1. Introduction
Adolescence is a time of rapid neural changes, with neural alterations during this developmental period being reported in a number of brain regions and with a number of neurotransmitter systems, including dopamine, glutamate, GABA, and serotonin [for review see 32]. Brain regions undergoing particularly marked changes in human adolescents and their counterparts in other species include the prefrontal cortex [16, 37, 49] and limbic and mesolimbic brain systems [15, 18], regions that form important substrates for modulating responsiveness to alcohol and other drugs of abuse. The risk of extensive alcohol use is increased during adolescence, with 12% of 8th graders, 22% of 10th graders, and one fourth of 12th graders (25%) reporting binge drinking (i.e., five or more drinks per occasion) during the past 2 weeks [17]. Elevated levels of ethanol intake are not restricted to human adolescents but can also be observed in adolescents of other species, with intake of ethanol being at least 2 times higher in adolescent rats than in their more mature counterparts under a number of test circumstances [3, 7, 20]. One of the potential contributors to heavy drinking during adolescence may be an age-related insensitivity to various adverse effects of ethanol that serve as cues to terminate drinking. For instance, relative to their adult counterparts, adolescent rats are less sensitive to ethanol-induced motor impairment [13, 28, 47], sedation [21, 23, 27], and “hangover”-associated anxiety [8, 41].
Whereas numerous studies have explored effects of chronic ethanol exposure during fetal or adult life, little research has been conducted to date assessing consequences of repeated exposure to ethanol during adolescence. The issue of consequences and adaptations to ethanol during adolescence is of particular importance, given that acquired tolerance to certain desired and adverse effect of ethanol could serve to escalate further ethanol use during this developmental period. However, results from the few experimental studies that have compared consequences of chronic ethanol exposure in adolescent and adult rats are inconsistent. Chronic exposure to high doses of ethanol (4 g/kg twice a day for 7 days) administered intragastrically was reported to induce more tolerance in adolescent than adult rats when indexed in terms of ethanol-induced hypothermia and sedation [34]. However, when animals were chronically exposed to ethanol via vapor inhalation, tolerance to hypothermic effects of ethanol was found to develop faster in adult rats than in their younger counterparts [25]. In a study equating initial functional motor impairment across age by dose adjustments, equivalent levels of tolerance were observed across the two ages [28]. Taken together, these findings suggest that relative rate of ethanol adaptation during adolescence may vary as a function of the response measure under investigation, as well as dose administered.
In contrast to these early ontogenetic studies that assessed consequences of repeated exposure to relatively high doses of ethanol, the present study was designed to investigate the impact of chronic exposure (7 days) to a relatively low dose of 1 g/kg ethanol, using ethanol-induced alterations in social behavior as a response measure [39]. Given the critical importance of interactions with peers during adolescence [see 32 for references and review], it is not surprising that human adolescents report that they often drink with peers to become more relaxed and sociable [2, 4, 31]. Adolescent rats, like their human counterparts, are also particularly sensitive to activating effects ethanol on social behavior [39, 40, 42, 44]. Typically, only adolescent but not adult animals demonstrate increases in social activity in response to low doses of ethanol when tested in a familiar, nonanxiogenic environment [39]. With higher doses of ethanol, both adolescents and adults demonstrate social inhibition, as indexed by reduced social activity and avoidance of the peer, although adolescents require higher doses for the emergence of these inhibitory effects of ethanol than adults [39]. Taking into account the remarkable importance of social interactions for adolescents, the present study was designed to test the hypothesis that adolescent rats will develop more pronounced chronic tolerance to the social consequences of ethanol than their adult counterparts. To assess tolerance development to ethanol-induced alterations of social behavior, animals were challenged across a range of ethanol doses capturing both activating (i.e., social facilitation in adolescent animals) and suppressing (i.e., social inhibition at both ages) effects of ethanol on social behavior. Chronically treated saline animals, having the same amount of handling and injection experience, were used as ethanol-naïve controls. Therefore, a between-subject measure of tolerance was used, with chronic tolerance being defined at each age as a shift of the dose-response curve to the right in animals chronically exposed to ethanol when compared to the curve of their ethanol-naïve (saline-exposed) age-mates.
2. Methods
2.1. Subjects
Adolescent and adult male and female Sprague-Dawley rats bred and reared in our colony at Binghamton University were used in these experiments. A total of 40 litters provided 200 male and female offspring to serve as experimental subjects and 200 to serve as partners. All animals were housed in a temperature-controlled (22°C) vivarium maintained on a 14-/10-hr light/dark cycle (lights on at 07:00 hr) with ad libitum access to food (Purina Rat Chow, Lowell, MA) and water. Pups were housed until weaning with their mothers in standard maternity cages with pine shavings as bedding material. Litters were culled to 10 (5 males and 5 females) pups within 24 hr after birth on postnatal day (P) 0. Rats were weaned on P21 and placed into standard plastic cages with same-sex littermates (5 animals in a cage). In all respects, maintenance and treatment of the animals were in accord with guidelines for animal care established by the National Institutes of Health, using protocols approved by the Binghamton University Institutional Animal Care and Use Committee.
2.2. Chronic Exposure and Testing Procedure
Experimental subjects received either 1 g/kg of ethanol or saline in a volume equal to the volume of ethanol administered (i.e., 1% body weight of experimental animal) intraperitoneally (i.p.) for 7 consecutive days (P27-P33 for adolescents and P62-P68 for adults). Ethanol was administered as a 12.6% (v/v) solution, a relatively low concentration that induced little (if any) tissue irritation at the site of injection. The solutions were administered at room temperature. All animals from a given litter received the same chronic treatment and were kept together in groups of 5 same sex littermates [33].
Behavioral testing occurred 48 hr after last exposure to ethanol (P35 or P70). One day before testing (P34 for adolescents and P69 for adults), all experimental animals were placed individually into the testing chamber for 30 min to make the experimental situation familiar for them. The test apparatuses consisted of Plexiglas (Binghamton Plate Glass, Binghamton, NY) chambers (30 x 20 x 20 cm for adolescents and 45 x 30 x 20 cm for adults) containing clean pine shavings. Each test apparatus was divided into two equally sized compartments by a clear Plexiglas partition that contained an aperture (7 x 5 cm for adolescents and 9 x 7 cm for adults) to allow movement of the animals between compartments [43, 44].
On the next day, each subject was injected i.p. with one of the five doses of ethanol (0, 0.25, 0.5, 0.75, or 1 g/kg). Dose was varied by altering the volume of the 12.6% ethanol solution. Control animals were injected with isotonic saline in a volume equal to that of the highest dose of ethanol administered (1% body weight). Immediately after ethanol administration, each experimental animal was marked by a vertical line on the back and placed individually in an opaque plastic holding cage (30 x 20 x 20 cm) for 30 min, given that pre-test social deprivation has been shown to increase baseline levels of social behavior from which inhibitory effects of ethanol (i.e., social inhibition) may be more readily detected [10]. Each animal was placed into the testing chamber simultaneously with a same age and sex test partner. Partners were always non-exposed animals that had not been socially isolated prior to testing and who were unfamiliar with both the test apparatus and the experimental animal with which they were paired for testing. Weight differences between test subjects and their partners were minimized as much as possible. The order of testing was counterbalanced for all treatment groups. During the 10 min test session, the behavior of the animals was recorded by a video camera (Panasonic model AF-X8, Secaucus, NJ), with real time being directly recorded onto the videotape for later scoring (Easy Reader II Recorder; Telcom Research TCG 550, Burlington, Ontario). After each test, the apparatus was wiped with 3% peroxide hydrochloride and the shavings were replaced with fresh ones. All testing procedures were conducted between 9:00 and 13:00 hr under dim light (15–20 lx). Tail blood samples were collected immediately after the test. For sampling, a small incision was made on the tail, and blood was allowed to flow into heparinized tubes.
2.3. Behavioral Observation
The frequency of a number of social activities from the ethogram of each test subject was analyzed from the video recordings [36, 38]. Overall social activity was scored as the sum of the frequencies of the following social behaviors: social investigation (sniffing of any part of the body of the partner), contact (crawling over and under the partner and social grooming), and play behavior (pouncing or playful nape attack, chasing, and pinning). Play fighting differs from serious fighting in the laboratory rat by target of attack: during play fighting, snout or oral contact is directed toward the partner's nape, while during serious fighting the object of the attack is the partner's rump. In the present experiments, subjects did not demonstrate serious fighting, and, hence, frequency of aggressive behavior was not scored.
Social preference/avoidance was analyzed by scoring the number of crossovers (movements between compartments) demonstrated by the experimental subject toward the non-manipulated ethanol-naive peer and the number of crossovers away from the peer [43, 44]. Social motivation was assessed by means of a coefficient of preference/avoidance [Coefficient (%) = (crossovers to – crossovers from) /(crossovers to + crossovers from) x 100]. Social preference was defined by positive values of the coefficient, while social avoidance was associated with negative values [43]. Total number of crossovers was used as an index of general locomotor activity.
2.4. Experimental Design and Data Analysis
Experimental subjects were chronically exposed either to isotonic saline or to 1 g/kg of ethanol and acutely challenged with one of five doses of ethanol (0, 0.25, 0.5, 0.75, and 1.0 g/kg) either on P35 (adolescents) or on P70 (young adults). Equal number of males and females were placed into each treatment and age group to allow analysis of sex effects across ontogeny. Therefore, the design of the present study was a 2 (chronic treatment) x 5 (ethanol challenge dose) x 2 (age) x 2 (sex) factorial, with 5 animals being placed into each of the 40 experimental groups defined by this factorial design. To eliminate the possible confounding of litter with treatment effects, no more than one subject from a given litter was assigned to a particular treatment group [14]. Animals were assigned randomly to the testing conditions and order of testing was counterbalanced across the experiment.
Using a real-time-event-recording program on a personal computer, behavioral data were scored from the videotape records by two observers without knowledge of the chronic exposure condition or acute challenge of any animal. Agreement between observers scoring the same videotape was in excess of 90% for each measure of social behavior and social preference. Overall social activity, coefficient of social preference/avoidance, and overall locomotor activity (i.e., total number of crossovers to and from the partner) were examined by using separate 2 (chronic treatment) x 5 (ethanol challenge dose) x 2 (age) x 2 (sex) between-group analyses of variance (ANOVAs). Where significant main effects and interactions involving age and the other variables were evident, planned ANOVAs within each age group were conducted to explore age-dependent consequences of repeated ethanol exposure on responsiveness to acute ethanol challenge. Ethanol-induced changes of social behavior and social motivation were assessed by post hoc comparisons (Fisher’s planned least significant difference test) between ethanol-challenged groups and the saline-challenged controls within each chronic treatment/age condition.
Percentage body weight gain from day 1 (P27 for adolescents and P62 for adults) to day 7 (P33 for adolescents and P68 for adults) of ethanol or saline exposure was analyzed using a 2 (chronic treatment) x 2 (age) x 2 (sex) ANOVA followed by separate for each age 2 (chronic treatment) x 2 (sex) ANOVAs. For calculation of percent body weight gain, mean body weights for animals of each sex within each litter were used.
2.5. Blood Ethanol Determination
Tail blood samples were collected immediately after behavioral testing into heparinized tubes, rapidly frozen, and maintained at –80°C until analysis of blood ethanol content (BEC). Samples were assessed for BEC via headspace gas chromatography using a Hewlett Packard (HP) 5890 series II Gas Chromatograph (Wilmington, DE). At the time of assay, blood samples were thawed and 25-μl aliquots were placed in airtight vials. Vials were placed in a HP 7694E Auto-Sampler, which heated each individual vial for 8 min, and then extracted and injected a 1.0 ml sample of the gas headspace into the gas chromatograph. Ethanol concentrations in each sample were determined using HP Chemstation software, which compares the peak area under the curve in each sample with those of standard curves derived from reference standard solutions. BECs were analyzed using a 2 (chronic treatment) x 4 (acute ethanol dose) x 2 (age) x 2 (sex) ANOVA.
3. Results
3.1. Body Weight Gain
A 2 (chronic treatment) x 2 (age) x 2 (sex) ANOVA for percent body weight gain from day 1 to day 7 of chronic exposure revealed significant main effects of age, F(1,32) = 1174.52, p < 0.0001, and sex, F(1,32) = 6.56, p < 0.05. Animals tested during the adolescent period demonstrated significantly and substantially higher increases in body weight across the exposure period than did their adult counterparts, whereas percent body weight gain in female subjects was lower than in males (see Table 1). However, animals chronically exposed to ethanol did not differ from those exposed to saline in terms of percent body weight gain at either age. Further ANOVA analyses, performed separately for adolescent and adult animals, revealed that adult males and females did not differ in terms of percent body weight gain, whereas female adolescents gained significantly less weight across the exposure period than did their male counterparts [a significant main effect of sex, F(1,16) = 6.35, p < 0.05).
Table 1.
Percent body weight gain from day 1 to day 7 of chronic exposure to saline or ethanol for adolescent and adult male and female rats.
| Age | Chronic Saline | Chronic Ethanol | ||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| Adolescent | 50.0 ± 2.3 | 43.5 ± 3.2 | 47.1 ± 2.3 | 42.9 ± 1.3 |
| Adult | 5.1 ± 1.2 | 4.9 ± 1.3 | 5.4 ± 0.6 | 4.1 ± 1.0 |
3.1. Overall Social Activity
Considerable age-related differences in effects of ethanol on overall levels of social activity were observed [age x acute ethanol dose interaction, F(4, 160) = 5.10, p < 0.001], with only adolescents showing ethanol-induced social facilitation and adolescent animals being less sensitive to the suppressing effects of ethanol on social behavior than their adult counterparts. Given these age-related differences in the acute effects of ethanol on overall social activity, data were analyzed separately for adolescents and adults to further assess the consequences of repeated ethanol exposure. Tolerance emerged to both stimulatory and inhibitory effects of ethanol on social activity in adolescent animals [chronic treatment x acute ethanol dose interaction, F(4, 80) = 4.20, p < 0.01]. As seen in Figure 1, adolescent animals showed an increase in social activity at low doses of ethanol. This ethanol-induced social facilitation was evident in chronic saline-exposed adolescents following 0.5 g/kg ethanol, whereas adolescents chronically exposed to ethanol required a dose of 0.75 g/kg. Following chronic saline exposure, adolescents demonstrated a significant decrease in social behavior following the challenge with 1 g/kg, whereas adolescents chronically exposed to ethanol demonstrated no ethanol-induced social inhibition. Similarly, overall social activity of adult animals differed as a function of chronic treatment and acute ethanol challenge dose, F(4, 80) = 2.61, p < 0.05. Chronically exposed to saline adults demonstrated significant decreases in social behavior following 0.75 and 1 g/kg of ethanol, whereas repeated ethanol exposure shifted this dose-response curve to the right, with chronically ethanol-exposed adults showing a significant decrease in overall social activity only after a dose of 1 g/kg (see Figure 1).
Figure 1.
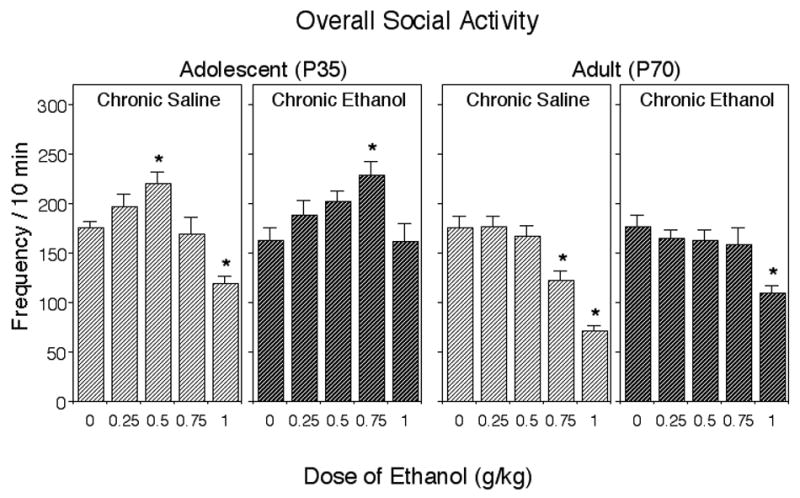
Effects of acute ethanol challenge (0, 0.25, 0.5, 0.75, and 1.0 g/kg) on overall social activity of adolescent and adult rats chronically exposed to saline or ethanol (1 g/kg). Asterisks (*) indicate significant ethanol-induced changes relative to corresponding saline (0 g/kg ethanol) control animals within each chronic treatment/age condition (p < 0.05), with data collapsed across sex (n = 10 per group).
3.3. Social Preference
Dramatic age-related differences in the effects of ethanol on social preference were observed in both chronic saline- and ethanol-exposed animals, as evidenced by a significant age x acute ethanol dose interaction, F(4, 160) = 3.19, p < 0.05 (see Figure 2). The 2 (chronic treatment) x 5 (ethanol challenge dose) x 2 (sex) ANOVA comparing the values of the coefficient for adolescent animals revealed a significant main effect of chronic treatment, F(1,80) = 8.06, p < 0.01, and a significant chronic treatment x acute ethanol dose interaction, F(4,80) = 4.09, p < 0.01. No effects of acute ethanol challenge on this measure of social motivation were seen in chronic saline-exposed adolescent rats, whereas adolescents chronically exposed to ethanol exhibited a marked enhancement of social preference when challenged with doses of 0.5, 0.75, and 1 g/kg. This was associated with a reduction in baseline preferences for social stimuli in adolescents chronically exposed to ethanol and examined in the absence of ethanol – i.e., chronic exposure to ethanol during adolescence significantly lowered preference coefficients relative to their saline-exposed counterparts when both groups were acutely challenged with 0 g/kg ethanol (see # in Figure 2). Coefficients of social preference in adults differed as a function of chronic treatment and acute ethanol challenge, F(4,80) = 5.42, p < 0.05, with 0.75 and 1 g/kg ethanol diminishing social preference in chronically saline-exposed adults, whereas acute ethanol challenge produced no significant alterations in social preference of adults following history of repeated ethanol exposure.
Figure 2.

Effects of acute ethanol challenge on social preference/avoidance of adolescent and adult rats chronically exposed to saline or ethanol (1 g/kg). * - significant ethanol-induced changes relative to corresponding saline (0 g/kg ethanol) control animals within each chronic treatment/age condition (p < 0.05), # - significant changes in chronically ethanol-exposed adolescent animals relative to chronically saline-exposed animals under the 0 g/kg challenge dose (p < 0.05), with data collapsed across sex (n = 10 per group).
3.4. Overall Locomotor Activity
Analysis of total number of crossovers to and from the partner, used as an index of overall locomotor activity in the present study, revealed only a significant main effect of acute ethanol dose, F(4, 160) = 14.44, p < 0.001. As shown in Figure 3, the dose of 1 g/kg ethanol significantly decreased the total number of movements between compartments in adolescent and adult animals chronically treated with either saline or ethanol. Acute effects of ethanol on overall locomotor activity did not differ as a function of age, nor was tolerance seen to these effects.
Figure 3.

Effects of acute ethanol challenge on locomotor activity (overall number of crossovers) of adolescent and adult rats chronically exposed to saline or ethanol (1 g/kg). * - significant changes relative to corresponding saline (0 g/kg ethanol) control animals within each chronic treatment/age condition (p < 0.05), with data collapsed across sex (n = 10 per group).
3.5. Blood Ethanol Concentration
A 2 (age) x 2 (chronic treatment) x 4 (acute ethanol dose) x 2 (sex) ANOVA conducted on BEC data revealed a significant age x chronic treatment x acute ethanol dose interaction, F(3, 128) = 2.77, p < 0.05. Given this three-way interaction, BECs were analyzed separately for adolescents and adults. BECs increased in a dose-dependent fashion in both adolescent [F(3, 64) = 487.31, p < 0.001] and adult rats [F(3, 64) = 235.30, p < 0.001], with age- and chronic exposure-related differences emerging at the challenge dose of 1 g/kg (see Figure 4). In general, blood ethanol levels did not differ as a function of chronic treatment in adolescent rats, whereas adults exposed to chronic ethanol demonstrated significantly lower BECs than their chronically saline-treated controls [chronic treatment x acute ethanol dose interaction, F(3, 64) = 2.92, p < 0.05] at the 1 g/kg dose (p < 0.05), with a similar trend seen at the 0.75 g/kg ethanol dose (p = 0.08). Adults challenged with the 1 g/kg dose of ethanol had significantly higher BECs than adolescents following chronic saline (p < 0.05), but not chronic ethanol exposure.
Figure 4.

Blood levels of ethanol in chronically exposed to saline or ethanol (1 g/kg) adolescent and adult animals following acute ethanol challenge, with data collapsed across sex (n = 10 per group).
4. Discussion
Regardless of considerable age-related differences in acute effects of ethanol on overall social activity, both adolescents and adults developed chronic tolerance to the social consequences of ethanol. Although the dose-response curves for ethanol-induced changes in social behavior were shifted similarly to the right at each age following chronic ethanol exposure, this tolerance in adolescent animals was only functional and not metabolic in nature, given that BECs were not lower following the chronic ethanol exposure. In contrast, adult animals responded to chronic ethanol exposure, at least in part, by developing metabolic tolerance, as indexed by a decrease in BECs after chronic ethanol at this age. Adolescents and adults also differed in the consequences of chronic ethanol on baseline social preference, with repeated ethanol exposure diminishing social preference of adolescents but not adults when sober. This social deficit was reversed by acute ethanol challenge in adolescent animals. In adults, a decrease in social preference was seen at higher doses of ethanol following chronic saline exposure, with chronic ethanol exposure making them tolerant to these effects. These findings suggest that mechanisms underlying adaptations to repeated ethanol may vary with age.
The decrease in social preference when sober among adolescents with a history of ethanol exposure may be related, at least partly, to ethanol-induced disruption in neural substrates underlying social behavior. Experimental studies that detail neural regions critical for peer-directed social interactions, especially under familiar, non-anxiogenic test conditions, are limited. However, recent research has revealed that frontal regions including medial prefrontal cortex, orbitofrontal cortex, amygdala, and ventral hippocampus, as well as dopamine input to these regions [6, 9, 11, 19, 24, 26], play an important role in peer-directed social interactions. These brain regions are among those that undergo considerable remodeling during adolescence [1, 15, 16, 18, 35, 37, 48, 49] and, hence are likely targets for contributing to the disruption in social preference following chronic adolescent exposure to ethanol. Indeed, there are a number of reports that ethanol-induced damage in these and other brain regions may be more pronounced during adolescence than in adulthood [5, 22]. For instance, Crews et al. [5] demonstrated that 4 days of “binge” exposure to high doses of ethanol (9–10 g/kg/day) produces cell death, which is more evident in adolescent than adult rats in a number of frontal-anterior brain regions.
Social deficits in adolescent animals following repeated ethanol exposure were associated with the emergence of ethanol-induced enhancement of social motivation, as indexed by a significant increase in the preference/avoidance coefficient in response to acute ethanol challenge in these animals. This effect was not seen in chronically saline-exposed adolescents at any ethanol dose or in adults in either chronic exposure group. These findings of an enhanced sensitivity of adolescent rats as a result of chronic ethanol exposure are reminiscent of work by White et al. [46]. In that study, adolescent rats given 5 g/kg ethanol i.p. every other day over a 20-day period and tested 20 days after the last injection demonstrated enhanced sensitivity to ethanol-induced disruption in a spatial working memory task than animals exposed to ethanol in adulthood or saline-exposed controls. Therefore, chronic exposure to ethanol during adolescence may not only result in adult-typical tolerance to some ethanol effects, with adolescents becoming even less sensitive to ethanol effects to which they are normally insensitive (e.g., ethanol-induced social inhibition), but may also induce the emergence of a unique hypersensitivity to other effects of ethanol (e.g., ethanol-induced enhancement of social motivation).
This adolescent-specific enhancement of sensitivity to ethanol-induced facilitation of social motivation following chronic ethanol exposure might be determined, at least partly, by ethanol-associated alterations in ongoing processes of neural development [see 32 for references and review]. Indeed, a number of studies have reported that ethanol exposure during adolescence produces pronounced alterations in neural functioning. For instance, Grobin et al. [12] assessed possible alterations in GABAA receptor function produced by chronic ethanol either during adolescence or adulthood and established that intermittent ethanol exposure (5 g/kg, i.p., 5 injections across a 20-day treatment period) results in greater enhancement of neocortical GABAA receptor sensitivity to a neurosteroid 3 α, 21-dihydroxy-5α– pregnan-20-one (THDOC) in animals exposed to ethanol as adolescents than as adults. Similarly, Slawecki et al. [29] found that exposure to ethanol vapor for 5 or 10 days during adolescence has long-lasting effects on brain activity, as indexed by a number of electrophysiological measures. Enhanced prepulse inhibition, a neurobehavioral index of sensorimotor gating, was observed following adolescent but not adult exposure to ethanol vapor for 14 days [30]. Taken together, these results support the conclusion that, at least under some circumstances, the neural alterations associated with chronic ethanol exposure may be particularly pronounced when this exposure occurs during adolescence.
In the present study, animals repeatedly exposed to ethanol developed chronic tolerance to ethanol-induced social inhibition regardless of age. This finding is reminiscent of previous results reported by Silveri and Spear [28], although in that study the chronic ethanol dose was varied across age (2.6 g/kg for adolescents and 2.2 g/kg for adults) to equate initial ethanol-induced motor impairment, whereas in the present experiment the same chronic dose of 1 g/kg was used for both adolescents and adults. However, no evidence of metabolic tolerance emerged at either age in the Silveri and Spear [28] study, whereas adults exposed to chronic ethanol in the present experiment demonstrated significantly lower BECs than their chronically saline-treated counterparts, results providing evidence for the emergence of metabolic tolerance in adults chronically exposed to ethanol. In contrast to the present findings, in the ontogenetic study of Swartzwelder et al. [34] adolescent rats were found to show greater tolerance to the hypothermic effects of ethanol than their adult counterparts, with only adolescents demonstrating chronic tolerance to the sedative/hypnotic effects of ethanol. The exacerbated tolerance development seen in adolescents in the Swartzwelder et al. study [34] could have been due to a greater degree of perturbation associated with the chronic exposure procedures. In that study, experimental subjects were administered intragastrically 4 g/kg ethanol twice daily, and their temperatures were recorded every other day using a rectal probe, whereas in the present experiment, animals were injected i.p. with ethanol or saline in a volume of 1% body weight once a day. Together, these results raise the possibility that greater amount of experimental perturbation/stress may foster greater expression of chronic tolerance in adolescents, whereas chronic tolerance may emerge similarly across age under less perturbing exposure/test circumstances. With a minimally invasive route of ethanol administration via vapor inhalation and indwelling telemetry probes for temperature recording, adolescents showed even less chronic tolerance to the hypothermic effects of ethanol than adults [25]. This finding is consistent with the postulation that age-related differences in ethanol adaptations (or the lack thereof) may be related, at least in part, to the degree of experimental perturbation/stress involved.
To the extent that these data are applicable to humans, the results of the present study along with other research employing animal models of adolescence [e.g., 5, 12, 25, 29, 30, 34, 45, 46] provide further evidence that adolescents may respond and adapt differently to repeated episodes of ethanol exposure than their more mature counterparts, with mechanisms underlying these adaptations also being age-specific. In addition to further characterizing adolescent-specific neurobehavioral alterations following repeated ethanol exposure, studies are needed to explore whether these adolescent-specific adaptations to and consequences of chronic ethanol might put them at higher risk for extensive alcohol use and the eventual emergence of alcohol abuse disorders.
Acknowledgments
The research presented in this study was supported by grants from the National Institute of Alcohol Abuse and Alcoholism R01 AA12453 to Elena I. Varlinskaya and R37 AA12525 to Linda P. Spear.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andersen SL, Dumont NL, Teicher MH. Developmental differences in dopamine synthesis inhibition by (+/−)-7-OH-DPAT. Naunyn-Schmiedeberg's Arch Pharmacol. 1997;356:173–181. doi: 10.1007/pl00005038. [DOI] [PubMed] [Google Scholar]
- 2.Beck KH, Treiman KA. The relationship of social context of drinking, perceived social norms, and parental influence to various drinking patterns of adolescents. Addict Behav. 1996;21:633–644. doi: 10.1016/0306-4603(95)00087-9. [DOI] [PubMed] [Google Scholar]
- 3.Brunell SC, Spear LP. Effects of stress on the voluntary intake of sweetened ethanol solution in pair-housed adolescent and adult rats. Alcohol Clin Exp Res. 2005;29:1641–1653. doi: 10.1097/01.alc.0000179382.64752.13. [DOI] [PubMed] [Google Scholar]
- 4.Cooper ML, Frone MR, Russell M, Mudar P. Drinking to regulate positive and negative emotions: a motivational model for alcohol use. J Pers Soc Psychol. 1995;69:990–1005. doi: 10.1037//0022-3514.69.5.990. [DOI] [PubMed] [Google Scholar]
- 5.Crews FT, Braun CJ, Hoplight B, Switzer RC, III, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000;24:1712–1723. [PubMed] [Google Scholar]
- 6.Daenen EWPM, Wolterink G, Gerrits MAFM, Van Ree JM. The effects of neonatal lesions in the amygdala or ventral hippocampus on social behaviour later in life. Behav Brain Res. 2002;136:571–582. doi: 10.1016/s0166-4328(02)00223-1. [DOI] [PubMed] [Google Scholar]
- 7.Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- 8.Doremus TL, Brunell SC, Varlinskaya EI, Spear LP. Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacol Biochem Behav. 2003;75:411–418. doi: 10.1016/s0091-3057(03)00134-5. [DOI] [PubMed] [Google Scholar]
- 9.Espejo EF. Prefrontocortical dopamine loss in rats delays long-term extinction of contextual conditioned fear, and reduces social interaction without affecting short-term social interaction memory. Neuropsychopharmacol. 2003;28:490–498. doi: 10.1038/sj.npp.1300066. [DOI] [PubMed] [Google Scholar]
- 10.File SE. The social interaction test of anxiety. Neurosci Protocol. 1993;10:1–7. [Google Scholar]
- 11.Flores G, Silva-Gómez AB, Ibáñez O, Quirion R, Srivastava LK. Comparative behavioral changes in postpubertal rats after neonatal excitotoxic lesions of the ventral hippocampus and the prefrontal cortex. Synapse. 2005;56:147–153. doi: 10.1002/syn.20140. [DOI] [PubMed] [Google Scholar]
- 12.Grobin AC, Matthews DB, Montoya D, Wilson WA, Morrow AL, Swartzwelder HS. Age-related differences in neurosteroid potentiation of muscimol-stimulated36Cl− flux following chronic ethanol treatment. Neurosci. 2001;105:547–552. doi: 10.1016/s0306-4522(01)00232-9. [DOI] [PubMed] [Google Scholar]
- 13.Hollstedt C, Olsson O, Rydberg U. The effect of alcohol on the developing rat: II. Coordination as measured by the tilting-plane test. Med Biol. 1980;58:164–168. [PubMed] [Google Scholar]
- 14.Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- 15.Insel TR, Miller LP, Gelhard RE. The ontogeny of excitatory amino acid receptors in rat forebrain - I. N-methyl-D-aspartate and quisqualate receptors. Neurosci. 1990;35:31–43. doi: 10.1016/0306-4522(90)90117-m. [DOI] [PubMed] [Google Scholar]
- 16.Jernigan TL, Trauner DA, Hesselink JR, Tallal PA. Maturation of human cerebrum observed in vivo during adolescence. Brain. 1991;114:2037–2049. doi: 10.1093/brain/114.5.2037. [DOI] [PubMed] [Google Scholar]
- 17.Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future National Results on Adolescent Drug Use: Overview of Key Findings, 2003. National Institute on Drug Abuse; Bathesda, MD: 2004. [Google Scholar]
- 18.Kellogg CK, Awatramani GB, Piekut DT. Adolescent development alters stressor-induced Fos immunoreactivity in rat brain. Neurosci. 1998;83:681–689. doi: 10.1016/s0306-4522(97)00408-9. [DOI] [PubMed] [Google Scholar]
- 19.Kolb B, Pellis S, Robinson TE. Plasticity and functions of the orbital frontal cortex. Brain Cognit. 2004;55:104–115. doi: 10.1016/S0278-2626(03)00278-1. [DOI] [PubMed] [Google Scholar]
- 20.Lancaster FE, Brown TD, Coker KL, Elliott JA, Wren SB. Sex differences in alcohol preference and drinking patterns emerge during the early postpubertal period in Sprague-Dawley rats. Alcohol Clin Exp Res. 1996;20:1043–1049. doi: 10.1111/j.1530-0277.1996.tb01945.x. [DOI] [PubMed] [Google Scholar]
- 21.Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of ethanol in adolescent and adult rats. Alcohol Clin Exp Res. 1996;20:1346–1351. doi: 10.1111/j.1530-0277.1996.tb01133.x. [DOI] [PubMed] [Google Scholar]
- 22.Monti PM, Miranda R, Jr, Nixon K, Sher KS, Swartwelder HS, Tapert SF, White A, Crews FT. Adolescence: booze, brains, and behavior. Alcohol Clin Exp Res. 2005;29:207–220. doi: 10.1097/01.alc.0000153551.11000.f3. [DOI] [PubMed] [Google Scholar]
- 23.Moy SS, Duncan GE, Knapp DJ, Breese GR. Sensitivity to ethanol across development in rats: Comparison to [3H]zolpidem binding. Alcohol Clin Exp Res. 1998;22:1485–1492. [PubMed] [Google Scholar]
- 24.Rangel A, Gonzalez LE, Villarroel V, Hernandez L. Anxiolysis followed by anxiogenesis relates to coping and corticosterone after medial prefrontal cortical damage in rats. Brain Res. 2003;992:96–103. doi: 10.1016/j.brainres.2003.08.038. [DOI] [PubMed] [Google Scholar]
- 25.Ristuccia RC, Spear LP. Sensitivity and tolerance to autonomic effects of ethanol in adolescent and adult rats during repeated vapor inhalation sessions. Alcohol Clin Exp Res. 2005;29:1809–1820. doi: 10.1097/01.alc.0000183010.72764.cd. [DOI] [PubMed] [Google Scholar]
- 26.Shah AA, Treit D. Excitotoxic lesions of the medial prefrontal cortex attenuate fear responses in the elevated-plus maze, social interaction and shock probe burying tests. Brain Res. 2003;969:183–194. doi: 10.1016/s0006-8993(03)02299-6. [DOI] [PubMed] [Google Scholar]
- 27.Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;22:670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- 28.Silveri MM, Spear LP. Acute, rapid and chronic tolerance during ontogeny: Observations when equating ethanol perturbation across age. Alcohol Clin Exp Res. 2001;25:1301–1308. [PubMed] [Google Scholar]
- 29.Slawecki CJ, Betancourt M, Cole M, Ehlers CL. Periadolescent alcohol exposure has lasting effects on adult neurophysiological function in rats. Dev Brain Res. 2001;128:63–72. doi: 10.1016/s0165-3806(01)00150-x. [DOI] [PubMed] [Google Scholar]
- 30.Slawecki CJ, Ehlers CL. Enhanced prepulse inhibition following adolescent ethanol exposure in Sprague-Dawley rats. Alcohol Clin Exp Res. 2005;29:1829–1836. doi: 10.1097/01.alc.0000183024.47167.27. [DOI] [PubMed] [Google Scholar]
- 31.Smith GT, Goldman MS, Greenbaum PE, Christiansen BA. Expectancy for social facilitation from drinking: The divergent paths of high-expectancy and low-expectancy adolescents. J Abnormal Psychol. 1995;104:32–40. doi: 10.1037//0021-843x.104.1.32. [DOI] [PubMed] [Google Scholar]
- 32.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Behav Physiol. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 33.Spear LP, File SE. Methodological considerations in neurobehavioral teratology. Pharmacol Biochem Behav. 1996;55:455–457. doi: 10.1016/s0091-3057(96)00272-9. [DOI] [PubMed] [Google Scholar]
- 34.Swartzwelder HS, Richardson RC, Markwiese-Foerch B, Wilson WA, Little PJ. Developmental differences in the acquisition of tolerance to ethanol. Alcohol. 1998;15:311–314. doi: 10.1016/s0741-8329(97)00135-3. [DOI] [PubMed] [Google Scholar]
- 35.Terasawa E, Timiras PS. Electrophysiological study of the limbic system in the rat at onset of puberty. Am J Physiol. 1968;215:1462–1467. doi: 10.1152/ajplegacy.1968.215.6.1462. [DOI] [PubMed] [Google Scholar]
- 36.Thor DH, Holloway RW., Jr Social play in juvenile rats: A decade of methodological and experimental research. Neurosci Biobehav Rev. 1984;8:455–464. doi: 10.1016/0149-7634(84)90004-6. [DOI] [PubMed] [Google Scholar]
- 37.van Eden CG, Kros JM, Uylings HBM. The development of the rat prefrontal cortex: its size and development of connections with thalamus, spinal cord and other cortical areas. In: Uylings HBM, van Eden CG, De Bruin JPC, Corner MA, Feenstra MGP, editors. The Prefrontal Cortex: Its Structure, Function and Pathology. Vol. 85. Elsevier Science; Amsterdam: 1990. pp. 169–183. [DOI] [PubMed] [Google Scholar]
- 38.Vanderschuren LJMJ, Niesink RJM, Van Ree JM. The neurobiology of social play behavior in rats. Neurosci Biobehav Rev. 1997;21:309–326. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- 39.Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcohol Clin Exp Res. 2002;26:1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- 40.Varlinskaya EI, Spear LP. Changes in sensitivity to ethanol-induced social facilitation and social inhibition from early to late adolescence. Ann NY Acad Sci. 2004;1021:459–461. doi: 10.1196/annals.1308.064. [DOI] [PubMed] [Google Scholar]
- 41.Varlinskaya EI, Spear LP. Acute ethanol withdrawal (hangover) and social behavior in adolescent and adult male and female Sprague-Dawley rats. Alcohol Clin Exp Res. 2004;28:40–50. doi: 10.1097/01.ALC.0000108655.51087.DF. [DOI] [PubMed] [Google Scholar]
- 42.Varlinskaya EI, Spear LP. Differences in the social consequences of ethanol emerge during the course of adolescence in rats: social facilitation, social inhibition, and anxiolysis. Develop Psychobiol. 2006;48:146–161. doi: 10.1002/dev.20124. [DOI] [PubMed] [Google Scholar]
- 43.Varlinskaya EI, Spear LP, Spear NE. Social behavior and social motivation in adolescent rats: Role of housing conditions and partner's activity. Physiol Behav. 1999;67:475–482. doi: 10.1016/s0031-9384(98)00285-6. [DOI] [PubMed] [Google Scholar]
- 44.Varlinskaya EI, Spear LP, Spear NE. Acute effects of ethanol on behavior of adolescent rats: Role of social context. Alcohol Clin Exp Res. 2001;25:377–385. [PubMed] [Google Scholar]
- 45.White AM, Bae JG, Truesdale MC, Ahmad S, Wilson WA, Swartzwelder HS. Chronic-intermittent ethanol exposure during adolescence prevents normal developmental changes in sensitivity to ethanol-induced motor impairments. Alcohol Clin Exp Res. 2002;26:960–968. doi: 10.1097/01.ALC.0000021334.47130.F9. [DOI] [PubMed] [Google Scholar]
- 46.White AM, Ghia AJ, Levin ED, Swartzwelder HS. Binge pattern ethanol exposure in adolescent and adult rats: differential impact on subsequent responsiveness to ethanol. Alcohol Clin Exp Res. 2000;24:1251–1256. [PubMed] [Google Scholar]
- 47.White AM, Truesdale MC, Bae JG, Ahmad S, Wilson WA, Best PJ, Swartzwelder HS. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacol Biochem Behav. 2002;73:673–677. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]
- 48.Yurgelun-Todd DA, Killgore WDS, Cintron CB. Cognitive correlates of medial temporal lobe development across adolescence: A magnetic resonance imaging study. Percept Motor Skill. 2003;96:3–17. doi: 10.2466/pms.2003.96.1.3. [DOI] [PubMed] [Google Scholar]
- 49.Zecevic N, Bourgeois JP, Rakic P. Changes in synaptic density in motor cortex of rhesus monkey during fetal and postnatal life. Dev Brain Res. 1989;50:11–32. doi: 10.1016/0165-3806(89)90124-7. [DOI] [PubMed] [Google Scholar]


