Abstract
Carney complex (CNC) is a familial multiple neoplasia syndrome characterized by spotty skin pigmentation, cardiac and cutaneous myxomas and endocrine tumors. CNC is inherited as an autosomal dominant trait, and is transmitted with greater frequency by women vs men. Nearly two-thirds of CNC patients are heterozygous for inactivating mutations in the gene encoding the protein kinase A (PKA) type Iα regulatory subunit (RIα), PRKAR1. We report here that male mice heterozygous for the Prkar1a gene have severely reduced fertility. Sperm from Prkar1a heterozygous mice are morphologically abnormal and reduced in number. Genetic rescue experiments reveal that this phenotype results from elevated PKA catalytic activity in germ cells as early as the pachytene stage of spermatogenesis. Consistent with this defect in the male mutant mice, sperm from CNC patients heterozygous for PRKAR1A mutations were also found to be morphologically aberrant and decreased in number. We conclude that unregulated PKA activity in male meiotic or postmeiotic germ cells leads to structural defects in mature sperm and results in reduced fertility in mice and humans, contributing to the strikingly reduced transmission of PRKAR1A inactivating mutations by male patients with CNC.
INTRODUCTION
In CNC families, genetic studies have reported linkage to two loci(1, 2) and one of these loci, the locus on chromosome 17 (17q22–24), corresponds to the PRKAR1A gene(3, 4). PRKAR1A is the type 1α regulatory subunit (RIα) of cAMP-dependent protein kinase (PKA) and inhibits PKA activity in the absence of cAMP. To explain the multiple neoplasia in CNC patients, it was postulated that PRKAR1A is a tumor suppressor gene; a mutation in one allele and the subsequent loss of heterozygosity (LOH) of the normal allele results in unregulated PKA activity and tumor formation(4). However, other studies report that haplo-insufficiency of the PRKAR1A gene without LOH is associated with cardiac myxomas(3), primary pigmented nodular adrenocortical disease (PPNAD)(5) and thyroid tumors(6). Reduced levels of RIα can lead to unregulated C subunit basal activity ((7) and may also shift the predominant holoenzyme from one containing RIα to one containing the type II regulatory subunit (RII)(3). Since RII-containing PKA holoenzymes bind more tightly to many A-kinase anchoring proteins (AKAPs)(8, 9), a shift in the subcellular localization of PKA could be another factor in the changes in cell cycle control and tumor formation in CNC patients. Our studies with animals containing a null mutation in one allele of Prkar1a suggest that unregulated PKA activity and not relocalization of subcellular PKA contributes to the fertility defects that are observed in the males.
Transmission of CNC occurs more frequently through the female parent but the mechanism for this is unknown(10, 11). Our results demonstrate that male mice with a null mutation in one allele of Prkar1a are subfertile as a result of abnormal sperm morphology and reduced sperm count. Semen analyses from 7 male CNC patients with mutations in PRKAR1A also show a sperm morphology phenotype similar to the mutant mice. These data suggest a common mechanism underlying male infertility in mice and men with haploinsufficiency of PRKAR1A.
RESULTS
Fertility Analysis of RIα+/− Male Mice
The targeted deletion of the Prkar1a (RIα) gene in mice is embryonic lethal in the homozygous state. As described in a previous report, the RIα−/− embryos fail to develop a functional heart tube and are resorbed at embryonic day 10.5. This defect in development is largely due to unregulated C subunit activity since it could be rescued by a genetic depletion of C subunit in Rα−/−embryos (7). Mating of RIα heterozygotes, in order to generate RIα−/− embryos, revealed that RIα+/− males, but not females, had reduced fertility when back-crossed to a C57BL/6 background. A fertility study in which RIα+/− males on a high C57BL/6 background (94% or 99%) were mated with wild type C57BL/6 females confirmed that these RIα+/− males are severely subfertile (Table 1). Mature sperm from the cauda epididymides of these males were fragile with broken heads and ruptured tails (Fig. 1), were reduced in number, were less motile, and were defective in fertilizing zona pellucida (zp)-intact and zp-free eggs (Table 1). No significant differences were observed in testes or seminal vesicle weight or serum content of luteinizing hormone or testosterone. Follicle-stimulating hormone was decreased slightly (33%) but significantly.
Table 1.
Fertility Parameters of Prkar1a heterozygous male mice.
| Genotype | Pregnancy Rate | Average Litter Size | Sperm ‡ Motility (%) | Sperm ‡ Speed (um/sec) | % Fertilized eggs in ivf (+zp) | % Fertilized eggs in ivf (−zp) | Sperm Count (x106 / mouse) |
|---|---|---|---|---|---|---|---|
| Wild type (+/+) | 13/16 | 6±0 | 58±3 | 201±2 | 25±6 | 70±11 | 18.5 ±4.4 |
| Prkar1a heterozygotes (+/−) | 1/15 | 2* | 38±10 | 136±12* | 0* | 8±3* | 3.5±1.0* |
Mice are on a high (94 or 99%) C57BL/6 background. Values shown are mean±S.E.
Significantly different from wild type at p<0.05.
This measures flagellar motility and velocity even for headless sperm.
Zp=zona pellucida
Ivf= in vitro fertilization
Figure 1.
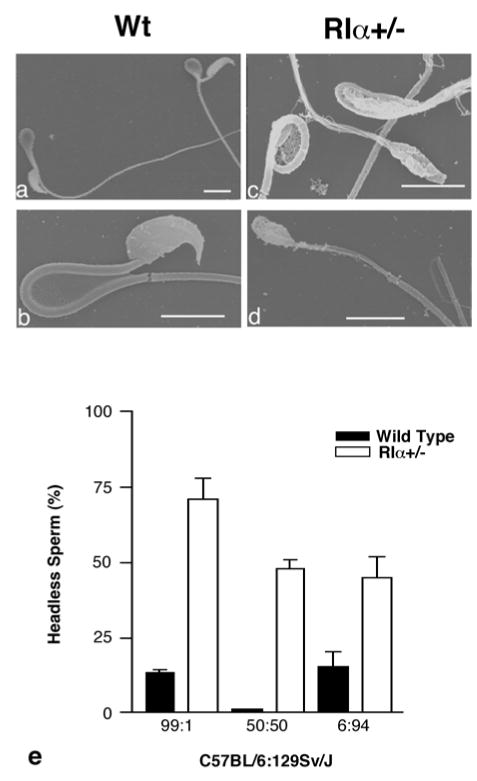
Sperm removed from the cauda epididymis of adult wild type and RIα+/− animals. SEM of wild type (a,b) sperm and RIα+/− (c,d) headless sperm with ruptured tails. Magnification bar=10um. (e) Percentage of sperm that are headless from wild type and RIα+/− males on different genetic backgrounds, shown as the ratio of C57BL/6:129Sv/J. N values are as follows: 99:1 (wt=10, RIα+/−=7), 50:50 (wt=1, RIα+/−=4), 6:94 (wt=3, RIα+/−=5). RIα+/− are significantly different from wild type on all backgrounds. RIα+/− on the 99:1 background are significantly different from RIα+/− on the 50:50 background. Significance level is p<0.05.
Breeding of RIα+/− males that were on a 50:50 background showed no deficits in their fertility as each plugged female produced a normal litter size (7±1 pups, mean± SEM). Although most severe on a C57BL/6 background, the fragility of the sperm was clearly evident on a mixed (50:50) 129Sv/J:C57BL/6 background and on mice backcrossed into the 129Sv/J background (Fig. 1e). The normal fertility of the RIα+/− males on the 50:50 background suggests that although approximately half of the sperm are headless, a sufficient percentage of sperm are morphologically normal and capable of fertilizing eggs. These data indicate that RIα+/− males on a high C57BL/6 background are subfertile due to sperm defects and reduced sperm number and motility. In addition genetic modifier(s) contribute to the severity of the phenotype rendering most RIα+/− males on a C57BL/6 background completely infertile.
We examined testes and epididymides of RIα+/− mice to determine the basis for the sperm abnormalities. RIα+/− and wild type testes sections were similar throughout most stages of spermatogenesis and spermiogenesis. However in testis sections of RIα+/− as young as 8 weeks of age, stage I round spermatids contained large clear areas in the nucleus devoid of chromatin (Fig 2a–d). A similar nuclear phenotype was observed in stage V–VI round spermatids in casein kinase 2α catalytic subunit (Csnk2a2)−/− mice (12) and, combined with nuclear envelope abnormalities and reduced epididymal sperm count, it was suggested that Ck2α’ deficiency impairs the phosphorylation of nuclear proteins resulting in cell-death. Similarly the reduced sperm count and nuclear abnormalities in RIα+/− animals may be a result of altered phosphorylation levels of nuclear proteins by PKA.
Figure 2.
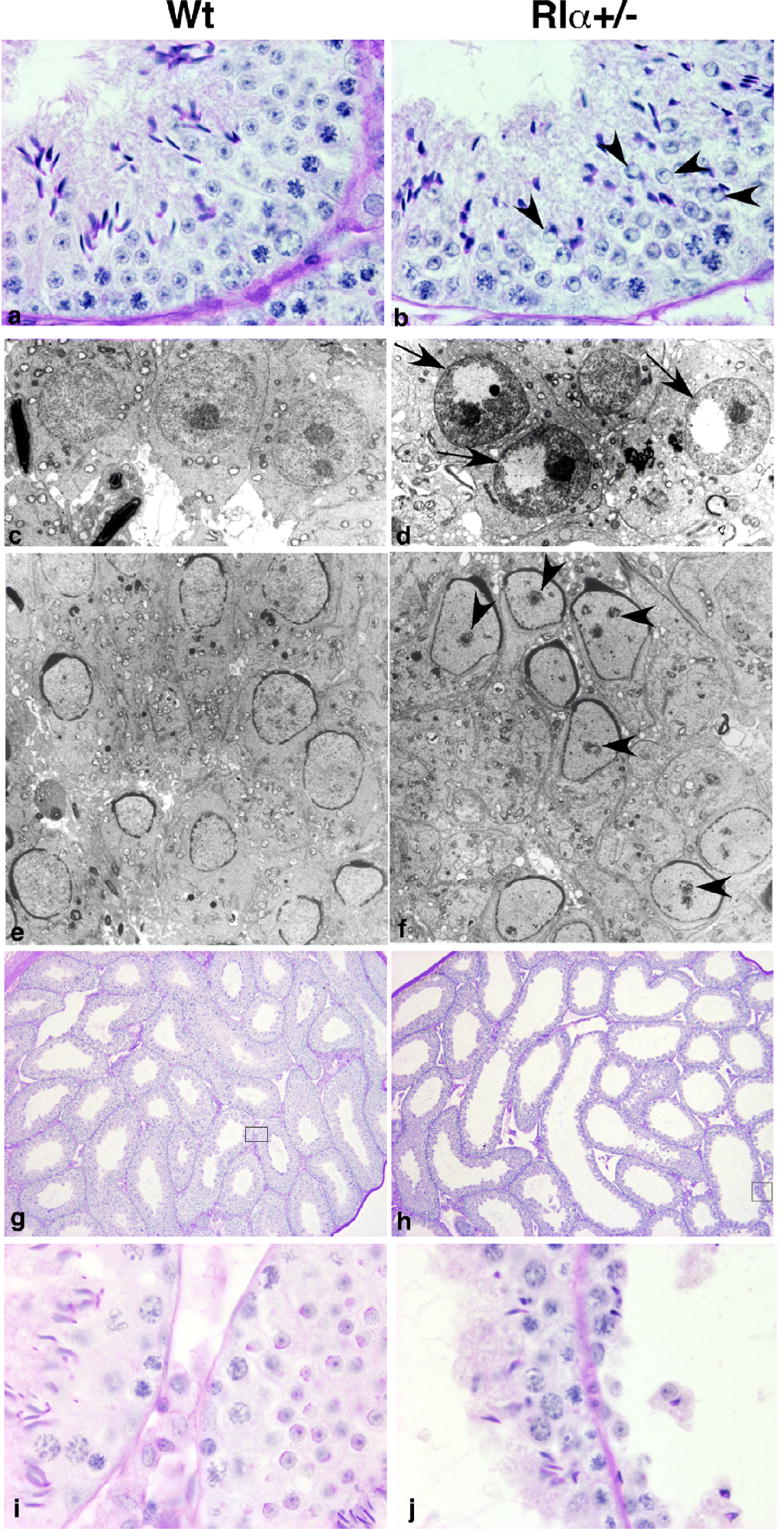
Histological analysis of testis of adult wild type and RIα+/− males. (a,b) Stage I testis showing that round spermatids (b, arrowhead) contain clear areas in the nucleus in RIα+/− males. (c,d) EM of stage I round spermatids displaying the nuclear clear area (arrow) devoid of chromatin in RIα+/− males. (e,f) EM of stage IX testis. Spermatids in RIα+/− males (f) contain premature chromatin focal condensations (arrowheads). (g,h) Testis showing enlarged seminiferous tubules in RIα+/− (h) with germ cell sloughing (j). (i,j) Higher magnification of seminiferous tubules in insets of (g,h), respectively.
Stage IX spermatids from RIα+/− contained premature chromatin focal condensations not found in wild type spermatids (Fig 2e,f). Precocious nuclear chromatin condensation can be triggered by premature synthesis of the nucleoprotein protamine 1 (13) leading to sterility in mice and it has been reported that infertility in a subset of human patients correlates with abnormal protamine content (14). We have found no change in protamine gene expression by array analysis (data not shown) and no change in protein expression in RIα+/− mice. However we can not exclude a more subtle temporal change in protamine expression that could result in the premature chromatin condensation observed in stage IX spermatids.
A third difference appeared in approximately 60% of older (at least 19 weeks old) RIα+/− animals: the seminiferous tubules of the testis were distended (Fig 2g–j) with germ cell sloughing in some of the tubules (Fig 2j). In addition, the corpus epididymis was enlarged (Fig 3a,b) and spermatic granulomas were present (Fig 3c,d). A similar phenotype is induced experimentally in laboratory animals by vasectomy or by injection of spermatozoa into connective tissues(15, 16). Since no obstructions of the vas deferens were observed in RIα+/− animals, we suggest that defects in the epididymis may leak spermatozoa from the ductal lumen, induce inflammatory cell responses and evoke formation of a spermatic granuloma and back pressure-induced testicular atrophy. Although some CNC patients have large-cell calcifying Sertoli cell tumors (LCCSCT), no evidence of LCCSCT has been observed in testes from RIα+/− mice.
Figure 3.
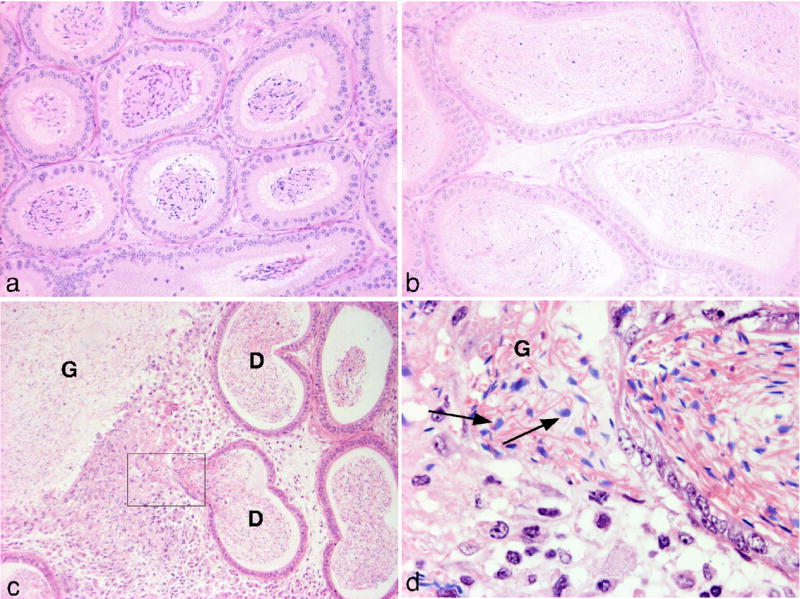
Histological analysis of epididymis of adult wild type and RIα+/− males. The corpus epididymis is enlarged in RIα+/− males (b) compared with wild type (a) and in some cases (c) contains a spermatic granuloma (G) adjacent to a duct (D). (d) Higher magnification of inset in (c) showing the presence of spermatids (arrows) in the interstitial space.
Genetic Rescue of RIα+/− Fertility Defects
We postulated that RIα haploinsufficiency causes fertility defects in male mice by a decrease in the content of RIα protein and a subsequent increase in constitutive (basal) PKA activity in germ cells. The R2C2 tetrameric holoenzyme is in the inactive state until cAMP levels are elevated and the active C subunit is released. The loss of one allele of the RIα gene lowers RIα mRNA and RIα protein synthesis and as a consequence, some C subunit might be left in the unbound and active state. Since RIα+/− is the major PKA regulatory subunit expressed in premeiotic and meiotic male germ cells(17, 18), this stage of spermatogenesis is the likely time when kinase activity is unregulated in RIα+/−. If elevated PKA results in defective sperm in RIα+/− animals, then genetically reducing the amount of kinase activity might be expected to partially rescue the phenotype. We crossed RIα+/− animals with Cα2+/− mice engineered to have reduced PKA activity only in meiotic and postmeiotic germ cells. The Cα2 isoform is an alternatively spliced product of the Prkaca gene (19, 20) and is expressed exclusively in male germ cells. Cα2 mRNA appears first at the mid-pachytene stage of spermatogenesis and is the only catalytic subunit found in mature sperm(19–21). Cα2 homozygous null males are infertile due to a complete loss of PKA activity in sperm although sperm morphology is normal. However, Cα2 heterozygous males are fertile with no apparent defects in sperm number, motility or morphology (21).
For the attempted rescue, RIα+/−;Cα2+/− adult males (97% C57BL/6) were generated by crossing RIα+/− females with Cα2+/− males. Sperm from these double heterozygote mice on a high C57BL/6 background were morphologically indistinguishable from wild type (Fig 4a,b) demonstrating a rescue of the RIα+/− mutant “fragile sperm” phenotype. Moreover, the sperm were capable of fertilizing eggs and generating offspring as efficiently as wild type sperm (data not shown). However, spermatic granulomas in the epididymis and testicular atrophy were still observed in approximately 60% of double heterozygote males that is similar to their incidence in RIα+/− animals. Since Cα2 is not expressed in the epididymis, the genetic rescue of the spermatic granulomas phenotype was not expected or observed.
Figure 4.
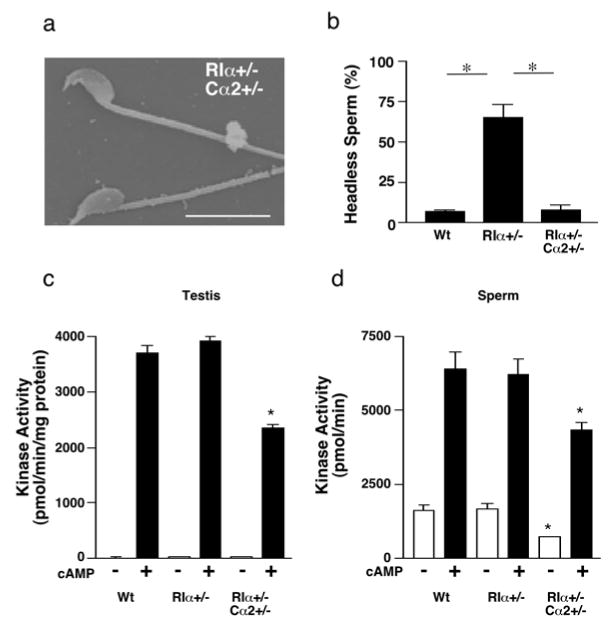
Analysis of RIα+/−,Cα2+/− animals (97% C57BL/6). (a) SEM of sperm removed from the cauda epididymis of RIα+/−,Cα2+/− adult male. (b) Percentage of sperm that are headless from wild type (n=12), RIα+/− (n=13), and RIα+/−,Cα2+/− (n=8) males. (c,d) Basal (-cAMP) and total (+cAMP) PKA activity in testis (c) and sperm (d) from wild type (n=4 or 5), RIα+/− (n=5) and RIα+/−,Cα2+/− (n=3) adult mice. * p<0.05
Total PKA activity was significantly reduced in both testis and sperm and basal PKA activity was significantly reduced in sperm in RIα+/−;Cα2+/− animals (Fig 4c,d). This reduction in PKA activity correlates with the restoration of normal sperm morphology and fertility and supports the hypothesis that elevated PKA activity exclusively in a subset of germ cells as early as the mid-pachytene stage of spermatogenesis causes sperm and fertility defects. However, we were unable to detect biochemically an increase in basal PKA activity in either adult sperm or testes from RIα+/−animals (Fig. 4c,d). The adult testis contains germ cells at all stages of development and we postulated that basal PKA activity is elevated in RIα+/− in a subpopulation of developing germ cells where RIα is the primary regulatory of PKA activity. Therefore, we measured PKA activity in testis from juvenile animals (P10 and P25) before the appearance of RIIα in elongated spermatids. These assays were also unable to detect an increase in basal PKA activity in juvenile RIα+/− testis (Supplemental Fig. 1).
Compensation by other Regulatory Subunits in RIα+/−
Compensation by the other regulatory subunits may regulate PKA activity when RIα levels are reduced and contribute to the infertility phenotype. In developing germ cells, RIα and RIIα are the primary regulatory subunits expressed (17). RIIα mRNA is normally induced in elongating spermatids (18) but could be prematurely induced in order to regulate PKA activity in RIα+/− developing germ cells. In RIα+/− testes but not in mature sperm, RIα protein levels were reduced and RIIα protein levels were elevated (Fig 5a,b), suggesting that RIIα may compensate and partially regulate basal kinase activity in RIα+/− immature germ cells. However, Northern analysis indicated that the shorter (2.4 kb) testis-specific RIIα mRNA is not prematurely induced in RIα+/− testis but is expressed at a time point coincident with the appearance of elongating spermatids and is similar to wild type expression (Fig 5c). The larger (6.0 kb) RIIα transcript was evenly expressed across all time points and was no different between wild type and RIα+/−. Since RIIα mRNA levels are unchanged but protein levels are elevated in RIα+/− testes, it is likely that posttranscriptional stabilization of RIIα protein may be involved and the increased RIIα protein levels may serve to help regulate PKA activity when RIα levels are reduced.
Figure 5.
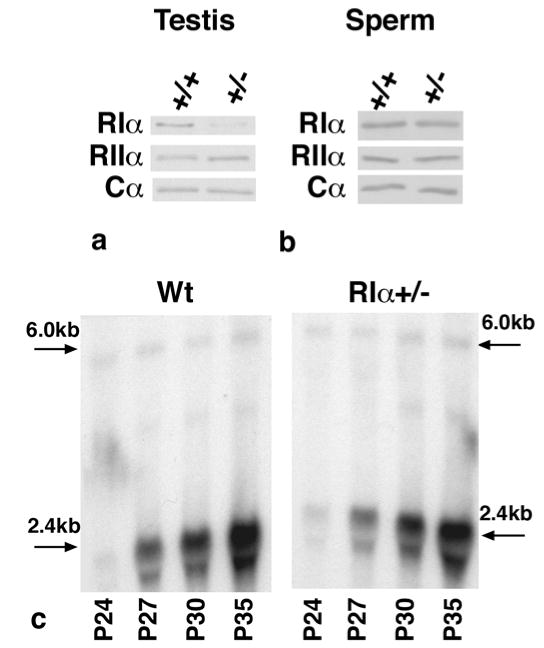
PKA subunit levels from wild type and RIα+/− mice. (a,b) Western blot analysis for RIα, RIIα, and Cα subunits in adult testis (a) and sperm (b). (c) Northern blot analysis of RIIα mRNA in wild type and RIα+/− testis at postnatal days (P) 24, 27, 30, and 35.
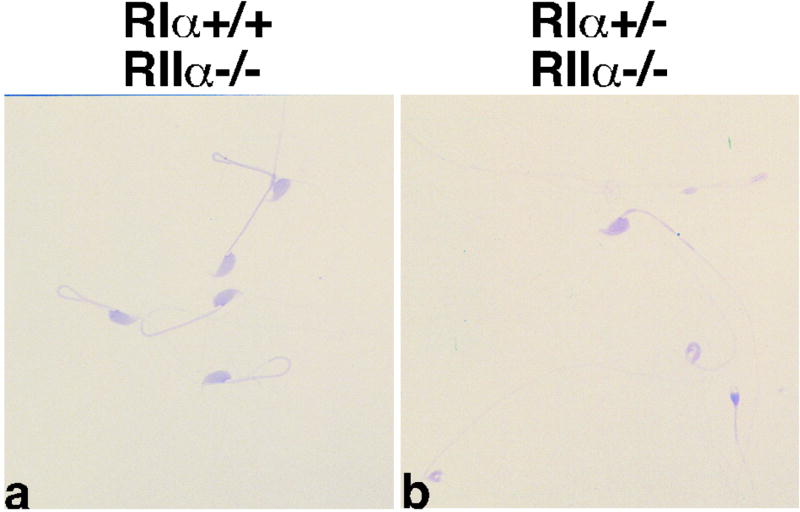
We therefore asked whether RIIα compensation contributes in any way to the abnormal morphology of RIα+/− sperm and the infertility phenotype. We had previously established a line of mice with a null mutation in the RIIα gene and these animals were shown to have normal sperm morphology and motility and fertility(22). RIα+/− animals were crossed with RIIα deficient mice and RIα+/−/RIIα−/− males were generated. The RIα+/− males on the RIIα null background exhibited the same sperm abnormalities (Fig 6a,b) and infertility as RIα+/− animals. RIα+/−/RIIα−/− males (n=2) each plugged 4 wild type females but generated one pup total compared with 74 pups from similarly mated wild type males (n=2). These data indicate that the subfertility and abnormal sperm morphology of RIα+/− animals are not dependent on the presence of RIIα and rule out a compensation mechanism as a cause of the subfertility phenotype. This finding coupled with the genetic rescue experiment with Cα2+/− animals suggests that reduced levels of RIα protein in the RIα+/− animals result in inappropriate C subunit activity and sperm fragility.
Figure 6.
Sperm morphology in RIIα−/− background. Cauda epididymal sperm from RIα+/+;RIIα−/− (a) and RIα+/−;RIIα−/− (b).
Semen Analysis of CNC Patients
Haploinsufficiency of PRKAR1A has been reported to occur in nearly two-thirds of CNC patients and male CNC patients have a significantly reduced fertility compared with female patients(3, 23, 24). We investigated whether this reduced fertility in male CNC patients with PRKAR1A mutations, like the RIα+/− mouse, results from abnormal sperm morphology and reduced sperm count. Ejaculates from CNC patients contained a high percentage of abnormal sperm characterized by head or tail defects. The presence of immature germ cells was also observed (Fig 7). Almost half (3/7) of the patients were azoo- or oligospermic. The sperm defects were observed in 4 unrelated probands and in all 3 CNC patients analyzed in family YZZ. These observations indicate that male CNC patients with haploinsufficiency of the PRKAR1A gene have reduced fertility and consequently reduced transmission of the mutation as a result of sperm morphology defects and azoo- or oligospermia. CNC patients without PRKAR1A mutations were not available for study. However, no evidence of male infertility was observed in a large CNC family carrying a mutation in the perinatal myosin heavy-chain gene (MYH8) (25).
Figure 7.
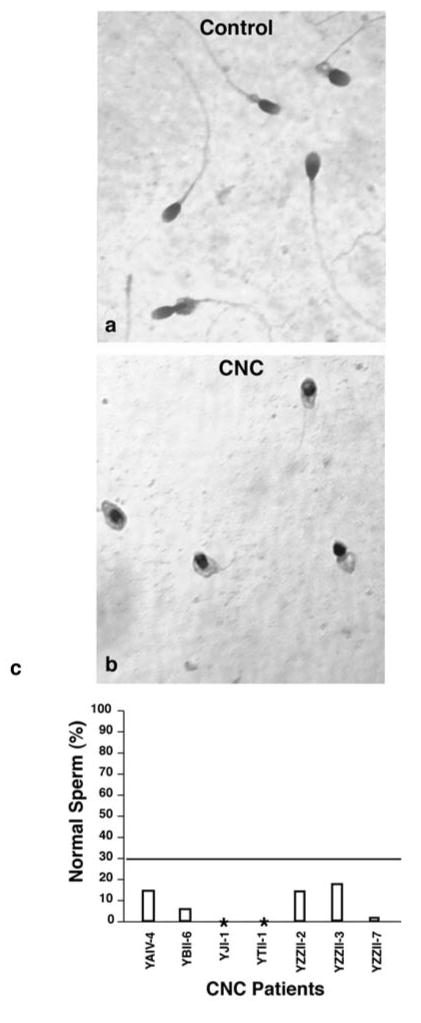
Sperm from CNC patients. Normal sperm (a) and sperm from a CNC patient (b). (c) Sperm morphology from seven CNC patients. Values less than 30% fall below the criteria for normal morphology ((40)). *Patients YJI-1 and YTII-1 are azoospermic.
Although there are similarities between the sperm defects and oligospermia found in ejaculates from CNC patients and in cauda epididymal sperm from RIα+/− mice, the sperm morphology changes were not identical. The majority of sperm from RIα+/− mice were too fragile to isolate intact, whereas most sperm from CNC patients were intact. These differences might be species specific or a result of the differences in isolation for mouse sperm (cauda epididymal dissection) versus the human sperm (ejaculate).
DISCUSSION
The targeted deletion of one allele of the Prkar1a gene in mice leads to a dramatic reduction in male fertility. Morphologically, the sperm defects in these animals are similar to those in sperm from CNC patients with RIα+/− mutations. Genetic rescue experiments demonstrate that elevated PKA activity and not relocalization of the kinase in germ cells produces the sperm defects in mice. This increase in PKA activity interferes with a developmental pathway required for the integrity of the sperm head and tail. In male germ cells, PKA affects gene expression (26), nucleosomal protein activity (27, 28), and motility(29). The nuclear changes evident in the stage I round spermatids (chromatin condensation at the margins of the nuclei) and premature focal chromatin condensation (stage IX) suggest that PKA is altering nuclear function.
The genetic rescue experiment supports the hypothesis that elevated PKA activity exclusively in germ cells as early as the mid-pachytene stage of spermatogenesis, when Cα2 is expressed, causes fragile sperm. The inability of the kinase assay to detect a change in PKA activity in adult or juvenile RIα+/− testis is consistent with the finding that free C subunit is unstable and rapidly degraded when not tightly complexed with R subunit (30, 31). This is supported by previous results from our lab in which a mutation was made in the C subunit to lower its binding affinity for the R subunit resulting in constitutive C subunit activity (32). When this constitutively active C subunit was expressed in the liver, no change in basal PKA activity was observed in liver extracts even though a phenotype predicted by elevated PKA activity (altered glucose homeostasis, glycogen storage, and fructose 2,6-bisphosphate levels) was evident.
The inability of Cα2+/− mice to rescue the granulomas and testicular atrophy of the RIα+/− mouse suggests that the RIα+/− epididymis phenotype reflects a somatic cell defect. Whether this is due to an alteration of PKA activity or its subcellular localization is not known. Alterations in fluid absorption and/or secretion in the efferent duct, a phenotype of ERα−/−(33) males, may contribute to pressure changes that lead to spermatic granuloma formation and back-pressure atrophy of the testis. PKA inhibits the sodium/hydrogen exchanger 3 (NHE3) in epithelial cells of the proximal kidney(34). NHE3 is also expressed in the efferent duct and epididymis where it is involved in the absorption of tubular fluid (35). One possibility is that unregulated PKA in nonciliated cells of the efferent duct in RIα+/− mice phosphorylates and inhibits the activity of NHE3. Decreased absorption could then dilate epididymal tubules and initiate back-pressure atrophy of the testis.
Infertility affects about 5% of the male population and yet, in most cases, the molecular cause remains uncertain. We have shown that haploinsufficiency of the RIα gene in both mice (Prkar1a) and humans (PRKAR1A) is deleterious to the structural integrity of mature sperm and, in mice, dramatically reduces the ability of the sperm to fertilize eggs. The genetic rescue experiments support the hypothesis that unregulated PKA activity in germ cells leads to aberrations in sperm structure and the resulting infertility. These results reveal the toxicity of unregulated kinase activity in developing male germ cells and are in sharp contrast to the phenotype of Cα2-deficient male germ cells which undergo normal spermatogenesis and spermiogenesis yet are immotile(21). These findings demonstrate the significance of the PKA signaling pathway in male fertility and support previous work showing that components of this pathway, such as adenylyl cyclases(36, 37), and AKAPs (38), are likely candidates affecting male fertility in humans.
Materials and Methods
Animals
Wild type and RIα+/− mice were derived as previously described(7). Animals were 94% and 99% C57BL/6 with the remaining background as 129Sv/J with the exception that for the sperm morphology analysis on different C57BL/6 backgrounds, 50% and 6% C57BL/6 animals were additionally used. In all studies, wild type (control) animals and RIα+/− animals are on the identical genetic background, either 94% or 99% C57BL/6. Female 6–8 week old C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME) for fertility studies and 3 week old B6C3 mice were purchased from Taconic Farms (Germantown, NY) for ivf studies. All experiments were carried out in accordance with guidelines established by the Institutional Animal Care and Use Committee at the University of Washington.
Mouse Fertility Assessment, Sperm Swimout and Motility Assay
A fertility study and sperm motility analysis were performed as described previously(22). To assess sperm motility, mature spermatozoa were obtained from the cauda epididymis. The analysis was performed on video-taped sperm samples obtained from 10–20 week old mice using an automated Hamilton-Thorn Motility Analyzer. A slide chamber of 50 um depth was used and motile sperm were tracked at 60 frames/s. The observer was unaware of the genotype of the sperm. Twenty fields were recorded for 5 s each and percent motility was evaluated on a minimum of 100 sperm from the recorded sperm samples.
Sperm Morphology and Count
Sperm from the cauda epididymis were released into capacitating media as described above. A sperm suspension was placed on a glass slide (Superfrost Plus, VWR, West Chester, PA) by gently smearing 10 ul of the suspension across the top of the slide using the edge of a second glass slide. The slides were air dried. Sperm morphology was observed following histological staining or propidium iodide staining. For histological evaluation, sperm were stained (Diff-Quik Stain Set, Dade Behring Inc, Newark, DE) and viewed at 40x under a Nikon light microscope. One hundred sperm were counted per animal. Sperm head morphology was evaluated following propidium iodide staining (20 ug/ml) and DNA-containing heads were visualized with a Nikon fluorescent microscope at 40X. All heads stained with propidium iodide were normal in shape whether they were attached to a flagellum or not. Sperm count was estimated by counting the total number of flagellum removed from both cauda epididymides on a glass slide prepared as described for the histological evaluation.
Electron Microscopy
Sperm were isolated from the cauda epididymis as described above. Harvested sperm were pelleted at 600xg and the supernatant was removed. The sperm were fixed in 3% gluteraldehyde in 0.1 M phosphate-buffered saline for 24 hrs at 4°C. Sperm were collected on Nucleopore filters or poly-L-lysine-coated glass cover slips, dehydrated in a graded ethanol series, subjected to critical point drying, and coated with gold/palladium. Samples were examined with a JEOL JSM 6300F scanning electron microscope at an accelerating voltage of 15 KV at the Electron Microscopy Laboratory at the University of Washington.
Western Blot and Kinase Assay
Sperm were obtained from the cauda epididymides in phosphate-buffered saline (PBS) and diluted in sample buffer. Western blot analysis and kinase assay were performed on these samples as previously described(22, 39).
RNA Isolation and Analysis
Testes were removed from animals at postnatal days 24, 27, 30 and 35 and RNA was isolated by using the RNeasy Kit (Qiagen, California) according to the manufacturer’s protocol. The samples were denatured in 20 mM MOPS, pH 7.0, 1 mM EDTA, 5 mM sodium acetate, 2.2 M formaldehyde, and 50% formamide at 65°C for 15 min. The samples (5 ug total RNA) were loaded on a 1.2% agarose gel and run in the same buffer without formamide. The gel was then blotted to nitrocellulose and hybridized overnight with a nick-translated mouse cDNA probe (0.8 kb HindIII-SmaI fragment: all within the open reading frame for mouse RIIα). Equal loading was visualized and confirmed by staining the 28S and 18S ribosomal bands with 0.4% methylene blue
In Vitro Fertilization
Three week old B6C3 females were i.p. injected with pregnant mare serum (2.5 IU) followed 48 hrs later with human chorionic gonadotropin (2.5 IU). Thirteen hours after the second injection, the females were euthanized and their oviducts were removed. The oocyte-cumulus complexes were isolated and the cumulus was removed in hyaluronidase (10 mg/ml) in M2 medium (Sigma). A proportion of the eggs were subsequently treated with acidic Tyrode’s solution (Sigma) to remove the zona pellucida. Sperm were obtained as described above in human tubal fluid (HTF) media (Irvine Scientific, Irvine, CA) supplemented with 0.5% BSA. Ten microliters of sperm at 1x106 sperm/ml were added to a 250 ul drop of HTF containing 5 cumulus-free eggs and covered with light mineral oil (Sigma, embryo tested). Dishes were placed in an incubator with 5% C02/95% air at 37°C overnight. The next morning, the number of 2-cell embryos was scored.
Serum Hormone Measurements
Blood was collected from euthanized animals by cardiac puncture and serum was analyzed by radioimmunoassay (RIA) for mouse LH, mouse FSH, and testosterone by the University of Virginia Center for Reproduction Ligand Assay and Analysis Core Facility.
Semen Analysis from CNC Patients
After providing informed consent per institutional guidelines, men with known PRKAR1A mutations provided semen specimens for analysis within 2 hours of collection by the Cornell University Medical College Infertility Laboratory or the IVF Unit at the North Karelian Central Hospital in Joensuu, Finland. Manual light microscopic evaluation of sperm concentration, motility, and morphology was performed. Analysis was performed by technicians unaware of the patient’s genotype or medical history.
Statistical Analysis
Unpaired t tests were performed when comparing between wild type and mutant groups.
Acknowledgments
We acknowledge the excellent contributions of Thomas Su, Kathy Kafer, Darius Paduch, Helena Kaariainen, and Esa Korkeela. This research was supported by NICHD/NIH U54HD12629 (GSM, KAB) through cooperative agreement as part of the Specialized Cooperative Centers Program in Reproduction Research, by the Royalty Research Fund (UW), and by NIHR01HL61785 (CTB).
Footnotes
The authors have nothing to declare.
Molecular Endocrinology. First published ahead of print May 25, 2006 as doi:10.1210/me.2006-0060
Publisher's Disclaimer: This is an un-copyedited author manuscript copyrighted by The Endocrine Society. This may not be duplicated or reproduced, other than for personal use or within the rule of "Fair Use of Copyrighted Materials" (section 107, Title 17, U.S. Code) without permission of the copyright owner, The Endocrine Society. From the time of acceptance following peer review, the full text of this manuscript is made freely available by The Endocrine Society at http://www.endojournals.org/. The final copy edited article can be found at http://www.endojournals.org/. The Endocrine Society disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties. The citation of this article must include the following information: author(s), article title, journal title, year of publication and DOI.
References
- 1.Stratakis CA, Carney JA, Lin JP, Papanicolaou DA, Karl M, Kastner DL, Pras E, Chrousos GP. Carney complex, a familial multiple neoplasia and lentiginosis syndrome. Analysis of 11 kindreds and linkage to the short arm of chromosome 2. J Clin Invest. 1996;97:699–705. doi: 10.1172/JCI118467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casey M, Mah C, Merliss AD, Kirschner LS, Taymans SE, Denio AE, Korf B, Irvine AD, Hughes A, Carney JA, Stratakis CA, Basson CT. Identification of a novel genetic locus for familial cardiac myxomas and Carney complex. Circulation. 1998;98:2560–2566. doi: 10.1161/01.cir.98.23.2560. [DOI] [PubMed] [Google Scholar]
- 3.Casey M, Vaughan CJ, He J, Hatcher CJ, Winter JM, Weremowicz S, Montgomery K, Kucerlapati R, Morton CC, Basson CT. Mutations in the protein kinase A R1a regulatory subunit cause familial cardia myxomas and Carney Complex. J Clin Invest. 2000;106:R31–R38. doi: 10.1172/JCI10841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirschner LS, Sandrini F, Monbo J, Lin JP, Carney JA, Stratakis CA. Genetic heterogeneity and spectrum of mutations of the PRKAR1A gene in patients with the carney complex. Hum Mol Genet. 2000;9:3037–3046. doi: 10.1093/hmg/9.20.3037. [DOI] [PubMed] [Google Scholar]
- 5.Groussin L, Kirschner LS, Vincent-Dejean C, Perlemoine K, Jullian E, Delemer B, Zacharieva S, Pignatelli D, Carney JA, Luton JP, Bertagna X, Stratakis CA, Bertherat J. Molecular analysis of the cyclic AMP-dependent protein kinase A (PKA) regulatory subunit 1A (PRKAR1A) gene in patients with Carney complex and primary pigmented nodular adrenocortical disease (PPNAD) reveals novel mutations and clues for pathophysiology: augmented PKA signaling is associated with adrenal tumorigenesis in PPNAD. Am J Hum Genet. 2002;71:1433–1442. doi: 10.1086/344579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandrini F, Matyakhina L, Sarlis NJ, Kirschner LS, Farmakidis C, Gimm O, Stratakis CA. Regulatory subunit type I-alpha of protein kinase A (PRKAR1A): a tumor-suppressor gene for sporadic thyroid cancer. Genes Chromosomes Cancer. 2002;35:182–192. doi: 10.1002/gcc.10112. [DOI] [PubMed] [Google Scholar]
- 7.Amieux PS, Howe DG, Knickerbocker H, Lee DC, Su T, Laszlo GS, Idzerda RL, McKnight GS. Increased basal cAMP-dependent protein kinase activity inhibits the formation of mesoderm-derived structures in the developing mouse embryo. J Biol Chem. 2002;277:27294–27304. doi: 10.1074/jbc.M200302200. [DOI] [PubMed] [Google Scholar]
- 8.Huang LJ, Durick K, Weiner JA, Chun J, Taylor SS. Identification of a novel protein kinase A anchoring protein that binds both type I and type II regulatory subunits. J Biol Chem. 1997;272:8057–8064. doi: 10.1074/jbc.272.12.8057. [DOI] [PubMed] [Google Scholar]
- 9.Burton KA, Johnson BD, Hausken ZE, Westenbroek RE, Idzerda RL, Scheuer T, Scott JD, Catterall WA, McKnight GS. Type II regulatory subunits are not required for the anchoring-dependent modulation of Ca2+ channel activity by cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1997;94:11067–11072. doi: 10.1073/pnas.94.20.11067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stratakis CA. Mutations of the gene encoding the protein kinase A type I-a regulatory subunit (PRKAR1A) in patients with the “complex of spotty skin pigmentation, myxomas, endocrine overactivity, and schwannomas” (Carney Complex) Ann N Y Acad Sci. 2002;968:3–21. doi: 10.1111/j.1749-6632.2002.tb04323.x. [DOI] [PubMed] [Google Scholar]
- 11.Veugelers M, Wilkes D, Burton KA, McDermott D, Son Y, Vaughan CJ, La Perle K, Goldstein M, Kligfield P, O’Hagan A, JP Moore S, Bennett K, Lavyne M, Neau JP, Richter G, Kirali K, Stapleton K, Morelli P, Norio R, Kartunnen M, Takanashi Y, Noszian I, Eitelberger F, Manfroi W, Meyer B, Mochizuki Y, Imai T, Legius E, Goldmuntz B, Edelberg J, Eccles D, Irvine AD, MG S, Basson CT. Comparative PRKAR1A genotype-phenotype analyses in humans with Carney complex and prakar1a haploinsufficient mice. Proc Natl Acad Sci U S A. 2004 doi: 10.1073/pnas.0405535101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Escalier D, Silvius D, Xu X. Spermatogenesis of mice lacking CK2alpha’:failure of germ cell survival and characteristic modifications of the spermatid nucleus. Mol Reprod Dev. 2003;66:190–201. doi: 10.1002/mrd.10346. [DOI] [PubMed] [Google Scholar]
- 13.Lee K, Haugen HS, Clegg CH, Braun RE. Premature translation of protamine 1 mRNA causes precocious nuclear condensation and arrests spermatid differentiation in mice. Proc Natl Acad Sci U S A. 1995;92:12451–12455. doi: 10.1073/pnas.92.26.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aoki VW, Carrell DT. Human protamines and the developing spermatid: their structure, function, expression and relationship with male fertility. Asian J Androl. 2003;5:315–324. [PubMed] [Google Scholar]
- 15.Kennedy SW, Heidger PM., Jr Fine structure of the spermatic granuloma of the rat vas deferens following vasectomy. Anat Rec. 1980;198:461–474. doi: 10.1002/ar.1091980308. [DOI] [PubMed] [Google Scholar]
- 16.Ball RY. Experimental production of spermatic granuloma in BALB/c mice. Andrologia. 1984;16:342–349. doi: 10.1111/j.1439-0272.1984.tb00293.x. [DOI] [PubMed] [Google Scholar]
- 17.Oyen O, Myklebust F, Scott JD, Cadd GG, McKnight GS, Hansson V, Jahnsen T. Subunits of cyclic adenosine 3′,5′-monophosphate-dependent protein kinase show differential and distinct expression patterns during germ cell differentiation: alternative polyadenylation in germ cells gives rise to unique smaller-sized mRNA species. Biol Reprod. 1990;43:46–54. doi: 10.1095/biolreprod43.1.46. [DOI] [PubMed] [Google Scholar]
- 18.Oyen O, Scott JD, Cadd GG, McKnight GS, Krebs EG, Hansson V, Jahnsen T. A unique mRNA species for a regulatory subunit of cAMP-dependent protein kinase is specifically induced in haploid germ cells. FEBS Letters. 1988;229:391–394. doi: 10.1016/0014-5793(88)81163-3. [DOI] [PubMed] [Google Scholar]
- 19.Agustin JT, Wilkerson CG, Witman GB. The unique catalytic subunit of sperm cAMP-dependent protein kinase is the product of an alternative Calpha mRNA expressed specifically in spermatogenic cells. Mol Biol Cell. 2000;11:3031–3044. doi: 10.1091/mbc.11.9.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desseyn JL, Burton KA, McKnight GS. Expression of a nonmyristylated variant of the catalytic subunit of protein kinase A during male germ-cell development. Proc Natl Acad Sci U S A. 2000;97:6433–6438. doi: 10.1073/pnas.97.12.6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nolan MA, Babcock DF, Wennemuth G, Brown W, Burton KA, McKnight GS. Sperm-specific protein kinase A catalytic subunit Cα2 orchestrates cAMP signaling for male fertility. Proc Natl Acad Sci U S A. 2004;101:13483–13488. doi: 10.1073/pnas.0405580101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burton KA, Treash-Osio B, Muller CH, Dunphy EL, McKnight GS. Deletion of Type IIa regulatory subunit delocalizes protein kinase A in mouse sperm without affecting motility or fertilization. J Biol Chem. 1999;34:24131–24136. doi: 10.1074/jbc.274.34.24131. [DOI] [PubMed] [Google Scholar]
- 23.Veugelers M, Wilkes D, Burton KA, McDermott DA, Song Y, Vaughan CJ, Hahn R, Goldstein MM, La Perle K, McKnight GS, Basson CT. Comparative Genotype-Pheotype Analyses of Human Carney Complex and Murine prkar1a Haploinsufficiency. Circulation. 2003;108:IV-83. [Google Scholar]
- 24.Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, Cho YS, Cho-Chung YS, Stratakis CA. Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat Genet. 2000;26:89–92. doi: 10.1038/79238. [DOI] [PubMed] [Google Scholar]
- 25.Veugelers M, Bressan MA, McDermott D, Weremowicz S, Morton CC, Mabry CC, Lefaivre JF, Zunamon A, Destree A, Chaudron JM, Basson CT. Mutation of perinatal myosin heavy chain associated with a Carney complex variant. The New England Journal of Medicine. 2004;351 doi: 10.1056/NEJMoa040584. [DOI] [PubMed] [Google Scholar]
- 26.Don J, Stelzer G. The expanding family of CREB/CREM transcription factors that are involved with spermatogenesis. Mol Cell Endocrinol. 2002;187:115–124. doi: 10.1016/s0303-7207(01)00696-7. [DOI] [PubMed] [Google Scholar]
- 27.Meetei AR, Ullas KS, Vasupradha V, Manchanahalli R, Satyanarayana R. Involvement of protein kinase A in the phosphorylation of spermatidal protein TP2 and its effect on DNA condensation. Biochemistry. 2002;41:185–195. doi: 10.1021/bi0117652. [DOI] [PubMed] [Google Scholar]
- 28.Pirhonen A, Linnala-Kankkunen A, Menpaa PH. P2 protamines are phosphorylated in vitro by protein kinase C, whereas P1 protamines prefer cAMP-dependent protein kinase. A comparative study of five mammalian species. Eur J Biochem. 1994;223:165–169. doi: 10.1111/j.1432-1033.1994.tb18979.x. [DOI] [PubMed] [Google Scholar]
- 29.Lindemann CB. A cAMP-induced increase in the motility of demembranated bull sperm models. Cell. 1978;13:9–18. doi: 10.1016/0092-8674(78)90133-2. [DOI] [PubMed] [Google Scholar]
- 30.Hemmings BA. cAMP mediated proteolysis of the catalytic subunit of cAMP-dependent protein kinase. FEBS Letters. 1986;196:126–130. doi: 10.1016/0014-5793(86)80226-5. [DOI] [PubMed] [Google Scholar]
- 31.Richardson JM, Howard P, Massa JS, Maurer RA. Post-transcriptional regulation of cAMP-dependent protein kinase activity by cAMP in GH3 pituitary tumor cells. Evidence for increased degradation of catalytic subunit in the presence of cAMP. J Biol Chem. 1990;265:13635–13640. [PubMed] [Google Scholar]
- 32.Niswender CM, Willis BS, Wallen A, Sweet IR, Jetton TL, Thompson BR, Wu C, Lange AJ, McKnight GS. Cre recombinase-dependent expression of a constitutively active mutant allele of the catalytic subunit of protein kinase A. Genesis. 2005;43:109–119. doi: 10.1002/gene.20159. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Q, Clarke L, Nie R, Carnes K, Lai L-W, Lien Y-HH, Verkman A, Lubahn D, Fisher JS, Katzenellenbogen BS, Hess RA. Estrogen action and male fertility: roles of the sodium/hydrogen exchanger-3 and fluid reabsorption in reproductive tract function. Proc Natl Acad Sci U S A. 2001;98:14132–14137. doi: 10.1073/pnas.241245898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinman E, Minkoff C, Shenolikar S. Signal complex regulation of renal transport proteins: NHERF and regulation of NHE3 by PKA. Am J Physiol Renal Physiol. 2000;279:F393–F399. doi: 10.1152/ajprenal.2000.279.3.F393. [DOI] [PubMed] [Google Scholar]
- 35.Bagnis C, Marsolais M, Biemesderfer D, Laprade R, Breton S. Na+/H+ -exchange activity and immunolocalization of NHE3 in rat epididiymis. Am J Physiol Renal Physiol. 2001;280:F426–F436. doi: 10.1152/ajprenal.2001.280.3.F426. [DOI] [PubMed] [Google Scholar]
- 36.Esposito G, Jaiswal BS, Xie F, Krajnc-Franken MA, Robben TJ, Strik AM, Kuil C, Philipsen RL, van Duin M, Conti M, Gossen JA. Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect. Proc Natl Acad Sci U S A. 2004;101:2993–2998. doi: 10.1073/pnas.0400050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Livera G, Xie F, Garcia MA, Jaiswal B, Chen J, Law E, Storm DR, Conti M. Inactivation of the mouse adenylyl cyclase 3 gene disrupts male fertility and spermatozoon function. Mol Endocrinol. 2005;19:1277–1290. doi: 10.1210/me.2004-0318. [DOI] [PubMed] [Google Scholar]
- 38.Miki K, Willis WD, Brown PR, Goulding EH, Fulcher KD, Eddy EM. Targeted disruption of the Akap4 gene causes defects in sperm flagellum and motility. Dev Biol. 2002;248:331–342. doi: 10.1006/dbio.2002.0728. [DOI] [PubMed] [Google Scholar]
- 39.Clegg CH, Correll LA, Cadd GG, McKnight GS. Inhibition of intracellular cAMP-dependent protein kinase using mutant genes of the regulatory type I subunit. J Biol Chem. 1987;262:13111–13119. [PubMed] [Google Scholar]
- 40.(WHO) WHO. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interation. Cambridge: Cambridge University Press; 1992. [Google Scholar]