Abstract
The emergence of parallel MRI techniques and new applications for real-time interactive MRI underscores the need to evaluate performance gained by increasing the capability of MRI phased-array systems beyond the standard four to eight high-bandwidth channels. Therefore, to explore the advantages of highly parallel MRI a 32-channel 1.5 T MRI system and 32-element torso phased arrays were designed and constructed for real-time interactive MRI. The system was assembled from multiple synchronized scanner-receiver subsystems. Software was developed to coordinate across subsystems the real-time acquisition, reconstruction, and display of 32-channel images. Real-time, large field-of-view (FOV) body-survey imaging was performed using interleaved echo-planar and single-shot fast-spin-echo pulse sequences. A new method is demonstrated for augmenting parallel image acquisition by independently offsetting the frequency of different array elements (FASSET) to variably shift their FOV. When combined with conventional parallel imaging techniques, image acceleration factors of up to 4 were investigated. The use of a large number of coils allowed the FOV to be doubled in two dimensions during rapid imaging, with no degradation of imaging time or spatial resolution. The system provides a platform for evaluating the applications of many-channel real-time MRI, and for understanding the factors that optimize the choice of array size.
Keywords: real-time MRI, parallel MRI, multi-channel MRI, 32 channels
The ability to perform real-time interactive MRI, with rapid or instantaneous translation and optimization of scan planes, is a central requisite for image-guided intervention, as well as a valuable tool for monitoring dynamic physiologic processes including function and perfusion. Riederer and colleagues (1,2) first implemented real-time MRI acquisition and reconstruction with interactive redirection of the pulse sequence from the operator’s console keyboard during continuous scanning (3). Implementation of high-speed pulse sequences soon followed, including those using interleaved echo-planar (EPI) (4), spiral (5), and radial (6) k-space trajectories. In parallel, graphic tools (7-11) and hardware (10) have been developed to improve the interactivity of real-time scan-plane prescription, and all of these tools have been applied to image the chest and the abdomen (7-11).
MRI frame rates can be further increased with parallel sensitivity-encoding techniques employing multiple surface-coil or phased-array detectors (12-17). Parallel MRI provides manifold increases in imaging speed by replacing some of the gradient spatial-encoding steps with spatial information derived from the distinct sensitivity profiles of the array coils (18). For example, the SMASH method (12) combines signals from each coil in the array to create spatial harmonics to fill in the lines of k-space that are lost when phase-encoding gradient steps are skipped. On the other hand, the SENSE (13) technique works in the spatial domain by using the different coil sensitivities to unfold images that otherwise exhibit severe aliasing artifacts due to omission of the gradient phase-encoding steps. These methods and variations (12-17) in theory provide maximum image acceleration factors that approach, the number of coils in the array. When lesser acceleration factors are deployed, some measure of the traditional signal-to-noise ratio (SNR) advantage of surface coils over volume coils (18) is realized.
Recently, parallel imaging with MRI phased arrays was extended to interactive, real-time MRI (19). This combination provides a route to the dramatic gains in imaging speed that are critical for interventional procedures. It is also directly applicable, for example, to the evaluation of myocardial function during stress, or minimizing cardiac, respiratory, and peristaltic motion during body imaging. The inevitable trade-off is that the acceleration of image speed by parallel MRI techniques sacrifices SNR, which is also critical for successful real-time imaging. Increasing the number of phased-array coil elements can potentially improve the SNR and/or provide larger fields of view (FOVs) and/or increase the imaging speed to extents that are both interchangeable and substantial. Because each phased-array element requires the expense and complexities of fabricating and processing an entire MRI receiver channel, the number of elements that have been commercially implemented for parallel acquisition had been limited, until now (20), to eight or fewer channels.
Increases to higher channel counts have begun to appear in conference reports (20-24). For example, a 32-channel experimental MRI system was developed on a small-bore animal scanner and tested with a 4-channel array (21). This was subsequently scaled to 64 channels and used with a small 64-channel array to demonstrate single-echo-acquisition MRI, yielding some low-resolution phantom images from very shallow depths (22). This system (21,22) has not been implemented in humans or with real-time continuous MRI. Nevertheless, large numbers of channelsoperated in true parallel fashion offer the promise of highly accelerated imaging at truly unprecedented rates. A central question for real-time in vivo parallel MRI is, What is the optimal number of channels? Without a highly parallel whole-body MRI system to investigate potential applications, the practical merits and limitations of multichannel systems for human MR imaging remain unknown.
We report here the design and construction of a 32-coil torso array and 32-channel MRI system for interactive real-time MRI and its application to high-speed, large-FOV 2D imaging of the torso and abdomen. The system was constructed to evaluate the feasibility, practicality, and performance advantages of a highly parallel MRI system (20).
MATERIALS AND METHODS
Two 32-channel MRI torso coil arrays were fabricated. The first was designed for parallel MRI and comprised two identical clamshell formers, each containing 4 rows of 4 coils, each 79 × 105 mm (23). Coils were spaced by 16 mm in the right/left (R/L) direction and overlapped by 18 mm in the superior/inferior (S/I) direction to provide optimal SNR for volumetric parallel imaging with order-of-magnitude accelerations (23). One former was positioned on the patient table and the other on top of the subject’s torso. The second torso array was designed primarily for conventional phased-array imaging and utilizes 10.6 cm diameter octagonal coil elements (24) overlapped to minimize mutual coupling in the usual manner (18). This array was also built on two clamshell formers, as pictured in Fig. 1a.
FIG. 1.

a: 32-element abdominal/torso phased array comprising two clamshell formers, each of which holds 16 active 10.6-cm-diameter octagonal coil elements overlapped to minimize mutual inductance. b: 32-channel scanner made up of four 8-channel systems with synchronized clocks.
Two custom 32-channel MRI systems were constructed to interactively excite, detect, and display the MRI data in real time. One was based on an older GE 1.5 T CV/i 4-channel scanner and the second was based on a current GE 1.5 T Excite 8-channel scanner (GE Medical Systems, Milwaukee, WI). Respectively for each system, 8 and 4 sets of MRI system electronics were assembled in cabinets from individual MRI systems. The resultant 32 MRI receivers and the digital data pipeline were integrated into a single MRI system (Fig. 1b). The auxiliary system cabinets con-taining the additional receiver channels were modified to permit triggering from the main system cabinet. Reference clock and local oscillator signals were similarly propagated from the main system to the auxiliary systems so that all receivers on the multiple cabinets operated synchronously with each other, effectively expanding the number of parallel receivers to a total of 32. Each cabinet retained the ability to independently demodulate the signal, thus enabling independent FOV-shifting in the frequency-encoding direction. Pulse sequences were modified to utilize the receiver synchronization, and to permit scan control and multichannel data acquisition.
A custom real-time-imaging software platform was developed to collect data from all 32 channels across the multiple scanner subsystems, reconstruct images, and display them on the scanner’s main monitor. The architecture for this platform comprised a number of interlinked processes. A real-time data-acquisition program running on each of the scanner subsystems communicates with that subsystem’s transceiver processor and reads data from scanner memory. The raw data are then sent in parallel from each scanner subsystem to a farm of Unix reconstruction engines. Each recon host computer holds the data in shared memory and runs separate programs to communicate data and to perform the reconstruction. Reconstructed images are then sent to the main scanner subsystem, where another program controls data flow and passes the images on to the display. A main program controls the overall real-time application and provides the user interface.
Figure 2 shows the data flow for this process. A “prepare” instruction from the real-time program initializes the scanner subsystems and starts the reconstruction process on the reconstruction hosts, with each host waiting for raw data from the scanners. A “start” instruction then initializes scanning and data is collected from the 32 channels across the subsystems. On each imaging loop the real-time program assigns a new reconstruction host to reconstruct the data. The chosen reconstruction host, in turn, extracts data from its input-data queue, reconstructs them, and places the resulting image in an output queue for transfer back to a display queue on the main scanner subsystem. Each image dataset is uniquely tagged to allow the display program to keep track of the image order.
FIG. 2.
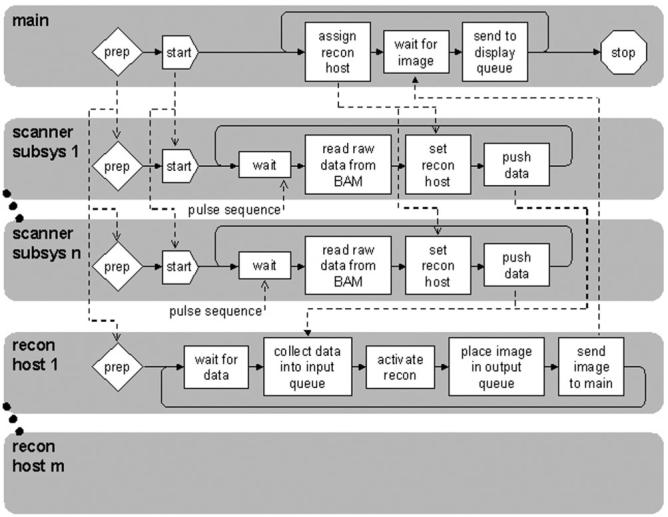
Real-time data flow for 32-channel scanner. Main program communicates with programs on each scanner sub-system and on each recon host to acquire, reconstruct, and display images in real time. “BAM,” scanner memory.
The 32-channel real-time imaging tool is shown in Fig. 3. This appears on the scanner console when a new pulse sequence is initiated. The tool has a real-time imagingwindow and a main panel, which displays scout images as well as image thumbnails associated with scan-plane locations that can be stored and recalled during real-time imaging (10). A number of buttons are provided to activate interactive moving of the scan plane by dragging icons across the real-time imaging window, selecting points on the real-time images, or by drawing lines on the scout image (10). Another display panel allows prospective or retrospective storing of real-time images to the scanner database.
FIG. 3.
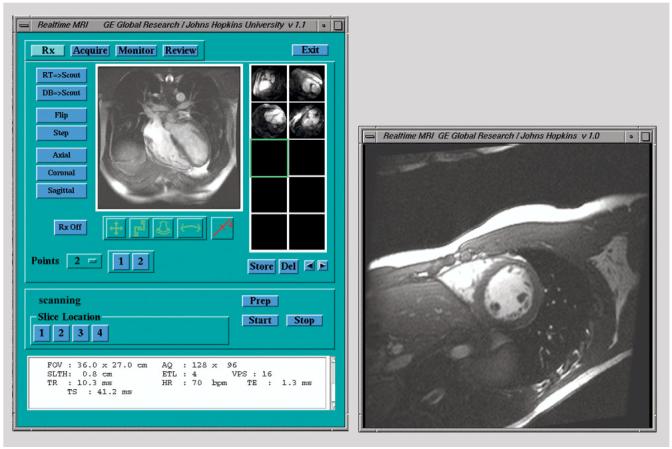
32-channel real-time MRI tool. During scanning, images are continuously displayed in window at right. Scan plane is interactively positioned during real-time imaging by lines placed on scout image at left, or by icons dragged across real-time window, or by points laid down on real-time window. Scan planes can be stored and retrieved as thumbnails to the right of the scout.
Real-time imaging was performed with the use of an interleaved echo-planar (EPI) pulse sequence. Typically, the echo-train length was 8, FOV was 40 cm, 3/4 phase FOV, 128 × 128 matrix size, bandwidth 125 kHz, and frame rate 10/sec. In addition, a large-FOV body-survey mode was developed, which employed a repeated single-shot-fast-spin-echo (SSFSE) sequence. Here the FOV was 36 cm, NEX 0.5, 128 × 128 matrix size, bandwidth 62.5 kHz, and the frame rate was 1-3/sec. Both pulse sequences were modified to allow simultaneous scanning on the main and auxiliary scanner cabinets, and to permit real-time scan-plane control.
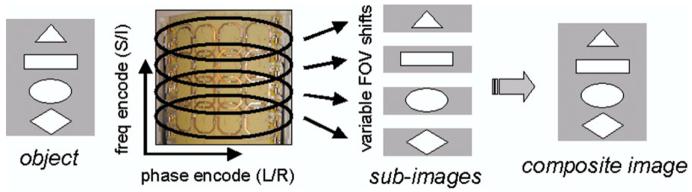
Higher-resolution SSFSE images were acquired as well (512 × 256, FOV 23-30 cm, TE 60 ms, bandwidth 83-125 kHz, imaging time 600-900 ms). To expand the FOV (e.g., for coronal imaging) while maintaining spatial resolution and imaging speed, a new form of parallel imaging (dubbed FASSET, for frequency-ASSET) was developed and implemented in the frequency-encoding direction (20). In this technique, the readout gradient was oriented in the superior/inferior orientation and limited-FOV images were acquired from each row of coils, as shown in Fig. 4. Each row of coils (including the 4 coils directly opposite on the other clamshell) had its FOV shifted in the frequency-encoding direction by offsetting the receiver frequency by an amount that centered the FOV under that row. The readout filter prevented aliasing of the signals from outside the FOV. Parallel encoding was then applied in the phase-encoding (left/right) direction, with a speedup factor of typically 2-3. A SENSE reconstruction was applied to the data acquired from each row to unfold the aliasing in the phase-encoding direction and create a “subimage” from that row. Composite large-FOV images (1024 × 512, FOV 52 cm) were then created by the combination of subimages from each row by shifting each subimage in the frequency-encoding direction and taking the square root of the sum-of-the-square of the signal in any overlapping regions of the subimages.
FIG. 4.
FASSET encoding scheme. Images are acquired with limited FOV and reduced matrix size in the frequency direction, resulting in accelerated imaging. The FOV from each row of coils is independently shifted during readout, so as to be centered under that row. In the reconstruction, images from each coil are shifted before optimal combination into a final composite image. This technique can be applied at the same time as traditional parallel imaging in the phase-encode direction, to provide additional acceleration factors.
Real-time MRI was performed in five healthy adult volunteers to demonstrate functionality. The protocol was approved by our institutional review board and informed consent was obtained from all subjects.
RESULTS
The body-survey mode, which employed a repeated SS-FSE sequence, was tested in several volunteers. Typical results are displayed in Fig. 5, which shows a series of still frames from a movie acquired during a coronal fly-through of the abdomen. The left side of each frame shows the scout image, with the current scan plane position marked by a line. The current scan plane is shown in the image onthe right of each frame. This was performed at the rate of 0.3 sec/image with all 32 channels.
FIG. 5.
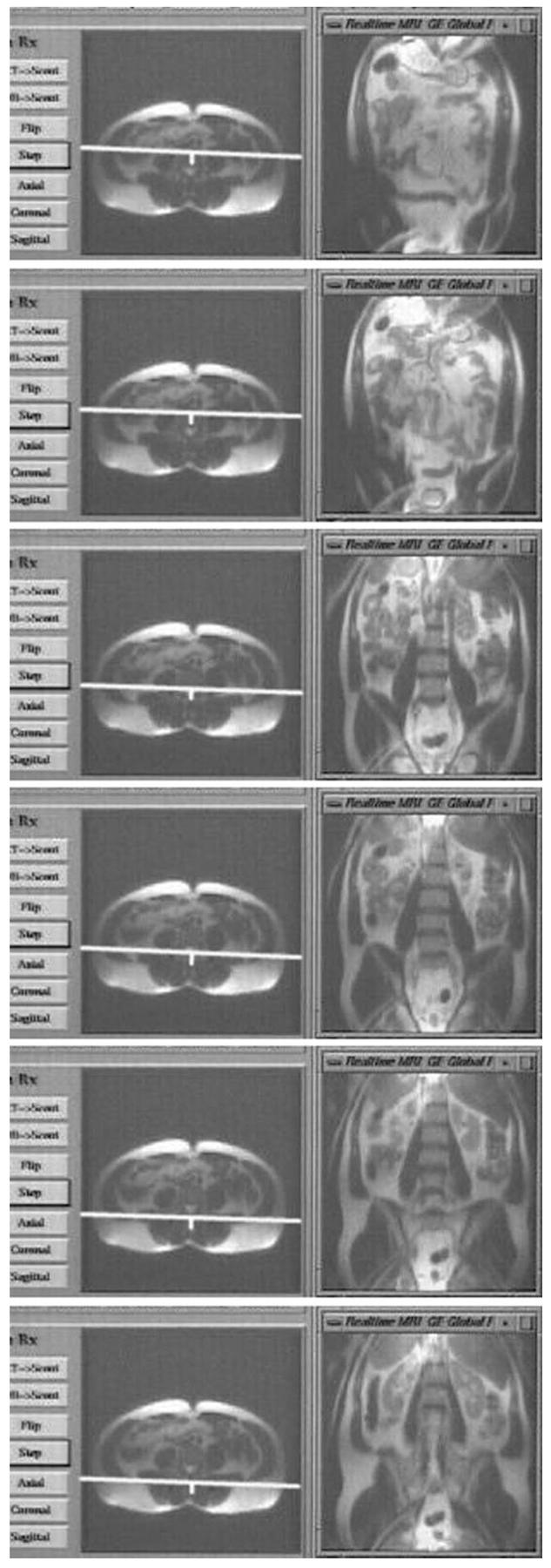
SSFSE fly-through of the abdomen. A series of still frames is shown from a real-time movie, with the real-time window on the right of each frame, and the scout window on the left. The scan plane is automatically stepped through a series of parallel slices defined in the scout window, with corresponding SSFSE images displayed in the real-time window.
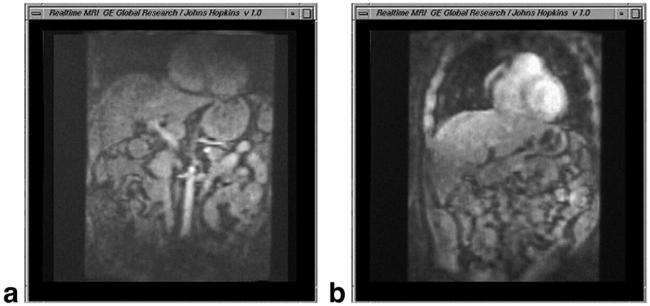
The interleaved EPI pulse sequence produced images at up to 15/sec, over an FOV of 40 cm, with 3 mm resolution. Figure 6 shows still frames from two movies acquired with interleaved EPI. In Fig. 6a a four-channel torso array was used, and in Fig. 6b data from the 32-channel torso phased array were acquired at a frame rate of 10/sec. With the 32-channel system, the movie on the right exhibits an effective FOV that is 1.5-times larger, measured as full-width half-maximum (FWHM) signal intensity. The SNR in the center of the FOV was 2 times higher than the movie on the left acquired with the conventional four-channel system and array.
FIG. 6.
Still frames from real-time inter-leaved-echo-planar movies acquired with (a) a 4-channel torso array (b) the 32-channel array of Fig. 3. Movies show cardiac, respiratory, and peristaltic motion. The movie from the 32-channel array exhibits larger effective FOV and doubled SNR.
Figure 7 shows SSFSE images of the abdomen of a normal volunteer acquired with the FASSET technique. In Fig. 7a, aliased and offset phased-array images acquired simultaneously from each row of coils (512 × 256, FOV 26 cm, TE 60 ms, bandwidth 83 kHz, imaging time 856 ms) are tiled vertically. In Fig. 7b, the images have been un-wrapped by a SENSE-type reconstruction in the phase-encoding direction (R/L) with use of a low-resolution reference and then shifted by integer multiples of 8 cm in the frequency-encoding (S/I) direction before root-of-the-sum-of-the-squares combination into an overall large 52 cm FOV image. This yielded a 512 × 1024 image. Here, we doubled the FOV in both the readout and phase-encoding directions, yielding four times the area of coverage, without sacrificing resolution or imaging speed.
FIG. 7.
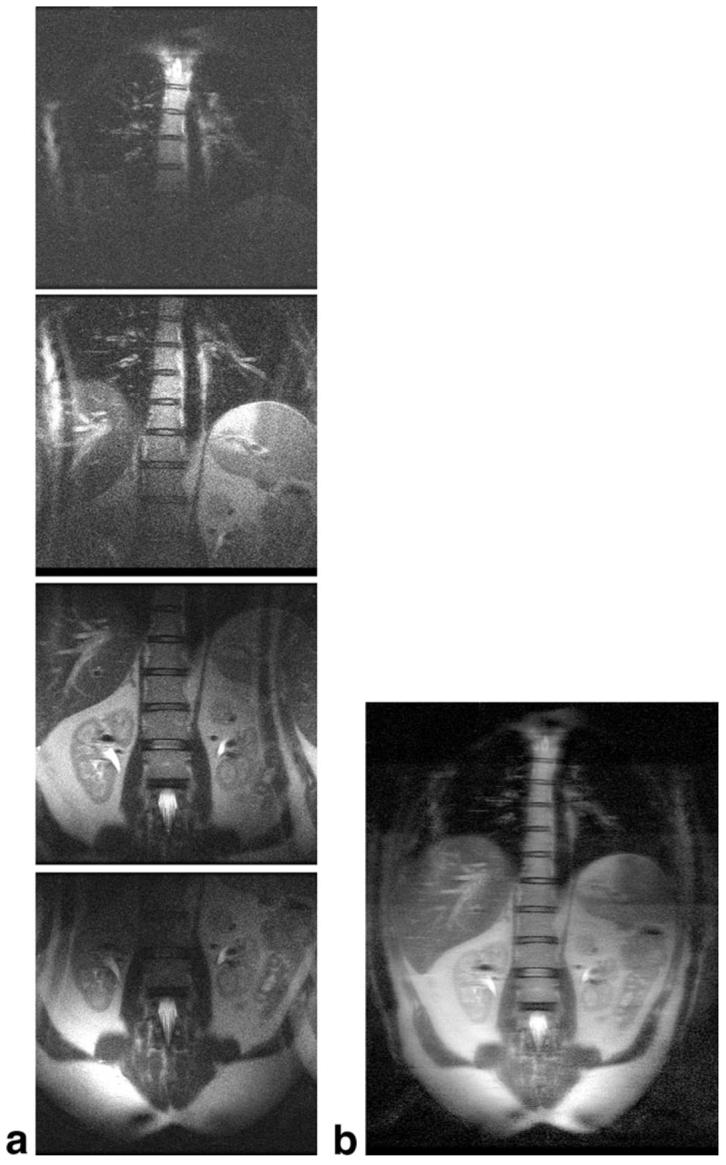
a: Reduced-FOV images acquired simultaneously from each row of 8 coils of the 32-element parallel array, with FOV shifted to be centered under that row. Aliasing is evident in the phase-encode direction. b: Combined FASSET image (512 × 1024, FOV 52 cm, imaging time 856 ms), obtained by unwrapping each image in the phase-encode direction, shifting the image by the appropriate amount in the frequency-encode direction, and taking the amplitude-weighted sum of the squares.
DISCUSSION
We have built and demonstrated a highly parallel, 32-channel real-time MRI system with 32-channel torso arrays. The use of a high number of coils allowed expansion of the FOV and doubling of the SNR compared with a 4-channel system during rapid 2D imaging while maintaining relatively high spatial resolution and short imaging times. This should be useful, in conjunction with the SSFSE sequence, for rapid surveys of the torso and abdomen, with potential applications in cancer screening and MR cholangiography. Large-FOV dynamic imaging at up to 15 frames/sec was performed with the interleaved EPI sequence running all 32 channels. This will permit continuous image monitoring for the purpose of guiding therapeutic interventions in oncology and the cardiovascularsystem. Note that the SSFSE sequence can be modified by incorporating driven equilibrium pulse sequences to preserve the contrast-to-noise ratio during rapid imaging from a single location (25). Also, in work in progress, acceleration factors as high as 16 have been obtained for 3D MRI, albeit not in real time, by applying parallel encoding in each of the phase-encoding directions (23,26).
FASSET provides a new means for accelerating imaging in the frequency-encoding direction when one has already reached the limits of receiver bandwidth. It can be applied in combination with other speed-enhancing parallel imaging techniques to provide additional acceleration. An important requirement for the FASSET technique is the availability of a multichannel system with receivers that are capable of being independently demodulated at different frequencies in order to enable mixed FOV-shifting. FASSET offers the greatest potential speed-up factor for efficient pulse sequences such as FSE and EPI, which have a relatively high readout duty cycle (that is, sequences in which a large percentage of the total time is devoted to data acquisition). Contemporaneous with the present work (20), a similar method was proposed for spiral MRI, another sequence with a high data readout duty-cycle, at the same conference (27). In that case, the concept was demonstrated with serial imaging rather than with simultaneous multichannel imaging because independent demodulation was not available.
The FASSET technique is in some respects similar to PILS (partially parallel imaging with localized sensitivities) (14), except that it is applied in the frequency-encoding rather than phase-encoding direction, and it uses the readout filters in addition to the limited sensitivity range of the receiver coils to prevent aliasing. In the examples shown, parallel imaging was performed separately within each row of coils and the composite accelerated images combined to create an overall image. Further improvements in the SNR may be anticipated by rearranging the sequence to perform parallel imaging with all 32 coils to provide speed-ups in the R/L direction, while continuing to apply separate FOV shifts to each row.
Our approach to the problem of assembling a many-channel prototype MRI scanner using multiple scanner subsystems has resulted in a system that has exceptional receiver versatility and control. It enables, for example, the independent offsetting of receiver frequency across subsets of channels that forms the basis of the FASSET method, currently not available in commercial systems. This can serve as a platform for evaluating the application of this technique to clinical problems.
Finally, we anticipate the ready consolidation of the systems electronics associated with our prototype. Although advances in electronics can be expected to miniaturize and modularize the channels, their function remains essentially the same today as it was when Roemer et al. (18) introduced phased arrays for MRI in 1990. Thus, once the requisite hardware is assembled, the central problem for real-time parallel scanning with very large arrays is basically one of managing an enormous flux of data so as to seamlessly produce reconstructed images on a screen, essentially instantly, as we have described herein. We believe that our novel 32-channel research prototype will provide an important platform to evaluate the utility of highly parallel MRI in many disease states and provide valuable information relating to optimal MRI array sizes and geometries for many applications.
ACKNOWLEDGMENTS
The authors thank Ron Watkins and Ken Rohling for assistance with development of the 32-channel system and coils, and Graeme McKinnon and Dan Sodickson for useful discussions.
Footnotes
Grant sponsor: National Institutes of Health (NIH); Grant number: R01 RR15396.
This work was presented in part at the 11th Meeting of the ISMRM, Toronto, 2003.
REFERENCES
- Riederer SJ, Tasciyan T, Farzaneh F, Lee JN, Wright RC, Herfkens RJ. MR fluoroscopy: technical feasibility. Magn Reson Med. 1988;8:1–15. doi: 10.1002/mrm.1910080102. [DOI] [PubMed] [Google Scholar]
- Wright RC, Riederer SJ, Farzaneh F, Rossman PJ, Liu Y. Real-time MR fluoroscopic data acquisition and image reconstruction. Magn Reson Med. 1989;12:407–415. doi: 10.1002/mrm.1910120314. [DOI] [PubMed] [Google Scholar]
- Holsinger AE, Wright RC, Riederer SJ, Farzaneh F, Grimm RC, Maier JK. Real-time interactive magnetic resonance imaging. Magn Reson Med. 1990;14:547–553. doi: 10.1002/mrm.1910140312. [DOI] [PubMed] [Google Scholar]
- McKinnon GC. Ultrafast interleaved gradient-echo-planar imaging on a standard scanner. Magn Reson Med. 1993;30:609–616. doi: 10.1002/mrm.1910300512. [DOI] [PubMed] [Google Scholar]
- Meyer CH, Hu BS, Nishimura DG, Macovski A. Fast spiral coronary artery imaging. Magn Reson Med. 1992;28:202–213. doi: 10.1002/mrm.1910280204. [DOI] [PubMed] [Google Scholar]
- Rasche V, Holz D, Proska R. MR fluoroscopy using projection reconstruction multi-gradient-echo (prMGE) MRI. Magn Reson Med. 1999;42:324–334. doi: 10.1002/(sici)1522-2594(199908)42:2<324::aid-mrm15>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Hardy CJ, Darrow RD, Nieters EJ, Roemer PB, Watkins RD, Adams WJ, Hattes NR, Maier JK. Real-time acquisition, display, and interactive graphic control of NMR cardiac profiles and images. Magn Reson Med. 1993;29:667–673. doi: 10.1002/mrm.1910290514. [DOI] [PubMed] [Google Scholar]
- Debbins JP, Riederer SJ, Rossman PJ, Grimm RC, Felmlee JP, Breen JF, Ehman RL. Cardiac magnetic resonance fluoroscopy. Magn Reson Med. 1996;36:588–595. doi: 10.1002/mrm.1910360414. [DOI] [PubMed] [Google Scholar]
- Kerr AB, Pauly JM, Hu BS, Li KC, Hardy CJ, Meyer CH, Macovski A, Nishimura DG. Real-time interactive MRI on a conventional scanner. Magn Reson Med. 1997;38:355–367. doi: 10.1002/mrm.1910380303. [DOI] [PubMed] [Google Scholar]
- Hardy CJ, Darrow RD, Pauly JM, Kerr AB, Dumoulin CL, Hu BS, Martin KM. Interactive coronary MRI. Magn Reson Med. 1998;40:105–111. doi: 10.1002/mrm.1910400115. [DOI] [PubMed] [Google Scholar]
- Busse RF, Debbins JP, Kruger DG, Fain SB, Riederer SJ. Interactive three-point localization of double-oblique sections using MR fluoroscopy. Magn Reson Med. 1999;41:846–849. doi: 10.1002/(sici)1522-2594(199904)41:4<846::aid-mrm26>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Sodickson DK, Manning WJ. Simultaneous acquisition of spatial harmonics (SMASH): ultra-fast imaging with radiofrequency coil arrays. Magn Reson Med. 1997;38:591–603. doi: 10.1002/mrm.1910380414. [DOI] [PubMed] [Google Scholar]
- Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999;42:952–962. [PubMed] [Google Scholar]
- Griswold MA, Jakob PM, Nittka M, Goldfarb JW, Haase A. Partially parallel imaging with localized sensitivities (PILS) Magn Reson Med. 2000;44:602–609. doi: 10.1002/1522-2594(200010)44:4<602::aid-mrm14>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Heidemann RM, Griswold MA, Haase A, Jakob PM. VD-AUTO-SMASH imaging. Magn Reson Med. 2001;45:1066–1074. doi: 10.1002/mrm.1141. [DOI] [PubMed] [Google Scholar]
- McKenzie CA, Yeh EN, Ohliger MA, Price MD, Sodickson DK. Self-calibrating parallel imaging with automatic coil sensitivity extraction. Magn Reson Med. 2002;47:529–538. doi: 10.1002/mrm.10087. [DOI] [PubMed] [Google Scholar]
- Kyriakos WE, Panych LP, Kacher DF, Westin CF, Bao SM, Mulkern RV, Jolesz FA. Sensitivity profiles from an array of coils for encoding and reconstruction in parallel (SPACE RIP) Magn Reson Med. 2000;44:301–308. doi: 10.1002/1522-2594(200008)44:2<301::aid-mrm18>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Roemer PB, Edelstein WA, Hayes CE, Souza SP, Mueller OM. The NMR phased array. Magn Reson Med. 1990;16:192–225. doi: 10.1002/mrm.1910160203. [DOI] [PubMed] [Google Scholar]
- Guttman MA, Kellman P, Dick AJ, Lederman RJ, McVeigh ER. Real-time accelerated interactive MRI with adaptive TSENSE and UNFOLD. Magn Reson Med. 2003;50:315–321. doi: 10.1002/mrm.10504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy CJ, Darrow RD, Saranathan M, Giaquinto RO, Rohling KW, Dumoulin CL, Zhu Y, Bottomley PA. Real-time large-FOV MRI with a massively parallel 32-channel MRI system and detector array.. Proc 11th Annual Meeting ISMRM; Toronto. 2003.p. 471. [Google Scholar]
- Brown DG, McDougall MP, Wright SM. Receiver design for parallel imaging with large arrays; Proc 10th Annual Meeting ISMRM; Honolulu. 2002.p. 863. [Google Scholar]
- Wright SM, McDougall MP, Brown DG. Single echo acquisition (SEA) MR imaging; Proc 11th Annual Meeting ISMRM; Toronto. 2003.p. 23. [Google Scholar]
- Zhu Y, Hardy C, Giaquinto R, Rohling K, Dumoulin C, Sodickson D, Ohliger M, Darrow R, Kenwood G. Highly parallel volumetric imaging with accelerated spatial encoding along two dimensions; Proc 11th Annual Meeting ISMRM; Toronto. 2003.p. 22. [Google Scholar]
- Dumoulin CL, Giaquinto RO, Rohling KW, Rossi CJ, Watkins RD, Hardy CJ, Darrow RD. A 32-channel coil for phased array imaging at 1.5 Tesla; Proc 11th Annual Meeting ISMRM; Toronto. 2003.p. 431. [Google Scholar]
- Busse RF, Riederer SJ, Fletcher JG, Bharucha AE, Brandt KR. Interactive fast spin-echo imaging. Magn Reson Med. 2000;44:339–348. doi: 10.1002/1522-2594(200009)44:3<339::aid-mrm1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Hardy CJ, Sodickson DK, Gianquinto RO, Dumoulin CL, Kenwood G, Niendorf T, Lejay H, McKenzie CA, Ohliger MA, Rotsky NM. Highly parallel volumetric imaging with a 32-element RF coil array. Magn Reson Med. 2004;52:869–877. doi: 10.1002/mrm.20209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Pauly J, Nishimura D. Broadband multi-coil reconstruction using spiral trajectories; Proc 11th Annual Meeting ISMRM; Toronto. 2003.p. 2351. [Google Scholar]