Abstract
Cytoskeletal motors convert the energy from binding and hydrolyzing ATP into conformational changes that direct movement along a cytoskeletal polymer substrate. These enzymes utilize different mechanisms to generate long-range motion on the order of a micron or more that is required for functions ranging from muscle contraction to transport of growth factors along a nerve axon. Several of the individual cytoskeletal motors are processive, meaning that they have the ability to take sequential steps along their polymer substrate without dissociating from the polymer. This ability to maintain contact with the polymer allows individual motors to move cargos quickly from one cellular location to another. Many of the processive motors have now been found to utilize secondary binding sites that aid in motor processivity.
Keywords: motor, processivity, cytoskeleton, kinesin, dynein, dynactin, myosin, molecular tether
INTRODUCTION
From the initial powerstroke model of Huxley in 19571 to the papers published today, there has been an ever-expanding interest in the molecular basis of intracellular motor function. Cytoskeletal motors couple the chemical steps of ATP binding and hydrolysis to mechanical conformational changes that drive movement of the motor along its substrate. The conformational changes in the motor protein can be thought of as mechanical steps that occur during each ATP hydrolysis cycle. The mechanical steps include binding to the polymer substrate, the power stroke, release from the polymer and the recovery stroke. The direct relationships between chemical and mechanical steps into the mechanochemical cycle are becoming clearer for several cytoskeletal motors and have been the subject of other recent reviews (refs. 2–5). An essential point for understanding motor processivity is that in at least one step of the mechanochemical cycle, a motor head must dissociate from the polymer. By doing so, that motor domain can recock its head during the recovery stroke. If a motor domain did not dissociate, that motor domain would move back during the recovery stroke the same distance it just traveled forward. Such an ineffectual attempt to prepare for the next power stroke would lead to futile movement and needless burning of ATP in the cell. This leads to a critical question about processive cytoskeletal motor function. How do cytoskeletal motors take multiple steps along their polymer substrates if the motor heads have to dissociate once during each mechanochemical cycle?
STRATEGY 1: HIGH PROCESSIVITY OF AN INDIVIDUAL TWO-HEADED MOTOR
For a typical two-headed motor to be processive, it must always have at least one head bound to the polymer substrate. If both heads let go of the substrate at the same time, the motor would diffuse away as it would no longer have any connection to that substrate. Those two-headed motors that are processive utilize a coordinated motion to ensure that at least one head is always bound to the polymer. The prevailing view, supported by many indirect and a few direct observations, is that two-headed motors move along the polymer with a hand-over-hand motion. To understand how this works, let’s start with both heads of a motor bound to sequential subunits of the polymer track (Fig. 1). When the back head dissociates from the polymer it reaches forward to the next sequential binding domain on the polymer beyond the front head. This motion equals a single step along the polymer; different motors can have different step sizes. This hand-over-hand motion is repeated for the other head, moving the motor one more step along the polymer. Repeating this process, the motor will continue stepping until it reaches the end of the polymer or until it releases both heads from the polymer at the same time. In either case, the cargo of the motor has been transported a number of steps away from its initial location.
Figure 1.
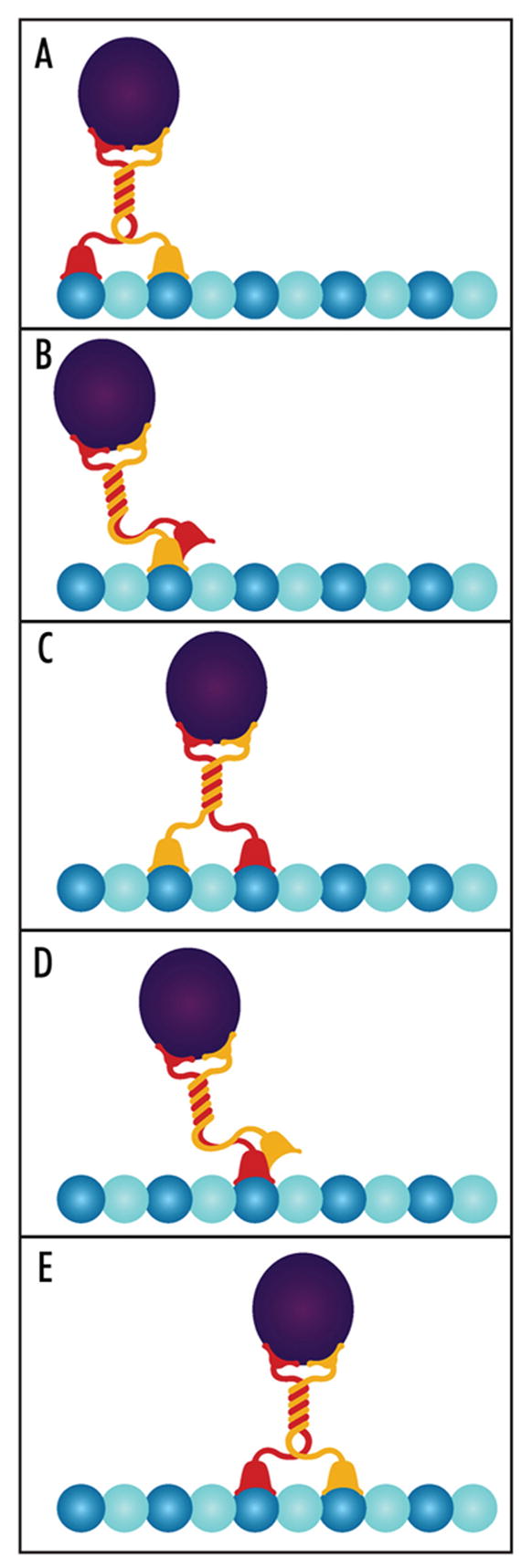
Hand-over-hand mechanism for a two-headed motor such as kinesin-1. (A) A two-headed motor binds to sequential binding sites (dark blue) along a polymer. The rear-most head (red) releases and swings forward (B) until it binds to the next binding site along the polymer (C). At this point, the cargo (purple) has moved forward one step. The other head (yellow) subsequently releases and swings forward (D) until it binds to the next available binding site (E). The cargo has now moved two steps along the polymer. The coordination of the two heads is apparent as the release of the rear head only occurs after the front head has bound tightly to the polymer and induced a strain upon the linkage between the two heads.
The fraction of time that each motor head is bound to the substrate is called the duty ratio of the motor.2,6,7 As described above, two-headed processive motors have each head bound to the substrate at least 50% of the time, so their duty ratio will be greater than 0.50. In addition to the numeric value of the duty ratio, it is also critical to consider the coordination (or gating) between the motor heads. In highly processive motors, the heads alternate steps and the timing of the individual steps of one motor head take into account the mechanochemical status of the other motor head bound to the polymer.
Kinesin-1, previously termed both conventional kinesin and KIF5, is a good example of a two-headed processive motor. Initial single molecule experiments with kinesin stuck to a coverslip surface showed that the individual kinesin motors can propel a bound micro-tubule along the coverslip surface,8 a process called microtubule gliding. These types of experiments have been augmented by single molecule experiments with fixed microtubules in which GFP-tagged kinesin or kinesin bound to beads moves along the microtubules.9–17 Collectively, these experiments show that individual kinesin molecules can take upwards of 100 sequential steps and can easily travel distances greater than one micron. For kinesin-1, each head spends over 50% of its time bound to the microtubule, giving duty ratios larger than 0.50.8,18
For kinesin-1, not only do the two heads each contact the micro-tubule at least 50% of the ATP cycle, recent work has shown that the heads coordinate their mechanochemical cycles so that at least one head is always bound to the microtubule.10,14 This is accomplished by an intricate communication between the two heads. The rear-most head does not dissociate from the microtubule until the front head has bound to the next sequential binding site and induced a strain upon the linkage between the front and rear heads. Furthermore, this strain can only occur if both heads are bound tightly to the micro-tubule; weak interactions with the microtubule are not sufficient to induce the necessary conformational strain. The conformation within the heads induced by the strain facilitates the dissociation of ADP from the rear head and its concomitant release from the microtubule for a forward step14 or the rare release of the front head for a backstep.10 This complicated interplay between the two heads ensures that at least one head is in contact with the polymer substrate at all times, preventing premature dissociation during the mechanochemical cycle. By continuing this coordinated stepping for multiple cycles, the kinesin-1 motor takes successive steps along the microtubule and therefore is a processive motor.
STRATEGY 2: MULTIPLE MOTORS WORK TOGETHER TO MOVE A COMMON CARGO
Using this strategy, two or more independent motors need to be bound to the same cargo (Fig. 2). Then, if one motor falls off at any point in the mechanochemical cycle, the additional motor(s) maintains contact with the microtubule and continues moving the cargo along the polymer. As long as there is at least one motor bound to the polymer at all times, the cargo will not diffuse away from the polymer. Therefore, the overall motion of the cargo may be driven by different individual motors instead of by a single continuously-active motor. The myosin II motors that form a sarcomere thick filament are a good example of this type of motor.19,20
Figure 2.
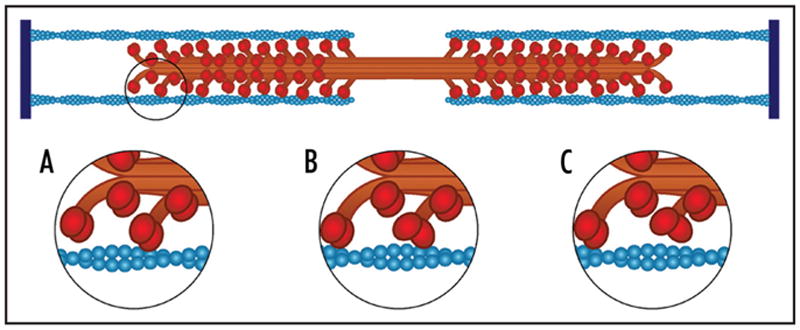
Multiple myosin motors work together in the sarcomere. At any given point only a small fraction of the myosin dimers are bound to actin thin filaments. (A) shows that a single head (red) of a myosin dimer has bound to the filament. In (B), that head has taken its power stroke and another myosin dimer has bound the filament. (C) shows that the release of the first head from the filament does not result in complete dissociation as the other myosin heads maintain contact with the filament.
From studies of myosin II in single molecule assays and in kinetic assays, it is clear that the myosin II mechanochemical cycle includes a step where the motor head dissociates from the polymer track.20–23 In myosin-II’s case, the time spent dissociated from the actin polymer constitutes the vast majority of the mechanochemical cycle, resulting in duty ratios estimated from 0.01 to 0.14.2 There does not appear to be any significant coordination of the mechanochemical cycles of the two heads of a myosin dimer,24–26 unlike what has been seen for kinesin-1. Therefore, during the time that one of the two heads is dissociated from the actin polymer, it is extremely likely that the other head will also dissociate from the polymer. Once both heads have dissociated, there remains no contact between that myosin-II dimer and the actin filament so the motility event of an individual myosin-II dimer is short lived and typically encompasses only a single step. Therefore individual myosin-II dimers are nonprocessive. In the cell, myosin-II motors are assembled into large arrays in the thick filament of the sarcomere (Fig. 2). In this case, the lack of processivity of any individual myosin dimer does not prevent the thick filament from generating long-range motility events on the order of 500 nm/sarcomere. This is because even as an individual myosin II dimer completely dissociates from the actin thin filament, many of the ~300 other motor heads27 present on the thick filament can independently bind and take an additional step along the actin thin filament. In this way, long-range processive motility occurs that is based on an innately nonprocessive motor.
ADDITIONAL STRATEGIES: UTILIZING SECONDARY BINDING SITES
For several years these two strategies were proposed as the only mechanisms used by cytoskeletal motors to achieve long-range motion. However, as more motors have been examined, it has become evident that additional mechanisms can also be utilized. In particular, studies of cytoplasmic dynein and of kinesin-3 have shown the importance of secondary binding sites in motor processivity.
In vitro studies have shown that individual cytoplasmic dynein motors can take multiple steps along the microtubule. However, the run lengths are generally less than one micron,28–30 distances that are significantly shorter than those observed for the conventional kinesin motors. Therefore, although single dynein molecules can take successive steps along the microtubule, they are not as processive as kinesin-1 motors. There has not been sufficient progress to determine if the lower processivity of dynein is due to a lower duty ratio of dynein, a lack of coordination between the two heads of dynein, or a combination of these two factors.
Studies of dynein function have also examined the dynein activator complex known as dynactin for potential roles in dynein motor processivity. Dynactin is a megadalton polypeptide complex that has three functional domains: microtubule-binding, dynein-binding, and cargo binding. In vitro motility studies of the role of dynactin in the actual movement of cytoplasmic dynein show that dynactin increases the processivity of the dynein motor two-fold to four-fold over the distance traveled by a single dynein molecule alone.28,31 The microtubule-binding ability of the p150 subunit of dynactin was found to be essential for this processivity enhancement.28 More recent work determined that the p150 subunit of dynactin actually has two sequential microtubule- binding domains. The N-terminus of p150 contains a CAP-Gly domain, a known microtubule interaction domain found in the CLIP-170-related family of proteins. The CAP-Gly domain is followed by the basic microtubule-binding domain, which has a high percentage of basic amino acids and shows conservation of charge and the organization of that charge across metazoan organisms. A recombinant dynactin p150 fragment that contains only the CAP-Gly domain actually inhibits dynein motility, apparently by binding tightly to the microtubule and acting much like an anchor to prevent dynein motility. In contrast, a recombinant fragment that contained only the basic microtubule-binding domain was able to enhance the processivity of dynein at least as well as native dynactin.31 This recombinant polypeptide exhibits an unusual property on its own: the ability to “skate” in vitro by one-dimensional diffusion along a microtubule lattice in the absence of a motor protein.31 Native dynactin molecules as well as all dynactin fragments that contained the basic domain were able both to enhance dynein processivity and to skate along microtubules. In contrast, the CAP-Gly microtubule-binding domain of p150 as well as the microtubule- binding domains of tau or CLIP-170 do not enhance dynein processivity and were unable to skate along microtubules.31 Within native dynactin molecules, the ability of the CAP-Gly domain to bind microtubules is regulated by phosphorylation.32 This provides a potential mechanism by which the dynactin CAP-Gly domain may be inactivated so that only the dynactin basic domain interacts with microtubules during processive dynein movements. Therefore the dynein/dynactin complex has a secondary binding site to the micro-tubule polymer provided by the dynactin basic domain, which acts as a second molecule processivity factor for cytoplasmic dynein.
The mechanism used by the basic domain has yet to be determined but appears likely to use multiple charge-charge interactions between the basic domain of dynactin p150 and the exposed acidic tail of tubulin dimers along the microtubule (S. King, unpublished data). In vertebrate species, p150 basic domains typically contain 13–15 basic amino acids whereas the C-terminus of alpha tubulin has 8 acidic amino acids and the C-terminus of β tubulin has 10 acidic residues. We propose (Fig. 3) that the dynein motor generates enough force to break some of the dynactin-microtubule charge-charge interactions at the same time that new sequential interactions are forming between the basic domain and the next tubulin dimer in the microtubule. We expect that the cumulative energy required to break all of the charge-charge interactions (as would occur if this domain completely dissociated from the microtubule) is significantly more than the energy required to break and remake individual charge-charge interactions as the polypeptide skates along the microtubule. This leads to a hypothesis that the basic domain of dynactin acts as a mobile tether between dynein and the microtubule; if the dynein motor dissociates from the microtubule while dynactin maintains contact between the motor, cargo and the microtubule, then the dynein motor can rebind the microtubule. The likelihood of dynein rebinding to the same (or a neighboring33) protofilament is extremely high because the motor is tethered to the microtubule.
Figure 3.
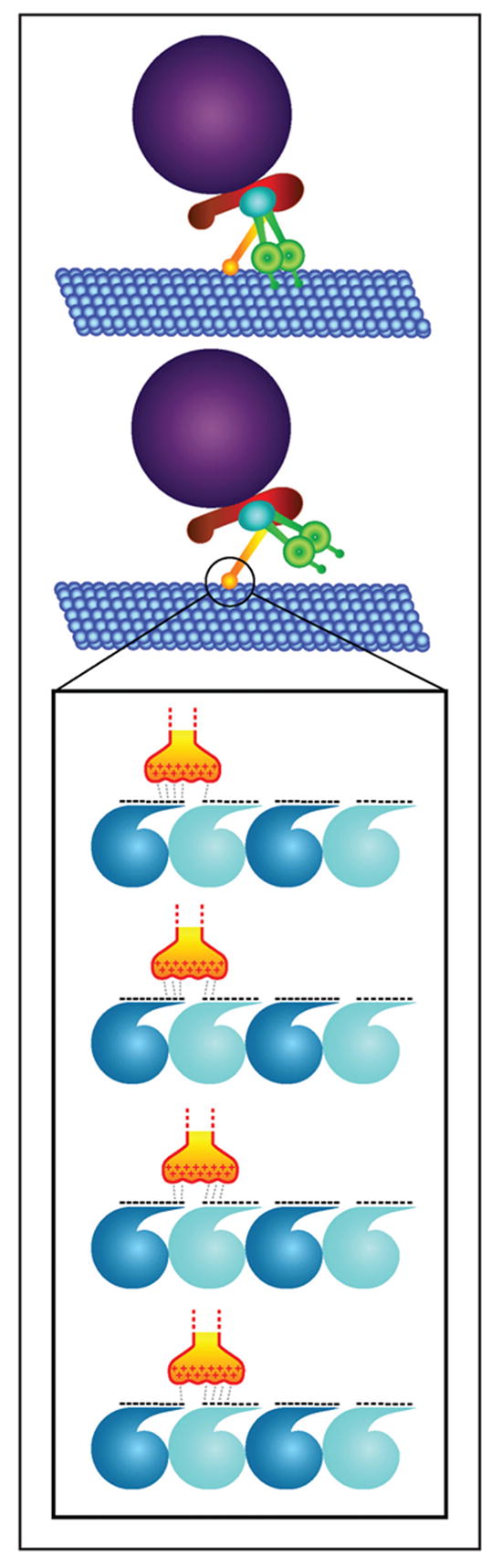
Dynactin acts as a molecular tether for cytoplasmic dynein. The base of dynactin (red) binds cargo (purple) whereas the p150 subunit of dynactin (yellow) binds to the microtubule (blue). If both cytoplasmic dynein motor domains (green) fall off the microtubule dynein, dynactin provides a secondary linkage between dynein, the cargo, and the microtubule. The inset shows a potential mechanism for how the dynactin secondary binding site could act as a tether. The basic amino acids of dynactin p150 (+) interact with the acidic amino acids found at the C-terminal tail of tubulin (−). The charge-charge interactions between the basic and acidic residues are sequentially formed and broken as the dynein motor moves, so that at any point in time, the basic domain maintains several charge-charge interactions with the microtubule. This mechanism provides a tether that is continually in contact with the microtubule even while dynein is propelling cargo along the microtubule.
Is there supporting evidence from other motors for a secondary binding site playing an important role in motor processivity? Studies with kinesin-1, kinesin-3, and myosin-V have also indicated the importance of secondary binding sites. The neck-linker region of kinesin-1 has been shown to be critical for processivity as deletions in this region decrease kinesin processivity about 10-fold.13 Other alterations of this region also enhanced or decreased processivity. Some of the more interesting changes to this region were alterations in the net charge of this normally basic region of the kinesin motor. Specifically, alterations that made this domain more basic either by adding in net basic heptads or by site-directed mutagenesis of selected amino acids increased the processivity of the motor whereas changes that decreased the net basic charge significantly decreased the motor processivity.13,15 Further support of a charge-charge interaction being important in the mechanism for kinesin-1 processivity comes from studies in which motor processivity was significantly reduced by increasing the ionic strength of the buffer15 or removing the acidic tail of tubulin.34
Kinesin-3 motors have been an enigma as there have been conflicting reports as to the processivity of these motors. Chimeric monomers of the kinesin-3 motor have been studied predominantly by Okada and Hirokawa. In their studies, the monomeric proteins (containing a single motor head) were capable of processive motility along microtubule tracks.35 This behavior posed a serious conundrum because as discussed earlier, the very act of stepping along the microtubule has been thought to include a dissociation from the polymer in order for the recovery stroke to occur. These researchers identified a particular loop that was required for the longer-range movement of the motor.36 This loop (called the K-loop) contains a large number of lysine residues that appear to contact the acidic tail of tubulin via charge-charge interactions.35,37,38 It is thought that the K-loop makes a weak interaction with the microtubule during the time when the strong-binding domain of the motor dissociates from the microtubule. In effect, the K-loop allows “semi-processive” biased Brownian motion of a single motor head along the microtubule.36 The secondary binding site mechanism used by kinesin-3 appears remarkably similar to the mechanism thought to be used by dynactin to boost dynein processivity. The main differences are in the number of heads of the motors and the presence of the secondary binding site on the same molecule (kinesin-3) or on a separate but physically linked molecule (dynactin).
Myosin V is a two-headed processive myosin that plays important roles in the transport of vesicles and other cellular cargoes. A recent study has shown that myosin V also utilizes a secondary binding site to enhance its processivity. Processive versions of myosin V contain a basic loop39 that binds to an acidic patch on the actin filament during the weak-binding stage of the ATPase cycle.40 The addition of this domain to less processive forms of myosin V that lack the basic amino acids increases the processivity of the motor along an actin filament.39 Similar to kinesin-1, the processivity of the chimera containing the basic loop decreases as the salt concentration of the buffer increases.39
CONCLUSIONS AND FUTURE QUESTIONS
The presence of secondary binding sites is becoming more apparent in processive two-headed motors. Are these sites actually essential for motor processivity? So far, the secondary binding sites appear to have several features in common, including a composition that includes several basic amino acids and the ability to exhibit weak binding interactions with acidic stretches along the polymer substrate. Furthermore, several of these weak interactions appear to maintain contact with the polymer in a manner that requires minimal energy investment/drag force upon the motor complex as the motor is moving. In these cases, the secondary contact sites may provide a tether or life-line for the motor to remain bound to the polymer. With such a tether in place, each motor has a greater likelihood of maintaining contact with the polymer and continuing motility even if the primary binding site located in the motor heads dissociates or “trips” while walking along the polymer. Further work will be needed to determine if other processive cytoskeletal motors utilize secondary binding sites to increase motor processivity.
Acknowledgments
S.J.K. is supported by a grant from the NIH (NS48501). The authors would like to thank Drs. M. Plamann, M. Ferrari, and L. Ehler for critical discussions of this work.
References
- 1.Huxley AF. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- 2.Howard J. Mechanics of motor proteins and the cytoskeleton. Sunderland, MA: Sinauer Associates Inc; 2001. [Google Scholar]
- 3.Krendel M, Mooseker MS. Myosins: Tails (and heads) of functional diversity. Physiology. 2005;20:239–51. doi: 10.1152/physiol.00014.2005. [DOI] [PubMed] [Google Scholar]
- 4.Vale RD. Myosin V motor proteins: Marching stepwise towards a mechanism. J Cell Biol. 2003;163:445–50. doi: 10.1083/jcb.200308093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vale RD, Milligan RA. The way things move: Looking under the hood of molecular motor proteins. Science. 2000;288:88–95. doi: 10.1126/science.288.5463.88. [DOI] [PubMed] [Google Scholar]
- 6.Dillon PF, Murphy RA. High force development and crossbridge attachment in smooth muscle from swine carotid arteries. Circ Res. 1982;50:799–804. doi: 10.1161/01.res.50.6.799. [DOI] [PubMed] [Google Scholar]
- 7.Howard J. Molecular motors: Structural adaptations to cellular functions. Nature. 1997;389:561–7. doi: 10.1038/39247. [DOI] [PubMed] [Google Scholar]
- 8.Howard J, Hudspeth AJ, Vale RD. Movement of microtubules by single kinesin molecules. Nature. 1989;342:154–8. doi: 10.1038/342154a0. [DOI] [PubMed] [Google Scholar]
- 9.Block SM, Goldstein LB, Schnapp BJ. Bead movement by single kinesin molecules studied with optical tweezers. Nature. 1990;348:348–52. doi: 10.1038/348348a0. [DOI] [PubMed] [Google Scholar]
- 10.Guydosh NR, Block SM. Backsteps induced by nucleotide analogs suggest the front head of kinesin is gated by strain. Proc Natl Acad Sci USA. 2006;103:8054–9. doi: 10.1073/pnas.0600931103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pierce DW, Hom-Booher N, Otsuka AJ, Vale RD. Single-molecule behavior of monomeric and heteromeric kinesins. Biochemistry. 1999;38:5412–21. doi: 10.1021/bi9830009. [DOI] [PubMed] [Google Scholar]
- 12.Pierce DW, Vale RD. Assaying processive movement of kinesin by fluorescence microscopy. Methods Enzymol. 1998;298:154–71. doi: 10.1016/s0076-6879(98)98016-8. [DOI] [PubMed] [Google Scholar]
- 13.Romberg L, Pierce DW, Vale RD. Role of the kinesin neck region in processive microtubule-based motility. J Cell Biol. 1998;140:1407–16. doi: 10.1083/jcb.140.6.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenfeld SS, Fordyce PM, Jefferson GM, King PH, Block SM. Stepping and stretching. How kinesin uses internal strain to walk processively. J Biol Chem. 2003;278:18550–6. doi: 10.1074/jbc.M300849200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thorn KS, Ubersax JA, Vale RD. Engineering the processive run length of the kinesin motor. J Cell Biol. 2000;151:1093–100. doi: 10.1083/jcb.151.5.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomishige M, Vale RD. Controlling kinesin by reversible disulfide cross-linking. Identifying the motility-producing conformational change. J Cell Biol. 2000;151:1081–92. doi: 10.1083/jcb.151.5.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vale RD, Funatsu T, Pierce DW, Romberg L, Harada Y, Yanagida T. Direct observation of single kinesin molecules moving along microtubules. Nature. 1996;380:451–3. doi: 10.1038/380451a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schnapp BJ, Crise B, Sheetz MP, Reese TS, Khan S. Delayed start-up of kinesin-driven microtubule gliding following inhibition by adenosine 5’-[{beta},{gamma}-Imido]Triphosp hate. PNAS. 1990;87:10053–7. doi: 10.1073/pnas.87.24.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harada Y, Sakurada K, Aoki T, Thomas DD, Yanagida T. Mechanochemical coupling in actomyosin energy transduction studied by in vitro movement assay. J Mol Biol. 1990;216:49–68. doi: 10.1016/S0022-2836(05)80060-9. [DOI] [PubMed] [Google Scholar]
- 20.Uyeda TQ, Kron SJ, Spudich JA. Myosin step size. Estimation from slow sliding movement of actin over low densities of heavy meromyosin. J Mol Biol. 1990;214:699–710. doi: 10.1016/0022-2836(90)90287-V. [DOI] [PubMed] [Google Scholar]
- 21.Finer JT, Simmons RM, Spudich JA. Single myosin molecule mechanics: Piconewton forces and nanometre steps [see comments] Nature. 1994;368:113–9. doi: 10.1038/368113a0. [DOI] [PubMed] [Google Scholar]
- 22.Ishijima A, Kojima H, Higuchi H, Harada Y, Funatsu T, Yanagida T. Multiple- and single-molecule analysis of the actomyosin motor by nanometer-piconewton manipulation with a microneedle: Unitary steps and forces. Biophys J. 1996;70:383–400. doi: 10.1016/S0006-3495(96)79582-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molloy JE, Burns JE, Kendrick-Jones J, Tregear RT, White DC. Movement and force produced by a single myosin head. Nature. 1995;378:209–12. doi: 10.1038/378209a0. [DOI] [PubMed] [Google Scholar]
- 24.Harada Y, Noguchi A, Kishino A, Yanagida T. Sliding movement of single actin filaments on one-headed myosin filaments. Nature. 1987;326:805–8. doi: 10.1038/326805a0. [DOI] [PubMed] [Google Scholar]
- 25.Ruff C, Furch M, Brenner B, Manstein DJ, Meyhofer E. Single-molecule tracking of myosins with genetically engineered amplifier domains. Nat Struct Biol. 2001;8:226–9. doi: 10.1038/84962. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka H, Ishijima A, Honda M, Saito K, Yanagida T. Orientation dependence of displacements by a single one-headed myosin relative to the actin filament. Biophys J. 1998;75:1886–94. doi: 10.1016/S0006-3495(98)77629-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bagshaw CR. Muscle contraction. London, New York: Chapman and Hall; 1993. [Google Scholar]
- 28.King SJ, Schroer TA. Dynactin increases the processivity of the cytoplasmic dynein motor. Nat Cell Biol. 2000;2:20–4. doi: 10.1038/71338. [DOI] [PubMed] [Google Scholar]
- 29.Mallik R, Carter BC, Lex SA, King SJ, Gross SP. Cytoplasmic dynein functions as a gear in response to load. Nature. 2004;427:649–52. doi: 10.1038/nature02293. [DOI] [PubMed] [Google Scholar]
- 30.Wang Z, Khan S, Sheetz MP. Single cytoplasmic dynein molecule movements: Characterization and comparison with kinesin. Biophys J. 1995;69:2011–23. doi: 10.1016/S0006-3495(95)80071-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Culver-Hanlon TL, Lex SA, Stephens AD, Quintyne NJ, King SJ. A microtubule-binding domain in dynactin increases dynein processivity by skating along microtubules. Nat Cell Biol. 2006;8:264–70. doi: 10.1038/ncb1370. [DOI] [PubMed] [Google Scholar]
- 32.Vaughan PS, Miura P, Henderson M, Byrne B, Vaughan KT. A role for regulated binding of p150(Glued) to microtubule plus ends in organelle transport. J Cell Biol. 2002;158:305–19. doi: 10.1083/jcb.200201029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reck-Peterson SL, Yildiz A, Carter AP, Gennerich A, Zhang N, Vale RD. Single-molecule analysis of dynein processivity and stepping behavior. Cell. 2006;126:335–48. doi: 10.1016/j.cell.2006.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Z, Sheetz MP. The C-terminus of tubulin increases cytoplasmic dynein and kinesin processivity. Biophys J. 2000;78:1955–64. doi: 10.1016/S0006-3495(00)76743-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okada Y, Hirokawa N. A processive single-headed motor: Kinesin superfamily protein KIF1A. Science. 1999;283:1152–7. doi: 10.1126/science.283.5405.1152. [DOI] [PubMed] [Google Scholar]
- 36.Okada Y, Hirokawa N. Mechanism of the single-headed processivity: Diffusional anchoring between the K-loop of kinesin and the C terminus of tubulin. Proc Natl Acad Sci USA. 2000;97:640–5. doi: 10.1073/pnas.97.2.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alonso MC, van Damme J, Vandekerckhove J, Cross RA. Proteolytic mapping of kinesin/ncd-microtubule interface: Nucleotide-dependent conformational changes in the loops L8 and L12. Embo J. 1998;17:945–51. doi: 10.1093/emboj/17.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woehlke G, Ruby AK, Hart CL, Ly B, Hom-Booher N, Vale RD. Microtubule interaction site of the kinesin motor. Cell. 1997;90:207–16. doi: 10.1016/s0092-8674(00)80329-3. [DOI] [PubMed] [Google Scholar]
- 39.Krementsova EB, Hodges AR, Lu H, Trybus KM. Processivity of chimeric class V myosins. J Biol Chem. 2006;281:6079–86. doi: 10.1074/jbc.M510041200. [DOI] [PubMed] [Google Scholar]
- 40.Volkmann N, Liu H, Hazelwood L, Krementsova EB, Lowey S, Trybus KM, Hanein D. The structural basis of myosin V processive movement as revealed by electron cryomicroscopy. Mol Cell. 2005;19:595–605. doi: 10.1016/j.molcel.2005.07.015. [DOI] [PubMed] [Google Scholar]


