients who had a single bone scan, 39 who had two bone scans, 21 who had three bone scans, and 24 who had more than three bone scans. The characteristics of our study set are listed in Table 1. Patient characteristics according to bone scan result are listed in Table 2. The minimum, median, mean, and maximum tPSA values were 0.1, 3.1, 13.4, and 494 ng/mL, respectively.
Table 2.
Patient Characteristics According to Bone Scan Result
| Positive Scan (n = 60) |
Negative Scan (n = 354) |
|||||
|---|---|---|---|---|---|---|
| Variable | No. | % | No. | % | ||
| Positive surgical margin | 25 | 42 | 160 | 45 | ||
| Extracapsular extension | 32 | 53 | 171 | 48 | ||
| Seminal vesicle invasion | 25 | 41 | 98 | 28 | ||
| Lymph node metastases | 8 | 13 | 35 | 10 | ||
| Pathology Gleason sum | ||||||
| 2-6 | 16 | 27 | 91 | 26 | ||
| 7 | 22 | 37 | 164 | 46 | ||
| 8-10 | 22 | 37 | 99 | 28 | ||
| Median preoperative PSA, ng/mL | 18.4 | 14.7 | ||||
| Months from operation to BCR, median | 13.3 | 16.6 | ||||
| Median PSA doubling time, months | 5.2 | 6.6 | ||||
| Median trigger PSA, ng/mL | 58.0 | 5.8 | ||||
| Median years from BCR to first bone scan, median | 3.5 | 3.9 | ||||
| Median PSA velocity, ng/mL/mo | 1.4 | 0.12 | ||||
| Median PSA slope, log (ng/mL)/mo | 0.13 | 0.09 | ||||
Abbreviations: PSA, prostate-specific antigen; BCR, biochemical recurrence.
Probability of Positive Bone Scans According to Pre- and Postoperative Variables
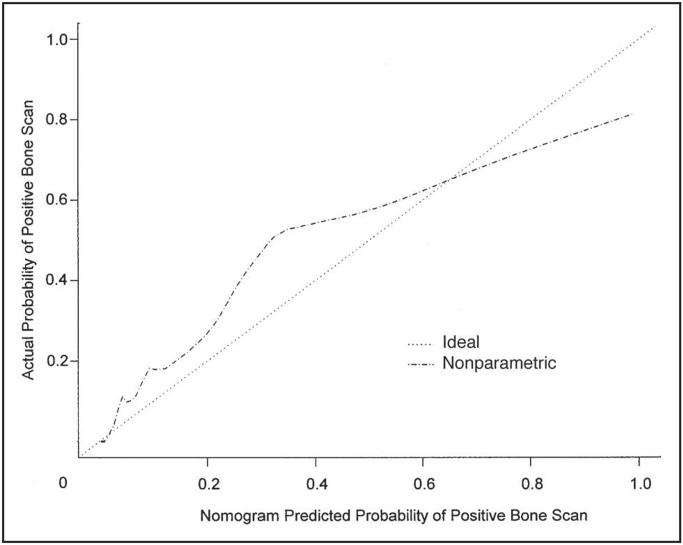
Of the 414 bone scans, 60 (14.5%) were positive for metastatic prostate cancer and 11 (2.7%) were abnormal but not indicative of cancer and were therefore classified as negative in the analysis. For the tPSA levels of 0 to 10, 10.1 to 20, 20.1 to 50, and above 50 ng/mL, bone scans were positive in 4% (median, 8.4 ng/mL), 36% (median, 13.2 ng/mL), 50% (median, 23.4 ng/mL), and 79% (median, 123.3 ng/mL), respectively. In univariate analysis, preoperative PSA (P = .04), seminal vesicle invasion (P = .02), PSA velocity (P < .001), and tPSA (P < .001) predicted a positive bone scan (Table 3). In multivariate analysis, PSA slope (odds ratio [OR], 2.71; P = .03), PSA velocity (OR, 0.93; P = .003), and tPSA (OR, 1.022; P < .001) predicted a positive bone scan (Table 4). A nomogram for predicting the bone scan result was constructed (Fig 1) and found to have a concordance index of 0.93 (Fig 1). In contrast, when we used a PSA cutoff level of 30 ng/mL at time of bone scan as the only predictor, the concordance index was 0.63. Figure 2 shows the calibration of the nomogram by plotting the nomogram-predicted probability for a positive bone scan against the actual observed probability. In general, the nomogram appears to be reasonably accurate.
Table 3.
Association of Variables With Positive Bone Scan in Univariate Analysis
| Variable | Odds Ratio | 95% CI | P |
|---|---|---|---|
| Preoperative PSA, ng/mL | 1.02 | 1.00 to 1.03 | .04 |
| Surgical margin (positive v negative) | 0.86 | .53 | |
| Extracapsular extension (positive v negative) | 1.14 | .58 | |
| Seminal vesicle invasion (positive v negative) | 1.81 | 1.12 to 2.94 | .02 |
| Lymph node involvement (positive v negative) | 1.30 | .45 | |
| Pathology Gleason sum | 1.10 | .38 | |
| Months from operation to BCR | 0.99 | .14 | |
| Years from BCR to bone scan | 1.00 | .95 | |
| PSA doubling time, months | 1.00 | .72 | |
| PSA slope, log (ng/mL/mo) | 2.29 | .06 | |
| PSA velocity, ng/mL/mo | 1.07 | 1.04 to 1.09 | < .001 |
| Trigger PSA, ng/mL | 1.015 | 1.012 to 1.019 | < .001 |
Abbreviations: PSA, prostate-specific antigen; BCR, biochemical recurrence.
Table 4.
Association of Variables With Positive Bone Scan in Multivariate Analysis
| Variable | Odds Ratio | 95% CI | P |
|---|---|---|---|
| Preoperative PSA, ng/mL | 1.00 | .82 | |
| Surgical margin (positive v negative) | 0.92 | .70 | |
| Extracapsular extension (positive v negative) | 1.05 | .84 | |
| Seminal vesicle invasion (positive v negative) | 1.39 | .20 | |
| Lymph node involvement (positive v negative) | 0.83 | .61 | |
| Pathology Gleason sum | 1.06 | .74 | |
| Months from operation to BCR | 1.00 | .46 | |
| Years from BCR to bone scan | 1.02 | .62 | |
| PSA doubling time, months | 1.02 | .83 | |
| PSA slope, log (ng/mL/mo) | 2.71 | 1.08 to 6.82 | .04 |
| PSA velocity, ng/mL/mo | 0.93 | 0.89 to 0.98 | .003 |
| Trigger PSA, ng/mL | 1.022 | 1.015 to 1.029 | < .001 |
Abbreviations: CI, confidence interval; PSA, prostate-specific antigen; BCR, biochemical recurrence.
Fig 1.
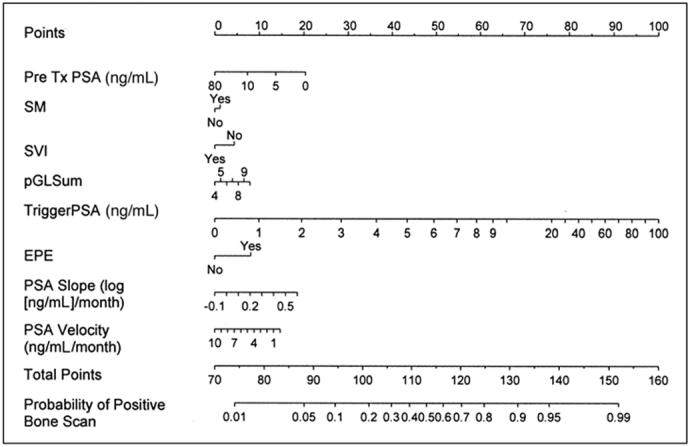
Bone scan nomogram based on 414 bone scans of 239 patients observed at our institution. Each scale position corresponds to points (top axis). Point values for all predictor variables are determined consecutively and summed to arrive at the total point value. This value is plotted on the total point axis, and directly below it is the predicted probability of a positive bone scan. For tPSA, use current serum PSA. Calculate PSA slope and velocity from the last three PSA values, the third of which is the tPSA. (PSA velocity is in units of ng/mL/mo and PSA slope is in units of log [ng/mL]/mo). Tools for the calculation of PSA velocity and slope are available at www.nomograms.org. Note: this nomogram is applicable only to a man who has developed biochemical failure after radical prostatectomy and has received no other prostate cancer therapy. SM, presence of a positive surgical margin; EPE, presence of extracapsular extension (either focal or established); SVI, presence of seminal vesicle invasion; pGLSum, the Gleason sum of the prostatectomy specimen; Tx, treatment (surgery). Examples: A man had a preoperative PSA of 10.1 ng/mL, radical prostatectomy specimen Gleason sum of 7, and was negative for ECE, SVI, and LNI. PSA at time of bone scan performance was 8 ng/mL, with a PSA velocity of 3 ng/mL/yr and PSA slope of 0.2. The total points according to the bone scan nomogram is 90, which predicts a 7% probability of a positive bone scan. A different man had a preoperative PSA of 8.1, radical prostatectomy specimen Gleason sum of 9, and was negative for ECE, SVI, and LNI. PSA at the time of bone scan performance was 63 ng/mL, with a PSA velocity of 5 ng/mL/yr and PSA slope of 0.5. The total points according the bone scan nomogram was 137, which predicts a 92% probability of positive bone scan.
Fig 2.
Nomogram calibration. An ideal nomogram would have predicted probabilities that match the actual probabilities (· · ·); (- · - · -) is overfit-corrected calibration of the nomogram using jackknife-predicted probabilities. These probabilities were obtained by leaving each patient out of the data set, forming a model based on the remaining patients, and then computing the prediction(s) for the omitted patient.
DISCUSSION
Diagnostic strategies for evaluating men with increasing PSA levels after radical prostatectomy vary among urologists in the United States and around the world. The choice of treatment among the options available for those patients (local treatment, systemic treatment, or observation) depends on the results of repeated diagnostic evaluations. Metastatic prostate cancer has a tendency to spread to bones, predominantly in the axial skeleton, and the imaging of choice is the radionuclide bone scan with 99mTc methylene diphosphonate.9,19 In a survey of urologists about the management of patients who experience biochemical failure after radical prostatectomy, 70% reported that they ordered a bone scan (the second most frequently ordered test after PSA), although the probability of a positive bone scan is relatively low when the decision to perform one is made by clinical judgment alone.20 More then a decade ago, Terris et al10 analyzed 264 bone scans in 118 patients after radical prostatectomy. In 97 of those patients, bone scans were performed with an undetectable PSA. Only four of these 97 patients were considered to have a positive bone scan. In retrospect, these seemed to be false positives because none of these four patients subsequently showed signs of disease progression. In contrast, in patients with an increasing PSA, 47% (10 of 21) of the scans were found to be positive. This observation led the authors to conclude that bone scans should be performed for all patients with elevated PSA after radical prostatectomy. The major limitation of that study was that the PSA levels at the time of bone scans, as well as other pre- and postoperative predictors, were not available, and thus risk factors for a positive bone scan could not be identified.
Two other studies showed much lower rates of positive bone scans among patients with increasing PSA after surgery. Cher et al14 evaluated 144 bone scans in 93 patients and found only five (4%) positive scans. Kane et al15 identified 12 positive scans (9.5%) of 127. In both series, the PSA level before bone scan (tPSA) and PSA velocity were significant variables in univariate analysis, but only the tPSA was a predictor in multivariate analysis.14,15
Our study analyzed a larger number of bone scans performed for patients with BCR after radical prostatectomy. As in previous studies, our analysis found tPSA to bea significant predictor for a positive bone scan.14,15,21 However, when we used tPSA of ≥ 30 ng/mL as the only predictor of a positive bone scan, its concordance index was only 0.63. Thus, we can conclude that tPSA alone, when dichotomized as 30 ng/mL and above versus below 30 ng/mL, does not serve as a reliable predictor for a positive bone scan.
In univariate analysis, we found that the presence of seminal vesicle invasion and PSA velocity (Table 3) predicted a positive bone scan, in addition to tPSA. In multivariate analysis, we were able to identify, apparently for the first time, that PSA slope and velocity were associated with a positive bone scan (Table 4). Those predictors were calculated by at least three values of PSA before the performance of bone scan and thus should reflect the kinetics of PSA in the months before the scan. In contrast, other predictors that have been associated with the probability of metastases, such as Gleason sum in the prostatectomy specimen and time from prostatectomy to biochemical failure, were not associated with positive bone scan in our multivariable analysis. Thus, our data support the concept that the patterns of PSA failure after radical prostatectomy, rather than pathologic parameters, are the more important predictors of metastatic progression to bone. PSA slope and PSA velocity appear to be superior to PSADT in predicting such progression. Our results are consistent with prior studies, suggesting that the characteristics of the increasing PSA are associated with prostate cancer— specific death22 or metastases.7
Prediction models in oncology in general, and for prostate cancer in particular, are complex because of the large number of prognostic factors that vary according to the different clinical disease states.23 Nomograms can combine a large amount of information from multiple predictors into a single probability model. We developed a nomogram that estimates the probability of a positive bone scan at any time after biochemical failure before the administration of hormonal therapy based on commonly available data, including the results of pathologic analysis of the operative specimen (status of surgical margin, presence of extracapsular extension, seminal vesicle invasion, and Gleason sum at time of radical prostatectomy) as well as postoperative follow-up (tPSA, PSA slope, and PSA velocity). The advantage of this approach is seen in the predictive ability of our model: bone scan results were predicted with a concordance index of 0.93. A strong inverse relationship was found between tPSA and both preoperative PSA and PSADT (s = -0.3). This means that as the values of PSADT decrease, the tPSA value tends to increase. Because of this inverse relationship, and because tPSA has the most pronounced association with bone scan results, the axes for PSA velocity and the preoperative PSA in the nomogram (Fig 1) appear to go in the wrong direction. Although it is tempting to simply remove these variables with insignificant and counterintuitive effects, doing so will tend to decrease the predictive ability of the model. It is important to recognize that moving a patient on one axis (eg, increasing his tPSA) will tend to move him on other axes as well (eg, his PSA velocity and preoperative PSA). Therefore, it is difficult to interpret the effect of a single variable in isolation.24
Important limitations do exist with this nomogram. The probability of a positive bone scan can be determined only for patients who were not treated with ADT, given that patients who received ADT were excluded from the analysis. Another important weakness of our study is the lack of standardization of the techniques and interpretation of the bone scans, given that some of them were performed by outside institutions. Finally, the bone scans were not performed at regular intervals, by protocol, but according to the discretion of the physician.
This nomogram will be useful in counseling patients with increasing PSA after radical prostatectomy before treatment with ADT. We plan to make this nomogram available as free software, as we do with all of our nomograms. These are available at http://www.nomograms.org. By using our bone scan nomogram, the treating physician would be able to predict the probability of a systemic progression according to the pre- and postoperative characteristics and pattern of PSA failure at any time during the patient’s follow-up after the detection of an increasing PSA. The use of multiple predictors makes this a more accurate approach than relying on a single categoric predictor, such as tPSA alone. Evaluation of patients with increasing PSA after local treatment remains controversial. On the basis of our results, we believe that patients with increasing PSA after local treatment can be evaluated accurately by using multiple predictors rather than relying on a single categoric predictor, such as tPSA alone. Predictors that describe the kinetics of PSA after biochemical failure had the most substantial impact on the probability of systemic progression. The nomogram adds an important, user-friendly, and accurate tool that will help physicians to identify both high- and low-risk patients for systemic progression. A randomized trial is needed to evaluate whether an early treatment for high-risk patients for systemic progression, before the development metastases, will lead to a reduction in the metastatic rate and improved survival.
Footnotes
Supported by the grant SPORE P50-CA58204 from the National Cancer Institute, by funds from The David H. Koch Foundation, and by the National Institutes of Health grant IRGICA76423-0IRI.
Authors’ Disclosures of Potential Conflicts of Interest
The following authors or their immediate family members have indicated a financial interest. No conflict exists for drugs or devices used in a study if they are not being evaluated as part of the investigation. Leadership Position: Michael W. Kattan, Oncovance. Concultant: Peter T. Scardino, Steba Pharm, Capcure. Stock Ownership: Peter T. Scardino, ProQuest, Honoraria: Peter T. Scardino, Astra Zeneca. Research Funding: Peter T. Scardino, Capcure. For a detailed description of these categories, or for more information about ASCO’s conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section of Information for Contributors found in the front of every issue.
REFERENCES
- 1.Holmberg L, Bill-Axelson A, Helgesen F, et al. A randomized trial comparing radical prostatectomy with watchful waiting in early prostate cancer. N Engl J Med. 2002;347:781–789. doi: 10.1056/NEJMoa012794. [DOI] [PubMed] [Google Scholar]
- 2.Hull GW, Rabbani F, Abbas F, et al. Cancer control with radical prostatectomy alone in 1,000 consecutive patients. J Urol. 2002;167:528–534. doi: 10.1016/S0022-5347(01)69079-7. [DOI] [PubMed] [Google Scholar]
- 3.Han M, Partin AW, Pound CR, et al. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy: The 15-year Johns Hopkins experience. Urol Clin North Am. 2001;28:555–565. doi: 10.1016/s0094-0143(05)70163-4. [DOI] [PubMed] [Google Scholar]
- 4.Dillioglugil O, Leibman BD, Kattan MW, et al. Hazard rates for progression after radical prostatectomy for clinically localized prostate cancer. Urology. 1997;50:93–99. doi: 10.1016/S0090-4295(97)00106-4. [DOI] [PubMed] [Google Scholar]
- 5.Han M, Partin AW, Zahurak M, et al. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol. 2003;169:517–523. doi: 10.1097/01.ju.0000045749.90353.c7. [DOI] [PubMed] [Google Scholar]
- 6.Roberts SG, Blute ML, Bergstralh EJ, et al. PSA doubling time as a predictor of clinical progression after biochemical failure following radical prostatectomy for prostate cancer. Mayo Clin Proc. 2001;76:576–581. doi: 10.4065/76.6.576. [DOI] [PubMed] [Google Scholar]
- 7.Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson JK, Oesterling JE, Lange PH, et al. Patient evaluation if prostate-specific antigen becomes elevated following radical prostatectomy or radiation therapy. Urol Clin North Am. 1994;21:677–685. [PubMed] [Google Scholar]
- 9.Nudell DM, Wefer AE, Hricak H, et al. Imaging for recurrent prostate cancer. Radiol Clin North Am. 2000;38:213–229. doi: 10.1016/s0033-8389(05)70157-3. [DOI] [PubMed] [Google Scholar]
- 10.Terris MK, Klonecke AS, McDougall IR, et al. Utilization of bone scans in conjunction with prostate-specific antigen levels in the surveillance for recurrence of adenocarcinoma after radical prostatectomy. J Nucl Med. 1991;32:1713–1717. [PubMed] [Google Scholar]
- 11.Oesterling JE, Martin SK, Bergstralh EJ, et al. The use of prostate-specific antigen in staging patients with newly diagnosed prostate cancer. JAMA. 1993;269:57–60. [PubMed] [Google Scholar]
- 12.Chybowski FM, Keller JJ, Bergstralh EJ, et al. Predicting radionuclide bone scan findings in patients with newly diagnosed, untreated prostate cancer: Prostate specific antigen is superior to all other clinical parameters. J Urol. 1991;145:313–318. doi: 10.1016/s0022-5347(17)38325-8. [DOI] [PubMed] [Google Scholar]
- 13.Lee CT, Oesterling JE. Using prostate-specific antigen to eliminate the staging radionuclide bone scan. Urol Clin North Am. 1997;24:389–394. doi: 10.1016/s0094-0143(05)70385-2. [DOI] [PubMed] [Google Scholar]
- 14.Cher ML, Bianco FJ, Jr, Lam JS, et al. Limited role of radionuclide bone scintigraphy in patients with prostate specific antigen elevations after radical prostatectomy. J Urol. 1998;160:1387–1391. [PubMed] [Google Scholar]
- 15.Kane CJ, Amling CL, Johnstone PA, et al. Limited value of bone scintigraphy and computed tomography in assessing biochemical failure after radical prostatectomy. Urology. 2003;61:607–611. doi: 10.1016/s0090-4295(02)02411-1. [DOI] [PubMed] [Google Scholar]
- 16.Ross PL, Scardino PT, Kattan MW. A catalog of prostate cancer nomograms. J Urol. 2001;165:1562–1568. [PubMed] [Google Scholar]
- 17.Ohori M, Wheeler TM, Dunn JK, et al. The pathological features and prognosis of prostate cancer detectable with current diagnostic tests. J Urol. 1994;152:1714–1720. doi: 10.1016/s0022-5347(17)32369-8. [DOI] [PubMed] [Google Scholar]
- 18.Ohori M, Scardino PT. Localized prostate cancer. Curr Probl Surg. 2002;39:833–957. doi: 10.1067/msg.2002.126335. [DOI] [PubMed] [Google Scholar]
- 19.Moul JW. Prostate specific antigen only progression of prostate cancer. J Urol. 2000;163:1632–1642. [PubMed] [Google Scholar]
- 20.Ornstein DK, Colberg JW, Virgo KS, et al. Evaluation and management of men whose radical prostatectomies failed: Results of an international survey. Urology. 1998;52:1047–1054. doi: 10.1016/s0090-4295(98)00403-8. [DOI] [PubMed] [Google Scholar]
- 21.Miller PD, Eardley I, Kirby RS. Prostate specific antigen and bone scan correlation in the staging and monitoring of patients with prostatic cancer. Br J Urol. 1992;70:295–298. doi: 10.1111/j.1464-410x.1992.tb15734.x. [DOI] [PubMed] [Google Scholar]
- 22.D’Amico AV, Chen MH, Roehl KA, et al. Preoperative PSA velocity and the risk of death from prostate cancer after radical prostatectomy. N Engl J Med. 2004;351:125–135. doi: 10.1056/NEJMoa032975. [DOI] [PubMed] [Google Scholar]
- 23.Scher HI, Heller G. Clinical states in prostate cancer: Toward a dynamic model of disease progression. Urology. 2000;55:323–327. doi: 10.1016/s0090-4295(99)00471-9. [DOI] [PubMed] [Google Scholar]
- 24.Di Blasio CJ, Rhee AC, Cho D, et al. Predicting clinical end points: Treatment nomograms in prostate cancer. Semin Oncol. 2003;30:567–586. doi: 10.1016/s0093-7754(03)00351-8. [DOI] [PubMed] [Google Scholar]