Abstract
Previous results have shown that CD4+CD25+ regulatory T cells (Tregs) control autoimmunity in a spontaneous model of type 1 diabetes, the nonobese diabetic (NOD) mouse. Moreover, anti-CD3 reverses diabetes in this setting by promoting Tregs that function in a TGF-β-dependent manner. This finding contrasts with a large body of work suggesting that CD4+CD25high Tregs act in a cytokine-independent manner, thus suggesting that another type of Treg is operational in this setting. We sought to determine the basis of suppression both in untreated NOD mice and in those treated with anti-CD3. Our present results show that a subset of foxP3+ cells present within a CD4+CD25low lymphocyte subset suppresses T cell immunity in spontaneously diabetic NOD mice in a TGF-β-dependent manner, a functional property typical of “adaptive” regulatory T cells. This distinct Treg subset is evident in NOD, but not normal, mice, suggesting that the NOD mice may generate these adaptive Tregs in an attempt to regulate ongoing autoimmunity. Importantly, in two distinct in vivo models, these TGF-β-dependent adaptive CD4+CD25low T cells can be induced from peripheral CD4+CD25− T lymphocytes by anti-CD3 immunotherapy which correlates with the restoration of self-tolerance.
Keywords: autoimmunity, immune tolerance, foxP3, nonobese diabetic mice, CD25+ T cells
Evidence has been accumulated to show the essential role of CD4+ T cells expressing CD25 and the foxP3 transcription factor in the maintenance of self-tolerance and in the control of various immune responses (1–5). Experimental evidence supports the hypothesis that regulatory T cells (Tregs) are delineated into two subsets (6). “Natural” CD4+CD25+ T cells emerge from the thymus as a distinct lineage (7–9), whereas “adaptive” CD4+CD25+ Tregs are induced at the periphery from CD4+CD25− T cells under particular conditions, i.e., antigenic stimulation and the presence of a cytokine environment (10–14). Most studies focused on brightly stained CD25+ T cells (CD25high) considering that they included the majority of Tregs, whereas CD25low T cells did not suppress and preferentially included activated T lymphocytes (15–17). However, recent studies in humans suggested that a population of Tregs can be defined based on expression of low levels of the IL-7 receptor (18). These Tregs are foxP3+ but express low levels of CD25, raising the question of whether alternative subsets of Tregs exist. Similarly, in normal mice, some authors described a subset of suppressive foxP3+ cells within the CD4+CD25low T cell subset (9).
We have previously reported that Tregs control autoimmunity in the nonobese diabetic (NOD) mouse strain that spontaneously develops autoimmune diabetes (19–21). Suppression in this model, especially after anti-CD3 therapy, depends on active immunoregulation that is TGF-β-dependent (21–23). This finding contrasts with a number of studies suggesting that natural Tregs suppress in a TGF-β-independent manner (3, 24). These results suggested that “classical” natural CD4+CD25high Tregs may not be solely involved in immunoregulation in this setting.
Therefore, in this study, we analyzed the in vitro and in vivo properties of CD4+CD25low T cells in the NOD mouse model. These cells express foxP3 and GITR and exhibit regulatory functions that, at variance with CD4+CD25low T cells recovered from normal mice, are highly TGF-β-dependent. Finally, not only do these Tregs play a critical role in attempts by the NOD mouse to regulate autoreactivity, they also account for the potent activity of anti-CD3 antibodies in the restoration of self-tolerance in vivo (22, 23, 25, 26). These results have important implications because, especially in adult hosts, such adaptive Tregs may be more amenable to manipulation both in vivo, for immunotherapy, and ex vivo, for cell-therapy purposes.
Results
Natural Tregs from the Thymus of NOD Mice Effectively Control Diabetogenic Effectors.
We first examined the capacity of natural Tregs from the thymus of prediabetic NOD mice to control in vivo diabetogenic effectors. CD25 is a good marker in the thymus for natural regulatory CD4+ T cells, allowing reliable purification of this subset (7, 8). CD4+CD8−CD25+ T cells isolated from the thymus of 6-week-old NOD mice were adoptively transferred into NOD–SCID mice to assess their capacity to prevent diabetes induced by splenocytes from diabetic mice. As shown in Fig. 1A, CD4+CD25+ thymocytes were protective and efficiently blocked diabetes. We then examined whether treating the recipients with antibodies blocking immunoregulatory cytokines affected the protection. After injection of antibodies to IL-4, the IL-10 receptor (data not shown), or TGF-β, no reversal of protection was observed (Fig. 1A). At variance, anti-TGF-β antibody abrogated the protection afforded by peripheral (splenic) CD4+CD25+ T cells isolated from prediabetic NODs (Fig. 1B). These latter data fit with results reported by various groups showing that neutralization of TGF-β could diminish the inhibition afforded in vitro by peripheral CD4+CD25+ Tregs (21, 27–31).
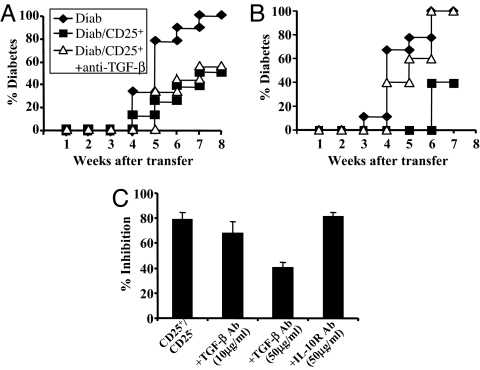
Fig. 1.
Regulatory capacities of thymic CD4+CD25+ T cells. Diabetes was monitored in NOD–SCID recipients injected with diabetogenic cells alone (spleen cells from diabetic NOD mice, 5 × 106, Diab) or with 1 × 106 CD4+CD25+ thymocytes (A) or CD4+CD25+ splenocytes (B) isolated from 6-week-old NOD mice. A significant protection was observed (P < 0.0001) with both regulatory populations. Administration of anti-TGF-β antibody abrogated diabetes protection afforded only by CD4+CD25+ T cells from the spleen but not from the thymus (P < 0.016). (C) Suppression of CD4+CD25− T cell proliferation by CD4+CD25+ thymocytes (n = 10). Antibodies to IL-10 receptor (50 μg/ml) or TGF-β (10 or 50 μg/ml) were added in the culture. Data were expressed as the percent inhibition.
The regulatory properties of the CD4+CD25+ thymocytes were also studied in vitro. Efficient inhibition of the proliferation of CD4+CD25− T cells (80%) was observed, which was not reversed on addition of anti-IL-4 or anti-IL-10 receptor antibodies or by low doses (10 μg/ml) of anti-TGF-β antibody (Fig. 1C). However, a ≈50% reversal of inhibition was observed with higher doses of anti-TGF-β (50 μg/ml) (Fig. 1C). This reversal is at variance with what was observed with peripheral CD4+CD25+ T cells in which in vitro suppressive activity was completely abrogated after addition of high-dose anti-TGF-β and was decreased by 50% at the low dose (10 μg/ml) (21). Finally, CD4+CD25+ thymocytes expressed high levels of CD62L and GITR, but membrane TGF-β was almost undetectable (Table 1).
Table 1.
Phenotype of CD25high and CD25low CD4+ T cells from NOD mice
| GITR | Membrane TGF-β | CD62L | CD103 | |
|---|---|---|---|---|
| CD4+CD25high | 96.3 ± 0.4 | 5.8 ± 0.8 | 52.3 ± 1.7 | 17.8 ± 1.1 |
| CD4+CD25low | 97.6 ± 0.3 | 12.1 ± 1.2 | 31.8 ± 2.7 | 16.2 ± 1.1 |
| Thymic CD25+ | 80.5 ± 0.6 | 2.5 ± 0.4 | 52.2 ± 1.4 | 16.3 ± 1.3 |
Expression of the IL-7 receptor was also measured and was not significantly detected in any cell population tested.
Analysis of foxP3+ Tregs That Control Diabetogenic Effectors in NOD Mice.
Based on our previous data showing the cytokine dependency of T cell-mediated immunoregulation in NOD mice (21–23) and also on the recent published evidence that, in mice and humans, alternative subsets of Tregs that do not belong to the natural CD4+CD25high compartment may exist in the periphery (9, 18), we analyzed in more detail the phenotypic and functional capacities of purified peripheral CD4+CD25low T cells from NOD mice (Fig. 2A).
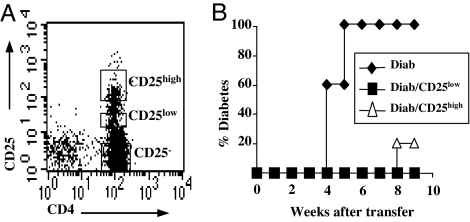
Fig. 2.
In vivo suppressive capacities of CD4+CD25high and CD4+CD25low T cells from NOD mice. (A) Splenic CD4+ T cells from 6-week-old NOD mice were labeled with CD4 and CD25 antibodies and sorted into CD4+CD25high (1.9 ± 0.1%) and CD4+CD25low (2.3 ± 0.1%) T cell populations by FACS. (B) NOD–SCID mice were adoptively transferred with diabetogenic cells (1 × 106, Diab) alone or in combination with CD25high or CD25low T cells (2.5 × 105 per recipient) recovered from 6-week-old NOD mice. Both populations significantly delay diabetes onset (P < 0.002).
In adoptive transfer experiments, CD25low T cells were as efficient as CD25high T cells in protecting NOD–SCID recipients from disease transfer (0% and 20% diabetes in CD25low and CD25high T cell-recipient animals, respectively; versus 100% in controls, 9 weeks after transfer) (Fig. 2B).
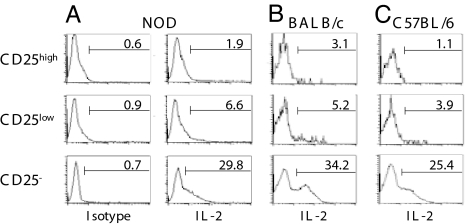
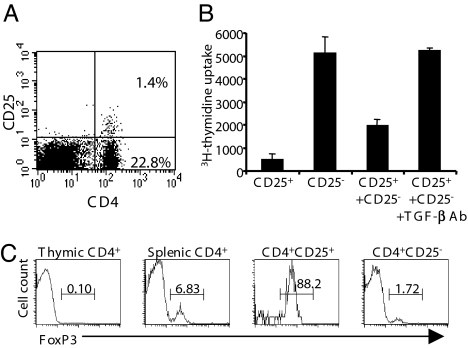
CD25low T cells included a high proportion (≈90%) of foxP3+ cells (Fig. 3A). A small fraction did not express foxP3 and represents activated/effector lymphocytes that are normally included in this compartment, as illustrated by the modest proportion of IL-2-producing cells present within the CD25low T cell subset (Fig. 4A) (9).
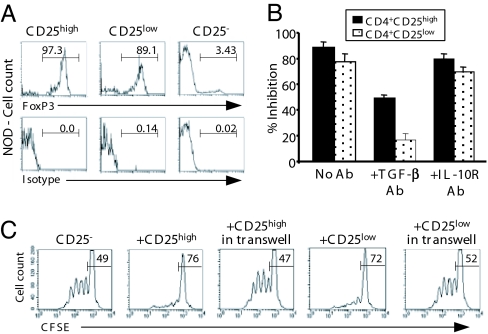
Fig. 3.
In vitro suppressive activity of CD4+CD25high and CD4+CD25low T cells from NOD mice. (A) Expression of foxP3 by FACS-sorted CD4+CD25−, CD4+CD25high, and CD4+CD25low T cells recovered from the spleen of prediabetic NOD mice. The numbers in each histogram represent the percentage of positively stained cells. (B) CD4+CD25− T cells from NOD mice were incubated with CD25high or CD25low T cells and stimulated with antigen-presenting cells (APCs) and anti-CD3 antibody for 72 h with or without antibodies to TGF-β or IL10 receptor (50 μg/ml) (n = 6). Data were expressed as the percent inhibition of proliferation. (C) The proliferation of carboxyfluorescein-diacetate-succinimidyl-ester-labeled CD4+CD25− T cells was analyzed by FACS after being incubated for 4 days with CD25low or CD25high T cells separated or not separated by a transwell membrane.
Fig. 4.
IL-2 production. CD4+CD25high, CD4+CD25low, and CD4+CD25− T cells T cells from 6-week-old NOD (A), BALB/c (B), or C57BL/6 (C) mice were stimulated with PMA/ionomycin for 4 h, fixed, and stained with anti-IL-2 antibodies. The numbers in each histogram represent the percentage of positively stained cells.
In vitro, CD4+CD25low T cells were as efficient as CD4+CD25high Tregs in inhibiting the proliferation of CD4+CD25− T cells in response to anti-CD3 stimulation (86.1 ± 4% versus 75.1 ± 6.1%, respectively; P < 0.006) (Fig. 3B). However, the addition of neutralizing TGF-β antibody preferentially abrogated inhibition by CD4+CD25low Tregs (80%) versus CD4+CD25high Tregs (50%) (Fig. 3B). Addition of an antibody blocking the IL-10 receptor had no effect (Fig. 3B). Importantly, preventing cell–cell contact by using a transwell device completely abrogated the suppressive capacities of both CD25+ subsets (Fig. 3C).
Although most CD25high and CD25low T cells expressed the activation marker GITR (Table 1), the homing receptor, L-selectin (CD62L), was more highly expressed on CD25high than CD25low T cells. No difference was found in the expression of the αE integrin subunit (CD103) or CD127. Importantly, membrane TGF-β was predominantly detected on CD25low T cells; a similar pattern was observed for the latency-associated peptide (data not shown).
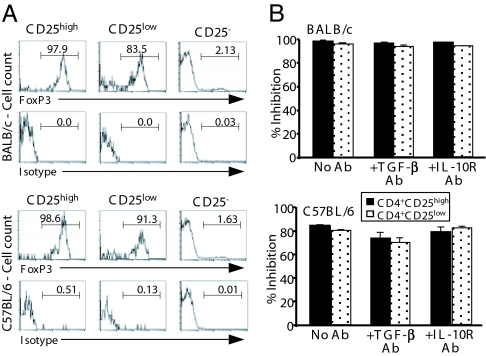
To further examine whether the presence of these TGF-β-dependent foxP3+CD4+CD25low Tregs was unique to the autoimmune situation, we analyzed the identical subset isolated from the spleen of two normal-prone strains, namely BALB/c and C57BL/6 mice. As shown in Fig. 5A, high proportions of foxP3+ cells were observed among the CD25low T cells isolated from both BALB/c and C57BL/6 mice, similar to those found in the equivalent subset in NOD mice (Fig. 3A). Small proportions of the CD25low populations were foxP3− and most probably correspond to activated lymphocytes (Fig. 4 B and C).
Fig. 5.
In vitro suppressive activity of CD4+CD25high and CD4+CD25low T cells from nonautoimmune-prone mice. (A) Expression of foxP3 by FACS-sorted CD4+CD25−, CD4+CD25high, and CD4+CD25low T cells recovered from the spleen of BALB/c or C57BL/6 mice. (B) Suppression of CD4+CD25− T cell proliferation to CD3 antibody by CD4+CD25high or CD4+CD25low T cells issued from BALB/c or C57BL/6 mice (n = 5). Each coculture was performed with or without anti-TGF-β or anti-IL10R antibodies (50 μg/ml). Data were expressed as the percent inhibition.
CD25low T cells from both BALB/c and C57BL/6 mice efficiently inhibited the anti-CD3-induced proliferation of autologous CD4+CD25− lymphocytes (Fig. 5B). Observed inhibition values were comparable to those observed with peripheral CD25high T cells. Interestingly, in contrast to what was observed in NOD mice, the addition of the anti-TGF-β antibody to the cultures did not have any effect in reversing the suppression. Antibodies neutralizing the biological activity of IL-10 (Fig. 5B) or IL-4 (data not shown) did not have any effect either. We also investigated the suppressive capacity of BALB/c CD4+CD25+ thymocytes. As expected, they were highly efficient at inhibiting the anti-CD3-induced proliferation of CD4+CD25− T cells. This suppressive effect was not reversed after neutralization of TGF-β [data from one representative experiment: no antibody, 97% inhibition; addition of anti-TGF-β antibody (2G.7) 10 μg/ml, 93.2% inhibition; addition of anti-TGF-β antibody (2G.7) 50 μg/ml, 90.2% inhibition]. Thus, as a whole, these results indicate that the ability of the peripheral CD4+CD25low Treg subset from normal mice to inhibit T cell activation was clearly not dependent on TGF-β.
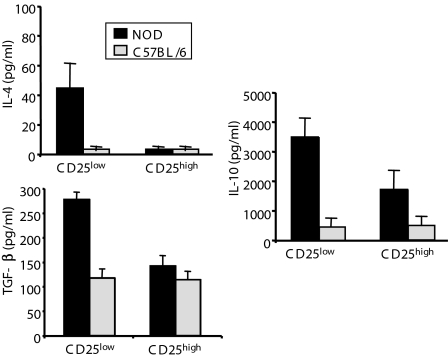
We next analyzed the capacity of CD4+CD25low T cells to produce immunoregulatory cytokines. Major differences were found between autoimmune-prone NOD mice and normal C57BL/6 mice. CD25low T cells from C57BL/6 mice produced low amounts of IL-10 and TGF-β and no IL-4 (Fig. 6). Conversely, CD25low T cells from NOD mice produced high levels of TGF-β, IL-10, and, to a lesser extent, IL-4. In addition, in NOD mice, the ability of the CD25low subset to secrete high levels of TGF-β clearly distinguished them from their counterparts, i.e., the CD25high subset, which, however, secreted quite substantial amounts of IL-10 (Fig. 6). Together, these results suggest that a novel Treg subset, based on low CD25 expression and TGF-β dependency, exists in NOD mice and may be critical to control disease.
Fig. 6.
Immunoregulatory cytokine production pattern by CD4+CD25high and CD4+CD25low T cells. CD4+CD25high and CD4+CD25low T cells recovered from the spleen of NOD or C57BL/6 mice were stimulated with coated anti-CD3 and soluble anti-CD28 antibodies for 48 or 72 h. Supernatants were harvested and the amount of IL-4, IL-10, and TGF-β secreted by each population was determined ELISA.
CD4+CD25low T cells are elicited in the periphery after anti-CD3 treatment.
We previously reported that a short low-dose treatment with anti-CD3 antibody applied to diabetic NOD mice induced permanent disease remission by restoring self-tolerance (25, 26). This effect is tightly dependent on the unique capacity of anti-CD3 to induce TGF-β-dependent CD4+CD25+ Tregs (22). We suggested that these cells did not derive from an expansion of thymic natural CD4+CD25+ Tregs (22). To obtain further insight into the phenotypic and functional characterization of these cells, we used two distinct in vivo models, CD28-deficient NOD mice and T cell-reconstituted NOD–SCID mice.
The NOD CD28−/− mouse model.
CD28/B7 interactions are critical for the homeostasis of CD4+CD25+ Tregs (19). Treatment of normal NOD mice with cytotoxic T lymphocyte antigen-4Ig, which blocks this interaction, leads to a major reduction of CD4+CD25+ T cells in both the thymus (32) and the periphery (19). Spontaneous diabetes is exacerbated in both B7-1/B7-2-double-deficient and CD28-deficient NOD mice: <1.5% of the CD4+ T cells expressed CD25 compared with 5–10% in wild-type controls (19).
Similar to previous observations in diabetic NOD mice, diabetic NOD CD28−/− mice treated with CD3-specific F(ab′)2 fragments (50 μg/d for 5 d) showed long-term remission of disease (22). The relative percentage of CD4+CD25+ T cells was higher in treated NOD CD28−/− mice than in untreated animals. Interestingly, the emerging CD25+ T cells were CD25low (mean fluorescence intensity, 33.1, range, 101–102 compared with untreated NOD mice, mean fluorescence intensity, 156.1, range, 101–103) (compare Fig. 7A with Fig. 2A).
Fig. 7.
Treatment of NOD CD28−/− mice with anti-CD3 antibody restores the number and the suppressive capacities of the CD4+CD25+ T cells. Diabetic NOD CD28−/− mice treated for 5 d with CD3-specific F(ab′)2 fragments enter long-term remission. (A) Expression of CD25 in the CD4+ T cell subset recovered from the spleen of NOD CD28−/− mice 3 weeks after the end of the treatment. (B) Analysis of the suppressive capacities of the CD4+CD25+ T cells from treated NOD CD28−/− mice on the anti-CD3-induced proliferation of CD4+CD25− T cells in the presence or absence of anti-TGF-β antibody. (C) Intracellular staining of foxP3 protein expressed by various T cell subsets isolated from the spleen or the thymus of treated NOD CD28−/− mice.
These CD25low splenocytes from treated NOD CD28−/− mice were suppressive in vitro, and as demonstrated in wild NOD mice, the inhibitory effect was TGF-β-dependent; the addition of anti-TGF-β antibody in the coculture completely reversed suppression (Fig. 7B). Interestingly, these CD25low induced Tregs expressed high levels of foxP3 and were observed in the spleen but not in the thymus of treated mice, suggesting that they were induced in the periphery (Fig. 7C). Importantly, as we had already observed in anti-CD3-treated wild-type NOD mice (22), TGF-β plays a central role in the long-term response observed in NOD CD28−/− mice because in vivo administration of a neutralizing anti-TGF-β antibody completely abrogated the anti-CD3-induced remissions (23).
The CD25− T cell-transferred NOD–SCID model.
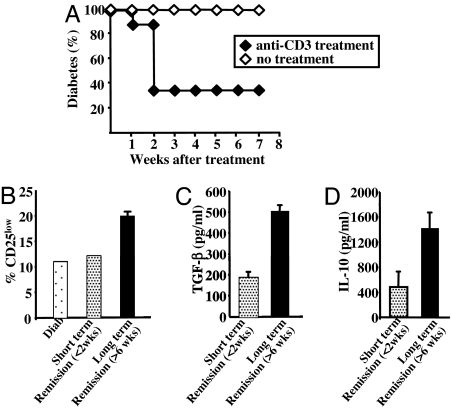
Based on these results, we wanted to confirm the role of the CD4+CD25low T cells in the tolerogenic capacities of the anti-CD3 antibody treatment in the diabetes model. CD4+CD25+ T cell-depleted splenocytes (CD25− cells) were adoptively transferred into NOD–SCID mice. On becoming diabetic, recipient animals were treated with anti-CD3 F(ab′)2 fragments. The vast majority of the mice entered remission within 1–2 weeks after the end of treatment (Fig. 8A). CD4+CD25+ T cells were induced in all recipients. However, the numbers of CD4+CD25+ T cells, as well as the intensity of expression of CD25 on the CD4+ T cells, were significantly different between the mice successfully treated and those not successfully treated (Fig. 8B). In particular, CD25 was detected in 25–30% of the CD4+ T cells recovered from recipients that entered long-lasting remission (6 weeks) as compared with 17% in untreated diabetic CD25−-injected NOD–SCID mice (data not shown). Interestingly, CD4+CD25low T cells represented ≈20% of the total CD4+ lymphocytes in mice that remained normoglycemic for at least 6 weeks compared with 10–12% in untreated diabetic recipients or recipients that entered short-lasting remission (Fig. 8B). Conversely, the proportion of CD4+CD25high T cells was not clearly modified after anti-CD3 treatment and rather represented in vivo-activated T cells.
Fig. 8.
Anti-CD3 antibody treatment preferentially induces CD4+CD25low T cells. (A) NOD–SCID mice were injected with CD25− T cells (2 × 106 per recipient), became diabetic and were treated with CD3-specific F(ab′)2 fragments. Long-term remission was induced in the majority of the mice. A small fraction of mice entered short-lasting remission (2 weeks). (B) Proportion of CD25low T cells within the CD4+ T cell population recovered from recipient NOD–SCID mice treated with anti-CD3 antibodies and that entered long-lasting remission (>6 weeks, black bars) or short-lasting remission (dotted bars). (C and D) TGF-β and IL-10 production by CD4+ T cells were measured in anti-CD3 antibody-treated recipient mice.
As shown in Fig. 8 C and D, CD4+ T cells isolated from mice that remained normoglycemic for >6 weeks produced significantly more IL-10 and TGF-β than cells isolated from mice that were normoglycemic for only 2 weeks. A 2.5- to 3-fold ratio was observed in both IL-10 and TGF-β productions when remission induced by anti-CD3 treatment was long-lasting.
Discussion
Natural Tregs, differentiating as an independent lineage of foxP3+ thymocytes, control self-tolerance in normal mice (1, 8, 33) and also in autoimmune-prone animals such as NOD mice. In fact, NOD mice in which the CD28 encoding gene was disrupted (NOD CD28−/−) lack natural CD4+CD25+ Tregs and exhibit accelerated diabetes (19). Here, we present evidence demonstrating that natural Tregs are not the only regulatory subset in the autoimmune setting. Another subset of Tregs that fulfills the definition of adaptive Tregs also plays an essential role in modulating diabetes. They express foxP3 but appear quite distinct from conventional natural CD25+ Tregs. One major distinctive characteristic is their cytokine dependency; they produce high amounts of TGF-β, express membrane TGF-β, and their regulatory activity is greatly impaired upon blockade of TGF-β but not of IL-4 or IL-10. Moreover, they have down-regulated CD62L and are phenotypically CD25low consistent with a recently activated cell-type distinct from natural Tregs that are CD62LhighCD25high (1, 8).
How can these results be reconciled with published data that suggests cytokine-independent, specifically TGFβ-independent, Treg activity in many disease settings (24, 33)? Much of this controversy emerged from attempts to develop a general, unifying view of CD25+ Tregs for the control of all immune responses that includes the notion that foxP3+ Tregs originate largely, if not exclusively, from natural CD4+CD25high Tregs. However, neither CD25 nor foxP3 appear to be exclusive markers for natural Tregs (8, 18). This implies that not all peripheral CD25+ Tregs described in the various situations are necessarily derived from the expansion of thymus-derived CD25highfoxP3+ Tregs. In fact, there is now compelling evidence to show that adaptive Tregs may be generated from peripheral CD4+CD25−foxP3− cells under well defined conditions (i.e., the type of antigenic stimulation, the nature of the APCs, and the cytokine milieu). In this regard, both IL-10 and TGF-β are two cytokines that preferentially promote adaptive CD25+foxP3+ Tregs (10, 11, 13, 14). In addition, phenotypic tools, including a foxP3-driven GFP mouse and a cell-surface marker, CD127 (IL-7 receptor), show that not all Treg activity is present within the CD25highfoxP3+ subset (18).
Quite interestingly, only CD25low T cells from NOD mice, and not those from normal mice, are TGF-β-dependent. Functional Tregs were detected within the CD25low subset in both C57BL/6 and BALB/c mice. However, their regulatory activity was clearly cytokine-independent. These results reproduce and extend the data by Fontenot et al. (9) in C57BL/6 mice, whereas they are at variance with those reported by Setoguchi et al. (34) in BALB/c mice. Such discrepancy may be caused by differences in the mouse lines used and/or their housing conditions, leading to different proportions of Treg versus effector T cells. In Setoguchi's report, BALB/c CD25low T cells included high proportions of activated T cells with no Tregs detectable (34), whereas in our case exactly the mirror image was found.
Thus, these TGF-β-dependent CD4+CD25low T cells appear to preferentially develop in the autoimmune background, most likely because of the chronic inflammatory environment and the teleological attempt to control the diabetogenic T cell activity.
Compared with peripheral CD4+CD25+ T cells, CD4+CD25+ thymocytes constitute a more homogeneous subset of predominantly CD25high T cells; they express high levels of CD62L and almost undetectable membrane TGF-β. CD25+ thymocytes effectively suppress diabetogenic effectors in vitro and in vivo. Their in vivo regulatory activity appeared TGF-β-independent, whereas in vitro, partial reversal of suppression (50%) was observed after addition of anti-TGF-β antibody but only at high concentrations (a condition in which 100% reversal of suppression is observed with peripheral CD25low T cells). Interestingly, the same type of in vitro response, i.e., partial reversal of suppression at high anti-TGF-β doses was observed for peripheral CD25high T cells. A trivial explanation for these results is that the effect observed in vitro is just an artifact due to the high doses of antibody that were used. One cannot exclude, however, that in the autoimmune setting both in the thymus and the periphery, CD25high T cells include a subset of TGF-β-dependent Tregs not present in normal individuals. The presence of such cells among CD25high thymocytes may be indicative of their thymic origin. Alternatively, they could represent a subset of cells recirculating from the periphery to the thymus.
Further evidence for the central role of this CD25low Tregs was obtained in two in vivo models in which remission of ongoing diabetes was achieved by using anti-CD3 antibody treatment. In the first model, we used NOD CD28−/− mice (19). We previously reported that short-term anti-CD3 antibody treatment induces durable disease remission by promoting T cell-mediated active tolerance implicating TGF-β-dependent Tregs (22, 35). Here, we show that in anti-CD3 antibody-treated NOD CD28−/− mice, these Tregs are exclusively CD25low and are foxP3+. They are TGF-β-dependent both in vitro and in vivo as the administration of a neutralizing antibody to TGF-β totally abrogated the therapeutic effects of anti-CD3 antibody as shown in wild-type NOD mice (22, 23). In the second model, CD25− splenocytes (CD25+ T cell-depleted) were adoptively transferred into NOD–SCID mice to induce diabetes. Recipients receiving the anti-CD3 antibodies at the time of diabetes onset went into sustained disease remission associated with the selective expansion of CD25low T cells and TGF-β-producing CD4+ T cells. Our data bring further support for the central role of TGF-β in T cell-mediated responses controlling autoimmunity, as already highlighted in a number of models (31, 36–40).
In conclusion, we demonstrated that natural suppressor CD4+CD25high foxP3+ T cells are not the only Tregs controlling autoimmunity. Rather, this subset more likely functions prominently to maintain self-tolerance in early life, as exemplified by the polyautoimmune syndrome that follows day-3 thymectomy in normal mice and the immunedysregulation, polyendocrinopathy, enteropathy, X-linked syndrome in humans (1). However, once self-tolerance is broken, T cells other than thymically derived CD25high T cells appear instrumental in regulating pathogenic effectors. This role is well in keeping with recent data by Billiard et al. (41) showing that in NOD mice, depletion of CD25high T cells exacerbates disease only when applied at the very early stages of the disease (3–4 weeks of age) but is without any effect later. Interestingly, depletion essentially targeted ≈80% of CD25high T cells. It is also important to mention that in NOD mice, thymectomy performed at up to 3 weeks of age (and not only up to day 3, as in normal mice) significantly accelerates disease onset (42).
In autoimmune-prone adult animals, adaptive Tregs that may arise from peripheral CD4+CD25− precursors become key players for regulating responses to self-antigens. In the diabetes setting, the CD25low T cells appear to be one of these adaptive Tregs. One may also mention that β-cell-specific (GAD-specific) Th2 and Tr1 cells endowed with regulatory capacities have been detected in unmanipulated NOD mice (43, 44).
It will be important to determine the level of overlapping (and perhaps identity) between CD4+CD25low Tregs, Th3, Tr1, and CD45RBlow cells. A better understanding of the mechanisms that drive adaptive Tregs will pave the way toward novel clinically applicable strategies. In this context, it is particularly encouraging to see that some therapeutic tools such as anti-CD3 antibodies, which proved effective in the clinic (45–47), exert their beneficial effect by up-regulating TGF-β-producing adaptive Tregs. In addition to other candidates that have been examined, future cell therapy approaches should consider adaptive Tregs as potentially interesting alternatives (2, 48, 49).
Materials and Methods
Mice.
BALB/c, C57BL/6, NOD, and NOD–SCID mice were bred in our animal facility under specific pathogen-free conditions. Glycosuria and glycemia were monitored by using colorimetric strips (Boehringer–Mannheim, Mannheim, Germany).
Antibodies and FACS Analysis.
Antibodies to TGF-β (2G.7) were purified and fluoresceinated in our laboratory. CD62L, CD25, CD4, CD8, and CD103 (αE integrin subunit) antibodies were obtained from PharMingen, San Diego, CA. Anti-GITR antibodies were kindly provided by S. Cobbold (Sir William Dunn School of Pathology, Oxford, U.K.). Intracellular staining was performed for foxP3 on freshly isolated cells according to the manufacturer's instructions (eBioscience, San Diego, CA). For intracellular cytokine staining, cells were incubated with PMA/ionomycin and brefeldin A for 4 h and fixed and stained in 0.1% saponin buffer (Sigma, St. Louis, MO) with anti-IL-2 antibodies or isotype controls (PharMingen).
Cell Preparations.
After depletion of B cells by magnetic bead cell sorting (Miltenyi Biotec, Auburn, CA), splenocytes were stained with CD4 and CD25 antibodies. CD4+CD25high, CD4+CD25low, and CD4+CD25− cells were purified on a FACS Vantage cell sorter (Becton Dickinson, San Jose, CA). Thymocyte suspensions were depleted of CD8+ T cells by magnetic bead cell sorting (Miltenyi Biotec) and total CD4+CD25+ T cells were purified by FACS sorting.
Adoptive Cell Transfers and in Vivo Antibody Treatments.
Six-week-old NOD–SCID mice were injected i.v. with either a single cell population or a mixture of two distinct populations. When needed, recipients were treated with antibodies to TGF-β (2G.7: mouse IgG2b for human TGF-β1, provided by C. J. M. Melief, Leiden, The Netherlands) at the dose of 1 mg per mouse i.p. three times a week starting at day 10 after cell transfers, for a duration of 4 weeks. Control recipients received purified mouse IgGs (Jackson ImmunoResearch Laboratories, West Grove, PA). In some experiments, NOD–SCID mice received CD25+ T cell-depleted splenocytes recovered from diabetic NOD mice (2 × 106 per recipient). On becoming diabetic, recipients were treated for 5 d with CD3-specific F(ab′)2 fragments (50 μg/d). Animals enter remission 1–2 weeks after the end of the treatment.
In Vitro Proliferation Assays.
Cells were cultured in complete RPMI medium 1640 supplemented with 10% FCS (Invitrogen, Carlsbad, CA). Total CD4+CD25+, CD4+CD25high, and CD4+CD25low T cells were cultured at a 1:1 ratio with syngeneic CD25− cells (2 × 104 each). Cells were stimulated with anti-CD3 antibody (2.5 μg/ml) in the presence of mitomycin-treated APCs for 72 h. Neutralizing antibodies to TGF-β or IL-10 receptor were added at 10 or 50 μg/ml. Data were expressed as the “% inhibition” deduced as follows: % Inhibition = [1 − (cpm (CD4+CD25− + CD4+CD25+) / cpm CD4+CD25−)] × 100.
Similar assays were performed by using carboxyfluorescein-diacetate-succinimidyl-ester-labeled CD4+CD25− T cells from 6-week-old NOD mice (2 × 105 cells per well) that were stimulated with anti-CD3 and APCs. CD4+CD25low or CD4+CD25high T cells (2 × 105 cells) were added in a transwell (Costar, Cambridge, MA) with APCs. After 4 d, the CD4+CD25− T cell proliferation was analyzed by FACS.
ELISA.
CD25low, CD25high, and CD25− T cells from 6-week-old mice were plated at 2 × 105 per well and stimulated with anti-CD3 (5 μg/ml) and anti-CD28 (2.5 μg/ml) antibodies. Supernatants were recovered after 48 or 72 h of culture. IL-4, IL-10, IFNγ, and TGF-β1 ELISA were performed by using DuoSet kit (R&D Systems, Abingdon, U.K.).
Anti-CD3 Treatment of NOD CD28−/− Mice.
NOD CD28−/− female mice presenting overt diabetes were treated i.v. with 50 μg of CD3-specific F(ab′)2 fragments per d on days 1–5. The animals entered remission 1–3 weeks after the end of the treatment.
Statistical Analysis.
The occurrence of diabetes was plotted by using the Kaplan–Meier method, i.e., a nonparametric cumulative survival plot. The statistical comparison between the curves was performed by using the log-rank (Mantel–Cox) test. In addition, when needed, results were analyzed by using the Student t test.
Acknowledgments
We thank F. Valette and M. Garcia for managing the animal facility and C. Gouarin, S. Barriot, and A. Esling for technical assistance. This work was supported by grants from Institut National de la Santé et de la Recherche Médicale, Association Claude Bernard, the Juvenile Diabetes Research Foundation, and the National Institutes of Health.
Abbreviations
- APCs
antigen-presenting cells
- Tregs
regulatory T cells
- NOD
nonobese diabetic.
Footnotes
The authors declare no conflict of interest.
References
- 1.Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, Shimizu J, Takahashi T, Nomura T. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 2.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 3.Shevach EM, DiPaolo RA, Andersson J, Zhao DM, Stephens GL, Thornton AM. Immunol Rev. 2006;212:60–73. doi: 10.1111/j.0105-2896.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- 4.Cobbold SP, Adams E, Graca L, Daley S, Yates S, Paterson A, Robertson NJ, Nolan KF, Fairchild PJ, Waldmann H. Immunol Rev. 2006;213:239–255. doi: 10.1111/j.1600-065X.2006.00428.x. [DOI] [PubMed] [Google Scholar]
- 5.Umetsu DT, DeKruyff RH. Immunol Rev. 2006;212:238–255. doi: 10.1111/j.0105-2896.2006.00413.x. [DOI] [PubMed] [Google Scholar]
- 6.Bluestone JA, Abbas AK. Nat Rev Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 7.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 8.Kim JM, Rudensky A. Immunol Rev. 2006;212:86–98. doi: 10.1111/j.0105-2896.2006.00426.x. [DOI] [PubMed] [Google Scholar]
- 9.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang S, Alard P, Zhao Y, Parnell S, Clark SL, Kosiewicz MM. J Exp Med. 2005;201:127–137. doi: 10.1084/jem.20041201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curotto de Lafaille MA, Lino AC, Kutchukhidze N, Lafaille JJ. J Immunol. 2004;173:7259–7268. doi: 10.4049/jimmunol.173.12.7259. [DOI] [PubMed] [Google Scholar]
- 13.Karim M, Kingsley CI, Bushell AR, Sawitzki BS, Wood KJ. J Immunol. 2004;172:923–928. doi: 10.4049/jimmunol.172.2.923. [DOI] [PubMed] [Google Scholar]
- 14.Cobbold SP, Castejon R, Adams E, Zelenika D, Graca L, Humm S, Waldmann H. J Immunol. 2004;172:6003–6010. doi: 10.4049/jimmunol.172.10.6003. [DOI] [PubMed] [Google Scholar]
- 15.Baecher-Allan C, Hafler DA. Immunol Rev. 2006;212:203–216. doi: 10.1111/j.0105-2896.2006.00417.x. [DOI] [PubMed] [Google Scholar]
- 16.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. J Exp Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baecher-Allan C, Wolf E, Hafler DA. Clin Immunol. 2005;115:10–18. doi: 10.1016/j.clim.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 18.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, de St Groth BF, et al. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 20.Boitard C, Yasunami R, Dardenne M, Bach JF. J Exp Med. 1989;169:1669–1680. doi: 10.1084/jem.169.5.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.You S, Belghith M, Cobbold S, Alyanakian MA, Gouarin C, Barriot S, Garcia C, Waldmann H, Bach JF, Chatenoud L. Diabetes. 2005;54:1415–1422. doi: 10.2337/diabetes.54.5.1415. [DOI] [PubMed] [Google Scholar]
- 22.Belghith M, Bluestone JA, Barriot S, Megret J, Bach JF, Chatenoud L. Nat Med. 2003;9:1202–1208. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
- 23.You S, Thieblemont N, Alyanakian MA, Bach JF, Chatenoud L. Immunol Rev. 2006;212:185–202. doi: 10.1111/j.0105-2896.2006.00410.x. [DOI] [PubMed] [Google Scholar]
- 24.Piccirillo CA, Letterio JJ, Thornton AM, Mchugh RS, Mamura M, Mizuhara H, Shevach EM. J Exp Med. 2002;196:237–246. doi: 10.1084/jem.20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chatenoud L, Thervet E, Primo J, Bach JF. Proc Natl Acad Sci USA. 1994;91:123–127. doi: 10.1073/pnas.91.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chatenoud L, Primo J, Bach JF. J Immunol. 1997;158:2947–2954. [PubMed] [Google Scholar]
- 27.Marie JC, Letterio JJ, Gavin M, Rudensky AY. J Exp Med. 2005;201:1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura K, Kitani A, Strober W. J Exp Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakamura K, Kitani A, Fuss I, Pedersen A, Harada N, Nawata H, Strober W. J Immunol. 2004;172:834–842. doi: 10.4049/jimmunol.172.2.834. [DOI] [PubMed] [Google Scholar]
- 30.Levings MK, Sangregorio R, Sartirana C, Moschin AL, Battaglia M, Orban PC, Roncarolo MG. J Exp Med. 2002;196:1335–1346. doi: 10.1084/jem.20021139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green EA, Gorelik L, McGregor CM, Tran EH, Flavell RA. Proc Natl Acad Sci USA. 2003;100:10878–10883. doi: 10.1073/pnas.1834400100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chatenoud L, Salomon B, Bluestone JA. Immunol Rev. 2001;182:149–163. doi: 10.1034/j.1600-065x.2001.1820112.x. [DOI] [PubMed] [Google Scholar]
- 33.Sakaguchi S. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 34.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. J Exp Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chatenoud L. Nat Rev Immunol. 2003;3:123–132. doi: 10.1038/nri1000. [DOI] [PubMed] [Google Scholar]
- 36.Gorelik L, Flavell RA. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 37.Seddon B, Mason D. J Exp Med. 1999;189:279–288. doi: 10.1084/jem.189.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bridoux F, Badou A, Saoudi A, Bernard I, Druet E, Pasquier R, Druet P, Pelletier L. J Exp Med. 1997;185:1769–1775. doi: 10.1084/jem.185.10.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khoury SJ, Hancock WW, Weiner HL. J Exp Med. 1992;176:1355–1364. doi: 10.1084/jem.176.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inobe JI, Chen Y, Weiner HL. Ann NY Acad Sci. 1996;778:390–392. doi: 10.1111/j.1749-6632.1996.tb21153.x. [DOI] [PubMed] [Google Scholar]
- 41.Billiard F, Litvinova E, Saadoun D, Djelti F, Klatzmann D, Cohen JL, Marodon G, Salomon BL. J Immunol. 2006;177:2167–2174. doi: 10.4049/jimmunol.177.4.2167. [DOI] [PubMed] [Google Scholar]
- 42.Dardenne M, Lepault F, Bendelac A, Bach JF. Eur J Immunol. 1989;19:889–895. doi: 10.1002/eji.1830190516. [DOI] [PubMed] [Google Scholar]
- 43.Tisch R, Wang B, Atkinson MA, Serreze DV, Friedline R. J Immunol. 2001;166:6925–6936. doi: 10.4049/jimmunol.166.11.6925. [DOI] [PubMed] [Google Scholar]
- 44.You S, Chen C, Lee WH, Brusko T, Atkinson M, Liu CP. J Immunol. 2004;173:6777–6785. doi: 10.4049/jimmunol.173.11.6777. [DOI] [PubMed] [Google Scholar]
- 45.Herold KC, Hagopian W, Auger JA, Poumian Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, et al. N Engl J Med. 2002;346:1692–1698. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- 46.Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, Rother K, Diamond B, Harlan DM, Bluestone JA. Diabetes. 2005;54:1763–1769. doi: 10.2337/diabetes.54.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, et al. N Engl J Med. 2005;352:2598–2608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 48.Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, Masteller EL, McDevitt H, Bonyhadi M, Bluestone JA. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bluestone JA, Tang Q. Proc Natl Acad Sci USA. 2004;101(Suppl 2):14622–14626. doi: 10.1073/pnas.0405234101. [DOI] [PMC free article] [PubMed] [Google Scholar]