Abstract
Sharp wave and associated fast oscillatory ripples (140–200 Hz) in the cornu ammonis 1 region are the most synchronous hippocampal patterns in the adult rat. Spike sequences associated with sharp waves are believed to play a critical role in transferring transient memories from the hippocampus to the neocortex and the emergence of super-fast ripples is pathognostic in temporal lobe epilepsy. Sharp waves in cornu ammonis 1 stratum radiatum are induced by a strong depolarization by the cornu ammonis 3 Schaffer collaterals, due to the synchronous discharge of cornu ammonis 3 pyramidal cells. Although during the first postnatal week, sharp-wave events are associated with hippocampal unit bursts in the pyramidal layer, ripple oscillations are absent. We investigated the emergence of fast-field oscillations in rat pups ranging from postnatal day 12–20 by recording with wire tetrodes in freely behaving pups and with 16-site linear silicon probes in head fixed animals. Cornu ammonis 1 pyramidal cell layer was determined by the presence of multiple unit activity and a reversal of the field potential in the deeper electrode sites. On-line verification of the recording sites was determined via an evoked response to commissural stimulation, showing a clear reversal in the field potential. Sharp wave-associated fast-field oscillations did not begin to emerge until the end of the second postnatal week and showed a gradual increase until day 18. Once ripples emerged, the intra-ripple frequency assumed adult values. The developmental time course of the ripple parallels the switch in the GABAA receptor-mediated signaling from excitation to inhibition. The time course may also reflect hitherto unidentified emergence of neuronal gap junctions.
Keywords: ripple, fast oscillation, EEG, gap junction, development
Large amplitude field potentials, or “sharp waves” (SPW), occur irregularly in the cornu ammonis (CA)1 stratum radiatum as a result of a strong depolarization by the CA3 Schaffer collaterals, due to the synchronous bursting of CA3 pyramidal cells. In the adult animal, SPW are associated with fast-field oscillations (~140–200 Hz), or “ripples” confined to the CA1 pyramidal cell layer (Buzsaki et al., 1992; O’Keefe and Nadel, 1978; Traub and Bibbig, 2000). SPW are considered to be self-organized endogenous events because they occur when the animal has no or minimal interaction with the environment, such as slow-wave sleep (SWS), immobility and consummatory behaviors (Buzsaki et al., 1983). The importance of the SPW derives from the observation that during its time window, 50,000–100,000 neurons discharge synchronously in the CA3–CA1–subicular complex–entorhinal complex (Chrobak and Buzsaki, 1996; Csicsvari et al., 1999a). Neuronal participation in the population discharge is not random but, instead, each SPW event represents an exhaustive “search” in the autoassociative CA3a-b-c recurrent network (Csicsvari et al., 2000; Ylinen et al., 1995). Importantly, the “content” of the SPW events is determined by previous experience of the animal (Buzsaki, 1989; Kudrimoti et al., 1999; Lee and Wilson, 2002; Nadasdy et al., 1999; Skaggs and McNaughton, 1996; Wilson and McNaughton, 1994). Because of the behavior-relevant content and because of the three-five-fold gain of network excitability during the SPW (Csicsvari et al., 1999a), this endogenous hippocampal output pattern, active during “offline” states of the hippocampus, may play a critical role in transferring transient memories from the hippocampus to the neocortex for permanent storage (Buzsaki, 1989, 1998; Buzsaki and Chrobak, 1995; Chrobak and Buzsaki, 1998; Hobson and Pace-Schott, 2002; Lorincz and Buzsaki, 2000; Siapas and Wilson, 1998).
The physiological role and mechanisms of the fast oscillatory ripple, accompanying most but not all SPW events (Csicsvari et al., 2000; Ylinen et al., 1995), are not well understood. Nevertheless, its importance is illustrated by the observation that the temporal structure of ripples changes in disease and the emergence of superfast ripples is pathognostic in the diagnosis of temporal lobe epilepsy in humans (Bragin et al., 1999; Staba et al., 2004). Three potential mechanisms have been put forward for the explanation of ripple initiation. According to the first model, the synchronous discharge of some CA3 and/or CA1 pyramidal cells leads to a transient synchronous discharge of CA1 basket and chandelier cells which, in turn, produce a rhythmic oscillatory pattern in CA1 pyramidal neurons (Brunel and Wang, 2003; Ylinen et al., 1995). The second model assumes that interneuron synchrony is brought about by dendrodendritic and dendrosomatic gap junctions between interneurons (Draguhn et al., 1998; Ylinen et al., 1995), although the recent finding that ripples remain unaltered in vivo in mice lacking gap junction-mediated coupling between interneurons decreases the relevance of this mechanism (Buhl et al., 2003). The third model postulates that gap junctions between the axons of pyramidal cells are responsible for ripple initiation (Draguhn et al., 1998; Schmitz et al., 2001; Traub et al., 1999, 2002, 2003; Traub and Bibbig, 2000).
In the present paper, we examined the emergence of the fast oscillation in postnatal developing rats. Previous investigations revealed that the first organized hippocampal pattern in the newborn rat is a SPW event (Leinekugel et al., 2002). However, SPW during the first week of life are not accompanied by structured fast oscillations. Identifying the postnatal time course of ripple emergence in the developing rat pup will be advantageous because it can be correlated with the developmental time course of different maturational processes. This correlational information then would allow, for example, targeted creation of transgenic mice lacking different sources of electrotonic coupling or receptor subtypes.
EXPERIMENTAL PROCEDURES
Animals and surgery: freely moving animals
A total of nine male Sprague–Dawley neonates were operated under isoflurane anesthesia on either postnatal day 11 (PND/P11, n = 3) or P13 (n = 6). PND were calculated from P0 as the reference to the day of birth. Holes above the right hippocampus (~1 mm in diameter) were drilled 1.5–2 mm posterior to Bregma and 1.5–2 mm lateral to the midline. Four wire electrodes (20 μm in diameter, California Fine Wire, Grover Beach, CA, USA) or two stereotrodes composed of the same wire were fixed to a movable microdrive with 100–300 μm vertical tip separation. Each complete turn of the microdrive moved the recording electrodes ~0.3 mm axially. Electrodes were then implanted into the neocortex and moved slowly so the deepest electrode reached the depth of 2.1 mm. The holes were then covered with a mixture of paraffin oil and wax. Two stainless steel screws were driven into the skull posterior to lambda to serve as ground and reference electrodes as well as anchors. Two screws were driven into the skull anterior to Bregma to serve as anchors. The microdrive was then fixed to the skull with grip cement (L.D. Caulk Division, Milford, DE, USA). The surgical wound was treated with Neopredef to make the animal more comfortable. After surgery, pups were given 0.1 ml of 10% glucose mixed in saline via s.c. injection and placed in an incubation chamber with two littermates. After recovery, the pups were reintroduced into their litter. The litter was culled to six animals in order to ensure the operated pups would have access to the mother for feeding. After 24 h of recover, daily recordings were performed for 1 to 2 h during immobility and SWS while the pup was ether isolated or with two pups from the litter.
Animals and surgery: head-fixed animals
A total of 24 male Sprague–Dawley neonates were handled for three days prior to surgery (P12, n= 3; P13, n= 4; P14, n= 3; P15, n= 4; P16, n= 3; P17, n= 2; P18, n= 2; P19, n= 1; P20, n= 2). Because of the lack of animals in the older age groups, data from P17 and P18 were collapsed to P18, and data from P19 and P20 were collapsed and referred to as P20. On the day of surgery, only pups that had a white band across the stomach area (i.e. those that were well-nourished) were selected for the experiment. Surgery was performed under isoflurane anesthesia. During surgery, stainless steel screws were placed posterior to lambda to serve as ground and reference electrodes and anchors. A hollow bar was mounted to the posterior skull and a screw was placed above the nose bone using grip cement to later serve to secure the animals head in the apparatus. Holes above the hippocampus (~1 mm in diameter) were drilled bilaterally 1.5–2 mm posterior to Bregma and 1.5–2 mm lateral to the midline and covered with a mixture of paraffin oil and wax. The surgical wound was treated with Neopredef (Pharmacia & Upjohn, Kalamazu, MI, USA) to make the animal more comfortable. After surgery, pups were given 0.1 ml of 10% glucose mixed in saline via s.c. injection and placed in an incubation chamber with two littermates for at approximately 1–2 h. Animals were placed into the apparatus and the body temperature was maintained at 37 °C with a heating pad. A 16-site linear probe (100 μm between recording sites; Bragin et al., 1995) was lowered into the hippocampus while a bipolar stimulating electrode was placed into the contra-lateral hippocampal CA1 stratum pyramidale. After the animals appeared comfortable, recordings were performed for approximately 2 h during SWS periods. All procedures conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and had been approved by the Institutional Animal Care and Use Committee of Rutgers University. The number of animals used were kept at the minimum necessary for demonstrating the developmental differences. If the animals appeared uncomfortable at any time, the experiment was terminated.
Data acquisition: freely moving animals
Electrical activity was recorded during the wake cycle and during SWS and immobility periods in an isolated recording chamber or when the pup was with two littermates. Only periods that the animal was immobile were used for analysis. An instrumentation amplifier, built in the female connector (four channels), was used to reduce cable movement artifacts. In most cases, unit activity characteristic with hippocampal pyramidal cell layer firing patterns were present on at least one of the electrodes. When units were not present, the microdrive was manipulated along the dorsoventral axis until such firing patterns were present. CA1 pyramidal cell layer was determined by anatomical location as well as the synchronous discharge of unit activity (Buzsáki et al., 1992). Wide band field potentials and unit activity were recorded after being amplified (5000–10,000×) and bandpass filtered (1 Hz to 5 kHz) (model 12–64 channel; Grass Instruments, Quincy, MA, USA), digitized with 14-bit resolution continuously at 20 kHz (PowerDaq PD2-MF-64-400/14H analog-to-digital converter; United Electronic Industries, Canton, MA, USA), and recorded on a personal computer using custom LabView software (National Instruments, Austin, TX, USA). Only animals where the SPW-related activity eventually appeared on the recording electrodes were used.
Data acquisition: head-fixed animals
Electrical activity was recorded during SWS periods. The tip of the silicon probe was lowered into the CA1 stratum pyramidale where the first recording sample was acquired. CA1 pyramidal cell layer was determined by anatomical location as well as the characteristic SPW-related synchronous discharge of unit activity (Buzsaki et al., 1992). The probe was then lowered so the tip was situated below the CA1 pyramidal layer in CA1 stratum lacunosummoleculare. After baseline recording, the commissural fibers were stimulated (100–400 μA for 100 μs, 10 s ISI; STG-1008, Multichannel Systems, Germany) in order to verify and calibrate the position of the recording sites. If a clear reversal was not observed between the recording site believed to be in CA1 stratum pyramidale and the site in CA1 lacunosummoleculare (Buzsaki and Eidelberg, 1982), the recordings were discarded and the probe was reinserted into a new location. After stimulation, baseline activity was evaluated again to ensure the stimulation did not alter the hippocampal activity. Wide band field potentials, unit activity, and evoked responses were recorded after being amplified (10,000×) and bandpass filtered (1 Hz to 5 kHz; model 12–64 channel; Grass Instruments), digitized with 14-bit resolution continuously at 20 kHz (PowerDaq PD2-MF-64-400/14H analog-to-digital converter; United Electronic Industries), and recorded on a personal computer using custom Labview software (National Instruments).
Data processing and analysis
All analyses and statistics were calculated using custom scripts written in C or for Matlab 7.0 for LINUX (MathWorks, Natick, MA, USA). All units extracted were considered as multiple unit activity. Multiple units were detected using methods previously described (Csicsvari et al., 1998, 1999b). Wide band data were decimated to 1.25 kHz for all electroencephalogram (EEG) analyses.
Detection of SPW-associated events
Only periods of SWS and immobility were used to detect SPW-associated events. When fast-field “ripple” oscillations were not present, an increase in the baseline unit activity in CA1 stratum pyramidale that is characteristic with SPW-associated events (Buzsaki et al., 1992) was used to classify such events (Leinekugel et al., 2002). In short, baseline unit activity was determined using a 200 ms sliding window over the entire session. An SPW-associated event was classified as a significant increase (>3 SD) in multiple unit activity. In the head-fixed animals, the presence of an SPW in CA1 stratum radiatum (Fig. 1, bottom trace) was detected separately from the increases in unit activity. Events were defined as periods that showed a clear reversal potential in reference to CA1 stratum pyramidale, which were then correlated with increases in unit activity. SPW in the CA1 stratum radiatum were detected by applying a variable amplitude threshold to the previously bandpass-filtered (1–20 Hz) EEG. A variable threshold was necessary as the overall power of EEG increases with age (Frank and Heller, 1997). For both experimental procedures, all computer-detected SPW-associated events were verified by eye.
Fig. 1.
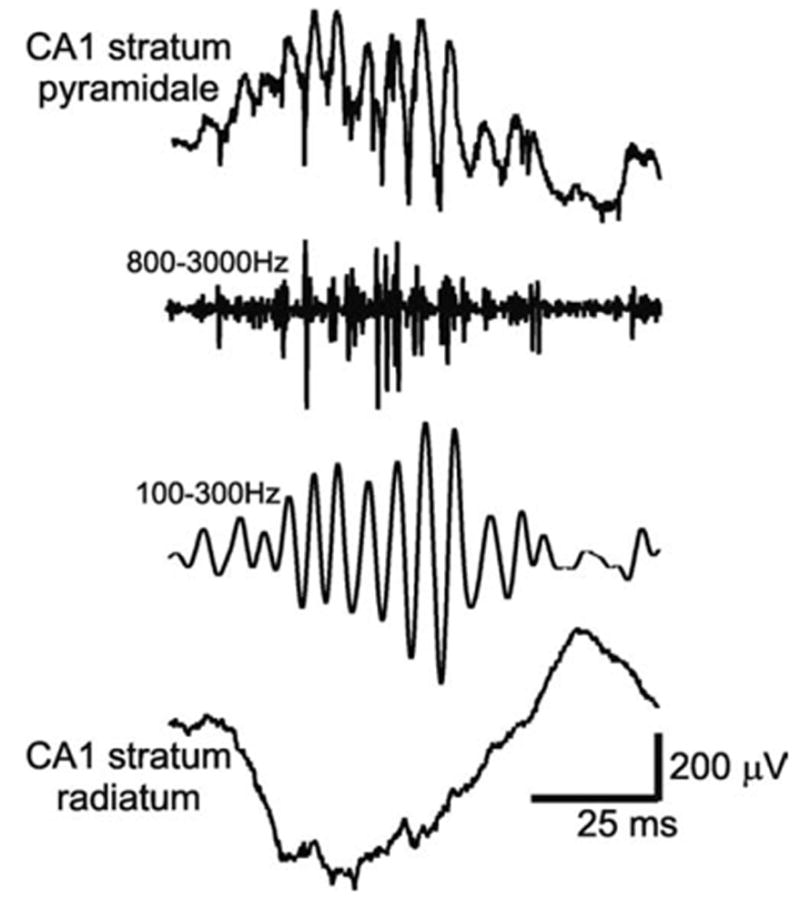
Example of an adult rat SPW event in the CA1 region of the dorsal hippocampus. Top and bottom traces are simultaneously recorded wide band traces from the pyramidal layer and stratum radiatum, respectively. Note oscillatory pattern in the pyramidal layer. These oscillations are accompanied by an increase in local unit activity (second trace, 0.5–5 kHz) and consist of 5–15 wavelets within ~70 ms (i.e. 140–200 Hz, filtered, third trace). The amplifications in the filtered traces are 2× that of the unfiltered ones.
Histology
After the collection of the data, rats were deeply anesthetized with a high dose of Nembutal (100 mg/kg). The animals were then perfused transcardially with saline (~15 ml) followed by 10% formalin (~60 ml). When possible, the electrodes were left in situ during the perfusion. The brains were extracted, blocked within range of the hippocampus, placed in fixative for 24–48 h, and finally cut into 80 μm-thick sections using a vibratome. For verification of electrode placement, sections were mounted onto gelatin-coated slides, stained with the Nissl method, dehydrated and covered with Depex for light microscopy.
RESULTS
Although ripple oscillations were not present in the younger animals, there was a clear increase in multiple unit activity during SPW-associated events in both freely moving and head-fixed preparations (Fig. 2). Only periods of SWS and immobility (in freely moving animals) were used for these analyses.
Fig. 2.
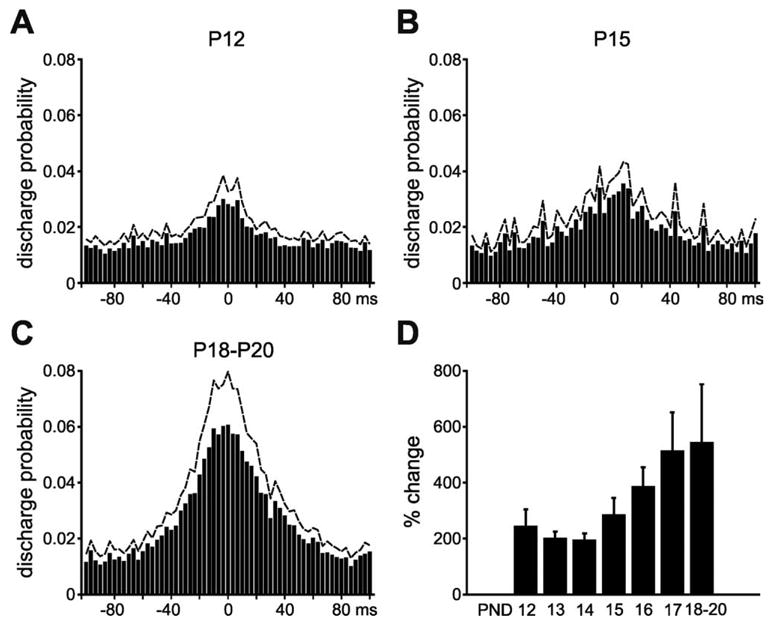
SPW events are accompanied by an increase in local multiple unit activity at all ages. Although ripple events were not observed in younger animals, there was a clear increase in unit activity in the CA1 str. pyramidale with a duration similar as in the adult and was consistent throughout development. (A) P12 animals, n= 6; (B) P15 animals, n= 12; (C) P18–P20 animals, n= 10. Histograms show discharge probability change from the background level (±100 ms from the detected peak of the SPW event). Dashed lines: +S.E.M. (D) Overall percent change in baseline unit activity for all animals (n = 33).
Development of hippocampal “ripple” oscillations in the freely moving neonate
The amplitude and peak frequency of the detected SPW-associated periods in CA1 stratum pyramidale were evaluated using methods previously described (Buhl et al., 2003; Buzsaki et al., 2003). In short, the multi-taper method was used on the wide band detected periods (±100 ms from the peak of the event). The characteristic 140–200 Hz field oscillations that are associated with the increase in unit activity did not begin to appear until approximately P14, and appeared to grow in amplitude as the animals grew older (Fig. 3). There was a significant increase in the amplitude of these oscillations as the age of the animal increased (F(6,21)= 4.11, P= 0.0069; Fig. 3D).
Fig. 3.
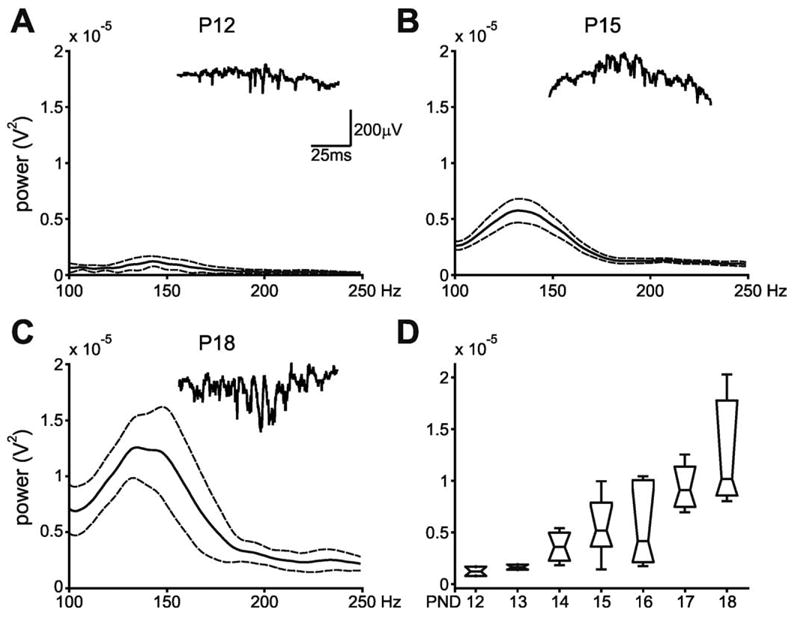
(A–C) Multi-taper analysis of wide band-detected SPW-associated activity for three selected age groups in freely moving animals. SPW-associated events were analyzed only during immobility and SWS periods. Note that fast field “ripple” oscillations do not begin to appear until after the second week of life. Solid/dotted lines: mean ± S.E.M. Insets: Wide band traces from CA1 str. pyramidale of detected SPW-associated events for each age in single rats. (D) Group results. Peak power in the 140–200 Hz frequency band across all age groups in freely moving animals. Note the increase in power of the peak ripple frequency as age increases. Horizontal lines indicate the mean at each age. Boxes show the distribution of the data. Error bars: ± S.E.M.
Development of hippocampal “ripple” oscillations in the head-fixed neonate
It is possible that the lack of ripples in the younger freely moving animals was due to variability in electrode placement, although the presence of multiple unit activity, detected in the pyramidal layer and the histological verification guarded against this possibility. In the head-fixed preparation, using the multisite probes and evoked responses, we could more accurately control the placement of the recording electrodes and at the same time monitor the concurrent SPW. All power comparisons were done from recordings when the tip was in or just below the pyramidal cell body layer. This is because of the possibility that the probe could damage local pyramidal cells, which could affect the presence of the field ripples. As observed in the freely moving animals, the characteristic 140–200 Hz field oscillations that are associated with the increase in unit activity in the adult did not begin to appear until approximately P14, and appeared to grow in amplitude as the animals grew older (Fig. 4). There was a significant increase in the amplitude of these oscillations as the age of the animal increased (F(6,20) = 4.81, P = 0.0032; Fig. 4D).
Fig. 4.
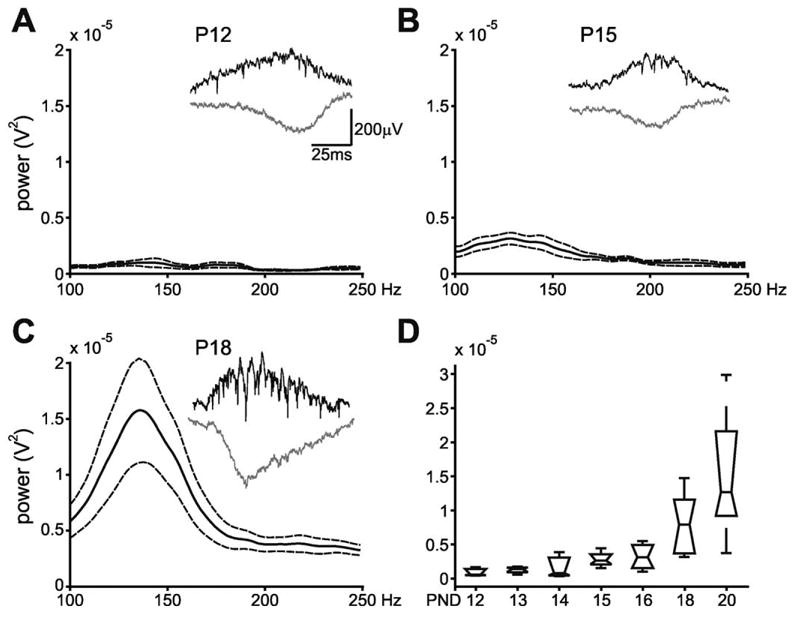
(A–C) Multi-taper analysis of wide band-detected SPW-associated activity for three selected age groups in head fixed animals. SPW-associated events were analyzed only during immobility and SWS periods. Note that fast field “ripple” oscillations do not begin to appear until after the second week of life as observed in the freely moving animals (Fig. 3). Solid/dotted lines: mean ± S.E.M. Insets: Examples of detected SPW-associated events for each age in single rats. Black traces: wide band recording from CA1 str. pyramidale; gray traces: wide band recording from CA1 str. radiatum. (D) Group results. Peak power in the 140–200 Hz frequency band across all age groups in head-fixed animals. Note the increase in power of the peak ripple frequency as age increases. Horizontal lines indicate the mean at each age. Boxes show the distribution of the data. Error bars: ± S.E.M.
Taking advantage of the linear layout of the multiple recording sites, we could observe the sink–source distribution of the spontaneously occurring events and evoked potentials in response to stimulation of the commissural fibers (Buzsaki and Eidelberg, 1982; Ylinen et al., 1995). To visualize these sink–source pairs, we used a triggered current source density analysis (CSD) of the evoked responses and of the detected SPW-associated events. The depth profile of the sink–source dipoles of the evoked responses and SPW was similar in all pups (data not shown), confirming that the major source of the current underlying SPW is the synchronous depolarization of the CA1 apical dendrites by the CA3 input.
In addition to the power change, we also examined the intra-ripple frequency of SPW events. In contrast to the power that increased daily from the incipience of ripples, its frequency was constant throughout the monitored developmental period, with a mean frequency of 143.73 ± 4.31 Hz for head-fixed animals and 142.82 ± 2.43 Hz for the behaving animals (Fig. 5). Using an arbitrary threshold (mean+3 SD), and the multitaper spectral method, we could establish that even at the earliest age, the peak power, albeit very small, occurred at a similar frequency to that of observed at older ages. The intraripple frequency for both groups of animals was not significantly different (F(6,20) = 1.44, P = 0.2477 and F(6,21) = 1.45, P = 0.2525 respectively).
Fig. 5.
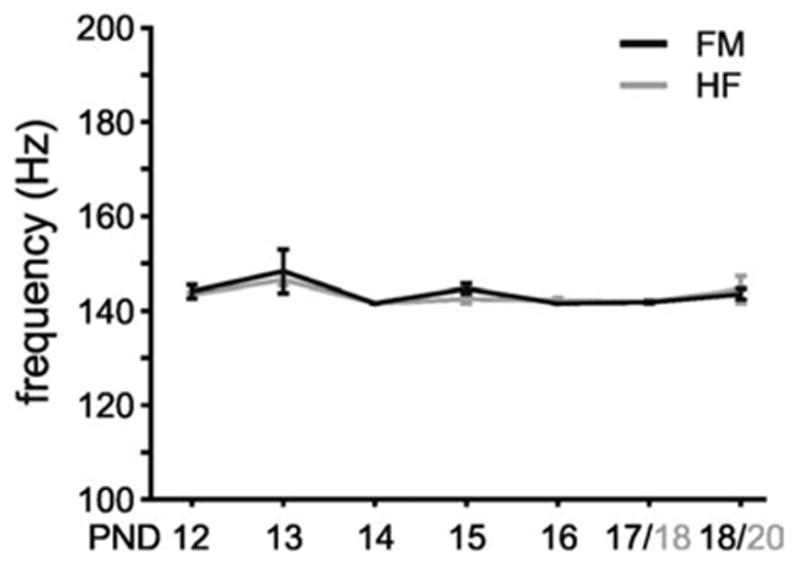
Intraripple frequency does not change during the time course of development. The frequency of the SPW-associated events was determined at the maximum power observed in the 100–200 Hz frequency band in the spectral analyses (see Figs. 3 and 4). Mean frequency of power spectral maxima in the ripple band in either freely moving (black lines) or head-fixed (gray lines) animals as a function of age. Note the invariance of ripple frequency with age. Error bars: ± S.E.M.
DISCUSSION
SPW bursts in the adult are the main endogenous, self-organized patterns of the hippocampus associated with a well-defined fast oscillatory event (“ripple”) confined to the CA1 pyramidal layer in the hippocampus. The ripple event in CA1 stratum pyramidale is associated with ripples of slower frequency in the subiculum–presubiculum–parasubiculum–deep entorhinal cortex (Chrobak and Buzsaki, 1998), and enhanced activity in at least two cortical targets, the perirhinal cortex (Collins et al., 1999) and the medial prefrontal cortex (Siapas and Wilson, 1998). It is this chain of activity that has been hypothesized to allow large ensembles of hippocampal neurons via SPW/ripple events to alter the synaptic connectivity of neocortical circuitry (Chrobak et al., 2000; Chrobak and Buzsaki, 1998).
Although SPW is the first detectable postnatal network pattern in the hippocampus, associated ripples begin to emerge only at the end of the second postnatal week, growing to near adult-like ripple oscillations by P20. Once they emerge their frequency remain invariant across development. These observations should help identify factors responsible for the generation of ripple oscillations, which in turn will allow us to study the physiological purpose of these oscillations in more detail.
The observation in the adult rat that halothane, a non-specific gap junction blocker, abolished hippocampal ripples prompted the suggestion that electrical coupling among neurons may be critical in the generation of super fast network oscillations (Ylinen et al., 1995). Subsequent findings indicated that connexin-36-type gap junctions, present between various classes of interneurons (Katsumaru et al., 1988a,b), are not critically involved (Buhl et al., 2003; Hormuzdi et al., 2001; Maier et al., 2002). On the other hand, ripple-like patterns, consisting of transient oscillation of mini population spikes without positive waves, have been described in vitro (Draguhn et al., 1998). These in vitro oscillations do not depend on synaptic transmission (see also Jones and Barth, 2002). These in vitro observations and computer simulations formed the basis of the hypothesis that ripple oscillations occur by electrical coupling between the axons pyramidal cells (Draguhn et al., 1998; Schmitz et al., 2001; Traub et al., 1999, 2002; Traub and Bibbig, 2000).
At least two factors contribute to the field ripples. Synchronous discharge of pyramidal neurons generates repetitive “mini populations spikes” (Buzsaki, 1986) that are responsible for the spike-like appearance of the troughs of ripples in the pyramidal cell layer. The positive “wave” component has been suggested to reflect synchronously occurring inhibitory post-synaptic potentials (IPSPs) in pyramidal cells (Grenier et al., 2001; Jones et al., 2000; Ylinen et al., 1995). Ripple-like patterns, consisting of transient oscillation of mini population spikes only, have been recently described in vitro (Draguhn et al., 1998). The in vitro oscillations persist after blockade of inhibition (see also Jones and Barth, 2002) or even after complete blockade of synaptic transmission. On the basis of these in vitro observations and computer simulations it was proposed that ripple oscillations were mediated by electrical coupling that occurred between the axons pyramidal cells (Draguhn et al., 1998; Schmitz et al., 2001; Traub et al., 1999; Traub and Bibbig, 2000). Although the in vitro ripple-like events in the absence of synaptic transmission share some features of the ripples, they may represent a different pattern. First, fast field oscillations with all major macroscopic characteristics have been recently reported in the mouse hippocampus (Maier et al., 2003) and from ventral slices of the rat hippocampus (Kubota et al., 2003), presumably because sufficient among of CA3–CA1 connections could be preserved in these preparations. Second, synaptic transmission-independent ripple-like events in vitro are present right after birth (Palva et al., 2000). This is in contrast to our present observations, which indicates that ripples in vivo first emerge during the late part of the second week of life in rats (see also Leinekugel et al., 2002). If gap junctions are critical in the emergence of ripple oscillations, their developmental profile is expected to match that of the physiological events underlying ripple generation. There is indication that non-connexin type electrical junctions are present in the hippocampus (Stebbings et al., 2002). Possible candidates are the recently described Pannexin 1 and 2 (Bruzzone et al., 2003). These new gap junctions have been found using in situ hybridization to be present in CA1 pyramidal and parvalbumin-containing basket cells (Vogt et al., 2004), and are potential candidates for the axoaxonic gap junctions proposed to exist between CA1 pyramidal cells (Draguhn et al., 1998; Schmitz et al., 2001; Traub et al., 1999, 2002; Traub and Bibbig, 2000). Further research will determine whether Pannexin 1 and/or 2 or other, hitherto unidentified electrical junctions, is essential for ripple oscillations.
Another well-studied developmentally regulated process is a change of the GABAA-receptor function from depolarizing to hyperpolarizing effect. In fact, during the first PND the depolarizing action of GABA through GABAAreceptors is considered to be a major excitatory force (Ben Ari et al., 1989). Recent observations indicate that the switch in GABAA signaling from depolarizing to inhibitory occurs between P13 and P15 in the Sprague–Dawley rat (Khazipov et al., 2004), which is consistent with the time course of the emergence of ripple oscillations reported here.
Acknowledgments
This research was supported by NIH (NS34994, MH54671). We thank E. Abercrombie, F. Nadim, D. Pare, J. M. Tepper and R. D. Traub for useful discussion and Danielle Mulder for helping with histological processing.
Abbreviations
- CA
cornu ammonis
- EEG
electroencephalogram
- P/PND
postnatal day
- SPW
sharp wave
- SWS
slow-wave sleep
References
- Ben Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol. 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr, Wilson CL, Fried I, Buzsaki G. High-frequency oscillations in human brain. Hippocampus. 1999;9:137–142. doi: 10.1002/(SICI)1098-1063(1999)9:2<137::AID-HIPO5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Bragin A, Jando G, Nadasdy Z, Hetke J, Wise K, Buzsaki G. Gamma (40–100 Hz) oscillation in the hippocampus of the behaving rat. J Neurosci. 1995;15:47–60. doi: 10.1523/JNEUROSCI.15-01-00047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunel N, Wang XJ. What determines the frequency of fast network oscillations with irregular neural discharges? I. Synaptic dynamics and excitation-inhibition balance. J Neurophysiol. 2003;90:415–430. doi: 10.1152/jn.01095.2002. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H. Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci U S A. 2003;100:13644–13649. doi: 10.1073/pnas.2233464100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl DL, Harris KD, Hormuzdi SG, Monyer H, Buzsaki G. Selective impairment of hippocampal gamma oscillations in connexin-36 knock-out mouse in vivo. J Neurosci. 2003;23:1013–1018. doi: 10.1523/JNEUROSCI.23-03-01013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. Hippocampal sharp waves: their origin and significance. Brain Res. 1986;398:242–252. doi: 10.1016/0006-8993(86)91483-6. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience. 1989;31:551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Memory consolidation during sleep: a neurophysiological perspective. J Sleep Res. 1998;7 (Suppl 1):17–23. doi: 10.1046/j.1365-2869.7.s1.3.x. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Buhl DL, Harris KD, Csicsvari J, Boldizsar C, Morozov A. Hippocampal network patterns of activity in the mouse. Neuroscience. 2003;116:201–211. doi: 10.1016/s0306-4522(02)00669-3. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Chrobak JJ. Temporal structure in spatially organized neuronal ensembles: a role for interneuronal networks. Curr Opin Neurobiol. 1995;5:504–510. doi: 10.1016/0959-4388(95)80012-3. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Eidelberg E. Convergence of associational and commissural pathways on CA1 pyramidal cells of the rat hippocampus. Brain Res. 1982;237:283–295. doi: 10.1016/0006-8993(82)90442-5. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Horvath Z, Urioste R, Hetke J, Wise K. High-frequency network oscillation in the hippocampus. Science. 1992;256:1025–1027. doi: 10.1126/science.1589772. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Leung LW, Vanderwolf CH. Cellular bases of hippocampal EEG in the behaving rat. Brain Res. 1983;287:139–171. doi: 10.1016/0165-0173(83)90037-1. [DOI] [PubMed] [Google Scholar]
- Chrobak JJ, Buzsaki G. High-frequency oscillations in the output networks of the hippocampalentorhinal axis of the freely behaving rat. J Neurosci. 1996;16:3056–3066. doi: 10.1523/JNEUROSCI.16-09-03056.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrobak JJ, Buzsaki G. Operational dynamics in the hippocampalentorhinal axis. Neurosci Biobehav Rev. 1998;22:303–310. doi: 10.1016/s0149-7634(97)00016-x. [DOI] [PubMed] [Google Scholar]
- Chrobak JJ, Lorincz A, Buzsaki G. Physiological patterns in the hippocampoentorhinal cortex system. Hippocampus. 2000;10:457–465. doi: 10.1002/1098-1063(2000)10:4<457::AID-HIPO12>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Collins DR, Lang EJ, Pare D. Spontaneous activity of the perirhinal cortex in behaving cats. Neuroscience. 1999;89:1025–1039. doi: 10.1016/s0306-4522(98)00396-0. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Czurko A, Buzsaki G. Reliability and state dependence of pyramidal cell-interneuron synapses in the hippocampus: an ensemble approach in the behaving rat. Neuron. 1998;21:179–189. doi: 10.1016/s0896-6273(00)80525-5. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Czurko A, Mamiya A, Buzsaki G. Fast network oscillations in the hippocampal CA1 region of the behaving rat. J Neurosci. 1999a;19:RC20. doi: 10.1523/JNEUROSCI.19-16-j0001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Czurko A, Mamiya A, Buzsaki G. Oscillatory coupling of hippocampal pyramidal cells and interneurons in the behaving rat. J Neurosci. 1999b;19:274–287. doi: 10.1523/JNEUROSCI.19-01-00274.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Mamiya A, Buzsaki G. Ensemble patterns of hippocampal CA3-CA1 neurons during sharp wave-associated population events. Neuron. 2000;28:585–594. doi: 10.1016/s0896-6273(00)00135-5. [DOI] [PubMed] [Google Scholar]
- Draguhn A, Traub RD, Schmitz D, Jefferys JG. Electrical coupling underlies high-frequency oscillations in the hippocampus in vitro. Nature. 1998;394:189–192. doi: 10.1038/28184. [DOI] [PubMed] [Google Scholar]
- Frank MG, Heller HC. Development of diurnal organization of EEG slow-wave activity and slow-wave sleep in the rat. Am J Physiol. 1997;273:R472–R478. doi: 10.1152/ajpregu.1997.273.2.R472. [DOI] [PubMed] [Google Scholar]
- Grenier F, Timofeev I, Steriade M. Focal synchronization of ripples (80–200 Hz) in neocortex and their neuronal correlates. J Neurophysiol. 2001;86:1884–1898. doi: 10.1152/jn.2001.86.4.1884. [DOI] [PubMed] [Google Scholar]
- Hobson JA, Pace-Schott EF. The cognitive neuroscience of sleep: neuronal systems, consciousness and learning. Nat Rev Neurosci. 2002;3:679–693. doi: 10.1038/nrn915. [DOI] [PubMed] [Google Scholar]
- Hormuzdi SG, Pais I, LeBeau FE, Towers SK, Rozov A, Buhl EH, Whittington MA, Monyer H. Impaired electrical signaling disrupts gamma frequency oscillations in connexin 36-deficient mice. Neuron. 2001;31:487–495. doi: 10.1016/s0896-6273(01)00387-7. [DOI] [PubMed] [Google Scholar]
- Jones MS, Barth DS. Effects of bicuculline methiodide on fast (> 200 Hz) electrical oscillations in rat somatosensory cortex. J Neurophysiol. 2002;88:1016–1025. doi: 10.1152/jn.2002.88.2.1016. [DOI] [PubMed] [Google Scholar]
- Jones MS, MacDonald KD, Choi B, Dudek FE, Barth DS. Intracellular correlates of fast (> 200 Hz) electrical oscillations in rat somatosensory cortex. J Neurophysiol. 2000;84:1505–1518. doi: 10.1152/jn.2000.84.3.1505. [DOI] [PubMed] [Google Scholar]
- Katsumaru H, Kosaka T, Heizmann CW, Hama K. Gap junctions on GABAergic neurons containing the calcium-binding protein parvalbumin in the rat hippocampus (CA1 region) Exp Brain Res. 1988a;72:363–370. doi: 10.1007/BF00250257. [DOI] [PubMed] [Google Scholar]
- Katsumaru H, Kosaka T, Heizmann CW, Hama K. Immunocytochemical study of GABAergic neurons containing the calcium-binding protein parvalbumin in the rat hippocampus. Exp Brain Res. 1988b;72:347–362. doi: 10.1007/BF00250256. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Khalilov I, Tyzio R, Morozova E, Ben Ari Y, Holmes GL. Developmental changes in GABAergic actions and seizure susceptibility in the rat hippocampus. Eur J Neurosci. 2004;19:590 – 600. doi: 10.1111/j.0953-816x.2003.03152.x. [DOI] [PubMed] [Google Scholar]
- Kubota D, Colgin L, Casale M, Brucher F, Lynch G. Endogenous waves in hippocampal slices. J Neurophysiol. 2003;89:81–89. doi: 10.1152/jn.00542.2002. [DOI] [PubMed] [Google Scholar]
- Kudrimoti HS, Barnes CA, McNaughton BL. Reactivation of hippocampal cell assemblies: effects of behavioral state, experience, and EEG dynamics. J Neurosci. 1999;19:4090–4101. doi: 10.1523/JNEUROSCI.19-10-04090.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AK, Wilson MA. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron. 2002;36:1183–1194. doi: 10.1016/s0896-6273(02)01096-6. [DOI] [PubMed] [Google Scholar]
- Leinekugel X, Khazipov R, Cannon R, Hirase H, Ben Ari Y, Buzsaki G. Correlated bursts of activity in the neonatal hippocampus in vivo. Science. 2002;296:2049–2052. doi: 10.1126/science.1071111. [DOI] [PubMed] [Google Scholar]
- Lorincz A, Buzsaki G. Two-phase computational model training long-term memories in the entorhinalhippocampal region. Ann N Y Acad Sci. 2000;911:83–111. doi: 10.1111/j.1749-6632.2000.tb06721.x. [DOI] [PubMed] [Google Scholar]
- Maier N, Guldenagel M, Sohl G, Siegmund H, Willecke K, Draguhn A. Reduction of high-frequency network oscillations (ripples) and pathological network discharges in hippocampal slices from connexin 36-deficient mice. J Physiol. 2002;541:521–528. doi: 10.1113/jphysiol.2002.017624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier N, Nimmrich V, Draguhn A. Cellular and network mechanisms underlying spontaneous sharp wave-ripple complexes in mouse hippocampal slices. J Physiol. 2003;550:873–887. doi: 10.1113/jphysiol.2003.044602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadasdy Z, Hirase H, Czurko A, Csicsvari J, Buzsaki G. Replay and time compression of recurring spike sequences in the hippocampus. J Neurosci. 1999;19:9497–9507. doi: 10.1523/JNEUROSCI.19-21-09497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The hippocampus as a cognitive map. Oxford: Oxford University Press; 1978. [Google Scholar]
- Palva JM, Lamsa K, Lauri SE, Rauvala H, Kaila K, Taira T. Fast network oscillations in the newborn rat hippocampus in vitro. J Neurosci. 2000;20:1170–1178. doi: 10.1523/JNEUROSCI.20-03-01170.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz D, Schuchmann S, Fisahn A, Draguhn A, Buhl EH, Petrasch-Parwez E, Dermietzel R, Heinemann U, Traub RD. Axo-axonal coupling: a novel mechanism for ultrafast neuronal communication. Neuron. 2001;31:831–840. doi: 10.1016/s0896-6273(01)00410-x. [DOI] [PubMed] [Google Scholar]
- Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21:1123–1128. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science. 1996;271:1870–1873. doi: 10.1126/science.271.5257.1870. [DOI] [PubMed] [Google Scholar]
- Staba RJ, Wilson CL, Bragin A, Jhung D, Fried I, Engel J., Jr High-frequency oscillations recorded in human medial temporal lobe during sleep. Ann Neurol. 2004;56:108–115. doi: 10.1002/ana.20164. [DOI] [PubMed] [Google Scholar]
- Stebbings LA, Todman MG, Phillips R, Greer CE, Tam J, Phelan P, Jacobs K, Bacon JP, Davies JA. Gap junctions in Drosophila: developmental expression of the entire innexin gene family. Mech Dev. 2002;113:197–205. doi: 10.1016/s0925-4773(02)00025-4. [DOI] [PubMed] [Google Scholar]
- Traub RD, Bibbig A. A model of high-frequency ripples in the hippocampus based on synaptic coupling plus axon-axon gap junctions between pyramidal neurons. J Neurosci. 2000;20:2086–2093. doi: 10.1523/JNEUROSCI.20-06-02086.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Draguhn A, Whittington MA, Baldeweg T, Bibbig A, Buhl EH, Schmitz D. Axonal gap junctions between principal neurons: a novel source of network oscillations, and perhaps epileptogenesis. Rev Neurosci. 2002;13:1–30. doi: 10.1515/revneuro.2002.13.1.1. [DOI] [PubMed] [Google Scholar]
- Traub RD, Pais I, Bibbig A, LeBeau FE, Buhl EH, Hormuzdi SG, Monyer H, Whittington MA. Contrasting roles of axonal (pyramidal cell) and dendritic (interneuron) electrical coupling in the generation of neuronal network oscillations. Proc Natl Acad Sci U S A. 2003;100:1370–1374. doi: 10.1073/pnas.0337529100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Schmitz D, Jefferys JG, Draguhn A. High-frequency population oscillations are predicted to occur in hippocampal pyramidal neuronal networks interconnected by axoaxonal gap junctions. Neuroscience. 1999;92:407–426. doi: 10.1016/s0306-4522(98)00755-6. [DOI] [PubMed] [Google Scholar]
- Vogt A, Hormuzdi SG, Monyer H. A developmental and immunohistochemial study of Pannexin 1 and Pannexin 2 expression in the central nervous system of the rat. Soc Neurosci Abst 2004 [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Ylinen A, Bragin A, Nadasdy Z, Jando G, Szabo I, Sik A, Buzsaki G. Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J Neurosci. 1995;15:30–46. doi: 10.1523/JNEUROSCI.15-01-00030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]


