Abstract
Plasma levels of cocaine (COC) and two of its principle metabolites, benzoylecgonine (BE) and ecgonine methyl ester (EME) were determined by liquid chromatography–tandem mass spectrometry (LC/MS/MS) in samples collected up to 3 h after a subcutaneous injection of cocaine (10 mg/kg) on six different days between days 4 and 24 postpartum, a period of dramatic change in the endocrine state of the female rat. Locomotor activity was measured in the same animals during this period using automated animal activity monitors. Additional measures in males provide a link to existing literature. We found that plasma levels of cocaine and its metabolites, as well as their respective time courses, are remarkably uniform across the postpartum period in female rats, as are the effects of cocaine on locomotor activity. Data from males show accord with prior published values. COC and BE, but not EME levels, were higher in males, and the time courses of COC and BE levels after injection varied somewhat between postpartum females and males; however, neither baseline nor cocaine-induced locomotor activity differed between postpartum females and males. We conclude that in the postpartum rat, there are no significant differences in the peripheral processing or general accessibility of cocaine to the brain to activate motor systems across the postpartum period. These data are critical to our understanding of differences in the reward salience of cocaine across the postpartum period and in other adult rat models [Mattson BJ, Williams S, Rosenblatt JS, Morrell JI. Comparison of two positive reinforcing stimuli: pups and cocaine throughout the postpartum period. Behav Neurosci 2001;115:683–94, Mattson BJ, Williams SE, Rosenblatt JS, Morrell JI. Preferences for cocaine- or pup-associated chambers differentiate otherwise behaviorally identical postpartum maternal rats.
Keywords: Cocaine, Subcutaneous, Benzoylecgonine, Ecgonine methyl ester, Locomotor activity, Postpartum, Plasma, Female, Male, Rat
Cocaine markedly impairs the expression of species-specific maternal behavior in postpartum rats when administered either systemically (Johns et al., 1994, 1997, 1998; Kinsley et al., 1994; Vernotica et al., 1996a,b; Zimmerberg and Gray, 1992) or centrally into discrete central nervous system sites (Vernotica et al., 1999). We recently investigated the effects of cocaine as a pharmacological challenge to natural motivational processes in the lactating, postpartum rat, which is known to be highly motivated to seek and care for her offspring (Fahrbach and Pfaff, 1982; Fleming et al., 1994; Hauser and Gandelman, 1985; Lee et al., 2000; Magnusson and Fleming, 1995; Mattson et al., 2001, 2003; Wilsoncroft, 1969). In a series of studies using place preference conditioning, we found that the motivation of the dam to seek pup-related cues or cocaine-related cues changes across the postpartum period. When given a choice between cues associated with cocaine, pups, or neutrality/ novelty, late postpartum (day 16) dams prefer cocaine-associated cues, whereas early postpartum (day 8) dams prefer pup-associated cues (Mattson et al., 2001, 2003). We have further shown that the change in the preference response in our choice paradigm is due to underlying independent changes in the reward salience of each of these stimuli (pups and cocaine) across the postpartum period (Wansaw et al., 2003a,b).
During the postpartum period the entire endocrine state of the female changes dramatically. The early postpartum period is characterized by low circulating levels of estradiol, moderate-to-high (increasing) levels of progesterone, and high levels of prolactin (Smith and Neill, 1977; Taya and Greenwald, 1982). In contrast, the late postpartum period is characterized by increasing levels of estradiol (which reach levels of the evening of diestrus-2 in the cycling female by day 20 postpartum), high but decreasing levels of progesterone, and low levels of prolactin (Smith and Neill, 1977; Taya and Greenwald, 1982). Because the endocrine profile of the dam changes so considerably across the postpartum period, it is possible that these endocrine changes alter effectiveness of injection routes in introducing cocaine into the circulation, or its metabolism. Either of these might then alter the effectiveness of cocaine on the CNS. Alternatively, transfer of cocaine into the circulation and its metabolism could be uniform across the postpartum period, suggesting the endocrine state of the CNS results in very specific differences in the effect of cocaine via CNS mechanisms not yet explored.
We carried out this study to determine whether peripheral processing might have a role in our findings of altered reinforcing salience of cocaine across the postpartum period. In the present study we also investigated whether the postpartum period causes changes in the general capacity of cocaine to affect simple behaviors such as locomotor activity.
We examined both plasma levels of cocaine (COC), and its major metabolites. Cocaine metabolism occurs through hydrolysis by esterases in blood and tissues (accounting for 80–90% of total elimination) and through oxidation by mixed-function oxidases, mainly in the liver (Shuster, 1992). The principle metabolites of cocaine in the rat include benzoylecgonine (BE) and ecgonine methyl ester (EME). BE is reportedly formed by nonenzymatic hydrolysis, whereas EME is formed by liver and serum esterases (Shuster, 1992). Both BE and EME are bioactive metabolites of cocaine. BE stimulates locomotor activity, whereas EME inhibits cocaine-stimulated activity (Misra et al., 1975; Schuelke et al., 1996).
Plasma cocaine and metabolite levels have been studied in four different adult rodent endocrine models including 1) pregnant, 2) postpartum and 3) virgin female rats, as well as 4) male rats. The least is known about the postpartum rat. Attempts to establish an animal model for the teratogenic effects of gestational cocaine exposure on the neurobiology and behavior of the offspring have yielded much information on plasma cocaine and metabolite levels in the pregnant female rat. Plasma cocaine and metabolite levels have been studied in pregnant rats and mice after subcutaneous (Collins et al., 1999; Spear et al., 1989; Vorhees et al., 1995), intraperitoneal (DeVane et al., 1989; Shah et al., 1980), intragastric (Dow-Edwards, 1990), and intravenous (Robinson et al., 1994) cocaine administration.
Our previous study comparing day 6 postpartum dams and virgin females found that plasma cocaine levels are comparable between postpartum dams and virgins and that cocaine-induced locomotor activity between these different endocrine models is only subtly different in pattern and peak values (Vernotica and Morrell, 1998). In the only other studies known to us that include postpartum females, Dwivedi et al. (1993, 1996) found that postpartum dams have higher brain concentrations of cocaine and lower serum-to-brain ratios of cocaine compared with pregnant and virgin female rats.
Plasma cocaine and metabolite levels have also been studied in virgin rats and mice after subcutaneous (Dow-Edwards et al., 1989; Vernotica and Morrell, 1998), intra-peritoneal (Benuck et al., 1987; Bowmann et al., 1999; Festa et al., 2004; Shah et al., 1980), and intravenous (Wiggins et al., 1989) cocaine administration, mostly using females without regard to stage of the estrous cycle. Additional, more specific work has now shown that hormonal fluctuations during the estrous cycle modulate cocaine metabolism (Quinones-Jenab et al., 1999) and behavioral responses to cocaine (Lynch et al., 2000; Quinones-Jenab et al., 1999; Roberts et al., 1989; Sell et al., 2000). Furthermore, estrogen alone has been shown to enhance the behavioral responsiveness of female rats to cocaine (Hu and Becker, 2003; Lynch et al., 2001; Sell et al., 2000).
Males offer a simpler endocrine model, as only one endocrine state exists in the intact male. Plasma cocaine and metabolite levels have been studied in male rats after subcutaneous (Misra, 1976; Nayak et al., 1976), intra-peritoneal (Bowmann et al., 1999; Festa et al., 2004; Lau et al., 1991), and intravenous (Misra, 1976; Nayak et al., 1976) cocaine administration.
All of these studies, regardless of gender or endocrine state, showed that peak blood levels and the time course of blood levels were highly dependent upon the route of administration and dosing regimen (acute vs. chronic) and they were in reasonable accord across a range of cocaine doses. Taken together these studies provided a context for our choice of dose and time frame for examining the post-injection plasma levels of cocaine and its metabolites.
Cocaine is a psychomotor stimulant that dramatically increases spontaneous locomotor activity. The effects of cocaine on the locomotor activity of rodents and its dose-dependent correlation to plasma cocaine levels have made it a widely used dependent measure of the behavioral effects of cocaine (Antoniou and Kafetzopoulos, 1996; Benuck et al., 1987; Lau et al., 1991; Post and Rose, 1976; Pradhan et al., 1978; Sell et al., 2000; Vernotica and Morrell, 1998; Yeh and Haertzen, 1991). Cocaine’s ability to increase locomotor activity and its ability to function as a positive reinforcer share overlapping neural components in the mesolimbic dopamine system (Wise and Bozarth, 1987). We therefore used locomotor activity measures as a behavioral correlate of plasma cocaine and metabolite levels to provide context for behavioral measures of motivation, such as those we make in our other studies using the place preference paradigm.
Many studies have examined sex differences in the behavioral effects of cocaine (Bowman and Kuhn, 1996; Festa et al., 2004; Glick et al., 1983; Sell et al., 2000; van Haaren and Meyer, 1991). These studies, regardless of gender, showed that peak levels of cocaine-induced locomotor activity and their time course are highly dependent on the route of administration, the dose of drug, and the dosing regimen (acute vs. chronic). Taken together these studies provided a context for our time frame for examining cocaine-induced locomotor activity.
In this study we examined lactating, fully maternal female rats on six different postpartum days between days 4 and 24. We included a group of male rats as a single reference point to data already in the literature and to our place preference examination of the reward salience of cocaine that extends to males (Wansaw et al., 2004). We chose to study a dose of 10 mg/kg because we established that this dose is sufficient to impair the expression of maternal behavior when present in the blood (Vernotica et al., 1996a,b) and that this was a reinforcing dose for the late postpartum but not the early postpartum dam (Mattson et al., 2001; Wansaw et al., 2002, 2003a). Furthermore we used a subcutaneous route of administration because our group and others have used this approach in pregnant and postpartum females to avoid confounding influences on fetal development or nursing processes (Vernotica et al., 1996a,b; Mattson et al., 2001, 2003; Johns et al., 1997, 1998). Concentrations of cocaine and its two principal metabolites, BE and EME, were determined by liquid chromatography–tandem mass spectrometry in plasma collected up to 3 h after a subcutaneous cocaine injection; locomotor activity was measured in the same animals using automated activity monitors.
1. Methods
1.1. Subjects
Subjects were male (n =9) and primiparous postpartum female (n =63) Sprague–Dawley rats obtained from our breeding colony maintained at the Research Animal Facility of Rutgers University, Newark Campus, which is accredited through the Association for Assessment and Accreditation of Laboratory Animal Care. The stock animals for the colony were originally purchased from Charles River Laboratories (Kingston, NY). Additional stud males and females are purchased periodically from the same vendor in order to keep the colony genetically consistent with the Charles River breeding stock. Nulliparous females 90–120 days old that were in behavioral estrus were mated with sexually active males according to the methods described by Mattson et al. (2001). After giving birth, females were housed individually with litters culled to eight pups each in polyethylene shoebox cages (41.9 cm long × 20.3 cm wide × 20.3 cm high) lined with fresh woodchip bedding (Beta chip, Northeastern Products Corp., Warrensburg, NY). Experimental males 67–72 days old were housed individually in similar cages. All animals were kept on a 12-h light/ dark cycle (with lights on at 0700 h) in a room maintained at 22 ± 1 °C. Animals had continuous access to water and food (postpartum females: Lab Diet, 2008; males: Lab Diet, 2001, PMI Nutrition International, LLC, Brentwood, MO) except during locomotor activity testing. All procedures were in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985).
1.2. Experimental Groups
Postpartum females were fully maternal dams (n =63, 250–350 g) that received cocaine injections on three separate days and were tested for locomotor activity and sacrificed 1–3 h after the third and final injection (3–7 days after the first injection). Dams received this third and final injection on day 4 (n =9), day 6 (n = 7), day 10 (n =13), day 12 (n =10), day 18 (n =14), or day 24 (n =10) postpartum. For day 4, n =3 at 60 min, n =3 at 120 min, and n =3 at 180 min. For day 6, n =2 at 60 min, n =2 at 120 min, and n =3 at 180 min. For day 10, n =4 at 60 min, n =5 at 120 min, and n =4 at 180 min. For day 12, n = 4 at 60 min, n = 3 at 120 min, and n = 3 at 180 min. For day 18, n =4 at 60 min, n =5 at 120 min, and n =5 at 180 min. For day 24, n =3 at 60 min, n =4 at 120 min, and n = 3 at 180 min.
Approximately half of the dams received their first and second injections of cocaine as part of a place conditioning study. Dams that did not participate in the place conditioning study received their first two injections of cocaine in the home cage, in the absence of pups. All dams, therefore, had the same drug history prior to being tested for locomotor activity after their third and final injection of cocaine. The third injection of cocaine was administered at least 48 h after the second injection. In postpartum and virgin female rats, plasma levels of cocaine are minimal (<50 ng/mL) 5 h after a 10 mg/kg subcutaneous injection (Vernotica and Morrell, 1998). In male rats, plasma levels of cocaine are barely detectable 12 h after a 20 mg/kg subcutaneous injection and are not detected 24 h after an injection (Nayak et al., 1976). Therefore, there was no concern for carryover effects on either plasma levels or locomotor activity.
Males (n =9, 350–425 g) also received cocaine injections on three separate days and were tested for locomotor activity and sacrificed 1–3 h after the third and final injection (5 days after the first injection). For the males, n =3 at 60 min, n =3 at 120 min, and n =3 at 180 min.
1.3. Cocaine administration
Cocaine hydrochloride in highly purified (>90%) powdered form was provided by the National Institute on Drug Abuse (Research Triangle Park, NC). Animals received subcutaneous injections of 10 mg/kg cocaine at a concentration of 4.5 mg/ml sterile 0.9% saline in the region of the dorsal–caudal flank. This dilution and the alternation of the injection site from left to right completely prevented skin necrosis.
1.4. Locomotor activity
Locomotor activity was measured using four Digiscan Animal Activity Monitors (Model RXYZCM8, Omnitech Electronics, Inc., Columbus, OH) connected to a Digiscan analyzer (Model DCM-4BBU). Each activity monitor is a framework of horizontal and vertical sensors that surrounds a Plexiglas animal cage (41.9 cm long × 41.9 cm wide ×30.5 cm high). The sensors create an invisible grid of infrared beams inside the cage. Eight horizontal beams (spaced 5 cm apart) traverse the cage from front to back and eight more beams traverse the cage from left to right; eight vertical beams traverse the cage from front to back. As an animal in the cage moves about, it breaks the beams. Beam breaks are converted to locomotor activity counts by the analyzer. Activity data, including total distance traveled (cm), rearing counts, and center time, were collected in 10 min bins for up to 3 h after injection. We report only total distance traveled because other activity measures covaried with it (Vernotica and Morrell, 1998).
1.5. Measures of general health and well-being
The general health and well being of each animal was assessed daily by physical examination and handling by the investigators, under the supervision of the veterinary staff of Rutgers University. No animals were found to be unhealthy or in discomfort. Dams treated with cocaine had weight changes that were identical to a group of dams not treated with cocaine; males gained weight normally during the experiment. Therefore, treatment with cocaine did not induce weight loss in our animals.
1.6. Injection and activity testing
Animals were removed from their home cages, given a subcutaneous injection, and within 2 min, were placed into the activity monitors for 3 h. Baseline locomotor activity was measured after an injection of vehicle (0.9% saline) on the day before locomotor testing with cocaine. On the day of the final cocaine injection, locomotor activity was measured continuously for up to 3 h after injection. Animals were then removed from the activity monitors and sacrificed 1, 2, or 3 h after cocaine administration for blood collection. All locomotor activity testing occurred between 0900 and 1700 h.
1.7. Blood sampling at sacrifice
Animals were deeply anesthetized with an overdose (1 mL, intraperitoneally) of sodium pentobarbital (Veterinary Laboratories, Inc., Lenexa, KS) before surgery. When corneal reflex and withdrawal to painful stimuli were no longer observed, the chest cavity was surgically opened to allow direct access to the heart. Heparin (0.2 mL, American Pharmaceutical Partners, Inc., Los Angeles, CA) was injected into the left ventricle to prevent clotting. Cardiac blood (7–10 mL) was collected from the left ventricle using a 3-mL syringe equipped with a 20-gauge needle (Becton Dickinson and Co., Franklin Lakes, NJ). Whole blood samples were centrifuged immediately (DYNAC II® Centrifuge, Becton Dickinson and Co., Franklin Lakes, NJ) at 2000 rpm for 12 min, and the plasma was transferred to cold Vacutainer® tubes containing 30 mg of sodium fluoride (Becton Dickinson and Co., Franklin Lakes, NJ). Two aliquots were made from the plasma of each animal.
1.8. Freezing and storage
We initially tested the effects of freezing method on plasma levels of cocaine and its metabolites and found no differences between samples rapidly frozen in liquid nitrogen and samples frozen more slowly in a non-frost-free freezer at −20 °C. All reported values are from plasma samples frozen and deliberately stored in our laboratory in a non-frost-free freezer at −20 °C (for no longer than 6 months) before being shipped frozen in dry ice for analysis.
1.9. Liquid chromatography–tandem mass spectrometry assay
Plasma samples were analyzed by Dr. Shen-Nan Lin at the Center for Human Toxicology at the University of Utah. Plasma samples were analyzed immediately upon receipt at the University of Utah. Plasma concentrations of COC and its two principal metabolites, BE and EME, were determined by liquid chromatography–tandem mass spectrometry (LC/MS/MS) as previously described by Lin et al. (2001). Plasma concentrations are expressed in ng/mL. The lower limit of quantitation for each analyte was 5 ng/mL.
The LC/MS/MS assay was remarkably stable and accurate over time. Mean plasma levels of COC, BE, and EME did not differ between biologically identical samples that were freshly collected from different animals and then analyzed on different days (different analysis batches) distributed over a period of two years. In addition, known quantities of cocaine added to drug-free plasma samples were verified accurately by the assay.
1.10. Differences between LC/MS/MS and GC/MS
In a subset of samples (n =22), plasma levels of COC and BE were determined by both LC/MS/MS and gas chromatography/mass spectrometry (GC/MS). These samples were obtained from dams sacrificed 1–3 h after their third and final injection of cocaine on day 6 (n =2), day 8 (n =4), day 11 (n =4), day 12 (n =4), day 14 (n =4), and day 15 (n =4) postpartum. Plasma COC and BE levels determined by LC/ MS/MS were 12% and 13% higher, respectively, than plasma COC and BE levels determined by GC/MS when the samples were analyzed by both methods (paired t-tests, both p’s< .0001) (Fig. 1).
Fig. 1.
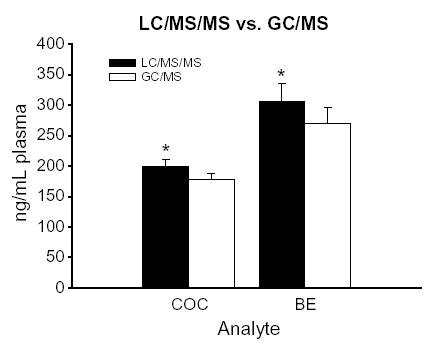
Plasma levels of COC and BE determined by LC/MS/MS (black bars) and GC/MS (white bars) for postpartum dams (n =22) sacrificed 1 –3 h after an injection of cocaine (10 mg/kg, subcutaneously). Plasma COC and BE levels were averaged over the 3-h post-injection period for direct comparison. Each bar represents the mean ± S.E.M. in units of ng/mL plasma. Plasma levels of COC and BE were significantly higher when the samples were analyzed by LC/MS/MS versus when they were analyzed by GC/MS. An asterisk denotes a statistically significant difference ( p <.0001) between the two analytical methods.
1.11. Impact of storage time on frozen plasma levels of COC, BE, and EME
Plasma samples stored for up to 6 months yielded stable assay results. Samples stored in the freezer for approximately 1–2 months (n =30) had average values of 187 ± 10 ng/ml COC, 342 ± 23 ng/ml BE, and 71 ± 5 ng/ml EME, virtually identical to those stored in the freezer for approximately 5–6 months (n =32), which had average values of 170 ± 12 ng/ml COC, 312 ± 25 ng/ml BE, and 71 ± 5 ng/ml EME [independent t-tests, ns].
Although Lin et al. (2001) reported stable levels of COC and BE in a much larger number of samples stored for up to 11 months, we found that levels of COC and BE were significantly elevated in a small number of samples reanalyzed after 7 to 30 months of storage. The differences may be due to our smaller sample size or differences in storage and shipping methods.
1.12. Data reduction and statistical analyses
Plasma cocaine and metabolite data were analyzed with two-factor ANOVA, with postpartum day (4, 6, 10, 12, 18, and 24) or endocrine model (postpartum female vs. male) as the between-groups factor and time after injection (60, 120, and 180 min) as the within-groups factor; Tukey’s HSD tests were done post hoc if F-values were statistically significant. Locomotor activity data after saline administration were analyzed with two-factor ANOVA and MANOVA with postpartum day (4, 6, 10, 12, 18, and 24) or endocrine model (postpartum female vs. male) as the between-groups factor and time after injection (30, 60, 90, 120, 150, 180 min) as a repeated factor. Individual one-way ANOVA followed by Tukey’s HSD tests were used to analyze differences in locomotor activity between postpartum days at each 30-min time point after cocaine administration. One-way repeated-measures ANOVA followed by Duncan’s tests were used to analyze differences in locomotor activity at each 30-min time point averaged across all postpartum days. Paired t-tests were used to analyze differences in locomotor activity between cocaine and saline treatments at each 30-min time point after injection. Finally, independent t-tests were used to analyze differences in plasma cocaine, metabolites, and locomotor activity between postpartum females and males. A significance level of p <.05 was used for all statistical tests. Statistical analyses were conducted using SAS for Windows (Version 8.2). All data are presented as mean ± standard error of the mean (S.E.M).
2. Results
2.1. Plasma cocaine and metabolite levels after cocaine injection on different postpartum days
2.1.1. Comparison across postpartum days
Plasma levels of COC were the same on different postpartum days as a function of time after injection of cocaine [postpartum day × time after injection interaction, F(10,45)= 0.52, ns] (Fig. 2A) and when averaged for the 3-h period after injection [main effect of postpartum day, F(5,45)= 1.49, ns] (Table 1). Plasma levels of BE and EME also did not differ between postpartum days as a function of time after injection of cocaine [postpartum day × time after injection interaction, F(10,45)= 0.65, ns for BE (Fig. 2B) and F(10,41)=0.84, ns for EME (Fig. 2C)], nor did they differ between postpartum days when averaged for the 3-h period after injection [main effect of postpartum day, F(5,45)= 0.53, ns for BE and F(5,41)=0.48, ns for EME (Table 1)].
Fig. 2.
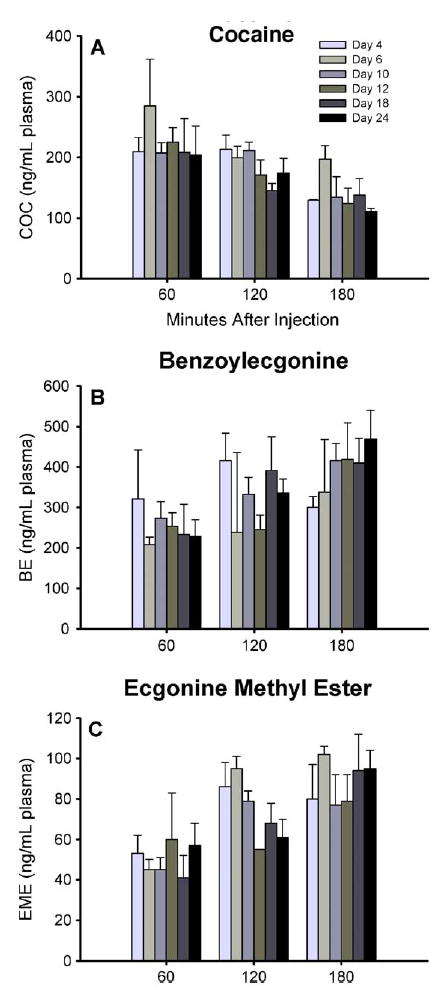
The time course of plasma levels of (A) COC, (B) BE, and (C) EME for dams sacrificed 1, 2, or 3 h after an injection of cocaine (10 mg/kg, subcutaneously) on different postpartum days from day 4 –24. Each bar represents the mean ± S.E.M. in units of ng/mL plasma. There are 2 – 5 dams per group, per time point. Plasma levels of COC, BE, and EME did not differ between postpartum days as a function of time after injection of cocaine. The time courses of plasma COC, BE, and EME were remarkably similar across postpartum days. Data are graphed in minutes after injection of cocaine.
Table 1.
Mean (± S.E.M.) plasma COC, BE, and EME levels averaged over the 3-hr period after subcutaneous injection of cocaine (10 mg/kg) in postpartum female rats and male rats
| Group | COC (ng/mL) | BE (ng/mL) | EME (ng/mL) |
|---|---|---|---|
| Day 4 | 184±17 | 346±45 | 73±8 |
| Day 6 | 223±25 | 272±70 | 84±10 |
| Day 10 | 186±15 | 340±28 | 68±6 |
| Day 12 | 178±19 | 300±38 | 65±8a |
| Day 18 | 161±19 | 353±45 | 70±10 |
| Day 24 | 164±19 | 344±40 | 70±8 |
| Postpartum Females | 179±8 | 330±17 | 71±3 |
| Males | 306±28b | 660±139b | 69±11 |
EME was measured in 6 of 10 day 12 postpartum animals.
Significantly different from postpartum females, p <.0001.
2.1.2. Time Course after Cocaine Injection
Whether examined on each postpartum day or averaged across all groups of postpartum dams, plasma levels of COC peaked at 60 min, were modestly lower by 120 min, and subsequently decreased to about half the peak value by 180 min [main effect of time after injection, F(2,45)= 11.65, p <.0001] (Fig. 4A, black bars). Plasma levels of COC at 180 min were significantly lower than those at 60 and 120 min (both p’s<.05). Nonetheless, plasma levels of COC at the 180-min time point remained substantial. Plasma levels of BE and EME increased gradually throughout the testing period and reached their highest levels within our analysis at 180 min [main effect of time after injection, F(2,45)=5.43, p <.05 for BE and F(2,41)=12.37, p <.0001 for EME] (Fig. 5B and C, black bars). Plasma levels of BE at 180 min were significantly higher than those at 60 min ( p < .05), whereas plasma levels of EME at both 120 and 180 min were significantly higher than those at 60 min (both p’s<.05).
Fig. 4.
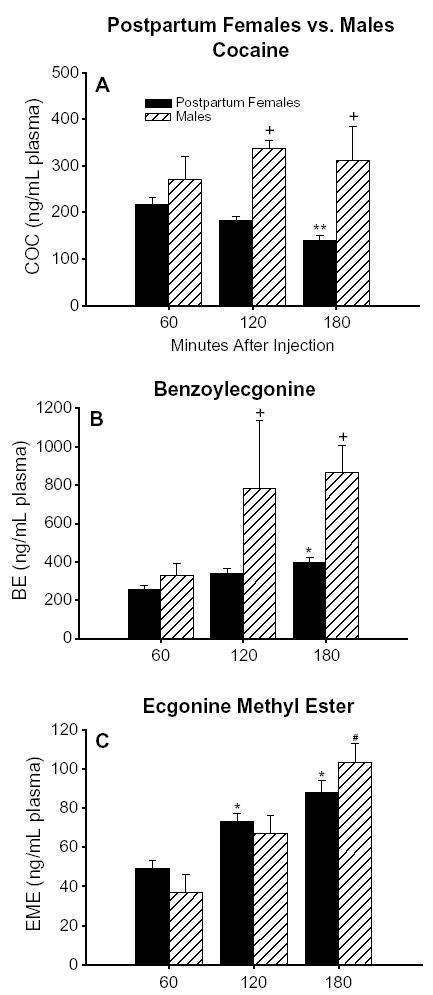
The time course of plasma levels of (A) COC, (B) BE, and (C) EME for all postpartum female rats (black bars) and male rats (hatched bars) sacrificed 1, 2, or 3 h after an injection of cocaine (10 mg/kg, subcutaneously). Each bar represents the mean±S.E.M. in units of ng/mL plasma. For postpartum females, n =20 at 60 min, n =22 at 120 min, and n =21 at 180 min. For males, n =3 at each time point up to 180 min. The time course of plasma levels of COC and BE, but not EME, differed significantly between postpartum female and male rats. A plus sign indicates a statistically significant difference between postpartum females and males at a single time point. An asterisk indicates a statistically significant difference from the postpartum females at the 60-min time point (postpartum females only). A double asterisk indicates a statistically significant difference from the postpartum females at the 60- and 120-min time points (postpartum females only). A number sign indicates a statistically significant difference from the males at the 60-min time point (males only).
Fig. 5.
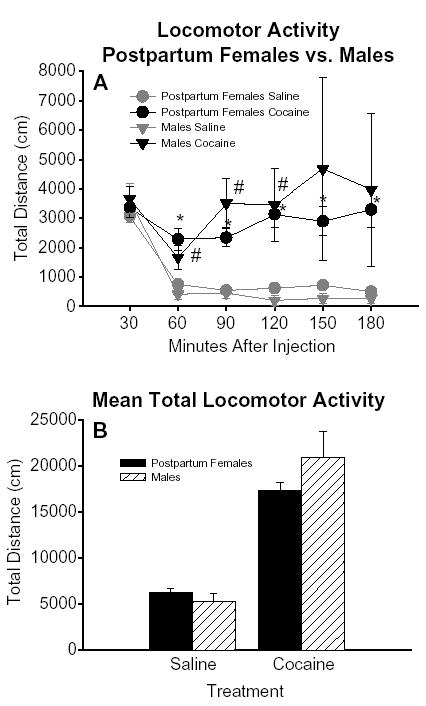
(A) The time course of locomotor activity for postpartum female and male rats tested up to 3 h after an injection of saline and cocaine. Each point represents the mean (±S.E.M.) total distance traveled (cm) in a 30-min interval. For the saline treatment n =9 males for each time point up to 180 min. For the cocaine treatment, n =9 males from 30– 60 min, n =6 males for 90– 120 min, and n =3 males for 150– 180 min. Female data are as described in Fig. 3C. No statistically significant differences were observed between postpartum females and males at any time point up to 3 h after an injection of either saline or cocaine. A number sign indicates a statically significant difference at a single time point between cocaine and saline treatments (male data only). (B) Mean total locomotor activity summed over the 3-h period after injections of saline and cocaine for postpartum female and male rats. Each bar represents the mean (±S.E.M.) total distance traveled (cm) summed over the 3-h period after injections of saline and cocaine. Total locomotor activity after saline and after cocaine, as well as the overall increase in locomotor activity after cocaine did not differ significantly between postpartum females and males.
2.2. Locomotor activity across the postpartum period
2.2.1. Baseline Locomotor Activity
The pattern of locomotor activity after saline injection did not differ across postpartum days [MANOVA Wilks lambda=.50, F(25,184)=1.52, ns] (Fig. 3A). Moreover, locomotor activity followed the expected pattern after saline injection and introduction into the activity monitor. After an initial period of normally increased exploration during the first 30 min, locomotor activity decreased markedly by 60 min, and remained significantly reduced throughout the remainder of the test period when averaged across all postpartum days [ F(5,290)=61.37, p <.0001, all p’s<.05] (Figs. 3C and 5A, gray circles).
Fig. 3.
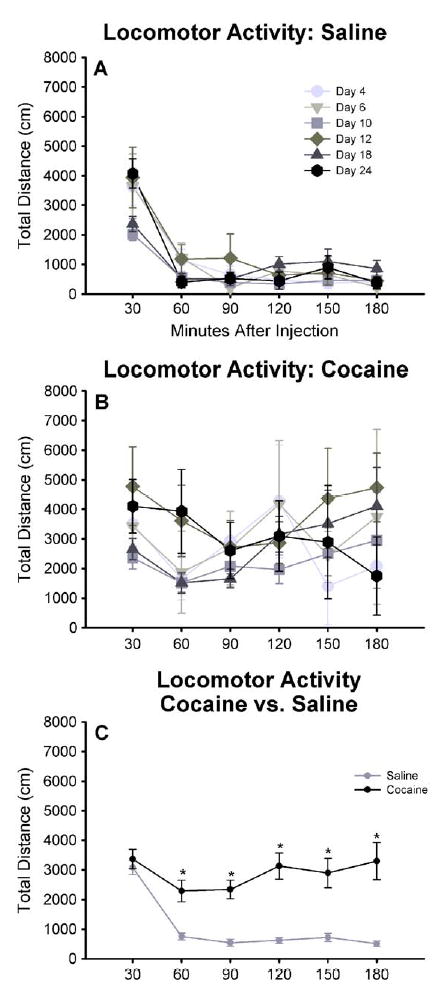
The time course of locomotor activity for dams tested up to 3 h after an injection of (A) saline and (B) cocaine (10 mg/kg, subcutaneously) on different postpartum days from day 4 –24. Each point represents the mean (±S.E.M.) total distance traveled (cm) in a 30-min interval. For the saline treatment, n =6 – 14 dams per group, per time point up to 180 min after injection. For the cocaine treatment, n =7 – 14 dams per group from 30–60 min, n =5 – 10 dams per group from 90–120 min, and n =3 – 5 dams per group from 150–180 min. No differences in the time course of locomotor activity after saline or cocaine injection were observed between postpartum days. (C) The time course of locomotor activity averaged across postpartum days up to 3 h after an injection of saline (gray circles) and cocaine (black circles). The mean and S.E.M for each time point was calculated from all individual dams regardless of postpartum day. For the saline treatment n =59 dams for each time point up to 180 min. For the cocaine treatment, n =63 dams from 30– 60 min, n =43 dams for 90– 120 min, and n =20 dams for 150–180 min. Locomotor activity after injection of cocaine was significantly higher than after saline injection at each time point from 60 to 180 min after injection. An asterisk denotes a statistically significant difference ( p <.05) between treatments.
2.2.2. Effect of Cocaine on Locomotor Activity
Cocaine increased total locomotor activity approximately 3-fold over baseline in postpartum females, regardless of the day on which it was administered. The pattern of increased locomotor activity after cocaine injection also did not differ across postpartum days [MANOVA Wilks lambda=.13, F(25,42)=1.25, ns; and by individual one-way ANOVA at each time point] (Fig. 3B). Whereas locomotor activity decreased dramatically after the initial exploratory period following saline injection, locomotor activity remained elevated throughout the test period after cocaine injection. Locomotor activity was slightly increased above saline (9%) during the initial exploratory period 30 min following cocaine injection; this became a substantial and significant increase of 205% above saline 60 min after cocaine injection (Fig. 3C) [t(62) = 4.12, p = .0001]. Locomotor activity remained significantly elevated compared with saline at 90 min [t(42)= 5.73, p <.0001, 331% above saline], 120 min [t(42)= 5.14, p <.0001, 405% above saline], and 150 min [t(19)= 4.54, p =.0002, 302% above saline] after injection of cocaine (Fig. 3C). At 180 min after cocaine injection, locomotor activity was increased 552% above saline levels [t(19)= 4.17, p =.0005] (Fig. 3C). Thus, 10 mg of subcutaneously administered cocaine produced a dramatic and prolonged (up to 3 h) increase in locomotor activity in postpartum dams.
2.3. Plasma cocaine and metabolite levels after cocaine injection in males compared with postpartum females
2.3.1. Plasma levels of cocaine and metabolites
Average plasma levels of COC were 71% higher in males than in postpartum females over the 3 h after injection [main effect of endocrine model, F(1, 66) = 36.96, p < .0001] (Table 1). Specifically, the plasma COC levels differed statistically at 2 and 3 h after injection. Plasma levels of COC remained high in males; however, in postpartum females they decreased steadily to about half the peak level by the 3-h time point [endocrine model × time after injection interaction, F(2, 66) = 3.27, p < .05] (Fig. 4A). Average plasma levels of BE were 100% higher in males than in postpartum females [main effect of endocrine state, F(1,66) = 30.88, p < .0001] (Table 1); the groups were significantly different at 2 and 3 h after injection [endocrine model × time after injection interaction, F(2, 66) = 4.65, p < .05] (Fig. 4B). Plasma levels of EME did not differ between males and postpartum females (Table 1; Fig. 4C).
2.3.2. Locomotor Activity
Locomotor activity after injection of saline was the same in postpartum females and males, both overall and at each time point (Fig. 5A and B). In each group, cocaine significantly increased activity over saline baseline [females t(62) = −4.67, p < .0001; males [t(8) = −1.87, p = .099]; however, the increase in activity was not statistically different in males compared with postpartum dams (Fig. 5A and B).
3. Discussion
3.1. Plasma cocaine levels, metabolite levels, and locomotor effects were uniform across the postpartum period
The present study established that plasma levels of cocaine and its metabolites, as well as their respective time courses, are similar across the postpartum period in female rats, as are the effects of cocaine on locomotor activity. The stability of the values is remarkable considering that they are taken from day 4 to day 24 postpartum when the females have very different levels of circulating hormones. Therefore, the marked differences in the reward salience of cocaine across the postpartum period that we have uncovered with our place preference conditioning paradigm (Mattson et al., 2001, 2003; Wansaw et al., 2003b, 2004) are not likely due to differences in the peripheral processing or general accessibility of cocaine to the brain to activate motor systems. Hence, we next will investigate whether the differences in the reward salience of cocaine across the postpartum period are likely to be due to changes in the effect of cocaine on specific neural structures that mediate the perception and prioritization of rewards (Mattson and Morrell, in press; Morrell et al., 2005).
3.2. Comparisons of plasma cocaine values across female endocrine models
The peak plasma level of cocaine for postpartum females in the present study (218±15 ng/mL) is comparable to that we previously reported for virgin females (~260 ng/mL) but is lower than that we reported using a small number of females on postpartum day 6 (~550 ng/ml) (Vernotica and Morrell, 1998). Both of our studies showed a similar time course of peak plasma cocaine levels across the female models, except that in the present study peak postpartum values of cocaine occurred within 1 h of injection and then diminished only slightly at 2 h. Our prior finding that the peak was at 2 h is probably due to the small number of subjects in the earlier study. Thus, our current data extend and strengthen the information about the time course and values of cocaine in plasma after subcutaneous injection previously available for the postpartum female.
The time course of plasma levels of any drug depends on the route of administration. In general, the absorption of a subcutaneously injected drug is slow and sustained (Benet and Sheiner, 1985). Other routes of administration provide faster peak and clearance times. Absorption of cocaine can be slowed from an injection site by the vasoconstriction caused by cocaine’s blocking the re-uptake of norepinephrine by adrenergic nerve endings in the walls of blood vessels (Shuster, 1992). In the pregnant female rat, plasma cocaine levels peak at 1 min after intravenous (Robinson et al., 1994) administration, at 15 min after intraperitoneal (mice) (Shah et al., 1980) or intragastric (Dow-Edwards, 1990) administration, and at 1 h after subcutaneous (Collins et al., 1999; Vorhees et al., 1995) administration. Plasma cocaine levels peak at 5 min in virgin females after intraperitoneal administration (Bowmann et al., 1999), at 30 min after intravenous administration (Wiggins et al., 1989) (the earliest time point measured in the study), and at 1 h after subcutaneous administration (Vernotica and Morrell, 1998). Thus, the time course of subcutaneously administered cocaine is longer compared to other routes of administration and peaks at 1 h in all three female rat endocrine models.
3.3. Comparisons of metabolite levels in female models
Our study is the first to report plasma levels of cocaine metabolites most extensively, including BE and EME in the postpartum rat. Peak plasma levels of these metabolites, in our data and all other studies utilizing female endocrine models, occur after peak plasma cocaine levels, in a route-dependent manner. Peak plasma levels of BE reported for postpartum females in this study (395 ±28 ng/mL) are much lower than those reported for pregnant females (1109±53 ng/mL) even considering that Collins et al. used a 20 mg/kg subcutaneous injection (Collins et al., 1999). This difference is probably because their females were chronically treated with cocaine, whereas our animals were acutely treated. The time course was also somewhat affected by these methodological differences: in the present study the peak plasma BE level occurred at 3 h (the last time point measured) whereas in the study by Collins et al. it occurred at 4 h after injection.
Cocaine metabolite levels have been studied in detail in virgin female rats after intraperitoneal cocaine administration. Plasma BE levels peak at 60 min, whereas plasma EME levels peak 15–60 min after intraperitoneal cocaine administration (Bowmann et al., 1999; Festa et al., 2004). In virgin female rats, plasma BE levels ranged from ~400–1400 ng/mL, whereas plasma EME levels ranged from ~200–400 ng/mL (Bowmann et al., 1999; Festa et al., 2004). In our postpartum females, metabolite levels after subcutaneous injection were considerably lower than the values after intraperitoneal injection, probably due to differences in dose and route of administration between this study and others (e.g., Bowmann et al., 1999; Festa et al., 2004), but a small contribution to these differences from the different endocrine models cannot be ruled out.
3.4. Comparisons of locomotor effects of cocaine across female endocrine models
There is correlation between the plasma level of cocaine and the onset of increased locomotor activity. Our data also demonstrate that the substantial but lower-than-peak values of cocaine found 3 h after injection are sufficient to maintain high levels of cocaine-induced locomotor activity. It is also possible that the bioactive metabolites of cocaine particularly BE, contribute to the prolonged locomotor effects.
In the present study, cocaine induced a 3-fold increase in locomotor activity, which is similar to the value we previously reported for virgin females (2.5-fold increase) and moderately lower than the value we reported for day 6 postpartum dams (5-fold increase) (Vernotica and Morrell, 1998). These differences in values in the postpartum dams could be a consequence of our prior small sample size and testing during the dark phase of the diurnal cycle in the prior study. Nonetheless, the two studies concur on the extended time course of the effect of cocaine on locomotor activity and suggest that a fairly stable range of locomotor responses is found with a single dose of cocaine.
We examined the data from our day 24 postpartum females particularly closely because at this point estrogen levels have increased again as the females wean their pups and resume cycling, although we did not establish that these females had resumed cycling. Sell et al. (2000) suggested that in the cycling virgin female rat estrogen levels and the locomotor activating effects of cocaine were positively correlated. Our data show that for the late postpartum female, this period was no different than any other day of the postpartum period in either plasma level of cocaine (or metabolites) or cocaine-induced locomotor activity. That is, when we compared days of the postpartum period when estrogen was lowest (days 4–12) to the days when estrogen was highest (day 24), there was no difference in our measures. Because the endocrine state of a female is not determined by the level of any single hormone, the effect of estrogen that others have seen within the cycle is likely modified by the endocrine context of the virgin and this very late postpartum period when the female is transitioning to a return to cycling.
3.5. Comparisons of plasma cocaine, metabolites, and locomotor activity in male rats
The peak plasma level of cocaine we report for males (337 ±18 ng/mL) is generally consistent with those reported by others for males after acute (490±60 ng/mL) and chronic (500±100 ng/mL) treatment with 20 mg/kg subcutaneous cocaine, taking the dose difference into consideration (Misra, 1976; Nayak et al., 1976). However, plasma levels of cocaine peaked earlier in our males (2 h after injection) than in the acutely treated males (4 h after injection) but peaked later than in the chronically treated males (1 h after injection) in the study by Nayak et al. Nonetheless, the generally similar outcomes across these studies is clear; for example, the males in our study had very similar levels of plasma cocaine (337±18 ng/mL) at 2 h after injection with a lower dose of drug than that used by Nayak et al. (1976) in chronically treated males (380±80 ng/mL) at 2 h after injection. Similar to the females, route of administration influences when the peak plasma level occurs in males. Plasma cocaine levels peak at 5 min in males after intraperitoneal cocaine administration (Bowmann et al., 1999), at 15 min after intravenous administration (the earliest time point examined) (Misra, 1976; Nayak et al., 1976), and at 4 h after subcutaneous administration (Misra, 1976; Nayak et al., 1976).
The peak plasma level of BE (866±142 ng/mL) in male rats was, as expected, lower than those reported for males given a single intraperitoneal injection of 15 mg/kg cocaine (~1400 ng/mL) (Bowmann et al., 1999) or 20 mg/kg cocaine (~1900 ng/mL) (Festa et al., 2004). The peak in plasma BE level was also, as expected, later in our animals (3 h vs. 45–60 min after injection) (Bowmann et al., 1999; Festa et al., 2004). Peak plasma level of EME (103~10 ng/ ml) was comparable with that in males administered 15 mg/ kg (~100 ng/mL) cocaine intraperitoneally (Bowmann et al., 1999) and lower than that of males administered 20 mg/ kg (~175 ng/mL) cocaine intraperitoneally (Festa et al., 2004). Similar to BE, peak plasma levels of EME occurred later in our males (3 h) compared with males administered cocaine intraperitoneally (45 min) (Festa et al., 2004). Plasma BE levels peak at 60 min in male rats after intraperitoneal (Bowmann et al., 1999) cocaine administration, whereas plasma EME levels peak at 45 min (Festa et al., 2004). Again, the delay in peak plasma levels of cocaine and its metabolites can be explained by the additive effects of the subcutaneous injection and cocaine’s vasoconstrictive properties.
The pattern of prolonged, increased locomotor activity in our male rats after subcutaneous cocaine administration is similar to that observed by others (Yeh and Haertzen, 1991). The prolonged availability of cocaine and its metabolites in the plasma is sufficient to support the substantial sustained increase of locomotor activity even 3 h after cocaine injection.
3.6. Limited comparison of the effect of cocaine on postpartum females, virgins, and males
In the present study, postpartum females had lower plasma levels of cocaine than males. Because others have found no differences in plasma cocaine levels between virgin females and males (Bowmann et al., 1999; Festa et al., 2004; van Haaren et al., 1997), we would expect that postpartum females would also have lower levels than virgins. In addition, plasma BE levels were lower in postpartum females compared with males, similar to the finding between virgin females and males (Bowmann et al., 1999; Festa et al., 2004). We found no differences in plasma levels of EME between postpartum females and males, whereas plasma levels of EME were higher in virgin females than in males (Bowmann et al., 1999; Festa et al., 2004).
There were no significant differences in baselines of locomotor activity after saline injection in postpartum females compared with males, as was the case in our prior comparison of virgins and postpartum females (Vernotica and Morrell, 1998). These dramatically different endocrine models have remarkably similar basic levels of exploration and activity. The impact of cocaine on the induction of locomotor activity in postpartum females and males was also similar in all dimensions, including peak and duration. The higher levels of cocaine and metabolites in males resulted in only a slightly greater locomotor response compared with the postpartum females, but this difference was not statistically significant. Other studies of virgin females and males found a greater locomotor effect of cocaine in virgin females than in males (Bowman and Kuhn, 1996; Festa et al., 2004; Glick et al., 1983; Sell et al., 2000; van Haaren and Meyer, 1991). These and other differences that have been documented in virgin females and males have recently been the subject of an excellent scholarly review (Festa and Quinones-Jenab, 2004) and so are not included in this discussion.
3.7. Variability in experimental groups
The variability in the plasma and activity data for a few of our groups was noticeably larger at some of the time points investigated. This is due to both the relatively small number of animals in each group at each time point (n ≤5 for plasma cocaine and metabolite levels) and the presence of extreme values in some of our groups. There is evidence, in the literature, that rodent populations consist of subsets of animals that are ‘‘high’’ and ‘‘low responders’’ in their locomotor response to novelty and that these characteristics impact behavioral responses to psychomotor stimulants (Piazza et al., 1989). It is possible that the extreme values in our locomotor activity data represent members of these subgroups and their characteristic diversity in the behavioral responses to stimulant drugs. We, therefore, chose to retain all individuals in our data analysis with the working hypothesis that this approach shows the natural (biological) variability in response to cocaine that is often overlooked. Nonetheless, inclusion of these animals and the increased variability they induced did not alter our general findings on the time courses of cocaine-induced locomotor activity or plasma cocaine and metabolite levels.
3.8. Overall conclusion
In our original work on the topic, we have shown that middle and late postpartum females prefer cocaine-associated cues, whereas early postpartum females prefer pup-associated cues (Mattson et al., 2001, 2003). In our subsequent work we have examined the salience of two hours of conditioning with subcutaneously injected cocaine (versus saline) independent of the pup-associated cues. We have found that late postpartum females have a modest preference for cocaine-associated cues and early postpartum dams have neither a preference nor aversion, while males and virgins females have a place aversion response with these conditioning parameters (Reiss et al., 2004; Wansaw et al., 2004). Those data combined with the findings of this study indicate that neither differences in peak plasma levels of cocaine and its metabolites nor differences in cocaine-induced locomotor activity explain the differences in preference for cocaine-associated cues across the postpartum period in lactating, maternal rats or in males.
Footnotes
This work was supported by March of Dimes Grant #12-FY02-05103 and NIH Grant R01 DA014025 awarded to J.I.M. and by NSF K-12 Teaching Fellowship awarded to M.P.W. Plasma samples were analyzed at the Center for Human Toxicology, University of Utah and supported by the U.S. Public Health Service, Contract N01-DA-6-7052.
References
- Antoniou K, Kafetzopoulos E. The pattern of locomotor activity after cocaine treatment in the rat. Behav Pharmacol. 1996;7:237 – 44. [PubMed] [Google Scholar]
- Benet LZ, Sheiner LB. Pharmacokinetics: the dynamics of drug absorption, distribution, and elimination. In: Gilman AG, Goodman LS, Rall TW, Murad F, editors. The pharmacological basis of therapeutics. 7th edition. New York: Macmillan Publishing Co.; 1983. pp. 3–34. [Google Scholar]
- Benuck M, Lajtha A, Reith ME. Pharmacokinetics of systemically administered cocaine and locomotor stimulation in mice. J Pharmacol Exp Ther. 1987;243:144–9. [PubMed] [Google Scholar]
- Bowman BP, Kuhn CM. Age-related differences in the chronic and acute response to cocaine in the rat. Dev Psychobiol. 1996;29:597– 611. doi: 10.1002/(SICI)1098-2302(199611)29:7<597::AID-DEV4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Bowman BP, Vaughan SR, Walker QD, Davis SL, Little PJ, Scheffler NM, et al. Effects of sex and gonadectomy on cocaine metabolism in the rat. J Pharmacol Exp Ther. 1999;290:1316 –23. [PubMed] [Google Scholar]
- Collins LM, Pahl JA, Meyer JS. Distribution of cocaine and metabolites in the pregnant rat and fetus in a chronic subcutaneous injection model. Neurotoxicol Teratol. 1999;21:639– 46. doi: 10.1016/s0892-0362(99)00037-9. [DOI] [PubMed] [Google Scholar]
- DeVane CL, Simpkins JW, Miller RL, Braun SB. Tissue distribution of cocaine in the pregnant rat. Life Sci. 1989;45:1271– 6. doi: 10.1016/0024-3205(89)90129-x. [DOI] [PubMed] [Google Scholar]
- Dow-Edwards D. Fetal and maternal cocaine levels peak rapidly following intragastric administration in the rat. J Subst Abuse. 1990;2:427 – 37. doi: 10.1016/s0899-3289(12)80003-4. [DOI] [PubMed] [Google Scholar]
- Dow-Edwards D, Fico TA, Osman M, Gamagaris Z, Hutchings DE. Comparison of oral and subcutaneous routes of cocaine administration on behavior, plasma drug concentration and toxicity in female rats. Pharmacol Biochem Behav. 1989;33:167–73. doi: 10.1016/0091-3057(89)90446-2. [DOI] [PubMed] [Google Scholar]
- Dwivedi C, Engineer FN, Vaughan SL. Alterations in biodistribution of cocaine may explain differential toxicity in pregnant and postpartum rats. Toxicol Appl Pharmacol. 1993;118:131– 4. doi: 10.1006/taap.1993.1018. [DOI] [PubMed] [Google Scholar]
- Dwivedi C, Eighmy AM, Singh KK. Biodistribution of cocaine during perinatal period in rats. Drug Chem Toxicol. 1996;19:313– 24. doi: 10.3109/01480549608998240. [DOI] [PubMed] [Google Scholar]
- Fahrbach SE, Pfaff DW. Hormonal and neural mechanisms underlying maternal behavior in the rat. In: Pfaff DW, editor. The physiological mechanisms of motivation. New York: Springer-Verlag; 1982. pp. 253–85. [Google Scholar]
- Festa ED, Quinones-Jenab V. Gonadal hormones provide the biological basis for sex differences in behavioral responses to cocaine. Horm Behav. 2004;46:509– 19. doi: 10.1016/j.yhbeh.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Festa ED, Russo SJ, Gazi FM, Niyomchai T, Kemen LM, Lin SN, et al. Sex differences in cocaine-induced behavioral responses, pharmacokinetics, and monoamine levels. Neuropharmacology. 2004;46:672– 87. doi: 10.1016/j.neuropharm.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Korsmit M, Deller M. Rat pups are potent reinforcers to the maternal animal: effects of experience, parity, hormones, and dopamine function. Psychobiology. 1994;22:44 – 53. [Google Scholar]
- Glick SD, Hinds PA, Shapiro RM. Cocaine-induced rotation: sex-dependent differences between left- and right-sided rats. Science. 1983;221:775– 7. doi: 10.1126/science.6879177. [DOI] [PubMed] [Google Scholar]
- Hauser H, Gandelman R. Lever pressing for pups: evidence for hormonal influence upon maternal behavior of mice. Horm Behav. 1985;19:454– 68. doi: 10.1016/0018-506x(85)90041-8. [DOI] [PubMed] [Google Scholar]
- Hu M, Becker JB. Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J Neurosci. 2003;23:693– 9. doi: 10.1523/JNEUROSCI.23-02-00693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns JM, Noonan LR, Zimmerman LI, Li L, Pedersen CA. Effects of chronic and acute cocaine treatment on the onset of maternal behavior and aggression in Sprague–Dawley rats. Behav Neurosci. 1994;108:107–12. doi: 10.1037//0735-7044.108.1.107. [DOI] [PubMed] [Google Scholar]
- Johns JM, Noonan LR, Zimmerman LI, Li L, Pedersen CA. Effects of short- and long-term withdrawal from gestational cocaine treatment on maternal behavior and aggression in Sprague– Dawley rats. Dev Neurosci. 1997;19:368– 74. doi: 10.1159/000111234. [DOI] [PubMed] [Google Scholar]
- Johns JM, Nelson CJ, Meter KE, Lubin DA, Couch CD, Ayers A, et al. Dose-dependent effects of multiple acute cocaine injections on maternal behavior and aggression in Sprague–Dawley rats. Dev Neurosci. 1998;20:525– 32. doi: 10.1159/000017353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsley CH, Turco D, Bauer A, Beverly M, Wellman J, Graham AL. Cocaine alters the onset and maintenance of maternal behavior in lactating rats. Pharmacol Biochem Behav. 1994;47:857– 64. doi: 10.1016/0091-3057(94)90288-7. [DOI] [PubMed] [Google Scholar]
- Lau CE, Imam A, Ma F, Falk JL. Acute effects of cocaine on spontaneous and discriminative motor functions: relation to route of administration and pharmacokinetics. J Pharmacol Exp Ther. 1991;257:444– 56. [PubMed] [Google Scholar]
- Lee A, Clancy S, Fleming AS. Mother rats bar-press for pups: effects of lesions of the mpoa and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behav Brain Res. 2000;108:215–31. doi: 10.1016/s0166-4328(99)00170-9. [DOI] [PubMed] [Google Scholar]
- Lin SN, Moody DE, Bigelow GE, Foltz RL. A validated liquid chromatography– atmospheric pressure chemical ionization– tandem mass spectrometry method for quantitation of cocaine and benzoylecgonine in human plasma. J Anal Toxicol. 2001;25:497– 503. doi: 10.1093/jat/25.7.497. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Arizzi MN, Carroll ME. Effects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacology (Berl) 2000;152:132– 9. doi: 10.1007/s002130000488. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Mickelberg JL, Carroll ME. Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacol Biochem Behav. 2001;68:641–6. doi: 10.1016/s0091-3057(01)00455-5. [DOI] [PubMed] [Google Scholar]
- Magnusson JE, Fleming AS. Rat pups are reinforcing to the maternal rat: role of sensory cues. Psychobiology. 1995;23:69– 75. [Google Scholar]
- Mattson BJ, Morrell JI. Preference for cocaine- versus pup-associated cues differentially activates neurons expressing either Fos or CART in lactating, maternal rodents. Neuroscience. doi: 10.1016/j.neuroscience.2005.06.045. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson BJ, Williams S, Rosenblatt JS, Morrell JI. Comparison of two positive reinforcing stimuli: pups and cocaine throughout the postpartum period. Behav Neurosci. 2001;115:683– 94. doi: 10.1037//0735-7044.115.3.683. [DOI] [PubMed] [Google Scholar]
- Mattson BJ, Williams SE, Rosenblatt JS, Morrell JI. Preferences for cocaine- or pup-associated chambers differentiates otherwise behaviorally identical postpartum maternal rats. Psychopharmacology (Berl) 2003;167:1– 8. doi: 10.1007/s00213-002-1351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra AL. Disposition and biotransformation of cocaine. In: Mule SJ, editor. Cocaine: chemical, biological, clinical, social, and treatment aspects. Cleveland: CRC Press; 1976. pp. 73–90. [Google Scholar]
- Misra AL, Nayak PK, Bloch R, Mule SJ. Estimation and disposition of [3H]benzoylecgonine and pharmacological activity of some cocaine metabolites. J Pharm Pharmacol. 1975;27:784– 6. doi: 10.1111/j.2042-7158.1975.tb09404.x. [DOI] [PubMed] [Google Scholar]
- Morrell JI, Mattson BJ, Wansaw MP, Smith KS, Seip KM. Medial Preoptic Area (MPOA) is a neural hot spot involved in the motivation to perform maternal behavior (abstract) Soc Neurosci. 2005 [Google Scholar]
- Nayak PK, Misra AL, Mule SJ. Physiological disposition and biotransformation of (3H) cocaine in acutely and chronically treated rats. J Pharmacol Exp Ther. 1976;196:556– 69. [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511– 3. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Post RM, Rose H. Increasing effects of repetitive cocaine administration in the rat. Nature. 1976;260:731– 2. doi: 10.1038/260731a0. [DOI] [PubMed] [Google Scholar]
- Pradhan S, Roy SN, Pradhan SN. Correlation of behavioral and neuro-chemical effects of acute administration of cocaine in rats. Life Sci. 1978;22:1737– 43. doi: 10.1016/0024-3205(78)90626-4. [DOI] [PubMed] [Google Scholar]
- Quinones-Jenab V, Ho A, Schlussman SD, Franck J, Kreek MJ. Estrous cycle differences in cocaine-induced stereotypic and locomotor behaviors in Fischer rats. Behav Brain Res. 1999;101:15– 20. doi: 10.1016/s0166-4328(98)00073-4. [DOI] [PubMed] [Google Scholar]
- Reiss J, Wansaw MP, Morrell JI. Soc Neurosci. 2004. Explorations of the reinforcing properties of cocaine throughout the postpartum period of the rat (abstract) [776.9] [Google Scholar]
- Roberts DC, Bennett SA, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology (Berl) 1989;98:408–11. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Robinson SE, Enters EK, Jackson GF, Chinchilli VM, Maher JR, McDowell KP, et al. Maternal and fetal brain and plasma levels of cocaine and benzoylecgonine after acute or chronic maternal intravenous administration of cocaine. J Pharmacol Exp Ther. 1994;271:1234– 9. [PubMed] [Google Scholar]
- Schuelke GS, Konkol RJ, Terry LC, Madden JA. Effect of cocaine metabolites on behavior: possible neuroendocrine mechanisms. Brain Res Bull. 1996;39:43 –8. doi: 10.1016/0361-9230(95)02040-3. [DOI] [PubMed] [Google Scholar]
- Sell SL, Scalzitti JM, Thomas ML, Cunningham KA. Influence of ovarian hormones and estrous cycle on the behavioral response to cocaine in female rats. J Pharmacol Exp Ther. 2000;293:879– 86. [PubMed] [Google Scholar]
- Shah NS, May DA, Yates JD. Disposition of levo-[3H]cocaine in pregnant and nonpregnant mice. Toxicol Appl Pharmacol. 1980;53:279– 84. doi: 10.1016/0041-008x(80)90427-5. [DOI] [PubMed] [Google Scholar]
- Shuster L. Pharmacokinetics, metabolism, and disposition of cocaine. In: Lakoski JM, Galloway MP, White FJ, editors. Cocaine: pharmacology, physiology, and clinical strategies. Boca Raton: CRC Press; 1992. pp. 1–14. [Google Scholar]
- Smith MS, Neill JD. Inhibition of gonadotropin secretion during lactation in the rat: relative contribution of suckling and ovarian steroids. Biol Reprod. 1977;17:255– 61. doi: 10.1095/biolreprod17.2.255. [DOI] [PubMed] [Google Scholar]
- Spear LP, Frambes NA, Kirstein CL. Fetal and maternal brain and plasma levels of cocaine and benzoylecgonine following chronic subcutaneous administration of cocaine during gestation in rats. Psychopharmacology (Berl) 1989;97:427–31. doi: 10.1007/BF00439542. [DOI] [PubMed] [Google Scholar]
- Taya K, Greenwald GS. Peripheral blood and ovarian levels of sex steroids in the lactating rat. Endocrinol Jpn. 1982;29:453– 9. doi: 10.1507/endocrj1954.29.453. [DOI] [PubMed] [Google Scholar]
- van Haaren F, Meyer ME. Sex differences in locomotor activity after acute and chronic cocaine administration. Pharmacol Biochem Behav. 1991;39:923–7. doi: 10.1016/0091-3057(91)90054-6. [DOI] [PubMed] [Google Scholar]
- van Haaren F, Garcea M, Anderson KG, Tebbett IR. Cocaine and benzoylecgonine in serum microsamples of intact and gonadectomized male and female Wistar rats. Pharmacol Biochem Behav. 1997;58:421–4. doi: 10.1016/s0091-3057(97)00294-3. [DOI] [PubMed] [Google Scholar]
- Vernotica EM, Morrell JI. Plasma cocaine levels and locomotor activity after systemic injection in virgin and in lactating maternal female rats. Physiol Behav. 1998;64:399–407. doi: 10.1016/s0031-9384(98)00092-4. [DOI] [PubMed] [Google Scholar]
- Vernotica EM, Lisciotto CA, Rosenblatt JS, Morrell JI. Cocaine transiently impairs maternal behavior in the rat. Behav Neurosci. 1996a;110:315–23. doi: 10.1037//0735-7044.110.2.315. [DOI] [PubMed] [Google Scholar]
- Vernotica EM, Rosenblatt JS, Morrell JI. Acute cocaine disrupts all components of established maternal behavior in the rat. Soc Neurosci Abstr. 1996b;22:1884. [Google Scholar]
- Vernotica EM, Rosenblatt JS, Morrell JI. Microinfusion of cocaine into the medial preoptic area or nucleus accumbens transiently impairs maternal behavior in the rat. Behav Neurosci. 1999;113:377– 90. doi: 10.1037//0735-7044.113.2.377. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Reed TM, Acuff-Smith KD, Schilling MA, Cappon GD, Fisher JE, et al. Long-term learning deficits and changes in unlearned behaviors following in utero exposure to multiple daily doses of cocaine during different exposure periods and maternal plasma cocaine concentrations. Neurotoxicol Teratol. 1995;17:253– 64. doi: 10.1016/0892-0362(94)00061-h. [DOI] [PubMed] [Google Scholar]
- Wansaw MP, Williams SE, Rosenblatt JS, Morrell JI. Cocaine- and pup-induced conditioned place preference in the lactating, maternal rat (abstract) Soc Neurosci. 2002 [185.15]. [Google Scholar]
- Wansaw MP, Reiss J, Morrell JI. Dynamic changes in motivation to seek either natural or pharmacological rewards occur as the postpartum period progresses in the rat (abstract) NIDA mini-convention. 2003a;62 [Google Scholar]
- Wansaw MP, Reiss J, Morrell JI. Varying the time dams are deprived of pups has minimal effects on preferences for pup-associated cues in the early, and substantial effects in the late postpartum period (abstract) Soc Neurosci. 2003b [937.12]. [Google Scholar]
- Wansaw MP, Reiss J, Morrell JI. Sex differences in the reinforcing properties of cocaine as revealed by conditioned place preference (abstract) Soc Neurosci. 2004 [776.8] [Google Scholar]
- Wiggins RC, Rolsten C, Ruiz B, Davis CM. Pharmacokinetics of cocaine: basic studies of route, dosage, pregnancy and lactation. Neurotoxicology. 1989;10:367–81. [PubMed] [Google Scholar]
- Wilsoncroft WE. Babies by bar-press: maternal behavior in the rat. Behav Res Meth Instrum. 1969;1:229– 30. [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–92. [PubMed] [Google Scholar]
- Yeh SY, Haertzen CA. Cocaine-induced locomotor activity in rats. Pharmacol Biochem Behav. 1991;39:723– 7. doi: 10.1016/0091-3057(91)90154-t. [DOI] [PubMed] [Google Scholar]
- Zimmerberg B, Gray MS. The effects of cocaine on maternal behaviors in the rat. Physiol Behav. 1992;52:379– 84. doi: 10.1016/0031-9384(92)90287-c. [DOI] [PubMed] [Google Scholar]


