Abstract
Pyridoxine (vitamin B6) intoxicated rodents develop a peripheral neuropathy characterized by sensory nerve conduction deficits associated with disturbances of nerve fiber geometry and axonal atrophy. To investigate the possibility that glucagon-like peptide-1 (7-36)-amide (GLP-1) receptor agonism may influence axonal structure and function through neuroprotection neurotrophic support, effects of GLP-1 and its long acting analog, Exendin-4 (Ex4) treatment on pyridoxine-induced peripheral neuropathy were examined in rats using behavioral and morphometric techniques. GLP-1 is an endogenous insulinotropic peptide secreted from the gut in response to the presence of food. GLP-1 receptors (GLP-1R) are coupled to the cAMP second messenger pathway, and are expressed widely throughout neural tissues of humans and rodents. Recent studies have established that GLP-1 and Ex4, have multiple synergistic effects on glucose-dependent insulin secretion pathways of pancreatic β-cells and on neural plasticity. Data reported here suggest that clinically relevant doses of GLP-1 and Ex4 may offer some protection against the sensory peripheral neuropathy induced by pyridoxine. Our findings suggest a potential role for these peptides in the treatment of neuropathies, including that associated with type II diabetes mellitus.
Keywords: GLP-1, exendin-4, behavior, diabetes, morphology, neuropathy, neuroprotection, neurotrophic, pyroxidine, rat, stereometry
Introduction
Endogenous GLP-1 is an insulinotropic peptide synthesized and secreted from the L-cells of the gastrointestinal tract in response to food. When given exogenously, GLP-1 improves glucostasis in type-2 diabetes patients, primarily by stimulating endogenous insulin secretion. Despite its promise, the use of GLP-1 as a therapeutic agent for the treatment of type-2 diabetes is critically undermined by its extremely short half-life (1.5 minutes in rodents and humans (Estall & Drucker, 2006; Holst 2006))
Exendin-4 (Ex4), a naturally occurring, more stable analogue of GLP-1, shares 53% sequence homology with GLP-1 (Figure 1), although it is the product of a uniquely non-mammalian gene. It binds at the GLP-1 receptor with greater affinity than GLP-1 (specifically due to the PSS sequence at the beginning of the 9-amino acid tail), is more potent at maintaining plasma insulin levels than GLP-1, and has a half-life of approximately 120 minutes in rodents (Wang et al., 1997; Greig et al., 1999).
Figure 1.

Amino acid sequences for GLP-1, Ex4 and the GLP-1 receptor antagonist, Exendin (9-39). The purple shading represents the amino acid substitutions in the Ex4 sequence relative to the GLP-1 sequence (shaded red). Replacement of the alanine with glycine in position 8 renders the peptide protease resistant and improves stability.
A growing body of published data confirm the central expression of GLP-1R mRNA in humans (Satoh et al., 2000) and rodents (Goke et al., 1995; Shughrue et al., 1996). GLP-1 has been shown to possess the ability to mediate central nervous system effects relating to satiety (Turton et al., 1996) and cognition (During et al., 2003). We have recently shown that GLP-1R activation induces neurite outgrowth in PC12 cells and SK-N-SH human neuroblastoma cells by a mechanism involving the second messenger cAMP (Perry et al., 2002a). Furthermore, GLP-1 and Ex4 possess anti-oxidant properties (Perry et al., 2003) and can protect central neurons against excitotoxicity (Perry et al., 2002b). During et al., (2003) also observed that GLP-1 is neuroprotective in a mouse model of excitotoxic brain damage caused by severe epileptic seizures. Thus, GLP-1 and related peptides are likely to possess central neural functions including neurotrophic and neoprotective effects, in addition to glucoregulatory and energy balance functions (Perry & Greig, 2003). These central effects may hold promise for the development of therapeutic agents to treat central neurodegenerative conditions, including Alzheimer’s disease, vascular dementia, post-stroke dementia and Parkinson’s disease (Greig et al., 2004; Greig & Perry, 2005) and peripheral neuropathies, such as that associated with type 2 diabetes mellitus.
GLP-1 receptor gene expression has also been demonstrated on neuronal cells of the nodose ganglion (Nakagawa et al., 2004), suggesting a possible peripheral nervous system role for this peptide. Sensory elements of the nodose ganglion (cell bodies of vagal afferents) are critical components of most visceral, respiratory, and cardiovascular autonomic reflexes. Sensory axons associated with the nodose ganglion innervate organs of the thorax and abdomen and relay afferent information concerning blood pressure, gastric distention, and blood oxygenation to the CNS. Trauma, tumors, disease (such as diabetes mellitus), toxins (arsenic) and drugs (cisplatin) can injure these visceral sensory nerves (Sima 2006; Lee & Swain, 2006).
Twenty percent of people over the age of 65 in the US suffer from diabetes mellitus. Peripheral neuropathy is a frequent complication diabetes mellitus, for which treatments are few other than maintaining tight control of blood glucose levels and symptom alleviation. Diabetic neuropathy interferes with autonomic reflexes. A component of this dysfunction is associated with altered visceral sensory nerve function. It is likely that afferents mediating these autonomic reflexes, which are disturbed in diabetic neuropathy, harbor GLP-1 receptors
Excess ingestion of pyridoxine (vitamin B6) causes peripheral sensory neuropathy in rodents (Xu et al., 1989), dogs (Schaeppi and Krinke, 1982) and humans (Albin et al., 1987). We have previously reported that the peripheral nerve degeneration associated with pyridoxine intoxication in rodents models at least one aspect of clinical diabetic peripheral neuropathy; specifically, damage to large fiber sensory neurons (Perry et al., 2004; Kuntzer et al., 2004). We have therefore begun to investigate any neurotrophic/neuroprotective capabilities of GLP-1R agonism in non-diabetic rodents with pyridoxine-induced sensory neuropathy, as part of efforts to investigate the value of GLP-1R agonists beyond the regulation of glucostasis.
Materials and methods
Animals and treatments
Behavioral (functional) evaluations were undertaken with adult male Sprague-Dawley rats weighing approximately 300-350 g each. Animals were housed under controlled light/dark and temperature conditions with food and water available ad libitum. Animals were lightly anaesthetized with isoflurane, and ALZET osmotic minipumps (ALZA Corp., Palo Alto, CA) implanted subcutaneously between the scapulae as previously reported (Perry et al., 2002b). The four treatment groups comprised: GLP-1 delivered at rates of 0.35, 3.5 and 35 pM/kg/min (n = 12 /group; designated: 0.35 GLP-1, 3.5 GLP-1 and 35 GLP-1, respectively) and Exendin (9-39), a competitive antagonist of the GLP-1 receptor (Figure 1), infused at a rate of 0.24 nM/kg/min in combination with 3.5 GLP-1 (n = 12; designated: 3.5 GLP-1 / Ex (9-39)). GLP-1 and Exendin (9-39) were delivered from separate pumps. A control group was infused with a scrambled inactive, 30-amino acid peptide (n = 12; designated: IAP). Incisions were sutured and the animals allowed to recover. The following day each infusion group was subdivided into two injection groups; one group received pyridoxine (n = 6; designated: PYR) and the second received saline injections (n = 6; designated: SAL). Pyridoxine hydrochloride (Sigma Chemicals) was diluted in sterile distilled water, pH adjusted to 7.2, warmed and administered by intraperitoneal (i.p.) injection at 400 mg/kg twice daily, for 14 days. Fresh pyridoxine solutions were prepared immediately before each injection.
Further animals were employed for the morphological evaluation of possible GLP-1 mediated neuroprotective effects using light microscopy and design-based stereology as we have previously reported (Perry et al., 2004). ALZET osmotic minipumps were implanted as described above. In the first treated group, GLP-1 was delivered at the rate of 3.5 pM/kg/min (n = 12; designated: 3.5 GLP-1). In a second group, Exendin-4 (Ex4) was delivered, also at the rate of 0.35 pM/kg/min (n = 12; designated: Ex4). Control animals were infused with the scrambled inactive peptide as before (n = 12; designated: IAP). Each infusion group was again subdivided into two, and received either 400 mg/kg pyridoxine (n = 6; designated: PYR) or saline (n = 6; designated: SAL) injections for 14 days.
Plasma GLP-1 levels
Blood samples were taken from rats, 48 hours after the implantation of the minipumps. Blood was drawn into heparinized tubes containing EDTA, Trasylol, and DPP-IV inhibitor (inhibits degradation of GLP-1 by the dipeptidyl peptidase IV enzyme present in serum) for GLP-1 determination. Samples (300 μl) were analysed by radioimmunoassay (Linco Research Inc., MO.).
Behavioural Evaluations
With knowledge that the functional deficits induced by PYR develop progressively with treatment, all behavioral (functional) tests were carried out between days 10 and 12 after initiation of pyridoxine administration.
Inclined screen
This test measured muscle tone, strength and balance. Each rat was placed in one of six separate compartments of a wire mesh screen that was tilted at an angle of 60° to the horizontal plane. Latency to fall from the screen (max score 15 minutes) was recorded. Those animals which fell / jumped off the screen in the first 60 seconds were placed back on and reassessed (Shukitt-Hale et al., 1998).
Rotarod
Fine motor coordination, balance and resistance to fatigue were quantified by measuring the number of falls that a rat made while standing on a rotating rod. The rod is a scored, plastic drum (about 15 cm in diameter), which rotates at about 3 rpm. The number of falls from the rotarod during a 3-minute total exposure time was recorded (Shukitt-Hale et al., 1998).
Histopathology
On day 14, all animals were sacrificed (excess isoflurane inhalation) and segments of sciatic nerve from just below the notch, and lumbar dorsal root ganglia (L4 - L6) were immediately excised for histological analyses and stereology. Tissues were post-fixed overnight in 4% paraformaldehyde prior to embedding in paraffin and serial sectioning at 4-6 μm in the transverse (coronal) plane. Representative sciatic nerve and dorsal root ganglion sections were deparaffinized and stained with luxol fast blue (for myelin) combined with a light cresyl violet counterstain. After deparaffinization, sections were boiled in citrate buffer, pH 6.0 for 20-25 minutes. After cooling, sections were washed in tap water, followed by several washes in phosphate-buffered saline and blocked in goat serum overnight at 4°C. Adjacent sections were stained immunocytochemically using the polyclonal rabbit anti-neurofilament antibodies (Chemicon, International Inc., Temecula, CA) at 1:500 dilution. Visualization of positive immunoreactivity was carried out using avidin-biotin/horse radish peroxidase and DAB as the chromagen. Stained sections were dehydrated in an ascending series of alcohols prior to mounting in D.P.X. (VWR Scientific Products, Willard, OH).
Sciatic nerve and dorsal root ganglion stereology
Morphometric analyses were performed with the aid of a computerized stereology system powered by Stereologer software (Systems Planning and Analysis Inc., Alexandria, VA) and hardware consisting of a Zeiss Axioskop microscope, motorized XYZ stage (Applied Scientific Instrumentation, Eugene, OR), Sony CCD video camera, TARGA video card, and personal computer/monitor. The parameters of interest within transverse (coronal) sciatic nerve sections taken from just above the notch were the number and area of myelinated axons stained with luxol fast blue (myelin + axon), and neurofilament-positive axons. The axonal area fraction and total area of axons for cross-sections of sciatic nerve biopsies were estimated using point counting stereology, as we have previously reported (Perry et al., 2004).
Dorsal root ganglion sections were stained with luxol fast blue and cresyl violet. Classifications for neurons in the DRG were based on their size, appearance and histochemical reactions, leading to the designation, A and B cells, as we have previously reported (Perry et al., 2004). The combined areas of the A- and B-cells were quantified in two sections, and the average value reported. The total mean area of DRG cells was estimated as the product of the area fraction for A- and B-cells at high magnification (63x) and the total DRG area estimated at low magnification (5x).
Data and statistical analysis
All data are presented as Mean ± SEM. Body weights were compared across time using a repeated measures ANOVA. All other functional and stereologic parameters were subjected to one-way analysis of variance (ANOVA). Tukey’s HSD post-hoc test was employed for comparisons of selected means subsequent to ANOVA. The level of significance was set at p < 0.05 (two-tailed) in all cases.
Results
Effects of pyridoxine intoxication
Animals were injected twice daily with either 400 mg kg-1 pyridoxine or vehicle for two weeks. Immediately following each pyridoxine injection, and as previously reported (Perry et al., 2004), animals showed signs of temporary (10-20 sec) discomfort, indicated by increased unsteadiness, vocalizing and aggressiveness towards cagemates. Saline injected animals did not show any signs of discomfort. Aside from the transient, acute, injection-related effects, there were no signs of pyridoxine-induced functional damage during the first week, with ± PYR treatment groups appearing indistinguishable. During the second week, functional deficits in the PYR animals became increasingly apparent, as expected from previous reports (Perry et al., 2004). The general unsteadiness, initially only evident post-injection, became continuous with a pronounced hindlimb deficit. The deficit progressed rapidly to all four limbs, impairing coordination and resulting in a severe walking abnormality. The observed functional deficits appeared to be less severe in the PYR animals receiving GLP-1.
Body weights
In spite of additional care to ensure that all animals could reach food and water easily, PYR animals had significantly reduced body weights (by approximately 15%; p<0.001) after 14 days, compared with SAL animals (Figure 2).
Figure 2.
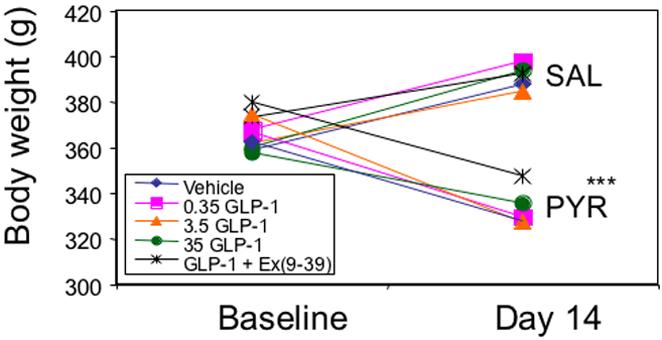
Body weight in grams of PYR - intoxicated or SAL animals receiving GLP-1 agonist/antagonist or inactive peptide (IAP) infusions for 14 days. * p≤0.05 PYR vs SAL.
Plasma GLP-1 levels
Plasma GLP-1 levels were assayed 48 hours after the start of infusions of GLP-1 or IAP to verify that pharmacologic levels of GLP-1 were being achieved. GLP-1 delivered at the rate of 3.5 pM/kg/min resulted in plasma levels of 138 ± 15.6 pM, compared with 2.3 ± 0.5 pM in IAP animals (normal fasting levels < 10 pM). Plasma GLP-1 levels were no different between PYR and SAL animals.
Inclined screen performance
As there were no significant differences in performance among any of the SAL groups (Figure 3), data for all SAL animals were pooled for statistical comparisons with PYR animals. Tukey’s HSD test, subsequent to ANOVA, showed that animals fell sooner in the PYR - IAP (p = 0.0001) and the PYR - 3.5 GLP-1 / Ex(9-39) (p < 0.001) groups, compared to SAL animals, indicating pyridoxine-induced functional impairment. GLP-1 (alone) at all doses restored inclined screen performance to levels that did not differ significantly from SAL animals.
Figure 3.
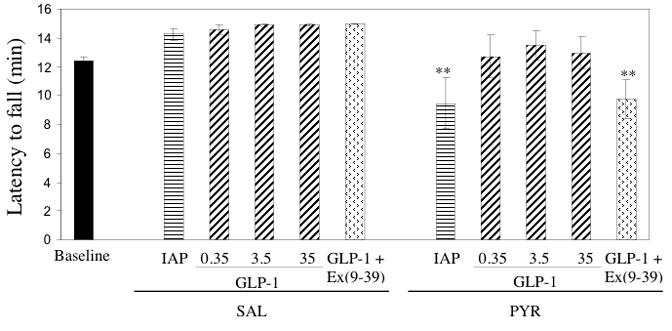
Latency to fall from the inclined screen in PYR - intoxicated or SAL animals receiving GLP-1 agonist/antagonist or inactive peptide (IAP) infusions for 14 days. *** p < 0.001 vs SAL (pooled)
Rotarod performance
Once again, no significant differences were observed in rotarod performance among the SAL groups, and data were pooled for comparison with the PYR animals. Animals in the PYR groups experienced more falls from the rotarod than SAL animals (Figure 4). Tukey’s HSD test, subsequent to ANOVA, showed that the number of falls from the rotarod was significantly higher in the PYR - IAP (p = 0.002), PYR - 0.35 GLP-1 (p = 0.01) and the PYR - 3.5 GLP-1 / Ex(9-39) (p = 0.025) groups compared to SAL animals. GLP-1 infusion produced dose-dependent reductions in fall number for pyridoxine-treated animals, although at the lowest GLP-1 dose (0.35 GLP-1), significantly more falls were still observed than in the SAL groups.
Figure 4.
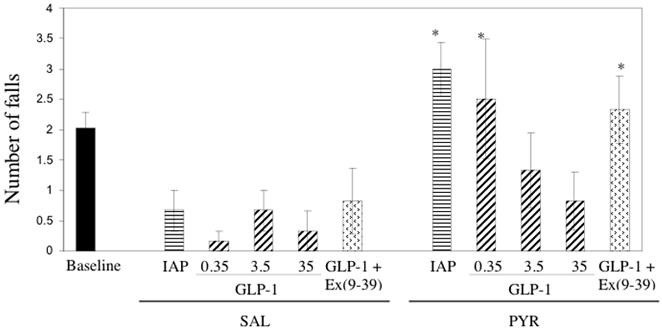
Number of falls during the rotarod test for PYR - intoxicated or SAL animals receiving GLP-1 agonist/antagonist or inactive peptide (IAP) infusions for 14 days. * p < 0.05; ** p ≤ 0.01 vs SAL (pooled)
Sciatic nerve and DRG morphology: light microscopy
After 14 days of pyridoxine treatment, sciatic nerve morphology was visualized using luxol fast blue histochemistry (LFB) and neurofilament-positive immunoreactivity (NFI). Observations were quantified with design-based stereology as described previously (Perry et al., 2004). Figure 5 illustrates the microscopic appearance of typical sciatic nerve sections from PYR and SAL animals. Sections stained with luxol fast blue from PYR - IAP (5B) rats showed fewer large diameter fibers and a relative increase in the number of smaller diameter fibers as compared to sections from SAL - IAP rats (5A). This resulted in an apparent decrease in nerve fiber density and an increased endoneurial area (interstitial connective tissue surrounding the fibers). Sections from PYR - 3.5 GLP-1 (5C) and particularly PYR - Ex4 (5D) treated rats showed fewer small diameter fibers than PYR - IAP rats (5B), and a large diameter axon profile and endoneurial area more similar to that observed in SAL - IAP animals (5A).
Figure 5.
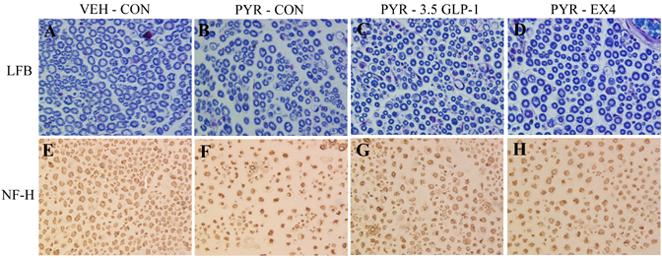
Transverse sections of sciatic nerve from PYR - intoxicated or SAL animals receiving GLP-1 agonist or inactive peptide (IAP) infusions for 14 days. Luxol fast blue staining (LFB) for myelin (A-D) and neurofilament-positive immunoreactivity (NFI) for the axon (E-H).
Figure 5E - H shows neurofilament-positive immunoreactivity (NFI), a specific axon marker. Evidence of axonal degeneration presents as an increase in the number of irregularly shaped axon profiles in the PYR - IAP group (5F) compared with the SAL - IAP group (5E). Consistent with the luxol fast blue stained sections, the endoneurial area appears larger in the PYR - IAP group (5F) than the SAL - IAP group (5E). The PYR - 3.5 GLP-1 (5G) and PYR - Ex4 (5H) groups showed axon profiles morphologically more similar to the SAL - IAP group than the PYR - IAP group.
Similar evidence of cellular degeneration was evident in the DRG of PYR rats (Figure 6). Degenerating profiles characteristic of apoptosis were observed in the PYR - IAP animals; specifically smaller cell bodies with evidence of intracytoplasmic vacuolation, and increased numbers of satellite cells (6B and 6C). The PYR - IAP animals also showed an abundance of irregular-shaped A- and B-cells (Figure 6C), and a more variable size distribution than the SAL - IAP animals. SAL-IAP animals showed the normal DRG phenotype (6A); large A-cells with well-defined Nissl granules and light nuclei, with one dark central nucleolus, and smaller and darker B-cells generally containing multiple nucleoli located peripherally. The PYR -3.5 GLP-1 group showed some evidence of morphologic normalization, with more uniform A- and B-cell morphologies and a reduced presence of apoptotic damage (6D) than in the PYR - IAP group.
Figure 6.
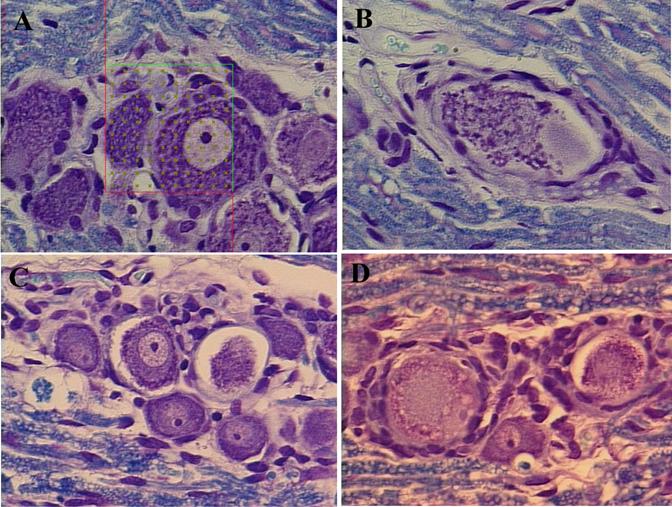
Transverse sections of L4 or L5 dorsal root ganglia from PYR - intoxicated or SAL animals receiving GLP-1 agonist or inactive peptide (IAP) infusions for 14 days. LFB staining.
Sciatic nerve morphology: stereology
Design-based stereology was employed to quantify the observations described above (Table 1). Multiple comparisons among the stereologic data and demonstration of statistically significant differences among groups were confounded by large data variability; however findings did mirror those from the light microscopy evaluations. PYR - IAP animals showed an approximate 44% greater myleinated fiber number as compared with the pooled data from the SAL animals. In animals receiving PYR - 3.5 GLP-1 and PYR - Ex4 there was some normalization of fiber number. The mean myelinated axon area was markedly reduced (41%) in the PYR - IAP animals as compared with pooled SAL data (p > 0.05 vs. SAL - IAP as well as SAL - pooled), while the mean area of all axons was reduced by 70%. The mean area of myelinated axons was not normalized by GLP-1 or Ex4, but for all axons, both GLP-1 and Ex4 returned values towards those of the SAL groups, and were not statistically different from the SAL - pooled value (p < 0.05). The total neurofilament area was significantly (48%) reduced in the PYR - IAP animals versus SAL animals (p = 0.002) and entirely normalized in PYR - 3.5GLP-1 and PYR - Ex4 groups. The changes in total neurofilament area appeared to occur in the absence of any changes in the total myelin area. Mean endoneural area was increased by 31% in the PYR - IAP animals as compared with pooled SAL data, with some normalization provided by GLP-1 and Ex 4. These data concur with the qualitative findings from light microscopy.
Table 1.
Stereologic parameters from sciatic nerve sections of PYR - intoxicated or SAL animals receiving GLP-1 agonist or inactive peptide (IAP) infusions for 14 days.
| Variable | SAL |
PYR |
|||||
|---|---|---|---|---|---|---|---|
| IAP | 3.5 GLP-1 | Ex4 | POOLED | IAP | 3.5 GLP-1 | Ex4 | |
| (n=7) | (n=5) | (n=6) | (n=18) | (n=7) | (n=7) | (n=4) | |
| Total number of myelinated axons Mean myelinated axon area (μm2) | 8174 | 10761 | 10218 | 9711 | 14070 | 11394 | 11426 |
| ± 1759 | ± 1617 | ± 1799 | ±943 | ± 1590 | ± 1720 | ± 3608 | |
| 242.73 | 202.74 | 221.80 | 222.42 | 131.17* | 123.27* | 136.14 | |
| ± 58.35 | ± 42.73 | ± 33.65 | ±25.29 | ± 14.12 | ± 13.71 | ± 22.90 | |
| Mean axon area (μm2) | 44.25 | 25.50 | 26.72 | 33.20 | 10.23** | 24.37 | 24.70 |
| ± 10.95 | ± 5.45 | ± 3.48 | ± 4.74 | ± 3.34 | ± 2.63 | ± 3.65 | |
| Total neurofilament area (μ2 × 106) | |||||||
| 0.26 | 0.25 | 0.25 | 0.25 | 0.13** | 0.26 | 0.26 | |
| ± 0.01 | ± 0.01 | ± 0.02 | ± 0.01 | ± 0.04 | ± 0.03 | ± 0.05 | |
| Total myelin area (μm2 × 106) | 1.62 | 1.78 | 2.11 | 1.83 | 1.52 | 1.11 | 1.22 |
| ± 0.32 | ± 0.35 | ± 0.63 | ± 0.24 | ± 0.34 | ± 0.22 | ± 0.42 | |
| Mean endoneurial area (μm2 × 106) | 0.71 | 0.89 | 0.93 | 0.84 | 1.10* | 0.97 | 0.94 |
| ± 0.15 | ± 0.06 | ± 0.07 | ±0.06 | ± 0.07 | ± 0.08 | ± 0.12 | |
No difference between SAL groups (p>0.05), hence all SAL data was pooled
p < 0.05
p < 0.01 vs. SAL – Pooled
Dorsal root ganglion: stereology
The mean area of the ganglion was unchanged following PYR treatment (Table 2). Data for mean areas of A and B cells from SAL groups was once again pooled for comparison with PYR groups. One-way analysis of variance demonstrated a significant reduction in the PYR - IAP group (p < 0.001) with trends towards normalization in PYR - 3.5 GLP-1 and PYR - Ex4 animals.
Table 2.
Stereological quantification of dorsal root ganglion morphology in PYR - intoxicated or SAL animals receiving GLP-1 agonist or inactive peptide (IAP) infusions for 14 days.
| Variable | >SAL | PYR | ||||
|---|---|---|---|---|---|---|
| IAP | 3.5 GLP-1 | Ex4 | IAP | 3.5 GLP-1 | Ex4 | |
| Mean ganglion area (μm2 × 106) | 1.02 | 0.95 | 1.04 | 0.99 | 0.87 | 1.0 |
| ± 0.19 | ± 0.15 | ± 0.19 | ± 0.3 | ± 0.33 | ± 0.18 | |
| Mean area of A-and B-cells (μm2 × 105) | 2.0 | 1.8 | 2.1 | 1.0*** | 1.5 | 1.6 |
| ± 0.4 | ± 0.3 | ± 0.5 | ± 0.3 | ± 0.5 | ± 0.5 | |
p<0.001 vs SAL
Discussion
In these investigations, evidence of neuroprotection mediated by agonism at the GLP-1 receptor was evaluated in animals with pyridoxine (PYR) induced peripheral sensory neuropathy. Data from functional (behavioral) evaluations and histological observations and analyses of nerve specimens, were referenced to similar data from saline-injected (SAL) animals without neuropathy. Compared to the SAL injected animals, PYR intoxicated animals exhibited a range of functional and morphological defects which were to varying degrees, ameliorated by treatment with GLP-1 or its longer-lasting analog Ex4.
We have previously reported (Perry et al., 2004) electrophysiologic dysfunction resulting from pyridoxine intoxication; including sensorimotor neuropathy, characterized by nerve conduction deficits and absence of the H wave (H waves confirm intact sensorimotor circuitry). These PYR-induced electrophysiologic impairments were duplicated in this series of investigations (data not shown). We are now additionally able to report significant functional (behavioral) deficits following PYR-treatment and some evidence of amelioration of these effects (neuroprotection) with GLP-1 treatment. As we have previously shown (Perry et al., 2004) pyridoxine intoxication is progressive, resulting in a pronounced hindlimb deficit leaving the animal unable to coordinate all four limbs simultaneously, resulting in severe ataxia. In spite of this, animals are able to move around the cage by adopting a shuffling gait, likely as a result of preserved muscle force and some residual proprioceptive function. The rotarod and inclined screen assessment paradigms represent measures of sensory and motor performance together with coordinating and integrative functions. Data indicate that GLP-1 treatment can restore incline screen performance in PYR-animals to levels observed in SAL treated controls. Furthermore, there was the suggestion of a dose-dependent protective effect of GLP-1 against PYR-induced functional deficits in rotarod performance.
Administration of exogenous of GLP-1 and Ex4 also appeared to support the maintenance of morphologic integrity of the individual axons within the sciatic nerve, and soma within the DRG of PYR-treated rats. Taken together with evidence that GLP-1 can enhance the survival and plasticity of neurons in the brain (Perry et al., 2002; During et al., 2003), these findings could indicate that GLP-1 has the ability to act at multiple targets to stimulate signaling pathways which enhance neuroprotection within the peripheral nervous system in addition to the central nervous system. As previously reported (Perry et al., 2004) pyridoxine toxicity results in the degeneration of peripheral sensory ganglia, particularly large neurons with long, heavily myelinated processes. High dose pyridoxine produces ataxia with necrosis of DRG neurons, while lower doses produce cell body and axonal atrophy without discernable functional sequelae. Between these ranges exists a spectrum of injury, with the earliest manifestations occurring at the level of the cell body. Cell body atrophy is manifest as cytoplasmic alterations including vacuolization, increased dense bodies, neurofilament aggregates, and chromatolysis. Our observations at the light microscope level suggest that GLP-1 and Ex4 treatment offers some protection against PYR-induced axonopathy within the sciatic nerve. Pyridoxine treatment appears to result in an increase in the total number of myelinated nerve fibers, mediated in part by a shift of the size distribution from large to small fibers, and an increased endoneurial area. This latter change is likely to be related to the axonal degeneration together with the increased frequency of small diameters fibers. Treatment with GLP-1 or its longer-lasting analog Ex4 appeared support the integrity of neurofilaments, suggesting a direct neuroprotective role for these peptides. We observed that the GLP-1 receptor agonists seemed to mediate quite complete normalization of the mean size of all axons in PYR animals, with a lesser effect in the large myelinated fibers. This may reflect the relatively short duration of treatment with GLP-1 or Ex4 which may not have been sufficient to support the placticity required to combat the pyridoxine insult, particularly in the larger fibers.
Within the DRG, A and B cell integrity and cytoplasmic appearance were improved in PYR animals that received concurrent GLP-1 or Ex4, with appearances under light microscopy similar to SAL animals. Pyridoxine is a toxin known to target large fiber sensory neurons (Albin et al., 1987); Krinke and Fitzgerald, 1988; Schaeppi and Krinke, 1982; Windebank et al., 1985; Xu et al., 1989). As such, we have previously demonstrated that pyridoxine intoxication causes a reduction in the mean area of A- and B-cells in the DRG (Perry et al., 2004). This was confirmed here, together with evidence that treatment with GLP-1 or Ex4 may offer some protection against the PYR-induced A and B cell area reductions and internal morphologic disruption. There appears to be a morphological correlation between the integrity of the DRG and the axonal dysruption in the sciatic nerve. Light microscopic observation of the neurofilament-positive immunoreactivity (NFI) was entirely supportive of the staining for myelin (LFB) in demonstrating a morphologic appearance in the PYR animals receiving GLP-1 or EX4 which more closely resembled the SAL treated animals than PYR - IAP .
The rationale for selecting pyridoxine to produce an animal model of large-fiber neuropathy is based on several factors, including the selective and severe neurotoxic actions of this compound on large DRG neurons in rodents (Xu et al., 1989), dogs (Schaeppi and Krinke, 1982), and humans (Albin et al 1987). Our interest in this particular animal model of sensory neuropathy is that the rapidly developing, large fiber neurodegeneration may be considered to model one aspect of clinical diabetic peripheral neuropathy. This model could be of use as a screen for evaluating neurotrophic / neuroprotective properties of novel compounds currently in development for type 2 diabetes mellitus.
GLP-1 receptor agonism has been characterized as a therapeutic option in diabetes. Ex4 (Exenatide; Amylin Pharmaceuticals Inc), is the first of a new class of pharmaceutics known as incretin mimetics, now in use for the treatment of type 2 diabetes (Estall & Drucker, 2006; Holst 2006). Ex4 binds at the putative GLP-1 receptor and is structurally similar to GLP-1, while providing a more long-lasting effect than GLP-1. Clinical data suggest that Ex4 treatment decreases blood glucose toward target levels, improves markers of beta cell function and is associated with weight loss. It has also been demonstrated to exhibit neurotrophic properties both in vitro and in vivo (Perry et al. 2002a, 2002b). Binding sites for Ex4 have been identified throughout the rat central nervous system (Goke et al 1995) which leads to speculation that sustainable central GLP-1 receptor agonism may have a therapeutic role in the treatment of a number of central and peripheral neurodegenerative disorders, such as Alzheimer’s disease, vascular and post-stroke dementia and Parkinson’s diseases and peripheral neuropathies, such as that associated with type 2 diabetes.
About 60-70% of type 2 diabetics have mild to severe forms of nervous system damage. The results of such damage include impaired sensation or pain in the hands and feet, slowed digestion of food in the stomach, carpel tunnel syndrome, and other nerve problems. Severe forms of diabetic neuropathy are a major contributing cause of lower-extremity amputations. Improved glycemic control and/or trophic support can help to reduce neural toxicity and minimize or eliminate subsequent diabetic complications, including distal symmetric polyneuropathy (UKPDS 33). However, no current therapy is capable of reversing the nerve degeneration induced by uncontrolled hyperglycemia. Aldose reductase inhibitors (ARI) have demonstrated beneficial effects on nerve function in rodent studies, by blocking neural accumulation of sorbitol and downstream toxic effects. Unfortunately, these findings have not been reproduced clinically for diabetic patients. The lack of success of ARI’s in the clinic may, in part, be related to the failure of a number of these compounds to penetrate the blood-nerve barrier. Similarly trials of the antioxidant, alpha-lipoic acid, have not demonstrated consistent beneficial effects in humans. To date, symptomatic relief is the only option available to diabetic neuropathy patients. It is possible that GLP-1 agonists such as Ex4, which hold the promise of neurotrophic or neuroprotective effects in addition to a favorable profile for glycemic and energy balance regulation may hold promise for the management of diabetic peripheral neuropathies.
In summary, we have presented preliminary evidence that GLP-1 receptor agonism can be neuroprotective in an experimental model of sensory neuropathy. Since it is has been demonstrated that GLP-1 receptor agonists can promote neural plasticity and protection in animal models of other neurological indications, we propose that GLP-1 agonists may hold promise as therapeutic agents for the treatment of many different neurodegenerative conditions throughout the central and peripheral nervous systems.
Acknowledgements
The authors thank Dr. Barry Warwick (Asympcom) for data analysis and his valuable editorial input. Animal studies were undertaken in full compliance with the guidelines for animal experimentation of the National Institutes of Health (DHEW publication 85-23, revised, 1995). The minimal possible number of animals was used and all efforts were made to minimize their suffering. This work was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albin RL, Albers JW, Greenberg HS, Townsend JB, Lynn RB, Burke JM, Jr., Alessi AG. Acute sensory neuropathy-neuronopathy from pyridoxine overdose. Neurology. 1987;37:1729–1732. doi: 10.1212/wnl.37.11.1729. [DOI] [PubMed] [Google Scholar]
- During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, Bland RJ, Klugmann M, Banks WA, Drucker DJ, Haile CN. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med. 2003;9:1173–9. doi: 10.1038/nm919. [DOI] [PubMed] [Google Scholar]
- Estall JL, Drucker DJ. Glucagon and glucagon-like peptide receptors as drug targets. Curr Pharm Des. 2006;12:1731–50. doi: 10.2174/138161206776873671. [DOI] [PubMed] [Google Scholar]
- Goke R, Larsen PJ, Mikkelsen JD, Sheikh SP. Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci. 1995;7:2294–2300. doi: 10.1111/j.1460-9568.1995.tb00650.x. [DOI] [PubMed] [Google Scholar]
- Greig NH, Holloway HW, De Ore KA, Jani D, Wang Y, Zhou J, Garant MJ, Egan JM. Once daily injection of exendin-4 to diabetic mice achieves long-term beneficial effects on blood glucose concentrations. Diabetologia. 1999;42:45–50. doi: 10.1007/s001250051111. [DOI] [PubMed] [Google Scholar]
- Greig NH, Mattson MP, Perry T, Chan SL, Giordano T, Sambamurti K, Rogers JT, Ovadia H, Lahiri DK. New therapeutic strategies and drug candidates for neurodegenerative diseases: p53 and TNF-alpha inhibitors, and GLP-1 receptor agonists. Ann N Y Acad Sci. 2004;20041035:290–315. doi: 10.1196/annals.1332.018. [DOI] [PubMed] [Google Scholar]
- Holst JJ. Glucagon-like peptide-1: from extract to agent. The Claude Bernard Lecture, 2005. Diabetologia. 2006;49:253–60. doi: 10.1007/s00125-005-0107-1. [DOI] [PubMed] [Google Scholar]
- Krinke GJ, Fitzgerald RE. The pattern of pyridoxine-induced lesion: difference between the high and the low toxic level. Toxicology. 1988;49:171–8. doi: 10.1016/0300-483x(88)90190-4. [DOI] [PubMed] [Google Scholar]
- Kuntzer T, Antoine JC, Steck AJ. Clinical features and pathophysiological basis of sensory neuronopathies (ganglionopathies) Muscle Nerve. 2004;30:255–68. doi: 10.1002/mus.20100. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Swain SM. Peripheral neuropathy induced by microtubule-stabilizing agents. J Clin Oncol. 2006;24:1633–42. doi: 10.1200/JCO.2005.04.0543. [DOI] [PubMed] [Google Scholar]
- Nakagawa A, Satake H, Nakabayashi H, Nishizawa M, Furuya K, Nakano S, Kigoshi T, Nakayama K, Uchida K. Receptor gene expression of glucagon-like peptide-1, but not glucose-dependent insulinotropic polypeptide, in rat nodose ganglion cells. Auton. Neurosci. 2004;110:36–43. doi: 10.1016/j.autneu.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Perry T, Greig NH. The glucagon-like peptides: a double-edged therapeutic sword? Trends Pharmacol Sci. 2003;24:377–83. doi: 10.1016/S0165-6147(03)00160-3. [DOI] [PubMed] [Google Scholar]
- Perry T, Greig NH. Enhancing central nervous system endogenous GLP-1 receptor pathways for intervention in Alzheimer's disease. Curr Alzheimer Res. 2005;2:377–85. doi: 10.2174/1567205054367892. [DOI] [PubMed] [Google Scholar]
- Perry T, Haughey NJ, Mattson MP, Egan JM, Greig NH. Protection and reversal of excitotoxic damage by glucagon-like peptide-1 and exendin-4. J Pharmacol Exp Ther. 2002b;302:1–8. doi: 10.1124/jpet.102.037481. [DOI] [PubMed] [Google Scholar]
- Perry T, Lahiri DK, Chen D, Zhou J, Shaw KT, Egan JM, Greig NH. A novel neurotrophic property of glucagon-like peptide 1: a promoter of nerve growth factor-mediated differentiation in PC12 cells. J Pharmacol Exp Ther. 2002a;300:958–966. doi: 10.1124/jpet.300.3.958. [DOI] [PubMed] [Google Scholar]
- Perry T, Lahiri DK, Sambamurti K, Chen D, Mattson MP, Egan JM, Greig NH. Glucagon-like peptide-1 decreases endogenous amyloid-beta peptide (Abeta) levels and protects hippocampal neurons from death induced by Abeta and iron. J Neurosci Res. 2003;72:603–612. doi: 10.1002/jnr.10611. [DOI] [PubMed] [Google Scholar]
- Perry TA, Weerasuriya A, Mouton PR, Holloway HW, Greig NH. Pyridoxine-induced toxicity in rats: a stereological quantification of the sensory neuropathy. Exp Neurol. 2004;190:133–44. doi: 10.1016/j.expneurol.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Satoh F, Beak SA, Small CJ, Falzon M, Ghatei MA, Bloom SR, Smith DM. Characterization of human and rat glucagon-like peptide-1 receptors in the neurointermediate lobe: lack of coupling to either stimulation or inhibition of adenylyl cyclase. Endocrinology. 2000;141:1301–1309. doi: 10.1210/endo.141.4.7420. [DOI] [PubMed] [Google Scholar]
- Schaeppi U, Krinke G. Pyridoxine neuropathy: correlation of functional tests and neuropathology in beagle dogs treated with large doses of vitamin B6. Agents Actions. 1982;12:575–582. doi: 10.1007/BF01965944. [DOI] [PubMed] [Google Scholar]
- Sima AA. Pathological mechanisms involved in diabetic neuropathy: can we slow the process? Curr Opin Investig Drugs. 2006;7:324–37. [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Glucagon-like peptide-1 receptor (GLP1-R) mRNA in the rat hypothalamus. Endocrinology. 1996;137:5159–5162. doi: 10.1210/endo.137.11.8895391. [DOI] [PubMed] [Google Scholar]
- Shukitt-Hale B, Mouzakis G, Joseph JA. Psychomotor and spatial memory performance in aging male Fischer 344 rats. Exp Gerontol. 1998;33:615–24. doi: 10.1016/s0531-5565(98)00024-2. [DOI] [PubMed] [Google Scholar]
- Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- Wang Y, Perfetti R, Greig NH, Holloway HW, DeOre KA, Montrose-Rafizadeh C, Elahi D, Egan JM. Glucagon-like peptide-1 can reverse the age-related decline in glucose tolerance in rats. J Clin Invest. 1997;99:2883–2889. doi: 10.1172/JCI119482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windebank AJ, Low PA, Blexrud MD, Schmelzer JD, Schaumburg HH. Pyridoxine neuropathy in rats: specific degeneration of sensory axons. Neurology. 1985;35:1617–1622. doi: 10.1212/wnl.35.11.1617. [DOI] [PubMed] [Google Scholar]
- Xu Y, Sladky JT, Brown MJ. Dose-dependent expression of neuronopathy after experimental pyridoxine intoxication. Neurology. 1989;39:1077–1083. doi: 10.1212/wnl.39.8.1077. [DOI] [PubMed] [Google Scholar]


