Abstract
Background
To understand the behavioral biology of Langerhans cells (LCs), we recently recorded time-lapse images of LCs in the knock-in mice expressing the I-Aβ chain tagged with the enhanced green fluorescence protein (EGFP). EGFP+ LCs showed relatively limited motility in the steady state, whereas topical application of dinitrofluorobenzene (DNFB) markedly augmented a unique movement of dendrites characterized by rhythmic extension and retraction, termed dSEARCH, and triggered amoeba-like lateral migration of cell bodies.
Objective
To define underlying mechanisms by which hapten treatment alters LC behaviors.
Methods
The I-Aβ-EGFP mice received subcutaneous (s.c.) injection of recombinant IL-1α or TNFα (50 ng/animal) and dynamic behaviors of EGFP+ LCs were recorded by time-lapse confocal microscopy at several time points to measure their dSEARCH activities and lateral migration. In a different sset of experiments, IL-1 receptor antagonist (IL-1Ra) or soluble TNF receptor-2 (sTNFR2) (0.5 μg/animal) was s.c. injected into the ear skin 30 minutes before topical application of DNFB, and LC behaviors analyzed thirty hours later.
Results
Local injection of IL-1α or TNFα induced significant, albeit modest, augmentation of both dSEARCH and lateral migration. Co-injection of TNFα and IL-1α further exacerbated motile activities in a synergistic manner by similar magnitudes observed after DNFB application. Conversely, DNFB-induced behavioral changes were inhibited completely by local injection of IL-1Ra or sTNFR2.
Conclusion
IL-1 and TNFα serve as equally important mediators of hapten-induced alteration of LC behaviors. Motile activities of epidermal LCs are reprogrammed by selected cytokines known to be produced by keratinocytes under pathological conditions.
1. Introduction
The outermost layer of skin, i.e., epidermis, serves as the first line of defense against a variety of environmental insults. Terminally differentiated keratinocytes form the stratum corneum, i.e., a physical barrier. The epidermis also functions as an immunological barrier by producing cytokines, antimicrobial peptides, and other mediators [1,2] and by hosting resident leukocytes, known as Langerhans cells (LCs), which represent an immature subset of the dendritic cell (DC) family of antigen presenting cells [3,4,5]. It is generally believed that LCs play dual or even counter-acting immuno-regulatory roles at this location depending on their states of maturation [6]. In the steady state, immature LCs maintain peripheral immune tolerance against self-antigens and innocuous environmental antigens. Upon sensing “danger” signals under pathological conditions, however, they differentiate into fully mature DCs capable of initiating T cell-mediated protective immunity against potentially harmful antigens. The allergic contact hypersensitivity response (CHR) to reactive haptens has long been used as a standard model for studying the above process of LC maturation and immuno-stimulatory function of mature LCs [7]. This classic view of LC biology, however, fails to explain the most recent findings that LCs are incapable of presenting microbial antigens to T cells directly [8,9,10] and that allergic CHR is inducible even in the complete absence of epidermal LC networks [11,12,13]. In other words, functional contributions of LCs to adaptive immunity still remain somewhat controversial at present.
The immunobiology of epidermal LCs may be elucidated not only by studying their functional properties, but also by monitoring their behaviors. Little information is available with regard to the latter aspect, except for the accelerated trafficking of LCs from the epidermis to the draining lymph nodes observed under pathological conditions, such as the sensitization phase of allergic CHR [14,15,16,17]. Even this well-known motile behavior has been studied only indirectly by examining the number, morphology, and phenotype of LCs in the epidermis, underlying dermis, and lymph nodes in fixed tissue samples harvested from hapten-painted animals at different time points. To directly visualize dynamic LC behaviors in living animals, we recently developed an intravital confocal imaging system using I-Aβ-EGFP knock-in mice, in which the endogenous major histocompatibility complex class II I-Aβ chain is replaced by an EGFP-tagged version [18]. All EGFP+ epidermal cells in the knock-in mice expressed CD11c as well as Langerin (CD207) and both of these LC markers were detected only in an EGFP+ subpopulation of epidermal cells, allowing us to identify epidermal LCs in the absence of tissue fixation or staining [19,20]. In situ behaviors of EGFP+ LCs were then visualized by recording three-dimensional confocal images every two minutes in the ear skin of anesthetized I-Aβ-EGFP knock-in mice. In the steady state, small numbers (5–10%) of EGFP+ LCs exhibited a unique behavior, termed the dendrite surveillance extension and retraction cycling habitude (dSEARCH), characterized by rhythmic extension and retraction of their dendrites. When monitored after topical application of dinitrofluorobenzene (DNFB), a majority of LCs showed robust dSEARCH activity, as well as amoeba-like lateral displacement of their cell bodies within the epidermal compartment [19]. These behavioral responses we observed after hapten painting resemble the dynamic movement of LCs recorded by Kissenpfennig et al., in the Langerin-EGFP knock-in mice after tape-stripping [12]. In the present study, we sought to define underlying mechanisms by which epidermal LCs reprogram their motile activities under pathological conditions.
2. Materials and Methods
2.1. Animals
Construction and characteristic features of I-Aβ-EGFP mice are described elsewhere [18]. All animal experiments were approved by the Institutional Animal Care and Use Committee at UT Southwestern Medical Center and carried out according to the NIH guidelines.
2.2. Treatment with DNFB, recombinant cytokines, or cytokine antagonists
Three days after hair removal from the ear skin, the I-Aβ-EGFP mice received topical application of 0.5% DNFB or subcutaneous (s.c.) injection (50 ng/animal) of recombinant murine IL-1α or TNFα (both purchased from R&D Systems, Minneapolis, MN). Control mice received topical application of vehicle alone (acetone/olive oil) or s.c. injection of PBS alone. In some experiments, recombinant murine IL-1 receptor antagonist (IL-1Ra) (0.5 μg/animal, R&D Systems), recombinant human soluble TNF receptor-2 (sTNFR2) (0.5 μg/animal, Etanercept, Amgen, Thousand Oaks, CA), or PBS alone was s.c. injected into the ear skin 30 minutes before topical application of 0.5% DNFB.
2.3. Intravital confocal imaging
Intravital imaging experiments were performed with 8–10 week-old I-Aβ-EGFP mice as described previously [19]. Briefly, anesthetized I-Aβ-EGFP mice were placed on the stage of a Zeiss LSM 510 META 2P confocal microscope with the tip of the ear mounted so as to record three-dimensional image stacks (20 sequential x-y planes separated by 1.0 μm) of EGFP+ LCs every 2 minutes (up to 1 hour). Maximum intensity projections of the x-y planes for each image stack were then generated using the MetaMorph (Universal Imaging) and ImageJ (NIH) software programs. Dendrite length was measured from the tip of the dendrite to the cell body using the Measure function of the ImageJ software. The dSEARCH index was then calculated for each EGFP+ LC by adding the absolute values of the changes in dendrite length for all dendrites recorded over the previous 6 minutes period. The x-y positions of the center of a cell body were tracked at different time points using the Manual Tracking function of the ImageJ software. The total traveled distance was then calculated by adding the linear distances in the x-y plane measured between adjacent frames throughout the observation period expressed as micrometers per hour [19].
2.4. Statistical analyses
The statistical significance of the difference observed in the dSEARCH index or total traveled distance values was assessed by the Mann-Whitney U-test. Potential synergy between IL-1α and TNFα was examined using a two-factor analysis of variance (ANOVA) by comparing the outcome of combined IL-1α plus TNFα with the effects of individual cytokines.
3. Results
3.1. Static demonstration of motile activities of LCs
Cellular movement is best demonstrated by recording the location and shape of a target cell at given intervals and showing the resulting time-lapse images as a video. However, cellular motility has been displayed in a static manner by pseudo-coloring the images of a target cell recorded at different time points in different colors [12]. To test the latter approach, we acquired images of EGFP+ LCs every 2 minutes in the ear skin of I-Aβ-EGFP knock-in mice after local application of vehicle alone or 0.5% DNFB. In complete agreement with our previous observations [19], time-lapse videos showed only modest LC motility in the control skin (see Supplemental Movie S1) and markedly exacerbated LC movement in the DNFB-painted skin (Movie S2). After pseudo-coloring the images recorded at time 0 (green), 30 minutes (red), and 60 minutes (blue), we overlaid them to generate a superimposed picture in the three-color scheme. EGFP+ LCs in the vehicle-painted skin were mostly seen in “white” due to the lack of significant lateral displacement of their cell bodies, whereas some of the dendritic processes appeared in different colors, representing the dSEARCH motion (Fig. 1a). By marked contrast, LCs in DNFB-painted skin appeared remarkably colorful, reflecting amplified dSEARCH activity and lateral migration. By comparing the static three-color displays versus the videos generated from the same datasets, we concluded that both approaches would be equally effective for illustrating motile activities of LCs.
Fig. 1. Combined IL-1α plus TNFα mimics the effect of hapten treatment on LC behaviors.
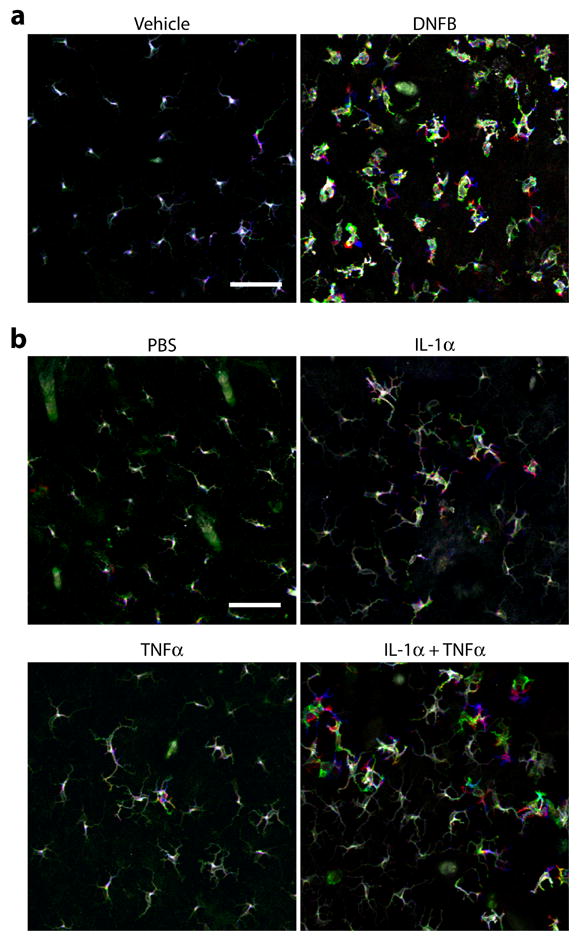
Dynamic behaviors of EGFP+ LCs were recorded in anesthetized I-Aβ-EGFP mice 30 hours after topical application of 0.5% DNFB or vehicle alone (a) or 6–8 hours after s.c. injection of a combination of IL-1α (50 ng/animal) and TNFα (50 ng/animal) or PBS alone (b). Each panel displays an overlay of three fluorescent images recorded at time 0 (pseudo-colored in green), time 30 minutes (red), and time 60 minutes (blue). Scale bar = 50 μm. The data are representative of at least three independent imaging experiments. Time-lapse images of LC behaviors produced from the same datasets can be viewed in Supplemental Movie S1 (vehicle painting), Movie S2 (DNFB painting), Movie S3 (PBS injection), Movie S4 (IL-1α injection), Movie S5 (TNFα injection), and Movie S6 (IL-1α + TNFα injection).
3.2. Impacts of locally injected IL-1α and TNFα on LC behaviors
Topical application of a reactive hapten is known to trigger expression of IL-1α and TNFα mRNA by keratinocytes [21,22,23]. Additionally, IL-1α and TNFα have been reported to mediate hapten-induced LC mobilization from the epidermis to the lymph nodes [14]. Thus, we sought to examine the potential contributions of these two proinflammatory cytokines to the observed behavioral responses of LCs to topically applied haptens. In the first set of experiments, we locally injected IL-1α or TNFα s.c. into the ear skin and recorded LC movement for 60 minutes at the early (1–3 hours), intermediate (6–8 hours), or late phase (24–26 hours). It should be noted that local injection of PBS alone caused no significant changes in the number, morphology, or motility of EGFP+ LCs (Fig. 1b and Movie S3). Locally injected IL-1α (50 ng/animal) augmented both dSEARCH activity and lateral migration of LCs with the most apparent changes observed during the intermediate phase (Fig. 1b and Movie S4). Local injection of TNFα (50 ng/animal) also elicited similar motile responses (Fig. 1b and Movie S5). Although the magnitude of such responses induced by individual cytokines was relatively modest, co-injection of IL-1α and TNFα produced robust dSEARCH and remarkable cellular migration (Fig. 1b and Movie S6).
3.3. Magnitudes and time-kinetics of cytokine-induced LC behavioral changes
To examine the dendrite movement in a more quantitative manner, we measured the “dSEARCH index”, representing the cumulative distances of dendrite extension and retraction observed for individual LCs [19]. Likewise, the extent of lateral migration of LCs was assessed by tracking their X-Y plane locations at different time points and calculating the “total traveled distance” during the 60-minute observation period [19]. Local injection of either IL-1α or TNFα elevated both parameters significantly above the control values observed after PBS injection (Fig. 2). Motile activities of LCs were further augmented by the combination of IL-1α and TNFα. Statistically significant synergy between the two cytokines was observed for dSEARCH index during the early and intermediate phases, as well as for total traveled distance during the intermediate and late phases.
Fig. 2. Magnitudes and time-kinetics of LC behavioral responses to IL-1α and/or TNFα.
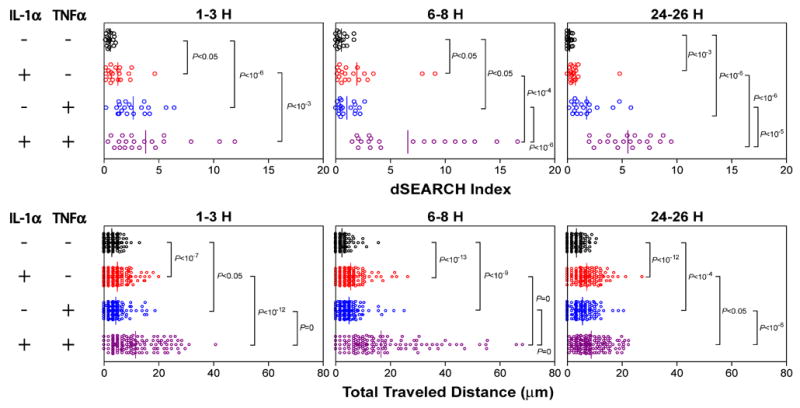
At the indicated time points after local cytokine injection, dynamic behaviors of EGFP+ LCs were recorded and the dSEARCH index (a) and the total traveled distance (b) values were measured. The dots represent the individual mean values measured for different EGFP+ LCs, whereas the bar represents the mean value over the entire panel. The P values indicate the level of statistically significant differences observed between the indicated panels as assessed by the Mann-Whitney U-test. Two-factor ANOVA revealed significant synergy between IL-1α and TNFα for dSEARCH index at 6–8 hours (P = 10−3) and 24–26 hours (P < 10−5) and for total travel distance at 1–3 hours (P < 10−9) and 6–8 hours (P < 10−8). Supplemental Material includes time-lapse images of LC behaviors recorded 6–8 hours after s.c. injection of PBS alone (Movie S3), IL-1α (Movie S4), TNFα (Movie S5), or IL-1α + TNFα (Movie S6).
Although the overall magnitude of motile activities of LCs induced by local injection of IL-1α plus TNFα was comparable to that observed after DNFB painting, the two stimuli caused such changes with different time-kinetics. Treatment with IL-1α plus TNFα augmented both dSEARCH index and total traveled distance rapidly with significant increases detectable within 1–3 hours and the peak responses observed at 6–8 hours. By contrast, DNFB-induced behavioral responses were most evident during a later period (24–30 hours). This delay may reflect the interval required for cytokine production because marked increases in IL-1α mRNA and TNFα mRNA expression were generally detected in the skin 2–12 hours after hapten application [21,22,23].
3.4. Inhibition of hapten-induced LC behavioral responses by cytokine antagonists
Having been able to duplicate hapten-induced behavioral responses of LCs by locally injecting IL-1α plus TNFα, we next determined whether one could prevent LC responses to hapten treatment with antagonists of the same two cytokines. For this purpose, we locally injected either recombinant IL-1Ra or sTNFR2 into the ear skin, applied 0.5% DNFB over the same skin site 30 min later, and recorded LC movement 30 hours after DNFB painting. In the control panel receiving PBS injection, EGFP+ LCs exhibited robust dSEARCH activity and lateral migration in response to DNFB application (Fig. 3a). By marked contrast, DNFB-induced behavioral responses were not noticeable in the panels receiving local injection of IL-1Ra or sTNFR2. This visual assessment was confirmed by measuring the two motile parameters; IL-1Ra and sTNFR2 both completely inhibited DNFB-induced increases in dSEARCH index and total traveled distance (Fig. 3b). These observations demonstrate that both IL-1 and TNFα are required for the observed motile responses of LCs to hapten treatment.
Fig. 3. Prevention of hapten-induced LC behavioral changes by local administration of a cytokine antagonist.
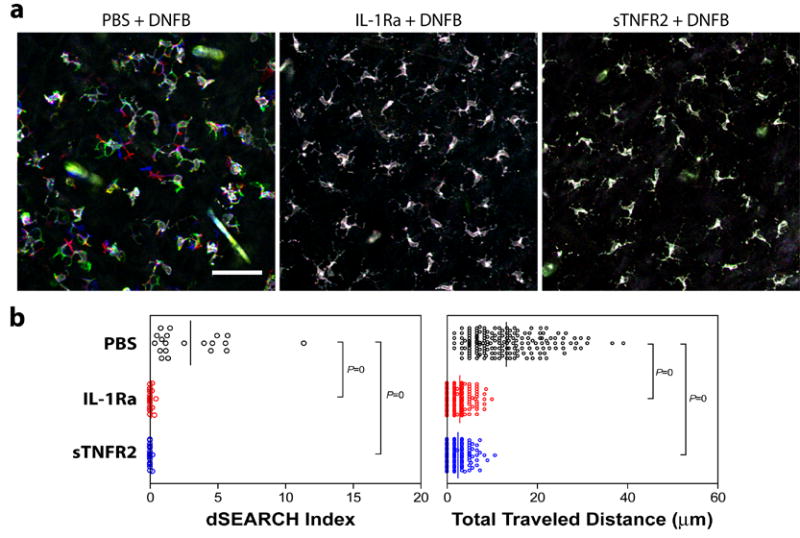
I-Aβ-EGFP mice received local injection of IL-1Ra (0.5 μg/animal), sTNFR2 (0.5 μg/animal), or PBS alone into the ear skin and, 30 minutes later, topical application of 0.5% DNFB on the same skin sites. Dynamic behaviors of EGFP+ LCs were recorded 30 hours after DNFB painting. (a) Each panel displays an overlay of confocal images recorded at time 0 (pseudo-colored in green), time 30 minutes (red), and time 60 minutes (blue). Scale bar = 50 μm. (b) The data were processed to calculate the dSEARCH index (left panel) and the total traveled distance (right panel). The dots represent the individual mean values measured for different EGFP+ LCs, whereas the bar represents the mean value of the entire panel. The P values indicate the statistical significance of the differences observed between the indicated panels as assessed by the Mann-Whitney U-test.
4. Discussion
The main objective of the present study was to define mechanisms by which pathological stimuli exacerbate LC movement. Our results indicate that both IL-1α and TNFα are required and sufficient for amplifying dSEARCH and triggering amoeba-like lateral migration of cell bodies. The same two cytokines have been postulated to mediate LC emigration from the epidermis under inflammatory conditions. This notion, however, is derived from the purely static observations that hapten-induced reduction in LC surface density was prevented by administrating selected antagonists of either IL-1 or TNFα and that LC density was diminished in the absence of hapten painting by injecting recombinant IL-1 or TNFα [14,24,25,26]. Our results now provide more direct evidence for the in vivo pharmacological activity of IL-1 and TNFα to reprogram LC motility and support the notion that the two cytokines play equally important roles in regulating LC behaviors in an interdependent fashion. It is likely that LC behaviors are controlled in a more complex manner by additional factors, including other cytokines, chemokines, Toll-like receptor ligands, and adhesion molecules [27]. The experimental approaches developed in the present study should be applicable to future studies for unveiling multiple layers of events that control in situ behaviors of LCs.
It is important to state major limitations of our present imaging study. First, although we visualized and measured dSEARCH motion of dendrites, it remains to be determined how (or even whether) this movement facilitates any immunological function of LCs. Two-color imaging experiments are in progress in our laboratory to study the spatial and temporal relationship between the extension and retraction movement of dendrites and the uptake of fluorescently labeled particles by LCs. Secondly, our confocal imaging system, which was optimized to maximize the X-Y plane resolution, is not most suitable for studying the process for vertical LC migration. Although EGFP+ LCs occasionally disappeared from the epidermal compartment during the 60 minutes imaging period after IL-1α plus TNFα treatment, no quantitative measurements could be made due to small sample numbers. This technical limitation must be overcome by improving the Z-axis resolution as well as the Z-axis depth for image acquisition. In other words, our findings should not be regarded to fully represent all the dynamic, three-dimensional motile activities of LCs.
In summary, we have demonstrated the roles of IL-1 and TNFα in hapten-induced augmentation of LC motility. Not only do our findings improve our understanding of the behavioral biology of LCs and the pathophysiology of allergic contact dermatitis, they also suggest a previously unrecognized mechanism of action for sTNFR2, Etanercept, which is now being administered successfully to patients with psoriasis [28,29].
Supplementary Material
Acknowledgments
We thank Dr. Hidde Ploegh for providing the I-Aβ-EGFP knock-in mice and Ms. Rachel Mohr for technical assistance. This study was supported by research grants from the NIH (A.T.).
Abbreviation list
- CHR
contact hypersensitivity response
- DNFB
dinitrofluorobenzene
- DC
dendritic cell
- dSEARCH
dendrite surveillance extension and retraction cycling habitude
- EGFP
enhanced green fluorescent protein
- IL-1Ra
IL-1 receptor antagonist
- LC
Langerhans cell
- s.c.
subcutaneous
- sTNFR
soluble TNF receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clark R, Kupper T. Old meets new: the interaction between innate and adaptive immunity. J Invest Dermatol. 2005;125:629–637. doi: 10.1111/j.0022-202X.2005.23856.x. [DOI] [PubMed] [Google Scholar]
- 2.Braff MH, Bardan A, Nizet V, Gallo RL. Cutaneous defense mechanisms by antimicrobial peptides. J Invest Dermatol. 2005;125:9–13. doi: 10.1111/j.0022-202X.2004.23587.x. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 4.Larrengina AT, Falo LD. Changing paradigms in cutaneous immunology: adapting with dendritic cells. J Invest Dermatol. 2005;124:1–12. doi: 10.1111/j.1523-1747.2004.23554.x. [DOI] [PubMed] [Google Scholar]
- 5.Romani N, Holzmann S, Tripp CH, Koch F, Stoitzner P. Langerhans cells – dendritic cells of the epidermis. Apmis. 2003;111:725–740. doi: 10.1034/j.1600-0463.2003.11107805.x. [DOI] [PubMed] [Google Scholar]
- 6.Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci USA. 2002;99:351–358. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aiba S, Katz SI. Phenotypic and functional characteristics of in vivo-activated Langerhans cells. J Immunol. 1990;145:2791–2796. [PubMed] [Google Scholar]
- 8.Allan RS, Smith CM, Belz GT, van Lint AL, Wakim LM, Heath WR, Carbone FR. Epidermal viral immunity induced by CD8α+ dendritic cells but not by Langerhans cells. Science. 2003;301:1925–1928. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- 9.Ritter U, Meissner A, Scheidig C, Korner H. CD8α- and Langerin-negative dendritic cells, but not Langerhans cells, act as principal antigen-presenting cells in leishmaniasis. Eur J Immunol. 2004;34:1542–1550. doi: 10.1002/eji.200324586. [DOI] [PubMed] [Google Scholar]
- 10.Zhao X, Deak E, Soderberg K, Linehan M, Spezzano D, Zhu J, Knipe DM, Iwasaki A. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J Exp Med. 2003;197:153–162. doi: 10.1084/jem.20021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett CL, van Rijn E, Jung S, Inaba K, Steinman RM, Kapsenberg ML, Clausen BE. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J Cell Biol. 2005;169:569–576. doi: 10.1083/jcb.200501071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kissenpfennig A, Henri S, Dubois B, et al. Dynamics and function of Langerhans cells in vivo dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23:611–620. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Cumberbatch M, Dearman RJ, Kimber I. Langerhans cells require signals from both tumour necrosis factor-alpha and interleukin-1 beta for migration. Immunology. 1997;92:388–395. doi: 10.1046/j.1365-2567.1997.00360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Love-Schimenti CD, Kripke ML. Dendritic epidermal T cells inhibit T cell proliferation and may induce tolerance by cytotoxicity. J Immunol. 1994;153:3450–3456. [PubMed] [Google Scholar]
- 16.Stoitzner P, Holzmann S, McLellan AD, et al. Visualization and characterization of migratory Langerhans cells in murine skin and lymph nodes by antibodies against Langerin/CD207. J Invest Dermatol. 2003;120:266–274. doi: 10.1046/j.1523-1747.2003.12042.x. [DOI] [PubMed] [Google Scholar]
- 17.Stoitzner P, Tripp CH, Douillard P, Saeland S, Romani N. Migratory langerhans cells in mouse lymph nodes in steady state and inflammation. J Invest Dermatol. 2005;125:116–125. doi: 10.1111/j.0022-202X.2005.23757.x. [DOI] [PubMed] [Google Scholar]
- 18.Boes M, Cerny J, Massol R, Op dB, Kirchhausen T, Chen J, Ploegh HL. T-cell engagement of dendritic cells rapidly rearranges MHC class II transport. Nature. 2002;418:983–988. doi: 10.1038/nature01004. [DOI] [PubMed] [Google Scholar]
- 19.Nishibu A, Ward BR, Jester JV, Ploegh HL, Boes M, Takashima A. Behavioral responses of epidermal Langerhans cells in situ to local pathological stimuli. J Invest Dermatol. 2006;126:787–796. doi: 10.1038/sj.jid.5700107. [DOI] [PubMed] [Google Scholar]
- 20.Vishwanath M, Nishibu A, Saeland S, Ward BR, Mizumoto N, Ploegh HL, Boes M, Takashima A. Development of Intravital Intermittent Confocal Imaging System for Studying Langerhans Cell Turnover. J Invest Dermatol. 2006 doi: 10.1038/sj.jid.5700448. [DOI] [PubMed] [Google Scholar]
- 21.Enk AH, Katz SI. Early molecular events in the induction phase of contact sensitivity. Proc Natl Acad Sci USA. 1992;89:1398–1402. doi: 10.1073/pnas.89.4.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang B, Zhuang L, Fujisawa H, et al. Enhanced epidermal Langerhans cell migration in IL-10 knockout mice. J Immunol. 1999;162:277–283. [PubMed] [Google Scholar]
- 23.Homey B, Assmann T, Vohr HW, Ulrich P, Lauerma AI, Ruzicka T, Lehmann P, Schuppe HC. Topical FK506 suppresses cytokine and costimulatory molecule expression in epidermal and local draining lymph node cells during primary skin immune responses. J Immunol. 1998;160:5331–5340. [PubMed] [Google Scholar]
- 24.Cumberbatch M, Fielding I, Kimber I. Modulation of epidermal Langerhans’ cell frequency by tumour necrosis factor-α. Immunology. 1994;81:395–401. [PMC free article] [PubMed] [Google Scholar]
- 25.Cumberbatch M, Dearman RJ, Kimber I. Interleukin 1β and the stimulation of Langerhans cell migration: comparisons with tumour necrosis factor α. Arch Dermatol Res. 1997;289:277–284. doi: 10.1007/s004030050193. [DOI] [PubMed] [Google Scholar]
- 26.Stoitzner P, Zanella M, Ortner U, et al. Migration of Langerhans cells and dermal dendritic cells in skin organ cultures: augmentation by TNF-α and IL-1β. J Leukoc Biol. 1999;66:462–470. [PubMed] [Google Scholar]
- 27.Jakob T, Ring J, Udey MC. Multistep navigation of Langerhans/dendritic cells in and out of the skin. J Allergy Clin Immunol. 2001;108:688–696. doi: 10.1067/mai.2001.118797. [DOI] [PubMed] [Google Scholar]
- 28.Leonardi CL, Powers JL, Matheson RT, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003;349:2014–2022. doi: 10.1056/NEJMoa030409. [DOI] [PubMed] [Google Scholar]
- 29.Gottlieb AB, Chamian F, Masud S, Cardinale I, Abello MV, Lowes MA, Chen F, Magliocco M, Krueger JG. TNF inhibition rapidly down-regulates multiple proinflammatory pathways in psoriasis plaques. J Immunol. 2005;175:2721–2729. doi: 10.4049/jimmunol.175.4.2721. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


