Abstract
Acquisition of classically conditioned eyeblink responses (CRs) in the rabbit critically depends on intermediate cerebellum-related neural circuits. A highly efficient method for determining possible sites of plasticity within eyeblink circuits is the reversible inactivation of circuit components during learning. Inactivation of either the HVI region of the cerebellar cortex or the cerebellar interposed nuclei (IN) during learning is known to prevent CR acquisition. In contrast, inactivating cerebellar efferent axons in the brachium conjunctivum (BC) with small injections of tetrodotoxin (TTX) has been reported to have no effect on CR acquisition. This suggested that the intermediate cerebellum is essential for learning CRs and that activity mediated by the BC is not required for this process. Since we previously found that BC inactivation blocks CR extinction we re-examined its role in CR acquisition. To ensure complete and long-lasting inactivation of the BC, we injected before each training session doses of TTX that were larger than those in the previous acquisition study. Contrary to the previous negative findings, we found that this temporary block of axons in the brachium conjunctivum prevented normal acquisition of CRs. Injecting TTX directly in the adjacent lateral lemniscus, which could possibly influence CR acquisition, had no effect on learning. In addition, a functional test of TTX diffusion around the BC indicated that the inactivation did not affect other known parts of eyeblink circuits, such as the cerebellar interposed nuclei, the middle cerebellar peduncle or the contralateral red nucleus. We conclude that this form of associative learning in the rabbit eyeblink system requires extra-cerebellar learning and/or cerebellar learning that depends on the operation of cerebellar feedback loops.
Keywords: Classical Conditioning, Cerebellum, Superior Cerebellar Peduncle, Associative Learning, Eye Blink
1. Introduction
During classical conditioning of eyeblink responses, rabbits learn to blink to a previously irrelevant stimulus (conditioned stimulus, CS) signaling an upcoming aversive stimulation (unconditioned stimulus, US) of the eye's surface. Some influential conceptions of eyeblink conditioning circuits (Attwell et al. 2002b; Krupa and Thompson 1995; Medina et al. 2000, 2002; Thompson 2005) assume that the motor command for learned conditioned responses (CR) is transmitted to eyeblink motoneurons via a serially connected chain involving the intermediate cerebellar cortex, cerebellar interposed nuclei and eyeblink premotoneurons. Currently, research is focused on determining where in this pathway, and in associated intermediate cerebellum-related circuits, learning occurs. Theoretically, any site receiving both CS and US information and having access to eyeblink motoneurons could undergo plastic changes responsible for CR acquisition and retention. It is known that neurons in multiple structures of eyeblink conditioning circuits, including the cerebellar cortex, cerebellar nuclei and eyeblink premotoneurons in the red nucleus, respond to presentations of the CS and US and exhibit neuronal activity that correlates temporally with behavioral CRs (Berthier and Moore 1986, 1990; Desmond and Moore 1991). Thus, multiple sites in eyeblink circuits receive information that is prerequisite for associative learning. Learning could be distributed among all or some of these structures, or it could be located at one dedicated site.
Several contemporary investigators suggest that critical eyeblink conditioning-related plasticity occurs in the cerebellum. Strong evidence for cerebellar learning comes from studies that inactivated individual components of cerebellar circuits during CR acquisition. It has been shown that inactivating the cerebellar cortex (Attwell et al. 2001) or cerebellar nuclei (Hardiman et al. 1996; Krupa and Thompson 1997) during learning prevents CR acquisition. On the other hand, Krupa and Thompson (1995) reported that small injections of the sodium channel blocker, tetrodotoxin (TTX), targeting the brachium conjunctivum (BC), did not prevent CR acquisition. The BC is the main cerebellar efferent pathway carrying axons of deep cerebellar nuclei neurons. Since inactivating neurons in the interposed nuclei prevented learning, but inactivating axons of these cells in the BC did not, it has been theorized that cerebellar inactivation during training disrupts plastic modifications within the cerebellum (Krupa and Thompson 1995; Christian and Thompson 2003). A notable feature of the original BC inactivation study (Krupa and Thompson 1995) was that the BC was targeted with relatively small injections of TTX (2 pmol in 90 nl). In our previous study, larger injections of TTX in the BC prevented the extinction of conditioned eyeblinks (Nilaweera et al. 2005). Did the differential involvement of the BC in CR acquisition and extinction reflect differences in their mechanisms or could it be that we disrupted CR extinction simply because of a more complete inactivation of the BC? To address this issue, we examined CR acquisition while inactivating the BC using a larger dose of TTX similar to that used in our extinction study. Our working hypothesis was that an even more complete inactivation of the BC will not prevent CR acquisition. In contrast to previous reports and to our expectation, we found that this larger inactivation of the BC disrupted CR acquisition. To address possible alternate explanations, two additional tests were performed. In the first test, we found that direct injections of TTX in the lateral lemniscus (LL, part of the auditory pathway adjacent to the BC) did not affect normal learning. In the second test, we determined that the effective diffusion of TTX used in our study did not exceed 2.2 mm. Both results suggest that inactivation of other known parts of eyeblink circuits is unlikely in our principal experiment. All these data indicate that the cerebellum's interaction with its efferent targets, via feedback loops, during learning is essential for normal acquisition of conditioned eyeblinks.
2. Results
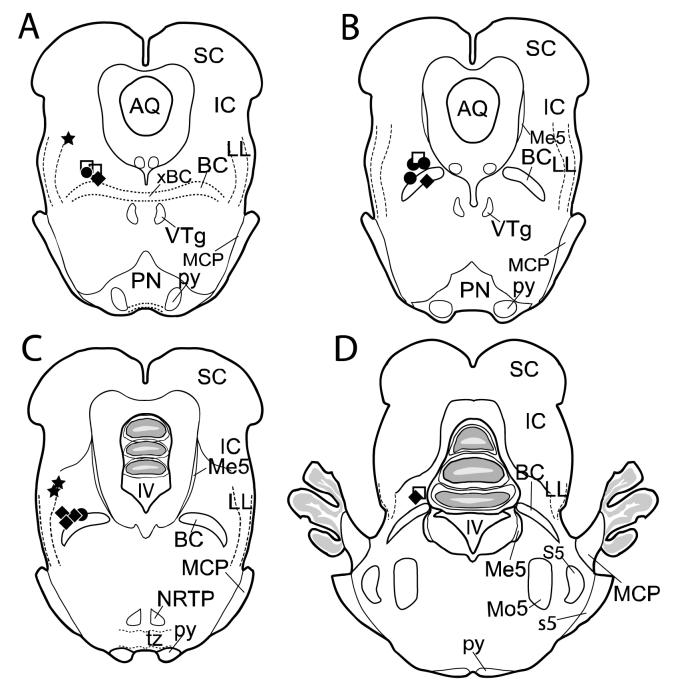
A total of 24 rabbits were used in this study. Six animals were eliminated from the data analysis either because their injection sites were too deep and close to the middle cerebellar peduncle, or because they failed to acquire more than 50% CRs by the fifth day of Acquisition Phase II (indicating likely BC damage). Eighteen animals were included in the final data analysis: 6 in the BC-targeted TTX group, 5 in the PBS (control) group, 3 in the LL-targeted group and 4 in the BC-targeted diffusion group. Histological analysis revealed that the injection sites were located close to the BC or to the LL in all included animals (Fig. 1).
Fig. 1.
Locations of injection sites for TTX (black diamonds, n=6), PBS control (black circles, n=5), LL (black stars, n=3), and diffusion (white squares, n=4) rabbits used in data analysis. All non-LL injection sites were within 1 mm from the BC. Anterior-posterior spread was 1.0 mm and all locations were between 0.5 mm rostral and 0.5 mm caudal to the caudal pole of the ventral tegmental nucleus. Injection sites in one TTX and one diffusion rabbit (D) were located 1.5 mm more caudal to the caudal pole of the ventral tegmental nucleus. The representative coronal sections through the region of interest are spaced 0.5 mm apart from (A) to (C), but (D) is 1 mm apart from (C), with (A) being most rostral. IV, fourth ventricle; AQ, aqueduct of Sylvius; BC, brachium conjunctivum; IC, inferior colliculus; LL, lateral lemniscus (dorsal portion); MCP, middle cerebellar peduncle; Me5, mesencephalic trigeminal nucleus; Mo5, motor trigeminal nucleus; NRTP, nucleus reticularis tegmenti pontis; PN, pontine nuclei; py, pyramidal tract; S5, sensory trigeminal nucleus; s5, root of the sensory trigeminal nerve; SC, superior colliculus; tz, trapezoid body; VTg, ventral tegmental nucleus; xBC, decussation of the brachium conjunctivum.
2.1 Effects of TTX on CR acquisition
2.1.1. The BC-targeted group
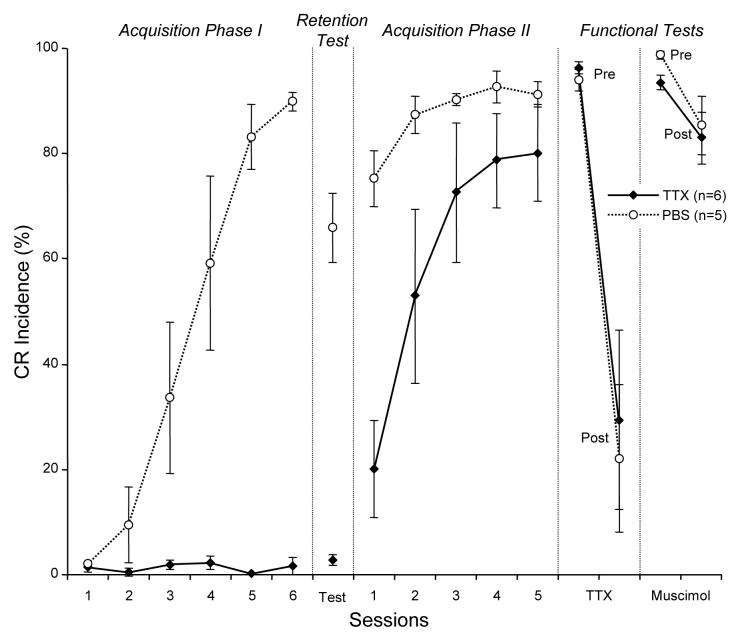
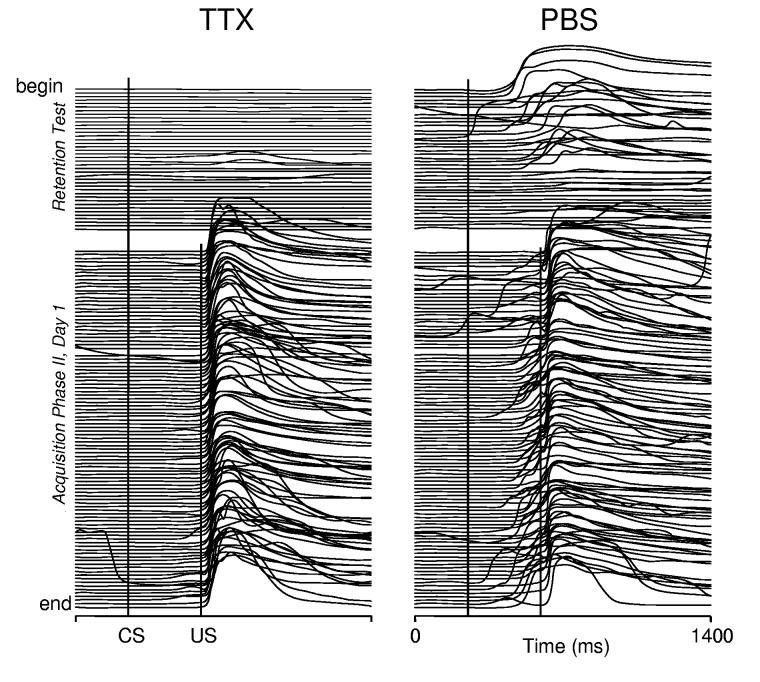
During Acquisition Phase I, rabbits receiving TTX injections did not produce CRs because inactivating the BC blocks expression of CRs (maximum 2.2±1.3% CRs, day 4, n=6). In contrast, the control PBS group gradually acquired CRs, showing significant acquisition by day 3 (33.6±9.6%), and by the sixth day of training they exhibited 89.8±1.8% (n=5) CR incidence (Fig. 2). Acquisition Phase I was followed by a retention test (40 CS-alone trials) designed to reveal if any CRs had been acquired during prior training with the BC inactivated. No drugs were injected on retention test day and this test was carried out after a one-day break to avoid any lingering TTX effects. This test was immediately followed by the first session of Acquisition Phase II to minimize long-term effects of extinction training on CR expression in subsequent training sessions. Examples of responses recorded during the retention test and the first conditioning session of Acquisition Phase II in one animal from the BC-targeted TTX group and one from the control group are shown in Fig. 3. Note during the retention test, (first 40 trials above the gap), the TTX animal produced only a few, small-amplitude eyelid movements. This contrasted with the control animal's high frequency of large-amplitude CRs that gradually extinguished toward the end of the test. Once training with paired trials resumed, the TTX animal exhibited no CRs. Small-amplitude CRs started to appear in the middle of the session, becoming more frequent toward the end of the experiment. On the other hand, the control animal quickly resumed the performance of large-amplitude CRs, suggesting that the extinction process initiated by the retention test had only a minor effect on subsequent acquisition training. At the group level, the BC-targeted TTX group exhibited low CR incidence (2.8±1.0%) during the retention test indicating that CRs were not acquired during the previous training phase with the BC inactivated (Fig. 2). In contrast to the BC-targeted TTX group, the control animals exhibited a much higher incidence of CRs during the retention test (66.0±6.6%, t9=10.45, p<0.0001), confirming robust CR acquisition during Acquisition Phase I. The sparse eyeblinks detected in TTX rabbits during the retention test had longer latencies (563±82 ms) when compared to controls (276±7.2 ms). Furthermore, retention test CRs in TTX rabbits appeared randomly in mid-to late-trial numbers during CS-alone sessions at a rate approximating that for spontaneous eyeblinks in rabbits.
Fig. 2.
Conditioned response incidence (mean±SEM) in the experimental (TTX) and control (PBS) groups during acquisition phases, retention and functional tests. The TTX group did not exhibit CRs in Acquisition phase I due to BC inactivation by TTX, whereas the control (PBS) group gradually acquired CRs to asymptotic levels. In the retention test, the TTX group had a very low incidence of CRs compared to the control group, indicating that essentially no learning occurred during inactivation. The TTX group achieved an asymptotic level during Acquisition phase II without BC inactivation. Before the TTX injection test (Pre), both groups exhibited over 90% CRs; after the injection (Post), CR incidence was dramatically reduced in both groups, indicating that injections during Acquisition Phase I were made to eyeblink-related parts of the BC. Compared to TTX, muscimol had a negligible effect on CR incidence. Sessions 1 through 6 are consecutive days of each respective phase of the experiment.
Fig. 3.
Individual examples of eyeblink mechanograms in a treatment (TTX) and control (PBS) rabbit during the CS-alone retention test and the first paired-stimuli session (100 trials) of Acquisition phase II. Each stack plot represents a complete printout from a single training session and each trace represents one trial. The uppermost trace (begin) is the first trial of the session. Upward deflections in the eyeblink traces indicate eye closure. The wide gap within each plot separates CS-alone from paired CS+US trials. Vertical lines in each plot denote CS and US onsets. Eyeblinks after the CS mark in the first 40 trials and those between the CS and US marks after trial 40 were considered CRs (see Methods section for details). During CS-alone trials, the TTX rabbit exhibited sparse, small-amplitude blinks, whereas the PBS rabbit expressed a much larger number of well-formed CRs at the beginning with extinction toward the end of session. During CS+US training, the PBS rabbit re-acquired a robust level of CRs. In contrast, the TTX rabbit had no CRs at the beginning of the session and then slowly acquired small-amplitude CRs.
Throughout Acquisition Phase II, control animals exhibited a higher CR incidence than did the TTX group (F4,6=6.63, p=0.02). This was most evident on day one of Acquisition Phase II, when the control group significantly outperformed TTX rabbits (TTX: 20.2±9.2% and PBS: 75.2±5.3% CRs; t9=4.89, p=0.0009). By the 5th day of Acquisition Phase II, CR incidence in the TTX and control groups was statistically similar (TTX: 80.2±9.2% and PBS: 91.2±2.4%, t9=1.07, p=0.3), indicating that TTX animals were able to acquire normal CR incidence. When compared to learning in naïve controls, the TTX group, without injections, initially acquired CRs faster (day one, 20.2±9.2% vs. 2.0±0.6% CRs, t3=1.78, p=0.11 see Fig.2). Repeated measures contrast analysis revealed this cross-phase effect to extend through days 2 (43.6±19.4%, t3=2.25, p=0.05) and 3 (39.1±19.6%, t3=1.99, p=0.08). Group performances had converged by day 5 (difference: 3.0±11.6%, t3=0.26, p=0.80). Overall, the cross-phase difference between learning curves of the TTX group during Acquisition Phase II and of controls during Acquisition Phase I was marginal (F4,6=3.63, p=0.08 ).
Both groups performed ≥90% CRs before the post-acquisition inactivation test (Fig. 2, Functional Tests). Injections of TTX dramatically decreased CR incidence in both TTX (t9=4.56 p=0.0014) and control groups (t9=4.48, p=0.0015) when compared to pre-injection levels, verifying that control injections were applied to a functionally relevant part of the eyeblink conditioning circuit. In contrast to TTX, muscimol injections had no effect on CR incidence (t8=0.4, p=0.7, see Fig. 2).
2.1.2. The LL-targeted group
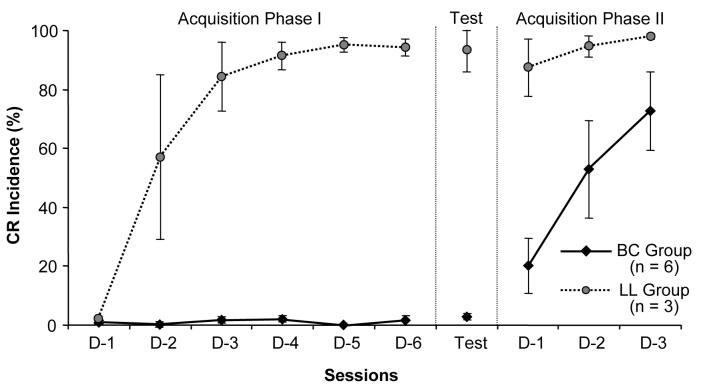
CR acquisition in the LL group was similar to that of control animals and significantly different from the BC-targeted TTX group. During Acquisition Phase I, LL-injected rabbits acquired CRs despite LL inactivation (Fig. 4). In the CS-alone retention test, the LL group had 93.0±7.0% CRs, showing that LL-targeted TTX injections had no effect on CR performance. During Acquisition Phase II, LL rabbits began with 87.3±9.6% CRs and reached 98.0±1.0% by the third training session (Fig. 4).
Fig. 4.
Mean CR incidence (±SEM) of BC-targeted (diamonds) and LL-targeted (circles) groups during individual stages of the experiment. While the BC group exhibited a negligible number of CRs during Acquisition Phase I, the LL animals rapidly acquired CRs. During the retention test, the BC group had no CRs, indicating that CRs were not acquired in sessions with the BC inactivated. On the other hand, the LL group exhibited high CR incidence, suggesting that normal CR acquisition occurred in sessions during which TTX was applied to the LL. During Acquisition Phase II, the LL group demonstrated a high frequency of CRs, while the TTX group acquired CRs gradually, as would be expected in animals that did not learn during Acquisition Phase I. D-1 through D-6: consecutive days of corresponding phases of the experiment.
2.2. Diffusion of TTX
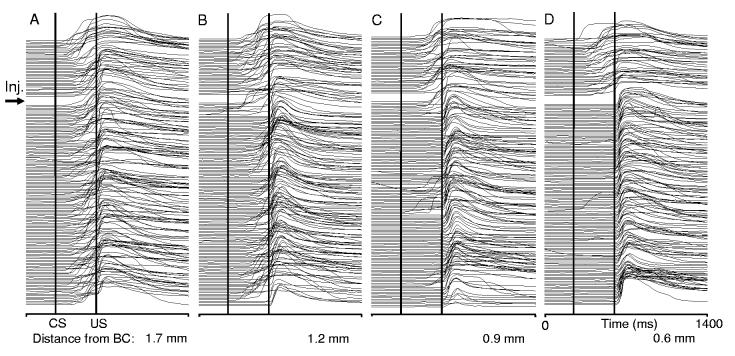
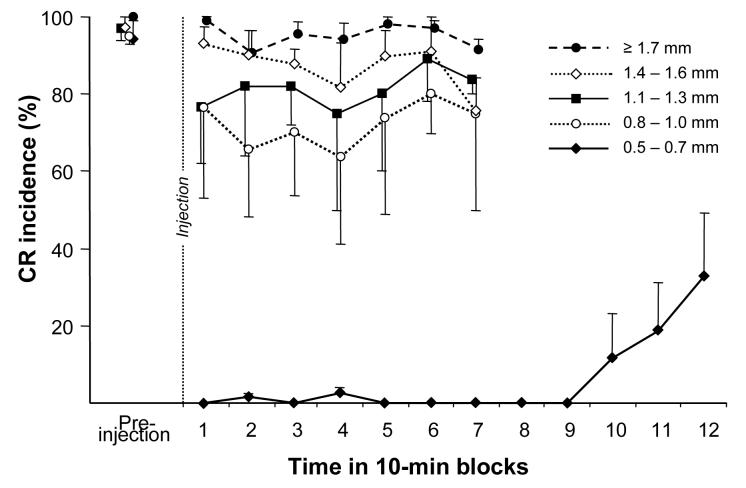
Figure 5 illustrates effects of injecting TTX at varying distances from the BC on CR performance in one animal before and 70 min post-injection. Injecting TTX 1.7 mm dorsal to the dorsal border of the BC had no effect on CR performance (Fig. 5A). Advancing the TTX injection needle nearer to the BC resulted in progressively larger, more complete CR performance deficits (Fig. 5B-D). At a distance of 1.2 mm from the BC, CRs affected by TTX became intermittent and their incidence decreased from 92% pre-injection to 68% post-injection. Injecting TTX 0.9 mm dorsal to the BC abolished CRs within several minutes following the injection. This effect, however, was incomplete and the animal intermittently produced CRs (100% before and 39% after) until recovery 74 min post-injection. When injected 0.6 mm from the BC, TTX completely abolished CRs. In this experiment CRs recovered 3 hours post-injection (recovery not shown in Fig. 5C and D). At the group level, when compared to pre-injection performance, only injections at distances <1.0 mm had statistically significant effects on CR incidence within the average 43 min time window corresponding to a CR acquisition experiment (10 min wait period plus ∼33 min training) (Fig. 6). Injections in the range of 1.1-1.6 mm had an insignificant tendency to decrease CR incidence. While injections in the 0.8 -1.0 mm range significantly decreased CR incidence for the first 40 min, injections at distances of 0.5-0.7 mm from the dorsal border of the BC completely abolished CRs in all 4 rabbits for 90 min post-injection. When comparing the first 70 min following TTX infusion, injections at distances <0.7 mm were significantly more effective in abolishing CRs than shallower injections (F4, 10=17.1, p=0.00018).
Fig. 5.
Individual examples of BC inactivation effects on CR performance when 2 μl of TTX were injected at progressively shorter distances from the BC. Each column represents a complete printout of eyeblink records in one experiment, with the first trial at the top and the last trial at the bottom of each stack. The first 40 trials (above the wide gap) represent normal CR incidence before the injection. The remaining trials (below the gap) cover 70 min following the TTX injection. Injecting TTX had no effect on CR performance at 1.7 mm dorsal to the BC (A). Injections at 1.2 mm (B) and 0.9 mm (C) partially abolished CRs, with effects on CR incidence, latency and amplitude. Only the deepest injection, 0.6 mm dorsal to the BC (D) completely abolished CRs. CS – conditioned stimulus onset. US – unconditioned stimulus onset.
Fig. 6.
Effect of 2 μl (1 ng/μl) TTX on CR incidence when injected at varying distances from the BC in the diffusion experiment (n=4). Injecting TTX completely abolished CRs only within 0.5-0.7 mm from the BC. At larger distances, CR incidence was only partially reduced. When TTX was injected 1.4 mm or farther from the BC, it had a negligible influence on CR expression.
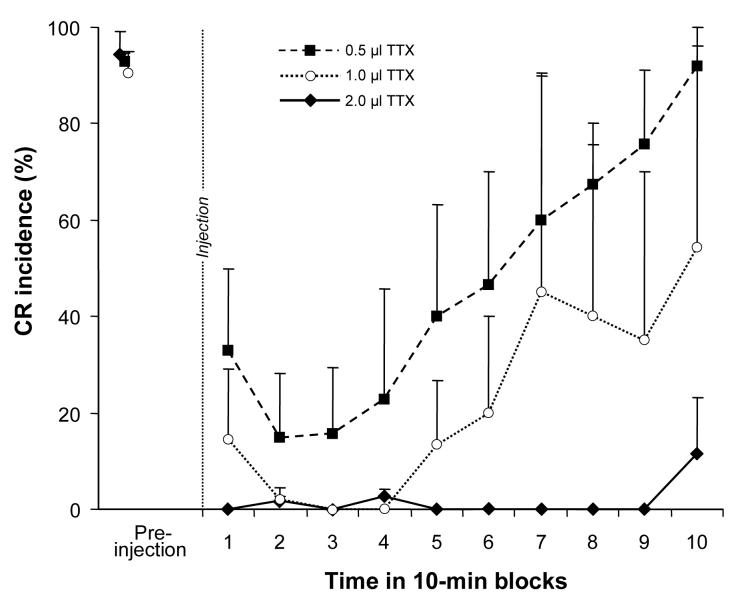
Our CR acquisition experiments utilized relatively large volumes of TTX. We selected the TTX volume based on our preliminary studies indicating insufficient BC inactivation with smaller volumes of TTX. Once all animals in the diffusion group had their most effective injection sites (0.5-0.7 mm dorsal to BC) mapped out with progressively deeper injections of 2 μl TTX, we subsequently halved injections (1 μl) to further examine volume effects on CR performance. Two rabbits received additional injections with the TTX volume halved again (0.5 μl). While injecting 2 μl of TTX produced immediate and long-lasting abolition of CRs, injecting 1 μl of the same TTX concentration abolished CRs after a 10 min delay, and this effect lasted only 30 min (Fig. 7). Injecting 0.5 μl of TTX did not abolish CRs completely and the maximum effect lasted ∼20 min before starting to recover.
Fig. 7.
Effect on CR incidence of reduced TTX volumes delivered to the site determined optimal for 2 μl (0.5-0.7 mm). While 2 μl of TTX produced a complete and long-lasting CR abolition, effects of 1.0 and 0.5 μl injections (also 1 ng/μl) had delayed onset and early recovery. Injecting 0.5 μl of TTX did not abolish CRs completely.
3. Discussion
The present study demonstrates that microinjections of TTX targeting the BC prevent CR expression during training and abolish CRs in well-trained animals. The CR retention test revealed that rabbits did not acquire CRs when trained with the BC inactivated. During additional training, after TTX injections were discontinued, rabbits acquired normal CR incidence. These results demonstrate that TTX injections temporarily disrupted CR acquisition. Injections of muscimol in the same area did not impair CR expression. Injections of TTX targeting the LL, but sparing the BC, did not prevent CR expression during learning and did not prevent CR acquisition. Most importantly, LL rabbits exhibited normal CRs during the retention test. These data indicate that the BC is involved both in CR acquisition and expression.
3.1. Effects of BC inactivation
The present study used a sodium channel blocker, TTX, to inactivate the BC. Although we targeted the BC, could an unintended inactivation of neurons or some fiber tract other than the BC cause the results witnessed here?
A previous in vivo study (Zhuravin and Bures 1991) demonstrated that even larger amounts of TTX (10 ng in 1μl; compared to the average of 1.4 ng in 1.4 μl in the present study) inactivated neural tissue at distances only up to 1.5 mm from the injection site in the rat. Our data are consistent with this finding. In our diffusion study, we found that injecting 2 μl of TTX 1.7 mm or farther dorsal from the BC had no effect on CR expression. On the other hand, injecting TTX 0.7 mm or closer to the BC's dorsal border produced complete, long-lasting CR abolition (Fig. 6). The latter distance is an estimate of the minimum radius of effective TTX spread in our experiments. To abolish CRs, TTX had to diffuse into the BC and inactivate eyeblink-related axons. Anatomical studies indicate that axons from the cerebellar interposed nuclei are contained predominantly in the lateral 2/3 of the BC (Voogd, 2004; Haroian et al., 1981). One could assess the maximum spread of TTX by assuming that it had to reach from the injection site to the farthest parts of the BC – its lateral edge. Under this circumstance, the maximum radius of the TTX spread was estimated to be approximately 2.2 mm. It should be noted that this could be an over-estimate, especially if the CR expression deficit was due to BC fibers closer to the injection site or to the dorsal-most portion of the rubro-spinal tract, which passes below the lateral part of the BC (Robinson et al., 2001). Even though our diffusion estimates are based on 2 μl of TTX it should be noted that we used 1.5 μl in our principal experiment. Our estimation that TTX spread does not exceed 2.2 mm is also in agreement with Krupa and Thompson (1995), who reported that using 43% of our TTX dose inactivated tissue within a 0.75 mm radius. Since our dose was 2.3 times larger, one could expect that it would affect 2.3 times larger tissue volume. Assuming spherical diffusion, this would correspond to the area within 1 mm from the injection site. This extrapolation from Krupa and Thompson's data is slightly larger than our estimate of minimum effective TTX diffusion. The above comparisons with data from other laboratories suggest that our estimate of the maximum TTX spread is cautious and that the physiologically effective spread of TTX was likely less than 2.2 mm.
Considering this maximum spread potential and the location of injections in the present experiments (Fig. 1), it is likely that TTX either completely or near completely inactivated the BC. The BC is a fiber tract that carries efferent axons of cerebellar nuclear neurons. Along its route, the BC comes close to other components of eyeblink conditioning circuits. To reduce the likelihood of affecting these structures, we targeted the BC at approximately 2/3 of the distance from the cerebellar interposed nuclei to the contralateral red nucleus. In all animals, injection sites were more than 4.5 mm from the caudal pole of the contralateral red nucleus and 5.5 mm from the rostral end of the ipsilateral interposed nucleus. These distances are well outside the estimated maximum radius of TTX spread, making rubral and interposed nuclear inactivation highly unlikely. At this BC location, there are no known eyeblink CR-related neuronal groups that could be affected by TTX injections. Negative results of control muscimol injections further support this notion, suggesting that effects of TTX on CR expression were related to inactivating the BC or other axons.
Besides the BC, the nearest known axonal components of eyeblink circuits to the injection sites are the rubro-spinal tract and the ipsilateral lateral lemniscus (LL). The rubro-spinal tract in the rabbit passes ventral to the lateral half of the BC (Robinson et al., 2001). Although the location of eyelid-related fibers in the rubro-spinal tract is not known, inactivating these fibers would not be expected to disrupt CR acquisition because inactivating the red nucleus directly had no effect on learning (Krupa et al., 1993). The LL is a component of the auditory system and it passes lateral to the BC en route to the inferior colliculus (Fig. 1). Because our paradigm utilized an auditory CS, possible involvement of the LL in explaining our present results has to be considered. Although the LL does not appear to be involved in pure tone discrimination (Cho et al. 2005), it can not be excluded that its inactivation could alter the neural representation of the bilateral acoustical CS and potentially retard the rate of CR acquisition, as shown in a study that examined effects of bilateral LL lesions (Steinmetz et al. 1987). Therefore we tested LL involvement in a separate group of animals. The placement of injections in these rabbits maximized LL inactivation while sparing the BC. Despite six consecutive training days with 2 μl TTX injected ∼1.8 mm from the optimal BC location, these LL-injected rabbits exhibited CRs during Acquisition Phase I, and exhibited a high CR incidence during the retention test in the absence of TTX.
These results indicate that the observed learning and performance deficits in the BC-targeted TTX group were not related to the inactivation of the LL. Furthermore, they re-confirm our conclusions regarding effective TTX spread by demonstrating the failure of repeated large doses ≤2.6 mm from a known eyeblink-related structure to disrupt CR expression. Other known eyeblink-related axonal pathways in this region, such as the middle cerebellar peduncle or rubro-spinal tract are located ventral to the BC, presumably beyond the estimated spread of TTX. We conclude that CR expression and acquisition deficits in the BC-targeted group were most likely induced by BC inactivation.
3.2. Discrepancy with prior study
Krupa and Thompson (1995) reported that small TTX injections targeting the BC blocked CRs during training but did not prevent CR acquisition. We confirm that inactivating the BC blocks CR expression in this study as well as in our previous studies (Nilaweera et al. 2002, 2005). We did not, however, replicate the lack of effect on CR acquisition. On the contrary, the present study demonstrates that inactivating cerebellar output axons prevents CR acquisition. Before discussing the implications of the present results, the possible reasons for the discrepancy with previous data should be considered.
Krupa and Thompson (1995) monitored eyeblinks by measuring movements of the nictitating membrane. The present study measured movements of the external eyelids. Could it be that inactivation of the BC selectively affects acquisition of the external eyelid component of the eyeblink? This seems unlikely since movements of the nictitating membrane and external eyelids in the rabbit are tightly coupled (McCormick et al. 1982), and also the sensor used in the present study (Ryan et al. 2006) would detect isolated nictitating membrane responses not coupled with external lid movements. The BC inactivation-induced deficit of CR acquisition in our experiments seems to involve both the external eyelid and the nictitating membrane components of the eyeblink. In contrast to our eyeblink detection method, the traditional recording of the nictitating membrane movements requires restraint of external eyelids. It has been shown in humans that mechanical loads on external eyelids induce non-associative motor learning (Schicatano et al., 2002). Possible implications of this factor for explaining differences between Krupa and Thompson (1995) and the present study are not clear.
Krupa and Thompson (1995) used paired CS and US trials as a retention test. To eliminate the involvement of new learning during the retention test, we presented the CS without the US (extinction protocol). One could speculate that animals injected with TTX during training were more sensitive to extinction effects than were controls, and for this reason they exhibited fewer CRs during post-injection training. While this can not be excluded, comparing the initial retention test trials (before extinction could occur) of the BC-targeted TTX group to controls shows that TTX injections disrupted CR acquisition in the present study.
An important difference between the original report and our experiments was the volume and amount of TTX used to block the BC. Krupa and Thompson (1995) used a small volume (90 nl) of vehicle with 639 pg of TTX. In our experience, microinjections of small volumes are difficult to administer reliably, especially in multi-injection protocols that induce the formation of scar tissue, which can impede drug diffusion. Moreover, we show here that smaller volumes (0.5 and 1.0 μl) can affect CRs, but this effect can recover relatively quickly (Fig. 7). These factors are critical in acquisition-during-inactivation experiments requiring complete inactivation for the duration of each training session. Our injections were in more rostral parts of the BC where axons are packed less densely than closer to the cerebellum. To ensure optimal spread of the drug and the appropriate duration of its effect, we injected a larger amount and volume of TTX averaging 1.4 ng (2.3 times that of the previous study). Extent of BC inactivation is the simplest and most likely factor explaining the difference between the present results and Krupa and Thompson's (1995) data. We surmise that the small TTX injections in their study either did not inactivate all BC axons involved in CR acquisition, or the TTX effect in some of their training sessions prematurely wore off.
3.3. Involvement of the BC in CR acquisition
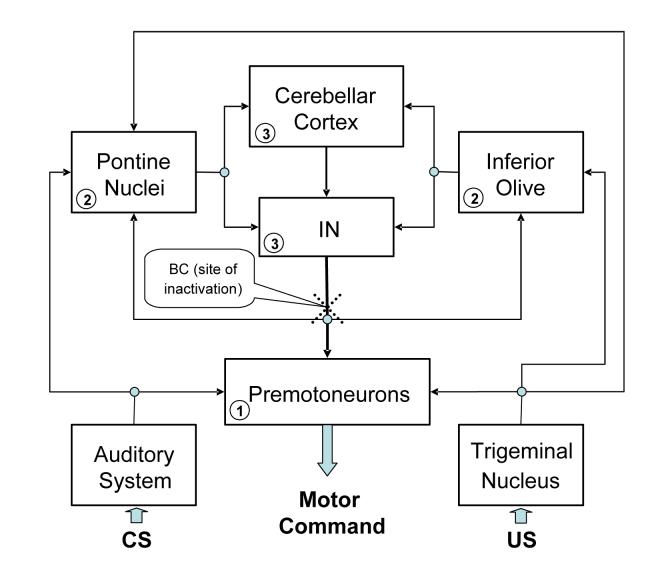
Early inactivation-during-learning studies assumed if inactivation of a certain part of the brain blocks learning, then plastic changes that underlie the learning are located either at the site of inactivation, or at one or more of its efferent targets (Krupa and Thompson, 1995, see also Christian and Thompson 2003 and Thompson 2005). Applying this reasoning to the results of our present study indicates that eyeblink conditioning-related plasticity has to occur at sites between the inactivation site and eyeblink motoneurons. Since inactivating eyeblink motoneurons during learning does not prevent CR acquisition (Krupa et al. 1996), eyeblink premotoneuronal pools seem to be the most likely site of learning (see Fig. 8). Interestingly, inactivating the most obvious group of cerebellar projection-receiving premotoneurons in the red nucleus also does not prevent learning (Clark and Lavond 1993). This leaves, by elimination, an as yet undetermined population of extra-rubral and cerebellar projection-receiving eyeblink premotoneurons as the likely site of eyeblink-related plasticity. The existence of a number of extra-rubral eyeblink premotoneuronal groups in the brainstem and cerebrum was recently documented by visualizing the transneuronal retrograde spread of rabies virus in the rat (Morcuende et al., 2002).
Fig. 8.
A conceptual block diagram of the cerebellum-related circuitry involved in acquisition and expression of classically conditioned eyeblinks in the rabbit. This block diagram is a highly simplified representation of relevant structures and connectivity. The CS and US information is supplied in parallel to the serially connected cerebellar cortex, cerebellar interposed nuclei (IN) and eyeblink premotoneurons. Output of eyeblink premotoneurons supplies motor commands to eyeblink motoneurons. Inactivating axons of cerebellar nuclear cells in the brachium conjunctivum (BC, dotted cross symbolizes the inactivation site) interrupts supply of cerebellar information to premotoneurons as well as to cerebellar afferent sources (pontine nuclei, inferior olive). Thus, the CR acquisition deficit in the present study is likely related to disruption of information processing at (1) premotoneurons (2) cerebellar afferent sources, and (3) in the cerebellum itself.
Concluding that extra-cerebellar eyeblink premotoneurons participate in eyeblink conditioning certainly does not exhaust the list of plausible causes explaining the BC inactivation-induced CR acquisition deficit. The reasoning outlined in the previous paragraph does not take into account cerebellar feedback pathways. It is known that the BC contains axons projecting to the inferior olive and pontine nuclei – structures that are major sources of cerebellar afferents and that supply the cerebellum with information about the CS and US (see Fig. 8). These projections to pre-cerebellar structures help to complete cerebellar feedback loops. Thus, blocking the BC is inevitably associated with disrupting the cerebellum's projections to its afferent sources, and this could affect information processing at pre-cerebellar sites and in the cerebellum itself. Importantly, Krupa and Thompson's (1995) study implied that the cerebellar feedbacks play no major role in CR acquisition. The results of the present study, together with several other recent reports, necessitate re-assessing this conclusion.
Our results are consistent with the proposition that blocking individual components of cerebellar circuits affects not only post-cerebellar components of the circuit, but also pre-cerebellar parts via feedback loops (Andersson et al., 1988; Bracha and Bloedel, 1996; Welsh and Harvey, 1998; Attwell et al., 2002b). The best described cerebellar feedback loop is the cerebello-olivary-cerebellar system that has been proposed to regulate cerebellar information processing during learning and also the tonic activity of Purkinje cells via negative feedback (Andersson et al., 1988; Bengtsson et al., 2004). Projections from the interposed nuclei to the inferior olive are inhibitory. Blocking these fibers in the BC is thus expected to disinhibit neurons in the IO (Lang, 2002). This in turn could suppress the firing rate of Purkinje cells in the cerebellar cortex and thus disinhibit the activity in deep cerebellar nuclei. The feasibility of this scenario is strongly supported by experimental evidence. Bengtsson et al. (2004) demonstrated that inactivating the BC suppresses Purkinje cell activity in the decerebrate ferret. In our laboratory we found that inactivating the BC in classically conditioned rabbits elevates the tonic activity of IN neurons (Nilaweera et al., 2003). It is highly likely that BC inactivation in the present study triggered the cascade of events described above, compromising the physiological state of structures within the cerebello-olivary-cerebellar loop. Both the cerebellar cortex and cerebellar nuclei have been shown to participate in CR acquisition in studies that minimized the interference with feedback loops during learning (Attwell et al. 2002a, Bracha et al., 1998). Therefore, malfunction of cortical and nuclear substrates could certainly contribute to or be primarily responsible for a BC inactivation-induced learning deficit.
Considerably less understood are other cerebellar feedbacks. Relative to eyeblink conditioning, cerebellar nuclear projections to pontine nuclei seem to be important (Teune et al., 2000). Cerebellar involvement in information processing in pontine nuclei was shown using pontine nuclear recording in a cerebellar inactivation study (Clark et al. 1997). Thus, BC inactivation-related information processing changes in pontine nuclei could interfere with CR acquisition. In summary, inactivating the BC could have disrupted learning-related processes at several levels, including post-cerebellar premotoneurons, pre-cerebellar afferent sites, and in the cerebellum itself. In our view, these mechanisms are not mutually exclusive and it is possible that BC inactivation hindered formation of plastic changes at all or most of these sites (Fig. 8).
The main contribution of the present study is that it demonstrates that inactivating the BC region in the mesencephalon severely impairs CR acquisition. This finding attributes to the BC a significantly larger role during CR acquisition than previously thought. It provides a strong impetus for further examinations of cerebellar feedback loop-dependent learning mechanisms, and it also points to a possible role for extra-cerebellar learning.
4. Experimental procedures
4.1. Surgical procedures
Male New Zealand White Rabbits (Oryctolagus cuniculus) (2.5-3.0 kg) were anesthetized with a mixture of Ketamine (50 mg/kg), Xylazine (6 mg/kg), and Acepromazine (1.5 mg/kg). Each rabbit was chronically implanted with a 28-gauge (thin-wall) guide cannula targeting the left BC in all acquisition and diffusion experiment rabbits. In the lateral lemniscus (LL) group, a second guide tube was implanted 1 mm lateral from the BC cannula. During surgery, lambda was positioned 1.5 mm ventral to bregma and the stereotaxic coordinates were taken from bregma. The BC injection guide tube was implanted 12-13 mm posterior and 2.8 mm lateral (in LL rabbits, the second cannula was 1 mm more lateral). The dorso-ventral coordinates differed in each type of experiment. In the BC-targeted acquisition group, the cannula was implanted 14.2 mm ventral to bregma, which was expected to be 1 mm dorsal to the BC. In both the LL-targeted acquisition and the BC-targeted diffusion groups, the cannulae were implanted 12.5 mm below bregma, and thus 2.7 mm above the BC. The cannula and a lightweight, small (25×10×12.5 mm) plastic stage, for attaching the air delivery nozzle and eyeblink recording system during experiments, were secured with dental acrylic to 3 anchoring screws implanted in the skull. Surgeries were performed under aseptic conditions. Rabbits were treated with antibiotics for 5 days. A 33-gauge stainless steel stylet and a light-weight cap protected the injection guide tube between experiments. The post-surgical recovery period was 1 week. All experiments were prepared and conducted in accordance with “Principles of Laboratory Animal Care” (NIH publication No. 86-23, revised 1985) and the protocol approved by the Institutional Animal Care and Use Committee of Iowa State University.
4.2. Training and injection procedures
One week post-surgery, rabbits were adapted to a standard rabbit restraining box (Plas-Labs Inc, Lansing, MI) inside a sound-attenuated chamber for 3 days (30 min/day). Prior to eyeblink conditioning, exploratory injections of lidocaine (4 μl, 4% solution) were administered to identify optimal sites for BC inactivation by observing behavioral signs empirically determined in previous experiments in this region. All injections described in this paper were administered at the rate of 0.75 μl/min and involved inserting in the guide cannula an injection needle (33-gauge) connected to a 10-μl Hamilton syringe (Hamilton Company, Reno, NV) by way of a transparent 1-μl graduated Tygon tubing. In our previous study we established that an effective inactivation of the BC at this mesencephalic site correlates with several easily observable behavioral effects (Nilaweera et al., 2005). Observing these effects allowed us to determine the correct injection placement in naïve animals. The BC injection site was considered optimal if lidocaine produced at least three of the following effects: (1) animal became unusually calm, (2) head hung low from the normal upright position, (3) head tilted to the left, (4) ipsilateral eye opened wider, occasionally with mild exophthalmus (Bracha et al., 2001), (5) ipsilateral ear drooped from upright position, (6) unconditioned photic eyeblink (UR) was abolished. If these effects were largely absent, then the injection was administered 0.3 mm deeper the following day. This approach was repeated until the desired behavioral effects were observed. For acquisition training, we used an 80-dB, 1-kHz, 450-ms tone CS and a 100-ms air puff US (210 kPa at the source) to the left cornea. The exhaust fan of the sound-attenuating chamber served as 65-dB white noise. The inter-stimulus interval was 350 ms and inter-trial intervals varied pseudo-randomly between 15 and 25 s. In all training sessions, rabbits received 100 paired (CS+US) trials.
4.2.1. CR acquisition in naïve rabbits during BC inactivation
Eyeblink conditioning during BC inactivation was initiated, once the optimal site for BC inactivation was identified. Before each training session, rabbits were microinjected with TTX (Calbiochem, 1 ng/μl = 3.1 pmol/μl). Conditioning with paired trials (CS+US) began 10 min post-injection to allow for TTX diffusion. This phase, Acquisition Phase I (conditioning with BC inactivation) consisted of 6 daily training sessions. The initial microinjection volume was 1.5 μl in all rabbits. The volume of injected TTX was adjusted individually depending on the presence of behavioral effects (see lidocaine injections above); the optimal volume averaged 1.43±0.13 (SD) μl. Controls were subjected to the same procedure, but were injected with 1.5 μl of phosphate buffered saline (PBS, pH 7.4). On training day 7, injections were discontinued and rabbits were tested for CR retention by presenting 40 CS-alone trials (retention test). Acquisition Phase I and the retention test were separated by a one-day break to avoid any lingering TTX effects on eyeblink CR performance. Retention testing was immediately followed by the first CS+US training session of Acquisition Phase II (conditioning without injections). Acquisition Phase II was continued for at least 5 days or until CR incidence reached 90% of trials. One of the uncertainties of this experiment was whether TTX injections in Acquisition Phase I were placed correctly to inactivate the BC. Since it is known that inactivating the BC abolishes CRs, we tested the previously injected sites of CR-expressing rabbits after completing Acquisition Phase II. Two different inactivating drugs, TTX and muscimol, were injected on separate days. In each of these tests, the injection needle was inserted at the beginning of the experiment, the drug was injected after 40 CS+US pre-injection trials, and ensuing effects were measured for at least an additional 100 CS+US trials. Correctly placed injections of TTX were expected to produce substantial BC inactivation and to abolish CR expression in the post-injection period. The GABA agonist, muscimol (MP Biochemicals, 0.4 g/L = 3.5 nmol/μl), injected at the same location and volume as TTX, was expected to disrupt CRs only if neurons in the vicinity of the injection site participate in CR expression.
4.2.2. CR acquisition in naïve rabbits during LL inactivation
In the LL injection group, procedures were similar to the BC group. First, the optimal depth for BC inactivation was determined by injecting lidocaine and monitoring behavioral effects of the inactivation. Once the optimal BC injection site was found, Acquisition Phase I (conditioning with LL inactivated) was initiated. To maximize LL inactivation while minimizing TTX effects on the BC, TTX was injected 1 mm lateral, using the lateral cannula, and 1.5 mm dorsal to the previously established optimal BC injection site. Thus, the LL injection site was effectively 1.8 mm from the optimal BC site. Two microliters of TTX (1 ng/μl) were injected and each animal was trained for 6 days. Two days after this training phase animals were tested without injections for CR retention in 40 CS-alone trials and then re-trained for 3 more days (Acquisition Phase II).
4.3. Examination of TTX diffusion
Four animals were used in the TTX diffusion group. These rabbits were implanted with an injection guide tube 2.7 mm dorsal to the BC (AP=12-13 mm posterior, ML=2.8 mm (left side) and DV=12.5 mm below bregma). Following post-surgical recovery and adaptation, rabbits were trained in the delay eyeblink conditioning paradigm until they reached 90% of CRs/session (100 CS+US paired trials per day). To assess the distance from which TTX could inactivate axons in the BC, trained rabbits were injected on separate days with TTX (2 μl, 1 ng/μl) at distances progressively closer to the BC. This was achieved by increasing the depth of the injection needle in small increments. Starting depth was estimated to be 2.7 mm from the BC. On the first two days, the needle was advanced in 0.5-mm increments and by 0.3 mm thereafter. The sequential steps of each injection experiment consisted of inserting the injection needle, presenting pre-injection trials, microinjecting TTX, and presenting post-injection trials. Presentation of post-injection trials resumed immediately after the injection and was continued for 70 min at least, regardless of the effect. If an effect on CR expression was observed, the experiment was continued until behavioral recovery, which took up to 2 hours or even longer. The first 100 post-injection trials (∼30 min) were presented continuously without a pause, and then we alternated every block of 10 trials with a 5 min pause. This step was taken to reduce eye irritation caused by the air puff during long experiments, especially while CRs were abolished. When complete CR abolition lasted at least 90 min with 2.0 μl of TTX, 1.0 μl and 0.5 μl were injected on subsequent days at the same depth to compare the effectiveness of reduced volumes.
4.4. Histology
Once all experiments were completed, deeply anesthetized rabbits were transcardially perfused with 1 L of PBS and 1 L of 10% buffered formalin after injecting 0.75 – 1 μl of tissue-marking dye at the injection sites. Brains were sectioned coronally (50 μm) on a freezing microtome. Sections were mounted on gelatin-coated slides and were stained with Neutral Red and Luxol Fast Blue.
4.5. Data Acquisition and Analysis
Eyeblink recording was performed using a custom made infrared detection system (Ryan et al. 2006). The output of the sensor was amplified, digitized (10-kHz, 12-bit A/D converter), and stored on a custom-made data acquisition system. Two surveillance video cameras (one infrared) were used to monitor both the positioning of the infrared sensor and the onset and progression of drug-induced behavioral effects during the experiment.
Eyeblink data were acquired for 1400 ms in each trial, starting 250 ms before CS onset. The data acquisition program measured alpha responses, CRs and URs. Baseline eyelid movements (before the CS) exceeding 0.1 mm were recognized as spontaneous blinks (SP) and any blink exceeding the CR-threshold (see below) within 80 ms after CS onset was recognized as an alpha response. Trials containing spontaneous eyeblinks and rare alpha responses were ignored in further statistical data analyses. If eyelid closure was more than 0.1 mm after the alpha period and before the onset of the US (between 330 and 600 ms) in paired trials then it was considered a CR. In the CS-alone trials, any blink exceeding this threshold with the onset after the alpha period was considered a CR. This low threshold was selected to capture even the smallest CRs, especially on the test day for the TTX group.
Multivariate and univariate repeated-measures analysis of variance (RMANOVA) were used to test for overall differences in CR incidence between TTX and PBS groups within and between phases of the multi-phase acquisition experiment. From these analyses, we also examined pre-planned contrasts (t-statistics) to address a priori hypotheses regarding specific acquisition days within Phase I and II as well as between Phase I PBS and Phase II TTX. Test Day performances for TTX and PBS groups were contrasted with a t-test. To test effects on acquisition of TTX injections targeting the LL, we conducted similar analyses (RMANOVAs and t-test) contrasting LL-injected rabbits to Phase I, Test Day, and Phase II of BC-injected rabbits. Separate RMANOVAs were used to output t-statistics and parameter values for Before/After TTX and muscimol experiments. All group data reported in the results section represent group means ± standard errors of the mean, unless otherwise stated. Data analyses were performed using StatSoft Statistica commercial software.
Acknowledgements
The authors would like to thank Mike Hord for assistance with the experimental set up, and Kristina Irwin for assistance with manuscript preparation. This research was supported by NIH grants R01 NS36210 and R01 NS21958.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson G, Garwicz M, Hesslow G. Evidence for a GABA-mediated cerebellar inhibition of the inferior olive in the cat, Exp. Brain Res. 1988;72:450–456. doi: 10.1007/BF00250590. [DOI] [PubMed] [Google Scholar]
- Attwell PJ, Cooke SF, Yeo CH. Cerebellar function in consolidation of a motor memory. Neuron. 2002a;34:1011–1020. doi: 10.1016/s0896-6273(02)00719-5. [DOI] [PubMed] [Google Scholar]
- Attwell PJ, Ivarsson M, Millar L, Yeo CH. Cerebellar mechanisms in eyeblink conditioning. Ann. N. Y. Acad. Sci. 2002b;978:79–92. doi: 10.1111/j.1749-6632.2002.tb07557.x. [DOI] [PubMed] [Google Scholar]
- Attwell PJ, Rahman S, Yeo CH. Acquisition of eyeblink conditioning is critically dependent on normal function in cerebellar cortical lobule HVI. J. Neurosci. 2001;21:5715–5722. doi: 10.1523/JNEUROSCI.21-15-05715.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson F, Svensson P, Hesslow G. Feedback control of Purkinje cell activity by the cerebello-olivary pathway. Eur. J. Neurosci. 2004;20:2999–3005. doi: 10.1111/j.1460-9568.2004.03789.x. [DOI] [PubMed] [Google Scholar]
- Berthier NE, Moore JW. Cerebellar Purkinje cell activity related to the classically conditioned nictitating membrane response. Exp. Brain Res. 1986;63:341–350. doi: 10.1007/BF00236851. [DOI] [PubMed] [Google Scholar]
- Berthier NE, Moore JW. Activity of deep cerebellar nuclear cells during classical conditioning of nictitating membrane extension in rabbits. Exp. Brain Res. 1990;83:44–54. doi: 10.1007/BF00232192. [DOI] [PubMed] [Google Scholar]
- Bracha V, Bloedel JR. The multiple pathway model of circuits subserving the classical conditioning of withdrawal reflexes. In: Bloedel JR, Ebner TJ, wise SP, editors. In Acquisition of motor behavior in vertebrates. MIT press; Cambridge, MA: 1996. pp. 175–204. [Google Scholar]
- Bracha V, Irwin KB, Webster ML, Wunderlich DA, Stachowiak MK, Bloedel JR. Microinjections of anisomycin into the intermediate cerebellum during learning affect the acquisition of classically conditioned responses in the rabbit. Brain Res. 1998;788:169–78. doi: 10.1016/s0006-8993(97)01535-7. [DOI] [PubMed] [Google Scholar]
- Bracha V, Zhao L, Irwin K, Bloedel JR. Intermediate cerebellum and conditioned eyeblinks. Parallel involvement in eyeblinks and tonic eyelid closure. Exp Brain Res. 2001;136:41–49. doi: 10.1007/s002210000563. [DOI] [PubMed] [Google Scholar]
- Cho TH, Fischer C, Nighoghossian N, Hermier M, Sindou M, Mauguiere F. Auditory and electrophysiological patterns of a unilateral lesion of the lateral lemniscus. Audiol. Neurootol. 2005;10:153–158. doi: 10.1159/000084025. [DOI] [PubMed] [Google Scholar]
- Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. Learn. Mem. 2003;10:427–455. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- Clark RE, Gohl EB, Lavond DG. The learning-related activity that develops in the pontine nuclei during classical eye-blink conditioning is dependent on the interpositus nucleus. Learn. Mem. 1997;3:532–544. doi: 10.1101/lm.3.6.532. [DOI] [PubMed] [Google Scholar]
- Clark RE, Lavond DG. Reversible lesions of the red nucleus during acquisition and retention of a classically conditioned behavior in rabbits. Behav. Neurosci. 1993;107:264–270. doi: 10.1037//0735-7044.107.2.264. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Moore JW. Single-unit activity in red nucleus during the classically conditioned rabbit nictitating membrane response. Neurosci. Res. 1991;10:260–279. doi: 10.1016/0168-0102(91)90083-b. [DOI] [PubMed] [Google Scholar]
- Hardiman MJ, Ramnani N, Yeo CH. Reversible inactivations of the cerebellum with muscimol prevent the acquisition and extinction of conditioned nictitating membrane responses in the rabbit. Exp. Brain Res. 1996;110:235–247. doi: 10.1007/BF00228555. [DOI] [PubMed] [Google Scholar]
- Haroian AJ, Massopust LC, Young PA. Cerebellothalamic projections in the rat: an autoradiographic and degeneration study. J. Comp. Neurol. 1981;197:217–36. doi: 10.1002/cne.901970205. [DOI] [PubMed] [Google Scholar]
- Krupa DJ, Thompson RF. Inactivation of the superior cerebellar peduncle blocks expression but not acquisition of the rabbit's classically conditioned eye-blink response. Proc. Natl. Acad. Sci. U. S. A. 1995;92:5097–5101. doi: 10.1073/pnas.92.11.5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupa DJ, Thompson RF. Reversible inactivation of the cerebellar interpositus nucleus completely prevents acquisition of the classically conditioned eye-blink response. Learn. Mem. 1997;3:545–556. doi: 10.1101/lm.3.6.545. [DOI] [PubMed] [Google Scholar]
- Krupa DJ, Weng J, Thompson RF. Inactivation of brainstem motor nuclei blocks expression but not acquisition of the rabbit's classically conditioned eyeblink response. Behav. Neurosci. 1996;110:219–227. doi: 10.1037//0735-7044.110.2.219. [DOI] [PubMed] [Google Scholar]
- Lang EJ. GABAergic and glutamatergic modulation of spontaneous and motor-cortex-evoked complex spike activity. J Neurophysiol. 2002;87:1993–2008. doi: 10.1152/jn.00477.2001. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Lavond DG, Thompson RF. Concomitant classical conditioning of the rabbit nictitating membrane and eyelid responses: correlations and implications. Physiol. Behav. 1982;28:769–775. doi: 10.1016/0031-9384(82)90192-5. [DOI] [PubMed] [Google Scholar]
- Medina JF, Christopher RJ, Mauk MD, LeDoux JE. Parallels between cerebellum- and amygdala-dependent conditioning. Nat. Rev. Neurosci. 2002;3:122–131. doi: 10.1038/nrn728. [DOI] [PubMed] [Google Scholar]
- Medina JF, Nores WL, Ohyama T, Mauk MD. Mechanisms of cerebellar learning suggested by eyelid conditioning. Curr. Opin. Neurobiol. 2000;10:717–724. doi: 10.1016/s0959-4388(00)00154-9. [DOI] [PubMed] [Google Scholar]
- Morcuende S, Delgado-Garcia JM, Ugolini G. Neuronal premotor networks involved in eyelid responses: retrograde transneuronal tracing with rabies virus from the orbicularis oculi muscle in the rat. J. Neurosci. 2002;22:8808–8818. doi: 10.1523/JNEUROSCI.22-20-08808.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilaweera WU, Irwin KB, Zenitsky GD, Aksenov DP, Bracha V. Are feedback loops involved in the cerebellar neuronal correlates of conditioned eyeblinks? Soc. Neurosci. 2002 Abstr. 79.6. [Google Scholar]
- Nilaweera WU, Zenitsky GD, Bracha V. Inactivation of the brachium conjunctivum prevents extinction of classically conditioned eyeblinks. Brain Res. 2005;1045:175–184. doi: 10.1016/j.brainres.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Robinson FR, Rice PM, Holleman JR, Berger TW. Projection of the magnocellular red nucleus to the region of the accessory abducens nucleus in the rabbit. Neurobiol. Learn. Mem. 2001;76:358–374. doi: 10.1006/nlme.2001.4028. [DOI] [PubMed] [Google Scholar]
- Ryan SB, Detweiler KL, Holland KH, Hord MA, Bracha V. A long range, wide field-of-view infrared eyeblink detector. J. Neurosci. Methods. 2006;152:74–82. doi: 10.1016/j.jneumeth.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Schicatano EJ, Mantzouranis J, Peshori KR, Partin J, Evinger C. Lid restraint evokes two types of motor adaptation. J. Neurosci. 2002;22:569–576. doi: 10.1523/JNEUROSCI.22-02-00569.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz JE, Logan CG, Rosen DJ, Thompson JK, Lavond DG, Thompson RF. Initial localization of the acoustic conditioned stimulus projection system to the cerebellum essential for classical eyelid conditioning. Proc. Natl. Acad. Sci. U. S. A. 1987;84:3531–3535. doi: 10.1073/pnas.84.10.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teune TM, van der Burg J, van der Moer J, Voogd J, Ruigrok TJ. Topography of cerebellar nuclear projections to the brain stem in the rat. Prog. Brain Res. 2000;124:141–172. doi: 10.1016/S0079-6123(00)24014-4. [DOI] [PubMed] [Google Scholar]
- Thompson RF. In search of memory traces. Annu. Rev. Psychol. 2005;56:1–23. doi: 10.1146/annurev.psych.56.091103.070239. [DOI] [PubMed] [Google Scholar]
- Voogd J. In: Cerebellum. In: The Rat Nervous System. Paxinos G, editor. Elsevier; Amsterdam: 2004. pp. 205–243. [Google Scholar]
- Zhuravin IA, Bures J. Extent of the tetrodotoxin induced blockade examined by pupillary paralysis elicited by intracerebral injection of the drug. Exp. Brain Res. 1991;83:687–690. doi: 10.1007/BF00229849. [DOI] [PubMed] [Google Scholar]