Abstract
Steroidogenic factor (SF1, NR5A1, Ad4BP) is an orphan nuclear receptor that is essential for steroid hormone-biosynthesis and endocrine development. Studies have found that the ability of this receptor to increase target gene expression can be regulated by post-translational modification, subnuclear localization, and protein-protein interactions. Recent crystallographic studies and our mass spectrometric analyses of the endogenous receptor have demonstrated an integral role for ligand-binding in the control of SF1 transactivation activity. Herein, we discuss our findings that sphingosine is an endogenous ligand for SF1. These studies and the structural findings of others have demonstrated that the receptor can bind both sphingolipids and phospholipids. Thus, it is likely that multiple bioactive lipids are ligands for SF1 and that these lipids will differentially act to control SF1 activity in a context-dependent manner. Finally, these findings highlight a central role for bioactive lipids as mediators of trophic-hormone stimulated steroid hormone biosynthesis.
Keywords: steroidogenic factor-1, CYP17, sphingosine, cAMP, adrenocorticotropin
1. Regulation of SF1 activity
Steroid hormone biosynthesis involves the concerted action of a group of proteins that regulate substrate delivery and metabolism. Both the activity and expression of these enzymes is controlled by the integration of signaling pathways. One of these signal transduction pathways involves the activation of the cAMP-dependent protein kinase (PKA). A consequence of PKA activation is increased transcription of steroidogenic genes. This increase in transcription is mediated by the binding of various transcription factors, including SF1, to cAMP responsive sequences in the promoters of steroidogenic genes (Sewer and Waterman, 2001; Sewer and Waterman, 2003). SF1 is a nuclear receptor that plays a key role not only in steroidogenesis (Bakke et al., 2001), but also in endocrine development and sex differentiation (Hammer et al., 2005; Parker et al., 2002).
Many laboratories have carried out research to determine the mechanism by which SF1 activates steroidogenic gene transcription. Although transcription of steroidogenic genes proceeds through activation of PKA, SF1 is not directly phosphorylated by PKA (Aesoy et al., 2002). PKA has been shown, however, to increase the half-life of SF1 (Aesoy et al., 2002). We have shown that phosphatase activity is required for SF1-dependent transcription of several steroidogenic genes (Sewer and Waterman, 2002a; Sewer and Waterman, 2002b) and that cAMP induces dephosphorylation of SF1 (Sewer and Waterman, 2002a). Phosphorylation of serine-203 is key for coactivator binding and the transactivation potential of the receptor (Hammer et al., 1999). The ability of SF1 to activate target gene expression is also regulated by SOMUylation (Chen et al., 2004; Komatsu et al., 2004; Lee et al., 2005), acetylation (Chen et al., 2005; Ishihara and Morohashi, 2005; Jacob et al., 2001), and interaction with various coregulatory proteins (Chen et al., 2005).
Previous studies to discern the role of ligand binding in the ability of SF1 to activate gene expression found that the ligand binding domain (LBD) of murine SF1 adopts an active conformation, with helices 1 and 12 packed against the predicted alpha-helical bundle, in the apparent absence of ligand, predicted ligand-independent activation (Desclozeaux et al., 2002). However, subsequent crystallographic studies carried out by three laboratories have shown that phospholipids are ligands for SF1 (Ingraham and Redinbo, 2005; Krylova et al., 2005; Li et al., 2005; Wang et al., 2005). The interaction of phosphatidylinositol phosphates (PIPs) with the LBD of murine SF1 has been shown to be required for maximal activity of the receptor (Krylova et al., 2005). Li et al. found that the receptor has a large LBD (approximately 1600 Å) that interacts with phospholipids that have fatty acid side chains between twelve and eighteen carbons and thus inferred that SF1 may readily exchange its ligands to respond to different phospholipid species (Li et al., 2005).
3. Bioactive sphingolipids in steroidogenesis
Sphingolipids are a diverse family of amphiphatic molecules that are comprised of a long-chain sphingoid base backbone, a polar head group, and an amide-linked, long-chain fatty acid. Due to the large concentration of sphingolipids such as sphingomyelin in the plasma membrane, it was held that these molecules only served structural roles. However, over the past few decades, many studies have demonstrated that these molecules, notably ceramide and sphingosine-1-phosphate (S1P), play integral roles in mediating varied cellular processes (Hannun and Obeid, 2002; Igarashi et al., 2003; Merrill Jr. et al., 1997; Spiegel and Milstien, 2002; Spiegel and Milstien, 2003; Strasberg and Callahan, 1988; Tilly and Kolesnick, 2002; Vesper et al., 1999). Ceramide is a second messenger for events as diverse as differentiation, senescence, proliferation, cell cycle arrest, and apoptosis (Hannun, 1994; Hannun et al., 2001; Kolesnick, 2002). S1P also modulates a wide variety of physiological functions, including cell proliferation and survival (Castillo and Teegarden, 2001; Olivera and Spiegel, 1993; Olivera A et al., 1999; Spiegel and Milstien, 2002), chemotaxis (Hla et al., 1999), and in protection against ceramide-mediated apoptosis (Cuvillier et al., 1996). In addition to the numerous roles for S1P as an intracellular effector, S1P also regulates cellular processes by binding to a subfamily of S1P G-protein-coupled receptors (Goetzl and An, 1998; Hla et al., 2001; Pyne and Pyne, 2000; Spiegel and Milstien, 2000).
We have identified a role for S1P in mediating adrenocorticotropin (ACTH)-stimulated CYP17 transcription (Ozbay et al., 2004; Ozbay et al., 2006). Treatment of H295R cells with ACTH or dibutyryl cAMP rapidly induces the catabolism (pathway shown in Figure 1) of complex sphingolipids such as sphingomyelin and ceramides and results in the secretion of S1P into the media (Ozbay T et al., 2004; Ozbay T et al., 2006). S1P activates a cascade of events culminating in the binding of the sterol regulatory element binding protein-1 (SREBP-1) to the promoter of CYP17, the activation of transcription, and increased cortisol biosynthesis. Others have shown that S1P stimulates cortisol secretion in zona fasciculata bovine adrenal cells in a PKC and Ca2+-dependent manner (Rabano et al., 2003). In addition to stimulating cortisol secretion, S1P has been recently found to increase aldosterone production via a protein kinase C and phospholipase D-dependent pathway (Brizuela et al., 2006).
Fig. 1.
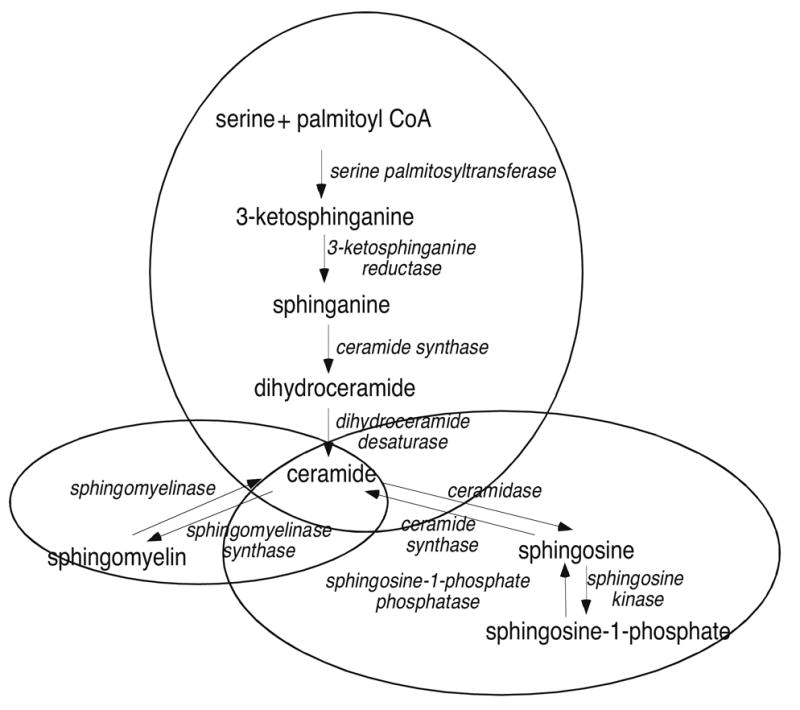
Sphingolipid metabolic pathway.
There have been many other reports of the stimulatory and inhibitory effects of bioactive sphingolipids on steroid hormone biosynthesis in both gonadal and adrenal cell lines (Budnick et al., 1999; Degnan et al., 1996; Kwun et al., 1999; McClellan et al., 1997; Meroni et al., 2000; Porn et al., 1991; Rabano et al., 2003; Santana et al., 1996). Increased ceramide levels stimulate progesterone synthesis in both MA-10 murine Leydig cells (Kwun et al., 1999) and JEG-3 human choriocarcinoma cells (McClellan et al., 1997). Tumor necrosis factor-α inhibits steroidogenic acute regulatory (StAR) protein expression and testosterone biosynthesis by increasing intracellular ceramide concentrations (Budnick et al., 1999). In rat granulosa cells, ceramide inhibits follicle stimulating hormone-stimulated progesterone biosynthesis and the mRNA expression levels of CYP11A1 and 3β-hydroxysteroid dehydrogenase (Santana et al., 1996). Collectively, these studies highlight the intimate relationship between sphingolipid metabolism and steroidogenesis.
4. Sphingosine is a ligand for SF1
Based on our previous findings demonstrating a role for S1P in activating SREBP1-mediated CYP17 transcription (Ozbay et al., 2004; Ozbay et al., 2006), we also characterized the role of S1P and other sphingolipids in SF1-dependent CYP17 expression. Although S1P had no effect on SF1-mediated CYP17 reporter gene expression, we did identify a role for sphingosine (SPH) as an endogenous ligand for SF1 (Urs et al., 2006). These studies were carried out by performing mass spectrometric analysis on SF1 that was immunoprecipitated from H295R cells. As shown in Table 1, SPH is bound to SF1 isolated from H295R cells. Interestingly, the amount of SPH bound to the receptor decreased when cells were stimulated with Bt2cAMP. SPH attenuated SF1-dependent CYP17 reporter gene expression and inhibited the ability of steroid receptor coactivator-1 (SRC-1) to stimulate CYP17 transcriptional activity (Urs et al., 2006). We also found that SPH significantly decreased cAMP-stimulated occupancy of SF1 on the CYP17 promoter and SPH CYP17 mRNA expression (Figure 2).
Table 1.
Mass spectrometric analysis of sphingolipids bound to SF1. H295R cells were treated for 4 h with 1 mM Bt2cAMP and SF1 immunoprecipitated. The purified receptor was subjected to lipid extraction and tandem mass spectrometric analysis.
| Amount of sphingolipid bound to SF1(pmol/mg total cellular protein ± STD) | ||
|---|---|---|
| Sphingolipid Molecular Species | control | Bt2cAMP |
| Sphingosine | 277 ± 62 | 154 ± 49 |
| Ceramides | 1 ± 0.5 | 0.5 ± 0.3 |
| Sphingosylphosphorylcholine | 321 ± 43 | 208 ± 37 |
| Sphingomyelin | 4 ± 0.9 | 10 ± 3 |
| Sphingosine-1-phosphate | 12 ± 0.3 | 4 ± 0.1 |
| Ceramide-1-phosphate | 14 ± 0.9 | 10 ± 2 |
Fig. 2.
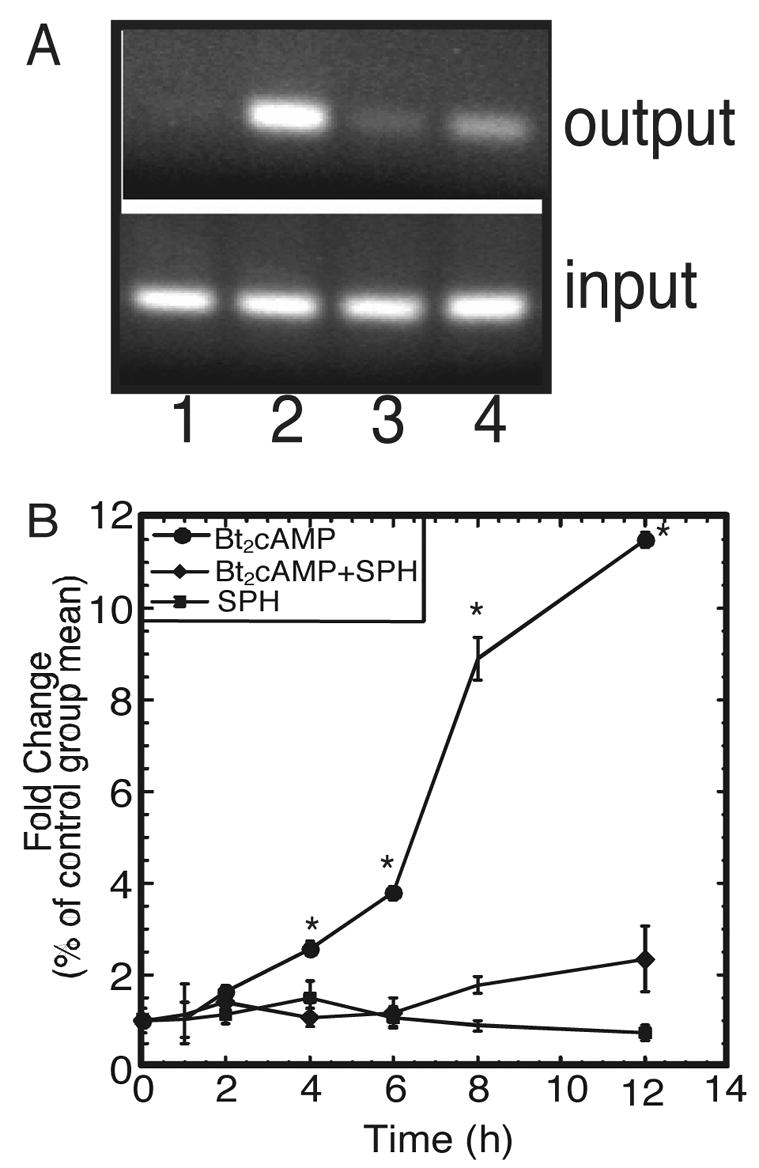
SPH inhibits the initiation of SF1-dependent transcription. A, H295R cells were treated with 1 mM Bt2cAMP in the presence and absence of 1 μM SPH for 1 h. Cells were incubated with 1% formaldehyde, harvested, sonicated, and immunoprecipitated with an anti-SF1 antibody. Cross-links were reversed and the purified DNA subjected to quantitative PCR using primers spanning the SF1 binding site on the human CYP17 promoter (−147/+25). PCR products were resolved on a 2% agarose gel and appear in the following order: lane 1, control; lane 2, Bt2cAMP; lane 3, Bt2cAMP +SPH; lane 4, SPH. B, Cells were treated for time points ranging from 1 h to 12 h with 1 mM Bt2cAMP in the presence and absence of 1 μM SPH. Total RNA was purified and subjected to real time RT-PCR using primers for CYP17 and β-actin. Data graphed represents mean ± SEM of four separate experiments performed in triplicate with CYP17 mRNA expression normalized to β-actin mRNA content. Asterisk (*) denotes statistically significant difference from untreated control p<0.05.
These findings led us to hypothesize that SPH was an antagonist for SF1 and acted to maintain the receptor in an inactive conformation. We further postulated that activation of the cAMP signal transduction pathway led to a series of events that altered the conformation of SF1, thereby promoting disassociation of SPH and activation of the receptor. As mentioned previously, since phosphorylation (Hammer et al., 1999) and acetylation (Chen et al., 2005; Ishihara and Morohashi, 2005; Jacob et al., 2001) of SF1 are key for receptor activation, it is probable that the binding of SF1 to target genes involves one or more post-translational modifications. Additionally, based on the receptor’s large ligand binding pocket (Li et al., 2005), it is also likely that cAMP promotes the exchange of SPH for an activating ligand. This hypothesis is supported by studies using the scintillation proximity assay in which we demonstrated that SF1 binds to various phospholipids and sphingolipids, including PE, phosphatidylcholine (PC), phosphatidic acid (PA), PIPs, and S1P. Various mixtures of PE, PA, and PIPs were identified as ligands in crystallographic studies (Krylova et al., 2005; Li et al., 2005; Wang et al., 2005), further supporting a potential role for multiple phospholipids and sphingolipids in regulating SF1 function.
4. Future Directions
Our findings demonstrating SPH as an endogenous ligand for SF1 give rise to several areas of research that need to be examined in the future. First, since we have found that ACTH (and Bt2cAMP) activate sphingolipid catabolism increase the activity of enzymes, such as sphingosine kinase, in the sphingolipid metabolic pathway (Ozbay et al., 2006), it is possible that trophic hormone stimulation may also regulate the expression of these enzymes. We have found that Bt2cAMP induces the transcription of sphingosine kinase-1 in H295R cells (unpublished observations), so it is plausible that ACTH/cAMP-stimulated signaling may regulate the transcription of other enzymes in the sphingolipid metabolic pathway. The potential transcriptional regulation of sphingolipid enzymes by trophic hormones may establish a feedback loop that maintains steroid hormone output at optimal levels
Our findings that both sphingolipids and phospholipids can bind to the receptor suggest that SF1 may have multiple ligands and that these ligands may be selectively produced in a tissue-, developmental-, species-, and/or target gene-specific manner. Studies directed at identifying activating ligands for SF1 are underway. Additionally, further research into the mechanism by which ACTH (or other trophic hormones) increase the production and degradation of these lipids is warranted. The liver homologue receptor-1 is also a member of the NR5 subfamily, and crystallographic analysis of this receptor also identified phospholipids in the ligand binding pocket (Wang et al., 2005), suggesting the SPH may also act to antagonize the actions of this receptor. Finally, since SF1 is also expressed in the pituitary and ventromedial hypothalamus, SPH and other bioactive ligands may play an important role in regulating the expression of SF1 target genes. In conclusion, our findings demonstrate and integral role for SPH in controlling SF1 activity and underscore the importance of integrating multiple signaling pathways and regulatory mechanisms in controlling steroid hormone biosynthesis.
Acknowledgments
This work was supported by NIH grants GM073241 (to M.B.S.) and PA02132 (to A.H.M.), NSF CAREER MCB0347682 and a Georgia Cancer Coalition Distinguished Scientist Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aesoy R, Mellgren G, Morohashi K, Lund J. Activation of cAMP-dependent protein kinase increases the protein level of steroidogenic factor-1. Endocrinology. 2002;143:295–303. doi: 10.1210/endo.143.1.8599. [DOI] [PubMed] [Google Scholar]
- Bakke M, Zhao L, Hanley NA, Parker KL. SF-1: a critical mediator of steroidogenesis. Mol Cell Endocrinol. 2001;171:5–7. doi: 10.1016/s0303-7207(00)00384-1. [DOI] [PubMed] [Google Scholar]
- Brizuela L, Rabano M, Pena A, Gangoiti P, Macarulla JM, Trueba M, Gomez-Munoz A. Sphingosine-1-phosphate: a novel stimulator of aldosterone secretion. J Lipid Res. 2006;47:1238–1249. doi: 10.1194/jlr.M500510-JLR200. [DOI] [PubMed] [Google Scholar]
- Budnick LT, Jahner D, Mukhopadhyay AK. Inhibitory effects of TNFalpha on mouse tumor Leydig cells: possible role of ceramide in the mechanism of action. Mol Cell Endocrinol. 1999;150:39–46. doi: 10.1016/s0303-7207(99)00029-5. [DOI] [PubMed] [Google Scholar]
- Castillo SS, Teegarden D. Ceramide conversion to sphingosine-1-phosphate is essential for survival in C3H10T1/2 cells. J Nutr. 2001;131:2826–2830. doi: 10.1093/jn/131.11.2826. [DOI] [PubMed] [Google Scholar]
- Chen WY, Lee WC, Hsu NS, Huang F, Chung BC. SUMO modification of repression domains modulates function of the nuclear receptor 5A1 (steroidogenic factor-1. J Biol Chem. 2004;279:38730–38735. doi: 10.1074/jbc.M405006200. [DOI] [PubMed] [Google Scholar]
- Chen WY, Juan LJ, Chung BC. SF-1 (nuclear receptor 5A1) activity is activated by cyclic AMP via p300-mediated recruitment to active foci, acetylation, and increased DNA binding. Mol Cell Biol. 2005;25:10442–10453. doi: 10.1128/MCB.25.23.10442-10453.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, Spiegel S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- Degnan BM, Bourdelat-Parks B, Daniel A, Salata K, Francis GL. Spingomyelinase inhibits in vitro Leydig cell function. Ann Clin Lab Sci. 1996;26:242–243. [PubMed] [Google Scholar]
- Desclozeaux M, Krylova IN, Horn F, Fletterick RJ, Ingraham HA. Phosphorylation and intramolecular stabilization of the ligand binding domain in the nuclear receptor steroidogenic factor 1. Mol Cell Biol. 2002;22:7193–7203. doi: 10.1128/MCB.22.20.7193-7203.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl EJ, An S. Diversity of cellular receptors and functions for the lysophospholipid growth factors lysophosphatidic acid and sphingosine 1-phosphate. FASEB J. 1998;12:1589–1598. [PubMed] [Google Scholar]
- Hammer GD, Krylova I, Zhang Y, Darimont BD, Simpson K, Weigel NL, Ingraham HA. Phosphorylation of the nuclear receptor SF-1 modulates cofactor recruitment: integration of hormone signaling in reproduction and stress. Mol Cell. 1999;3:521–526. doi: 10.1016/s1097-2765(00)80480-3. [DOI] [PubMed] [Google Scholar]
- Hammer GD, Parker KL, Schimmer BP. Minireview: transcriptional regulation of adrenocortical development. Endocrinology. 2005;146:1018–1024. doi: 10.1210/en.2004-1385. [DOI] [PubMed] [Google Scholar]
- Hannun YA. The sphingomyelin cycle and the second messenger function of ceramide. J Biol Chem. 1994;269:3125–3128. [PubMed] [Google Scholar]
- Hannun YA, Luberto C, Argraves KM. Enzymes of sphingolipid metabolism: from modular to integrative signaling. Biochemistry. 2001;40:4893–4903. doi: 10.1021/bi002836k. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM. The ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J Biol Chem. 2002;277:25847–25850. doi: 10.1074/jbc.R200008200. [DOI] [PubMed] [Google Scholar]
- Hla T, Lee MJ, Ancellin N, Liu CH, Thangada S, Thompson BD, Kluk MJ. Biochem Pharmacol. 1999;58:201–207. doi: 10.1016/s0006-2952(99)00086-6. [DOI] [PubMed] [Google Scholar]
- Hla T, Lee MJ, Ancellin N, Paik JH, Kluk MJ. Lysophospholipids--receptor revelations. Science. 2001;294:1875–1878. doi: 10.1126/science.1065323. [DOI] [PubMed] [Google Scholar]
- Igarashi J, Erwin PA, Dantas APV, Chen H, Michel T. VEGF induces S1P1 receptors in endothelial cells: Implications for cross-talk between sphingolipids and growth factor receptors. Proc Natl Acad Sci. 2003;100:10664–10669. doi: 10.1073/pnas.1934494100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingraham HA, Redinbo MR. Orphan nuclear receptors adopted by crystallography. Current Opinion in Structural Biology. 2005;15:708–715. doi: 10.1016/j.sbi.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Ishihara SL, Morohashi K. A boundary for histone acetylation allows distinct expression patterns of the Ad4BP/SF-1 and GCNF loci in adrenal cortex cells. Biochem Biophys Res Commun. 2005;329:554–562. doi: 10.1016/j.bbrc.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Jacob AL, Lund J, Martinez P, Hedin L. Acetylation of steroidogenic factor 1 protein regulates its transcriptional activity and recruits the coactivator GCN5. J Biol Chem. 2001;276:37659–37664. doi: 10.1074/jbc.M104427200. [DOI] [PubMed] [Google Scholar]
- Kolesnick R. The therapeutic potential of modulating the ceramide/sphingomyelin pathway. J Clin Invest. 2002;110:3–8. doi: 10.1172/JCI16127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu T, Mizusaki H, Mukai T, Ogawa H, Baba D, Shirakawa M, Hatakeyama S, Nakayama KI, Yamamoto H, Kikuchi A, Morohashi K. Small ubiquitin-like modifier 1 (SUMO-1) modification of the synergy control motif of Ad4 binding protein/steroidogenic factor 1 (Ad4BP/SF-1) regulates synergistic transcription between Ad4BP/SF-1 and Sox9. Mol Endocrinol. 2004;18:2451–2462. doi: 10.1210/me.2004-0173. [DOI] [PubMed] [Google Scholar]
- Krylova IN, Sablin EP, Moore J, Xu RX, Waitt GM, MacKay JA, Juzuniene D, Bynum JM, Madauss K, Montana V, Lebedeva L, Suzawa M, Williams JD, Williams SP, Guy RK, Thornton JW, Fletterick RJ, Willson TM, Ingraham HA. Structural analyses reveal phosphatidyl inositols as ligands for the NR5 orphan receptors SF-1 and LRH-1. Cell. 2005;120:343–355. doi: 10.1016/j.cell.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Kwun C, Patel A, Pletcher S, Lyons B, Abdelrahim M, Nicholson D, Morris E, Salata K, Francis GL. Ceramide increases steroid hormone production in MA-10 Leydig cells. Steroids. 1999;64:499–509. doi: 10.1016/s0039-128x(99)00013-6. [DOI] [PubMed] [Google Scholar]
- Lee MB, Lebedeva LA, Suzawa M, Wadekar SA, Desclozeaux M, Ingraham HA. The DEAD-box protein DP103 (Ddx20 or Gemin-3) represses orphan nuclear receptor activity via SUMO modification. Mol Cell Biol. 2005;25:1879–1890. doi: 10.1128/MCB.25.5.1879-1890.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Choi M, Cavey G, Daugherty J, Suino K, Kovach A, Bingham NC, Kliewer SA, Xu HE. Crystallographic identification and functional characterization of phospholipids as ligands for the orphan nuclear receptor steroidogenic factor-1. Mol Cell. 2005;17:491–502. doi: 10.1016/j.molcel.2005.02.002. [DOI] [PubMed] [Google Scholar]
- McClellan DR, Bourdelat-Parks B, Salata K, Francis GL. Sphingomyelinase affects hormone production in Jeg-3 choriocarcinoma cells. Endocrinol Metab. 1997;3:19–24. [Google Scholar]
- Meroni SB, Pellizzari EH, Canepa DF, Cigorraga SB. Possible involvement of ceramide in the regulation of rat Leydig cell function. J of Steroid Biochemistry and Molecular Biology. 2000;75:307–313. doi: 10.1016/s0960-0760(00)00188-6. [DOI] [PubMed] [Google Scholar]
- Merrill AH, Jr, Schmelz E-M, Dillehay DL, Spiegel S, Shayman JA, Schroeder JJ, Riley RT, Voss KA, Wang E. Sphingolipids--the enigmatic lipid class: biochemistry, physiology, and pathophysiology. Toxicol Appl Pharmacol. 1997;142:208–225. doi: 10.1006/taap.1996.8029. [DOI] [PubMed] [Google Scholar]
- Olivera A, Spiegel S. Spingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature. 1993;365:557–560. doi: 10.1038/365557a0. [DOI] [PubMed] [Google Scholar]
- Olivera A, Kohama T, Edsall L, Nava V, Cuvillier O, Poulton S, Spiegel S. Sphingosine kinase expression increases intracellular sphingosine-1-phosphate and promotes cell growth and survival. J Cell Biol. 1999;147:545–558. doi: 10.1083/jcb.147.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozbay T, Merrill AH, Jr, Sewer MB. ACTH regulates steroidogenic gene expression and cortisol biosynthesis in the human adrenal cortex via sphingolipid metabolism. Endocr Res. 2004;30:787–794. doi: 10.1081/erc-200044040. [DOI] [PubMed] [Google Scholar]
- Ozbay T, Rowan Leon A, Patel P, Sewer MB. Cyclic adenosine 5′-monophosphate-dependent sphingosine-1-phosphate biosynthesis induces human CYP17 gene transcription by activating cleavage of sterol regulatory element binding protein 1. Endocrinology. 2006;147:1427–1437. doi: 10.1210/en.2005-1091. [DOI] [PubMed] [Google Scholar]
- Parker KL, Rice DA, Lala DS, Ikeda Y, Luo X, Wong M, Bakke M, Zhao L, Frigeri C, Hanley NA, Stallings N, Schimmer BP. Steroidogenic factor 1: an essential mediator of endocrine development. Recent Prog Horm Res. 2002;57:19–36. doi: 10.1210/rp.57.1.19. [DOI] [PubMed] [Google Scholar]
- Porn MI, Tenhunen J, Slotte JP. Increased steroid hormone secretion in mouse Leydig tumor cells after induction of cholesterol translocation by sphingomyelin degradation. Biochim Biophys Acta. 1991;1093:7–12. doi: 10.1016/0167-4889(91)90131-g. [DOI] [PubMed] [Google Scholar]
- Pyne S, Pyne NJ. Sphingosine 1-phosphate signalling in mammalian cells. Biochem J. 2000;349:385–402. doi: 10.1042/0264-6021:3490385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabano M, Pena A, Brizuela L, Marino A, Macarulla JM, Trueba M, Gomez-Munoz A. Sphingosine-1-phosphate stimulates cortisol secretion. FEBS Lett. 2003;535:101–105. doi: 10.1016/s0014-5793(02)03882-6. [DOI] [PubMed] [Google Scholar]
- Santana PL, Lanes L, Hernandez I, Gonzalez-Robayna I, Tabraue C, Gonzalez-Reyes J, Quintana J, Estevez F, Ruiz de Galarretta CM, Fanjul LF. Interleukin-1beta stimulates sphingomyelin hydrolysis in cultured granulosa cells: evidence for a regulatory role of ceramide on progesterone and prostaglandin biosynthesis. Endocrinology. 1996;137:2480–2489. doi: 10.1210/endo.137.6.8641202. [DOI] [PubMed] [Google Scholar]
- Sewer MB, Waterman MR. Insights into the transcriptional regulation of steroidogenic enzymes and StAR. Rev in Endocrine and Metabolic Disorders. 2001;2:269–274. doi: 10.1023/a:1011516532335. [DOI] [PubMed] [Google Scholar]
- Sewer MB, Waterman MR. ACTH/cAMP-mediated transcription of the human CYP17 gene in the adrenal cortex is dependent on phosphatase activity. Endocrinology. 2002a;143:1769–1777. doi: 10.1210/endo.143.5.8820. [DOI] [PubMed] [Google Scholar]
- Sewer MB, Waterman MR. cAMP-Dependent transcription of steroidogenic genes in the human adrenal cortex requires a dual-specificity phosphatase in addition to PKA. J Mol Endocrinol. 2002b;29:163–174. doi: 10.1677/jme.0.0290163. [DOI] [PubMed] [Google Scholar]
- Sewer MB, Waterman MR. ACTH modulation of transcription factors responsible for steroid hydroxylase gene expression in the adrenal cortex. Microsc Res Tech. 2003;61:300–307. doi: 10.1002/jemt.10339. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Functions of a new family of sphingosine-1-phosphate receptors. Biochim Biophys Acta. 2000;1484:107–116. doi: 10.1016/s1388-1981(00)00010-x. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Sphingosine 1-phosphate, a key cell signaling molecule. J Biol Chem. 2002;277:25851–25854. doi: 10.1074/jbc.R200007200. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- Strasberg PM, Callahan JW. Lysosphingolipids and mitochondrial function. II. Deleterious effects of sphingosylphosphorylcholine. Biochem Cell Biol. 1988;66 doi: 10.1139/o88-153. [DOI] [PubMed] [Google Scholar]
- Tilly JL, Kolesnick RN. Sphingolipids, apoptosis, cancer treatments and the ovary: investigating a crime against female fertility. Biochim Biophys Acta. 2002;1585:135–138. doi: 10.1016/s1388-1981(02)00333-5. [DOI] [PubMed] [Google Scholar]
- Urs AN, Dammer E, Sewer MB. Sphingosine Regulates the Transcription of CYP17 by Binding to Steroidogenic Factor-1. Endocrinology. 2006 doi: 10.1210/en.2006–0355. Rapid Electronic Publication first published on Aug 3, 2006 . [DOI] [PubMed] [Google Scholar]
- Vesper H, Schmelz E-M, Nikolova-Karakashian MN, Dillehay DL, Lynch DV, Merrill AH., Jr Sphingolipids in food and the emerging importance of sphingolipids to nutrition. J Nutr. 1999;129:1239–250. doi: 10.1093/jn/129.7.1239. [DOI] [PubMed] [Google Scholar]
- Wang W, Zhang C, Marimuthu A, Krupka HI, Tabrizizad M, Shelloe R, Mehra U, Eng K, Nguyen H, Settachatgul C, Powell B, Milburn MV, West BL. The crystal structures of human steroidogenic factor-1 and liver receptor homologue-1. Proc Natl Acad Sci. 2005;102:7505–510. doi: 10.1073/pnas.0409482102. [DOI] [PMC free article] [PubMed] [Google Scholar]


