Abstract
Cytokine-induced sickness behavior was recognized within a few years of the cloning and expression of interferon-α, IL-1 and IL-2, which occurred around the time that the first issue of Brain, Behavior, and Immunity was published in 1987. Phase I clinical trials established that injection of recombinant cytokines into cancer patients led to a variety of psychological disturbances. It was subsequently shown that physiological concentrations of proinflammatory cytokines that occur after infection act in the brain to induce common symptoms of sickness, such as loss of appetite, sleepiness, withdrawal from normal social activities, fever, aching joints and fatigue. This syndrome was defined as sickness behavior and is now recognized to be part of a motivational system that reorganizes the organism's priorities to facilitate recovery from the infection. Cytokines convey to the brain that an infection has occurred in the periphery, and this action of cytokines can occur via the traditional endocrine route via the blood or by direct neural transmission via the afferent vagus nerve. The finding that sickness behavior occurs in all mammals and birds indicates that communication between the immune system and brain has been evolutionarily conserved and forms an important physiological adaptive response that favors survival of the organism during infections. The fact that cytokines act in the brain to induce physiological adaptations that promote survival has led to the hypothesis that inappropriate, prolonged activation of the innate immune system may be involved in a number of pathological disturbances in the brain, ranging from Alzheimers' disease to stroke. Conversely, the newly-defined role of cytokines in a wide variety of systemic co-morbid conditions, ranging from chronic heart failure to obesity, may begin to explain changes in the mental state of these subjects. Indeed, the newest findings of cytokine actions in the brain offer some of the first clues about the pathophysiology of certain mental health disorders, including depression. The time is ripe to begin to move these fundamental discoveries in mice to man, and some of the pharmacological tools are already available to antagonize the detrimental actions of cytokines.
Introduction
The first paper on sickness behavior was published in Brain, Behavior, and Immunity (BBI) by Aubert et al. (Aubert et al., 1995) in 1995. Since then, 39 papers on sickness behavior have been published in the journal, which represent 20 percent of the 192 papers published on cytokine-induced sickness behavior that are listed in PubMed. Despite some risk of overlap, it would be fair to add to these statistics the 14 papers on cytokines and depression that were published in BBI in 2002. Although the familiar phrase that "statistics lie and statistician's use statistics" is apropos in the sense that statistics can be used to describe nearly anything one wants them to tell, it is clear from this cursory quantitative review of the literature that BBI has been instrumental in promoting the concept of cytokine-induced sickness behavior. This conclusion becomes even more obvious when these figures are compared to the 52 papers on cytokines and the brain that were published by BBI and contrast with the 15,382 papers listed by PubMed in this field (as of July 27,2006). BBI has not been the home journal of those scientists who study the expression and actions of cytokines in the brain. Its niche is clearly at the interface between immunity and behavior in physiological and pathological conditions. The objective of this article is not to add another paper on cytokine-induced sickness behavior to the list of those already published by BBI but to show why it was and remains logical for BBI to play an instrumental role in the publication of the results on cytokine-induced sickness behavior.
Before 1987: The History of Sickness Behavior
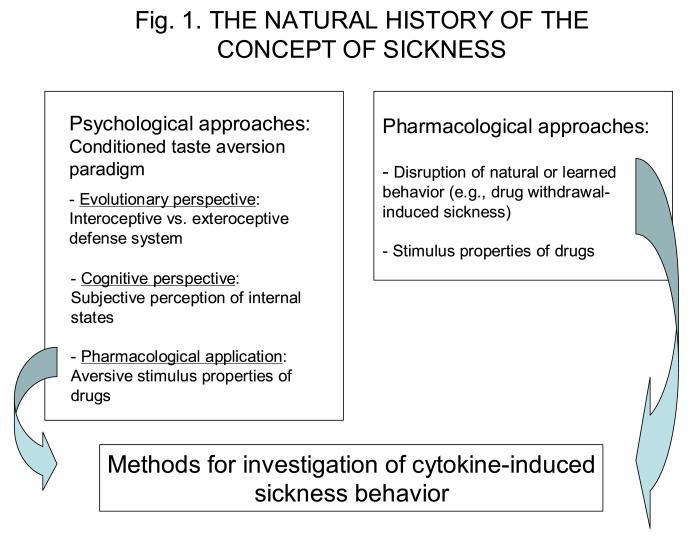
The study of sickness has a rich history in psychology and behavioral pharmacology (Fig. 1). In psychology, research on the concept of sickness started in the mid fifties with Garcia's work on bait shyness (Garcia et al., 1955). Garcia explained the fact that rodents cannot be poisoned very easily by their propensity to develop learned aversions to the new taste of any compound that induces gastro-intestinal malaise. At the time this research was carried out, the laws of learning were dominated by knowledge gained from the study of classical conditioning and operant conditioning. It was believed that learning could only take place if a very short interval, on the order of a few seconds, separated the conditioned stimulus (the taste) from the unconditioned stimulus (the malaise experienced in response to poisonous food). In operant conditioning, the operant response could be learned only if the reward immediately followed the operant response or if secondary reinforcers were present to maintain the conditioned response. Because conditioned taste aversions could still be learned despite the long delay - several hours - between the taste and illness, and illness could only be conditioned to interoceptive stimuli (e.g., a taste or a smell), but not to exteroceptive stimuli (e.g., a tone or a light), Garcia proposed that the laws of classical conditioning applied only to the exteroceptive defense system that developed to protect organisms from predators. In contrast, the laws of conditioned taste aversion apply to the interoceptive defense system that developed to protect organisms from ingested poisons (Garcia et al., 1974). According to this evolutionary perspective, the ability to form learned taste aversions is an important advantage for an eclectic gastronomic animal since it helps the animal to distinguish those foods that are noxious from those that are safe and possibly health promoting. Of course, this strategy is functional only if the eclectic gastronomic animal displays neophobia and ingests only very small amounts of a new food (i.e., a nibbler). This aspect of sickness was somewhat neglected in Garcia's theoretical elaboration. Since psychology often develops in waves of controversy, it is not surprising that this view was used to challenge the concept proposed by Garcia that food aversions are learned. It was argued by others that the so called conditioned taste aversions could just represent a summation of neophobic responses, the novelty of the sensory experience associated with the poisonous food and the new experience of gastro-intestinal malaise (Mitchell et al., 1975).
Figure 1.
Psychological and pharmacological roots of the concept of sickness behavior. In behavioral sciences, the ability of animals to relate sickness and malaise to the nature of the nutrients they ingest was studied within the context of the conditioned taste aversion paradigm. This ability was further elaborated in terms of advantages during evolution and cognitive abilities. Some pharmacologists used the conditioned taste aversion paradigm for studying the aversive stimulus properties of drugs. However, this type of approach was miniscule when compared to the huge scientific and industrial investment in the systematic study of the stimulating properties of psychoactive drugs based on operant conditioning technology. The sickness-inducing properties of certain treatments were mainly quantified indirectly, via the disruption of natural or learned behavior. These two different lines of research, especially the last one, gave rise to the methodology currently used for studying cytokine-induced sickness behavior.
Formation of a conditioned taste aversion implies that the subject is able to monitor its internal state and to associate with it not only an affective (bad versus good) but also a quantitative value (a little versus a lot). The malaise induced by lithium chloride is not necessarily the same as the malaise induced by the muscarinic cholinergic receptor agonist pilocarpine. In terms of cognition, this means that animals are able to discriminate internal states with a degree of precision that is much more advanced than just the difference between illness and no illness. For instance, rats can discriminate the illness-inducing effects of vasopressin from those of apomorphine but not from those of angiotensin II despite the fact these two peptides are hypertensive (Bluthe et al., 1985). In terms of subjective experience, these data indicate that the interoceptive cues which are responsible for the conditioned taste aversion induced by vasopressin and angiotensin II are related to the hypertensive action of these peptides. In terms of neural mechanisms, the possibility of discriminating between different sources of illness implies that different aversive internal states have a different neurobiological basis. As a typical example, we demonstrated early on that lesion of the area postrema abolishes conditioned taste aversions induced by lithium chloride in rats but not those caused by amphetamine (Ritter et al., 1980).
This brief summary of the history of conditioned taste aversion is important because it helps us to understand why scientists confronted with the question of the sickness-inducing properties of a given treatment were prone to use the methodology developed by bait shyness experiments. After having observed that lithium chloride induces conditioned taste aversion and decreases aggressive behavior, we asked whether the anti-aggressive effect of this compound was due to its sickness-inducing properties. For this purpose, we lesioned the area postrema and found that it not only blocked the aversive properties of lithium but also its anti-aggressive actions (McGlone et al., 1980). In the same manner, we used conditioned taste aversion methodology to investigate the aversive stimulus properties of vasopressin since many of the memory-like effects of this peptide had been proposed to be due to its peripheral visceral arousing effects (Dantzer et al., 1988).
Behavioral pharmacologists went along two alternative strategies to assess the aversive properties of drugs. The less sophisticated approach was based on the quantitative measurement of disruption in well-established behavioral responses. For instance, Gellert and Sparber trained rats to press a lever for a food reward according to a fixed-ratio 20 schedule of reinforcement (20 consecutive lever presses were needed to obtain a single pellet of food). Disruption of this operant behavior was used as a quantitative measure of withdrawal sickness induced by naloxone in morphine-dependent rats (Gellert and Sparber, 1977). More sophisticated approaches have been developed to study the stimulus properties of drugs. As a typical example, the stimulus properties of benzodiazepine anxiolytics can be measured by training rats to press one of two levers in a Skinner box in order to obtain a food reward when treated with the drug under investigation and the other lever when treated with saline or another drug with different stimulus properties (Dantzer and Perio, 1982). All of these reports confirm that sickness has a long and rich history in the psychological and pharmacological sciences.
From 1987 to 1996: The Merging of Sickness Behavior With Immunology
The way the concept of sickness behavior merged with immunology is summarized in Fig. 2. Prior to 1987, the biological activities of “endogenous pyrogen,” “leukocyte endogenous mediator,” and “lymphocyte activating factor” were suspected to be derived from a single protein. That protein was eventually purified, renamed as IL-1, and was shown to be active in the brain, as determined by induction of both fever in rabbits and EEG signals associated with slow-wave sleep in rats. However, the very first unequivocal evidence showing that recombinant interleukin-1, which was not cloned until 1984 by Dinarello's group (Auron et al., 1984), was active in the brain was not published until 1986 (Besedovsky et al., 1986). In this paper, Hugo Besedovsky and colleagues showed that administration of both purified and recombinant IL-1 to mice activated the hypothalamic-pituitary-adrenal (HPA) axis, as evidenced by an increase in plasma ACTH and corticosterone in IL-1-treated mice. As good immunophysiologists, Besedovsky et al. interpreted their results mechanistically in terms of a direct effect of IL-1 on the HPA axis, a hypothesis they later confirmed by showing that IL-1 activates corticotropin-releasing factor containing neurons in the paraventricular nucleus of the hypothalamus (Berkenbosch et al., 1987). However, for any psychobiologist knowing about the aversive properties of drugs, there was an obvious alternative interpretation. IL-1 could have simply made the animals feel sick and the HPA response could have just been the indirect result of this new experience of sickness. To test this possibility, Tazi et al. used the conditioned taste aversion paradigm to show that rats injected with IL-1, at the doses necessary to activate the HPA axis, formed an aversion to the taste of a saccharin solution (Tazi et al., 1988). The sickness inducing effects of IL-1 were further confirmed by the demonstration of the disruption of an operant fixed-ratio response for food and a spontaneous social exploration response to the introduction of a juvenile into the home cage of the test animal (Crestani et al., 1991; Kent et al., 1992a,b).
Figure 2.
Evolution of the concept of sickness behavior. This figure illustrates the different steps in formulation of the concept of cytokine-induced sickness behavior. It demonstrates that the concept was grounded initially in two different lines of research that focused on the possible aversive stimulus properties of immune activation on one hand and the metabolic constraints of the fever response on the other hand. Ultimately, the concept of cytokine-induced sickness behavior found its own momentum by borrowing from motivational theory. Whether this concept is sufficiently solid to encompass all aspects of the organism's response to stimulation of the innate immune system or must be included in another more basic motivational system, such as the pain defense system, is still disputed.
In 1989, we speculated that the sickness-inducing properties of cytokines were not an artifact but reflected the genuine action of these proteins in the brain (Dantzer and Kelley, 1989). This was done within the context of a review paper in which we tried to make biological sense of the vast amount of data that were already available at that time concerning the effects of stress on immunity. The concept developed in this review paper was that the immune system could not work independently of the brain to regulate the host response to pathogens and that this required communication systems not only from the brain to the immune system but also from the immune system to the brain. In this sense, brain-immune system communication was really just a new example of another systemic physiological regulatory system that is critical for maintaining survival.
This idea that communication systems exist between the immune and central nervous system and that this communication is biologically important was controversial. That was because, at that time, the main function for the immune system was considered to be only the production of cells and proteins that protect the body against pathogenic challenges. Immunologists could culture plasma cells in vitro, and these cells would produce protective antibodies. Cultured macrophages could phagocytize microbes and kill them. Indeed, the immune system was simply considered to be an autonomous system that functioned independently of other physiological systems (Kelley, 1985). Although Ed Blalock and colleagues were publishing at that time a substantial amount of data showing that there were real links between the brain and immune system (e.g., Blalock, 1984), the possibility that the immune system was regulated by hormones or neurotransmitters was not taken seriously by the scientific community. We went on in our review article to describe cytokines as newly-defined communication molecules that were capable of informing the brain about the occurrence of an infection and triggering the sickness behavior response. We directly compared the central nervous system effects of cytokines to non-specific symptoms of sickness, concluding that “...products from immune cells also mediate non-specific behavioral responses to infection, such as malaise, fatigue, sleepiness, anorexia, apathy and irritability.” Using the scant literature that was available on the psychobiological properties of recombinant cytokines injected into humans, mostly because of their promise for the treatment of various forms of cancers, we cited data from phase I clinical trials showing that injections of cytokines caused neurotoxic side effects leading to malaise, fatigue, weakness and lethargy. Opponents argued that these symptoms occurred simply because large, pharmacological amounts of cytokines were injected into these subjects. The possibility was not considered that either local or systemic proinflammatory cytokines that are induced at physiological levels during an infection or acute and chronic inflammatory conditions serve as true communication molecules between the immune system and brain to maintain homeostasis. This idea was considered to be a heretical concept. However, this premise is consistent with the original and current view of immunologists today that the innate immune system is non-specific. For example, macrophages and neutrophils phagocytize any microbe that is coated with specific antibody or specific components of complement. Similarly, clinical symptoms of sickness are non-specific in the sense that they are induced by a wide variety of clinical conditions.
Cytokine-induced sickness behavior was formulated independently in a similar way by Hart (Hart, 1988). His starting point was the febrile response rather than the behavioral change itself. He saw sickness behavior as an adaptive response to reduce energy consumption at a time of high energy demand that is necessary to maintain a fever and to fight infection. As pointed out by Kluger (1991), there is considerable evidence that fever is adaptive because it decreases both mortality and morbidity. But, we showed early on that IL-1-induced sickness behavior is independent of the febrile response since behavioral responses of mice with fever were normal (Kent et al, 1992b). Similarly, although endogenous pyrogen induces fever and slow-wave sleep, these two physiological responses are independent of one another, which established that slow-wave sleep is not secondary to fever (Krueger et al, 1984). Hart went on to point out that other behaviors, such as reduced grooming (which significantly reduces water loss in rodents), sleepiness and changes in physical appearance, certainly contribute to the ability of the body to rid itself of the infecting pathogen. Watkins and Maier entered the field later with still a different perspective, arguing that cytokines are responsible for the hyperalgesia that occurs during inflammation (Maier et al., 1993) and that the sickness response to cytokines is just an avatar of this necessity to deal with pain (Watkins and Maier, 2000). It could also be argued that sickness behavior evolved to reduce the probability of coming in contact with a predator, to minimize infection with conspecifics or to reduce reproductive behavior at a time when the body's immune defenses are being actively mobilized. But, it is too simplistic to view fever, hyperalgesia, aversion to noxious stimuli or sleepiness as THE single specific physiological response that is solely responsible for imparting the survival-promoting property of sickness behavior. Instead, the mind and body is an integrated unit that works together to promote survival and recovery during infectious episodes. An understanding of any of the physiological components of sickness behavior will promote a greater understanding of sickness behavior biology at all levels of organization.
What was determinant for the psychological aspect of cytokine-induced sickness behavior was the fortuitous encounter in 1990 of Robert Dantzer with Neal Miller at a conference organized on neuroimmunomodulation in Florence, Italy. Dantzer knew Miller on the basis of Miller's very elegant work on motivational competition (Bower and Miller, 1960; Miller, 1957). He was therefore very excited to explain to him the results of cytokines in sickness behavior and his speculation on the motivational properties of sickness. Dantzer's proposal on sickness as a motivational state was built on Bolles' views of motivations as central states that organize both perception and action (Bolles and Fanselow, 1980). The idea was that sickness takes precedence over other behavioral activities because the infected organism is at a life and death juncture that requires it to deal with infection in the most efficient possible way. Miller blatantly told Dantzer that there was nothing new about his ideas. Indeed, based on only empirical results, Miller had already proposed the same theory several years earlier. A few weeks later, when he returned to New York, Miller sent to Dantzer the paper he referred to during their conservation (Miller, 1964). Although this paper was published as a review paper in an obscure journal, its content was stunning since it contained all the ingredients of the modern history of cytokine-induced sickness behavior. In response to endotoxin, Miller speculated that rats produced an endogenous compound that acted on their brains to make them feel sick. This sickness always presented in the form of a change in operant responding independently of the nature of the reward. This change in operant responding was the result of motivational competition between sickness and the motivational state underlying the operant response since rats could either decrease or increase their operant response rate depending on the consequences of this response. More specifically, when rats submitted to forced wheel running were trained to obtain an episode of rest by pressing a lever, rats injected with endotoxin increased, rather than decreased, their lever pressing in order to obtain more rest periods.
The encounter with Miller was guided for the hypothesis that was tested by Aubert et al. on the motivational properties of sickness behavior. Ingenious experimental designs allowed Aubert to vary the strength of motivational competition, much as Neal Miller would have done, and to show that the actual form of sickness behavior depended on the respective level of each set of motivational factors (Aubert et al., 1997). These results have already been presented and summarized in various publications (Dantzer, 2001; Konsman et al., 2002) and it is not necessary to do this once more. However, it is worthwhile to emphasize that this view of sickness behavior as the expression of a motivational state has important implications in terms of homeostasis. Hans Selye was completely correct when he described infectious agents as stressors that trigger a counter regulatory response aiming at re-establishing homeostasis. However, as pointed out by Henri Laborit (Laborit, 1991; 1991), Selye's mistake was due to his belief in absolute homeostasis. In doing so, Selye remained in the same league as Claude Bernard and Walter Cannon. However, it is now apparent that homeostasis is not a single state. There are various systems and levels of homeostasis, each adapted to specific physiological conditions. Being infected requires a different set of responses than being pathogen free. The same is true for an organism confronted with a predator. Fear does not have the same physiological requirements as hunger or thirst. These physiological states do not depart from some hypothetical absolute level of homeostasis. On the contrary, each of these states is well-organized at the subjective, behavioral and physiological levels and has its own homeostatic regulatory systems. This is what relative homeostasis is about.
From 1997 to 2006: The Move From Sickness to Depression
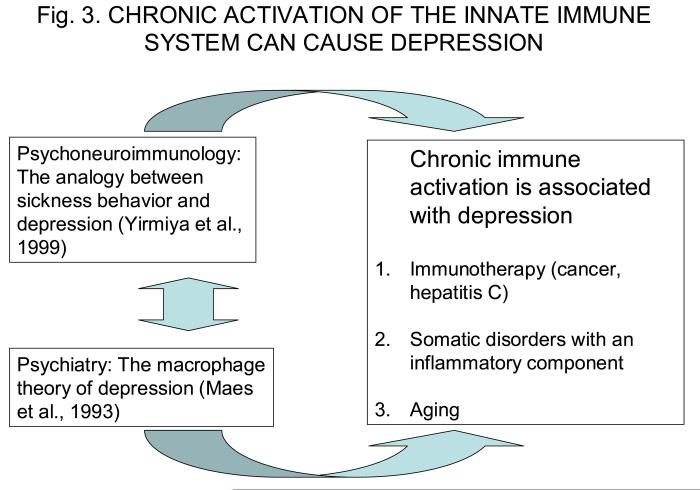
The way the concept of cytokine-induced sickness behavior merged with the field of mental health is illustrated in Fig. 3. Defining cytokine-induced sickness behavior as a motivational state implies that it belongs in the realm of physiology, just as fear, hunger, thirst and other motivational states. To be able to withdraw from the environment, seek rest and care for the body is as normal in response to infectious agents as being able to shift to a state of increased arousal and readiness for action when confronted with a potential exteroceptive threat, say in the form of a predator. This view on the evolutionary advantage of the behavioral effects of cytokines is not entirely new since a similar perspective had been elaborated earlier and put to test using febrile responses to infection (Kluger, 1991). Viewing sickness behavior as an adaptive response of the host to infectious microorganisms creates a new and important question: What happens when the acute sickness response is no longer adaptive either because it is out of proportion with the set of causal factors that were the trigger for or because the sickness response is prolonged and taxes the organism's resources? This condition actually occurs during a variety of chronic inflammatory diseases.
Figure 3.
From cytokine-induced sickness behavior to cytokine-induced depression. Similarities between some of the clinical signs of sickness behavior and some symptoms of depression prompted a series of mechanistic investigations at the experimental level. This took place at a time at which some investigators had already established a data base on immunity and mental health in depressed patients, and they had proposed that some forms of clinical depression could be just another manifestation of an acute phase response. Convergence between these two lines of research provided scientific justification for systematic studies on the effects of exogenously administered cytokines on mood of patients with cancer or subjects with chronic viral infections. This type of approach that investigates relationships between activation of the innate immune system and affective disorders is now being gradually extended to subjects whose innate immune system is chronically activated in pathological (e.g., obesity, coronary heart disease) and physiological (e.g., aging, persons with disabilities) conditions.
Raz Yirmiya was the first psychobiologist to draw the analogy between sickness behavior and depression. He showed that rats treated with cytokines are less sensitive to the rewarding properties of a saccharin solution or to the presentation of a sexually-active partner (Yirmiya et al., 1999). Some of these deficits can be prevented by chronic but not acute administration of antidepressant drugs that have little or no beneficial effects on sickness behavior. Although indirect, this type of pharmacological evidence is important since behavioral deficits observed in animals of which the innate immune system is activated could be simply due to sickness. Indeed, activation of the innate immune system has now been shown to induce depressive-like behavior in mice independently of sickness (Frenois et al., 2006).
These data are highly relevant to the clinical condition of depression. Michael Maes was the first psychiatrist to point to the analogies between the immune profile of depressed patients and the acute phase response that is characteristic of an activated innate immune system (Maes, 1993). Although Maes' results still remain highly controversial since they have not always been replicated by other investigators, Maes was very active in promoting the macrophage theory of depression (Maes et al., 1995). According to this theory, the proinflammatory cytokines responsible for the acute phase response act on the brain to induce depression. One key player in this action of cytokines in the brain is an enzyme known as indoleamine 2,3 dioxygenase (IDO) (Capuron et al., 2001). This enzyme degrades tryptophan, an essential amino acid that is the limiting factor for the synthesis of serotonin. Degradation of tryptophan generates neurotoxic metabolites such as quinolinic acid and 3-hydroxy-kynurenine that easily cross the blood-brain barrier and act as an agonist to the glutamatergic receptor. IDO is activated in cytokine-induced depressed patients, as shown by decreased plasma levels of tryptophan and the increased plasma levels of kynurenine. This increase in IDO activity has been confirmed in mice in which the immune system is activated (Moreau et al., 2005). An elevation in IDO activity is associated with alterations in brain serotonin neurotransmission and with development of depressive-like behavior.
The association between decreased serotonin levels and excessive glutamatergic activity forms the first biochemical basis for cytokine-induced depression. Most of the support for this theory comes from two different sources, clinical and experimental. Lucile Capuron was the first scientist to systemically investigate at the psychological level the symptoms of patients whose immune system is activated because of the chronic administration of cytokines for treatment of forms of cancer resistant to radiotherapy and chemotherapy (i.e., kidney cancer and metastatic melanoma) or for treatment of chronic viral infections (i.e., hepatitis C). She started her research as a graduate student in Bordeaux and continued as a post-doctoral associate and professor at Emory University. She was able to deconstruct the symptoms of depression experienced by patients treated by cytokines into two main dimensions (Capuron et al., 2002; Capuron et al., 2004). The neurovegetative dimension includes fatigue, loss of appetite and sleep disorders. These symptoms develop in all patients and are resistant to current, widely-used anti-depressants. The psychological dimension includes depressed mood, anxiety and cognitive dysfunction. These symptoms occur in 30 to 50 percent of the patients and are alleviated by anti-depressants. Vulnerability to development of depression can be identified at both the psychological (patients who are at risk have higher scores of depressed mood) and at the neuroendocrine levels (patients who are at risk of developing depression have a higher pituitary-adrenal response to cytokine treatment).
2007 and Beyond: The Move to Translational Research
Cytokine-induced sickness behavior is no longer a curiosity. In animal research, its measurement has become a standard procedure for assessing the mode of functioning of communication pathways from the immune system to the brain as well as the sensitivity of the brain to direct immune stimulation. At the clinical level, many investigators are now actively engaged in collecting the type of evidence that is necessary to test the possibility that many of the subjective complaints of patients afflicted with a variety of infectious, autoimmune and neoplastic diseases, as well as those with an inflammatory component that is intrinsic to the disease (e.g., atherosclerosis, obesity, inflammatory bowel disease) or iatrogenic (e.g., inflammation caused by cancer adjuvant therapy) are actually caused by cytokines acting in the brain (Kelley et al, 2003; Dantzer, 2004). This type of research is conducted by research groups with various levels of expertise in interdisciplinary approaches. This entire field would benefit greatly from standardization of appropriate markers of inflammation and a systematic approach for investigation of the risk factors. Furthermore, it is now possible to develop clinical trials that are aimed at blocking cytokine production or action, attenuating the production of second messengers or deactivating glial cells that produce excessive quantities of proinflammatory cytokines. Although this type of research can be initiated with compounds that are currently available on the market, there are a number of other possible targets such as those that include components of the signaling pathways that underlie the production and/or action of cytokines as well as effector molecules that mediate the effects of cytokines on their ultimate cell targets. Coordination of this type of research would greatly enhance its innovative potential and avoid the duplication of efforts that is likely to occur because of the diversity of pathological conditions that lead to non-specific clinical signs of sickness behavior.
Acknowledgements
Supported by the NIH to KWK (MH51569 and AI50442) and RD (MH71349).
Footnotes
Correspondence: Robert Dantzer, University of Illinois, 227 Edward R. Madigan Laboratory, 1201 West Gregory Drive, Urbana, IL 61801, dantzer@uiuc.edu
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aubert A, Vega C, Dantzer R, Goodall G. Pyrogens specifically disrupt the acquisition of a task involving cognitive processing in the rat. Brain Behav Immun. 1995;9:129–148. doi: 10.1006/brbi.1995.1013. [DOI] [PubMed] [Google Scholar]
- Aubert A, Goodall G, Dantzer R, Gheusi G. Differential effects of lipopolysaccharide on pup retrieving and nest building in lactating mice. Brain Behav Immun. 1997;11:107–118. doi: 10.1006/brbi.1997.0485. [DOI] [PubMed] [Google Scholar]
- Auron PE, Webb AC, Rosenwasser LJ, Mucci SF, Rich A, Wolff SM, Dinarello CA. Nucleotide sequence of human monocyte interleukin 1 precursor cDNA. Proc Natl Acad Sci U S A. 1984;81:7907–7911. doi: 10.1073/pnas.81.24.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkenbosch F, van Oers J, del Rey A, Tilders F, Besedovsky H. Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science. 1987;238:524–526. doi: 10.1126/science.2443979. [DOI] [PubMed] [Google Scholar]
- Besedovsky H, del Rey A, Sorkin E, Dinarello CA. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science. 1986;233:652–654. doi: 10.1126/science.3014662. [DOI] [PubMed] [Google Scholar]
- Blalock JE. The immune system as a sensory organ. J. Immunology. 1984;132:1067–1070. [PubMed] [Google Scholar]
- Bluthe RM, Dantzer R, Le Moal M. Peripheral injections of vasopressin control behavior by way of interoceptive signals for hypertension. Behav Brain Res. 1985;18:31–39. doi: 10.1016/0166-4328(85)90166-4. [DOI] [PubMed] [Google Scholar]
- Bolles RC, Fanselow MS. A perceptual-defensive-recuperative model of fear and pain. Behav Brain Sci. 1980;3:291–323. [Google Scholar]
- Bower GH, Miller NE. Effects of amount of reward on strength of approach in an approach-avoidance conflict. J Comp Physiol Psychol. 1960;53:59–62. doi: 10.1037/h0038938. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Miller AH, Dantzer R. Baseline mood and psychosocial characteristics of patients developing depressive symptoms during interleukin-2 and/or interferon-alpha cancer therapy. Brain Behav Immun. 2004;18:205–213. doi: 10.1016/j.bbi.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Gualde N, Bosmans E, Dantzer R, Maes M, Neveu PJ. Association between immune activation and early depressive symptoms in cancer patients treated with interleukin-2-based therapy. Psychoneuroendocrinology. 2001;26:797–808. doi: 10.1016/s0306-4530(01)00030-0. [DOI] [PubMed] [Google Scholar]
- Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- Crestani F, Seguy F, Dantzer R. Behavioural effects of peripherally injected interleukin-1: role of prostaglandins. Brain Res. 1991;542:330–335. doi: 10.1016/0006-8993(91)91587-q. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: where do we stand? Brain Behav Immun. 2001;15:7–24. doi: 10.1006/brbi.2000.0613. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Innate immunity at the forefront of psychoneuroimmunology. Brain Behav Immun. 2004;18:1–6. doi: 10.1016/j.bbi.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Perio A. Behavioural evidence for partial agonist properties of RO 15-1788, a benzodiazepine receptor antagonist. Eur J Pharmacol. 1982;81:655–658. doi: 10.1016/0014-2999(82)90355-7. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Stress and immunity: an integrated view of relationships between the brain and the immune system. Life Sci. 1989;44:1995–2008. doi: 10.1016/0024-3205(89)90345-7. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Bluthe RM, LeMoal M. Experimental assessment of drug-induced changes in cognitive function: vasopressin as a case study. Neurotoxicology. 1988;9:471–477. [PubMed] [Google Scholar]
- Frenois F, Moreau M, Micon C, Lestage J, Kelley KW, Dantzer R, Castanon N. Lipopolysaccharide induces delayed cellular activities within the mouse extended amygdala, hippocampus and hypothalamus that parallel the expression of depressive-like behavior in mice. 2006 doi: 10.1016/j.psyneuen.2007.03.005. In preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J, Kimeldorf DJ, Koelling RA. Conditioned aversion to saccharin resulting from exposure to gamma radiation. Science. 1955;122:157–158. [PubMed] [Google Scholar]
- Garcia J, Hankins WG, Rusiniak KW. Behavioral regulation of the milieu interne in man and rat. Science. 1974;185:824–831. doi: 10.1126/science.185.4154.824. [DOI] [PubMed] [Google Scholar]
- Gellert VF, Sparber SB. A comparison of the effects of naloxone upon body weight loss and suppression of fixed-ratio operant behavior in morphine-dependent rats. J Pharmacol Exp Ther. 1977;201:44–54. [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Kelley KW. Immunological consequences of changing environmental stimuli. In: Moberg GP, editor. Animal Stress. American Physiological Society; Bethesda, MD: 1985. pp. 193–233. [Google Scholar]
- Kelley KW, Bluthé RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, Broussard SR. Cytokine-induced sickness behavior. Brain, Behavior, and Immunity. 2003;17:S112–S118. doi: 10.1016/s0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- Kent S, Bluthé RM, Dantzer R, Hardwick AJ, Kelley KW, Rothwell NJ, Vannice JL. Different receptor mechanisms mediate the pyrogenic and behavioral effects of interleukin-1. Proc. Natl. Acad. Sci. USA. 1992b;89:9117–9120. doi: 10.1073/pnas.89.19.9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent S, Kelley KW, Dantzer R. Effects of lipopolysaccharide on food-motivated behavior in the rat are not blocked by an interleukin-1 receptor antagonist. Neurosci Lett. 1992a;145:83–86. doi: 10.1016/0304-3940(92)90209-p. [DOI] [PubMed] [Google Scholar]
- Kluger MJ. Fever: role of pyrogens and cryogens. Physiol Rev. 1991;71:93–127. doi: 10.1152/physrev.1991.71.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger JM, Walter J, Dinarello CA, Wolff SM, Chedid L. Sleep-promoting effects of endogenous pyrogen (interleukin-1) Am. J. Physiol. 1984;246(6 Pt 2):R994–999. doi: 10.1152/ajpregu.1984.246.6.R994. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 2002;25:154–159. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- Laborit H. The major mechanisms of stress. Methods Achiev Exp Pathol. 1991;15:1–26. [PubMed] [Google Scholar]
- Laborit H. Stress revisited. 2. Systemic effects of stress. Introduction. Methods Achiev Exp Pathol. 1991;15:XI–XV. [PubMed] [Google Scholar]
- Maes M. A review on the acute phase response in major depression. Rev Neurosci. 1993;4:407–416. doi: 10.1515/revneuro.1993.4.4.407. [DOI] [PubMed] [Google Scholar]
- Maes M, Smith R, Scharpe S. The monocyte-T-lymphocyte hypothesis of major depression. Psychoneuroendocrinology. 1995;20:111–116. doi: 10.1016/0306-4530(94)00066-j. [DOI] [PubMed] [Google Scholar]
- Maier SF, Wiertelak EP, Martin D, Watkins LR. Interleukin-1 mediates the behavioral hyperalgesia produced by lithium chloride and endotoxin. Brain Res. 1993;623:321–324. doi: 10.1016/0006-8993(93)91446-y. [DOI] [PubMed] [Google Scholar]
- McGlone JJ, Ritter S, Kelley KW. The antiaggressive effect of lithium is abolished by area postrema lesion. Physiol Behav. 1980;24:1095–1100. doi: 10.1016/0031-9384(80)90053-0. [DOI] [PubMed] [Google Scholar]
- Miller NE. Experiments on motivation. Studies combining psychological, physiological, and pharmacological techniques. Science. 1957;126:1271–1278. doi: 10.1126/science.126.3286.1271. [DOI] [PubMed] [Google Scholar]
- Miller NE. Some psychophysiological studies of the motivation and of the behavioral effects of illness. Bull British Psychol Soc. 1964;17:1–20. [Google Scholar]
- Mitchell D, Kirschbaum EH, Perry RL. Effects of neophobia and habituation on the poison-induced avoidance of exteroceptive stimuli in the rat. J Exp Psychol Anim Behav Process. 1975;1:47–55. [PubMed] [Google Scholar]
- Moreau M, Lestage J, Verrier D, Mormede C, Kelley KW, Dantzer R, Castanon N. Bacille Calmette-Guerin inoculation induces chronic activation of peripheral and brain indoleamine 2,3-dioxygenase in mice. J Infect Dis. 2005;192:537–544. doi: 10.1086/431603. [DOI] [PubMed] [Google Scholar]
- Ritter S, McGlone JJ, Kelley KW. Absence of lithium-induced taste aversion after area postrema lesion. Brain Res. 1980;201:501–506. doi: 10.1016/0006-8993(80)91061-6. [DOI] [PubMed] [Google Scholar]
- Tazi A, Dantzer R, Crestani F, Le Moal M. Interleukin-1 induces conditioned taste aversion in rats: a possible explanation for its pituitary-adrenal stimulating activity. Brain Res. 1988;473:369–371. doi: 10.1016/0006-8993(88)90868-2. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Maier SF. The pain of being sick: implications of immune-to-brain communication for understanding pain. Annu Rev Psychol. 2000;51:29–57. doi: 10.1146/annurev.psych.51.1.29. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Weidenfeld J, Pollak Y, Morag M, Morag A, Avitsur R, Barak O, Reichenberg A, Cohen E, Shavit Y, Ovadia H. Cytokines, “depression due to a general medical condition,” and antidepressant drugs. Adv Exp Med Biol. 1999;461:283–316. doi: 10.1007/978-0-585-37970-8_16. [DOI] [PubMed] [Google Scholar]