Abstract
Ca2+-calmodulin-dependent protein kinase II (CaMKII) is a serine/threonine protein kinase critically involved in synaptic plasticity in the brain. It is highly concentrated in the postsynaptic density fraction, exceeding the amount of any other signal transduction molecules. Because kinase signaling can be amplified by catalytic reaction, why CaMKII exists in such a large quantity has been a mystery. Here, we provide biochemical evidence that CaMKII is capable of bundling F-actin through a stoichiometric interaction. Consistent with this evidence, in hippocampal neurons, RNAi-mediated down-regulation of CaMKII leads to a reduction in the volume of dendritic spine head that is mediated by F-actin dynamics. An overexpression of CaMKII slowed down the actin turnover in the spine head. This activity was associated with β subunit of CaMKII in a manner requiring its actin-binding and association domains but not the kinase domain. This finding indicates that CaMKII serves as a central signaling molecule in both functional and structural changes during synaptic plasticity.
Keywords: cytoskeleton, plasticity, synapse
In hippocampal CA1 pyramidal cells, a transient burst of synaptic input potentiates the efficiency of subsequent transmission (1). This phenomenon, long-term potentiation (LTP), has been considered as a cellular counterpart of memory, and its molecular mechanisms have been intensively studied. A strong postsynaptic depolarization caused by high-frequency input induces a Ca2+ influx through postsynaptic NMDA receptors, which then triggers a series of biochemical processes, including an activation of a serine/threonine protein kinase, Ca2+/calmodulin-dependent protein kinase II (CaMKII). The activation of CaMKII is followed by a series of autophosphorylation events, which enables active CaMKII to stay activated until all subunits are dephosphorylated (2). This activated CaMKII phosphorylates AMPA receptor and various other postsynaptic proteins, which triggers a mechanism eventually leading to an enhancement of AMPA receptor-mediated synaptic transmission (2–9).
In addition to the functional change typically measured by electrophysiological recordings, recent works revealed another aspect of synaptic plasticity: the structural changes associated with the functional modifications (10–13). During LTP of hippocampal CA1 pyramidal cells, a dendritic spine, the tiny protrusion where most of excitatory synapses are harbored in this class of neurons, expands within ≈60 s after the LTP-inducing stimulation (12, 13). Antagonists for both NMDA receptor and CaMKII blocked the effect of tetanic stimulation; therefore, the activation of NMDA receptor and CaMKII are both important for structural plasticity (12, 13). This observation encouraged us to explore the role that CaMKII activity plays in the regulation of postsynaptic structure.
CaMKII is found in a large quantity in postsynaptic density fraction much more than any other signal transduction molecules, almost comparable with cytoskeletal components (14, 15). This knowledge led us and others to speculate that CaMKII may serve not only as a signal transduction molecule but also as a structural element at synapses (2, 14). In relation to this speculation, it has been demonstrated that CaMKII binds to F-actin through a specific sequence present in the β subunit (16–20), but this interaction has been considered to anchor CaMKII to the postsynaptic sites. However, considering that CaMKII is an oligomeric protein composed of 12–14 subunits with rotational symmetry (2), it is possible that one oligomer can simultaneously bind to multiple F-actin, thereby acting as an F-actin-bundling protein at postsynaptic sites.
Here, we show evidence that CaMKII is in fact not only a signaling protein, but also a structural protein that bundles F-actin through a specific and stoichiometric interaction mediated by the β subunit, and that such capacity is required for the maintenance of the postsynaptic architecture of the dendritic spine. This work ascribes a specific, previously undescribed role for CaMKII as a cytoskeletal component in the postsynaptic structure.
Results
F-Actin Is Bundled by CaMKII.
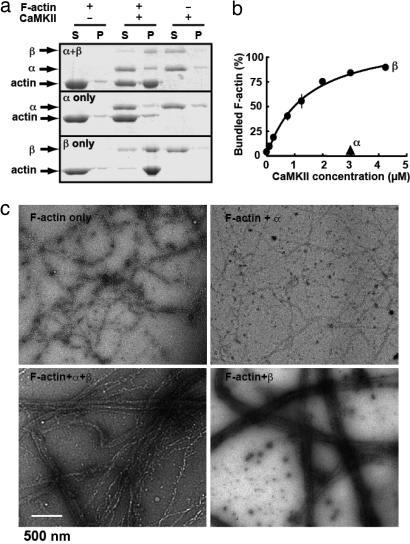
To demonstrate the F-actin-bundling activity of CaMKII, we first used the conventional F-actin cosedimentation assay (21). Purified non-muscle actin was polymerized and coincubated with purified CaMKII (heteromeric complex of α and β). The resultant complex was centrifuged at 10,000 × g, at which linear, unbundled F-actin stays in supernatant but bundled F-actin precipitates (21). Upon separation of both fractions on an SDS/PAGE, F-actin was detected in the supernatant when CaMKII was absent, but in the pellet when CaMKII was present (Fig. 1a). CaMKII without F-actin did not form a pellet, indicating that CaMKII itself did not form complexes that precipitate, and that an interaction between F-actin and CaMKII was indeed needed to form the pellet. This activity was associated with β subunit of CaMKII, but not with the α subunit, a finding consistent with the presence of an F-actin binding sequence in CaMKIIβ but not in α. The concentration response curve of CaMKIIβ at a fixed concentration of actin indicates that the maximum effect was attained at a molar ratio of actin to CaMKIIβ of higher than 1:1 (or 1:≈0.08, as CaMKIIβ holoenzyme; Fig. 1b). This stoichiometry of actin and CaMKII is reasonable in view of the amount of these two proteins present in the postsynaptic density fraction (15).
Fig. 1.
CaMKII bundles F-actin. (a) Cosedimentation of purified CaMKII and F-actin. F-actin formed in vitro was allowed to react with purified CaMKIIα/β, -α, or -β (all at 3 μM as monomer) for 30 min and sedimented at 10,000 × g for 10 min. Under this condition, the linear, unbundled F-actin stays in supernatant but bundled F-actin sediments. Both supernatant (S) and pellet (P) were separated on SDS/PAGE and stained with CBB. (b) Dose-dependent bundling of F-actin by CaMKIIβ. F-actin (3 μM as monomer) was incubated with increasing concentrations of CaMKIIβ (0.1–4.25 μM as monomer) and actin-bundling assays were performed. The amount of bundled F-actin was plotted against the concentration of CaMKIIβ. Mean± SEM. from three independent experiments is shown. (c) Negatively stained electron micrographs of F-actin in the presence or absence of purified CaMKII, all at 3 μM as monomer.
We next attempted to visualize the F-actin bundling by CaMKII. We negatively stained F-actin filaments formed in the presence or absence of CaMKII and observed them using electron microscopy (Fig. 1c). In the absence of CaMKII, F-actin did not show bundling. In contrast, in the presence of CaMKII, F-actin formed clear bundles. Again, this activity was associated with CaMKIIβ; homomeric CaMKIIα failed to show such activity. This assay, in conjunction with the cosedimentation assay, establishes that CaMKII is an F-actin-bundling protein, consistent with a recent report (20).
CaMKII Does Not Affect Kinetics of Polymerization and Depolymerization of Actin.
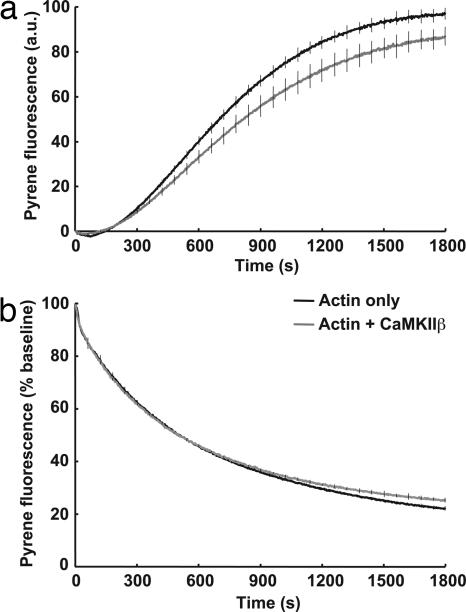
Given the bundling activity of CaMKIIβ, we wondered whether binding of CaMKII affects the kinetics of actin polymerization or depolymerization. To test this hypothesis, we used standard fluorescence measurement of actin polymerization/depolymerization using pyrene conjugated actin (22). The polymerization was initiated by the addition of actin polymerization buffer to G-actin in the presence and absence of CaMKII. A sigmoidal curve showing the lag phase, the log phase and the plateau phase of polymerization was observed. The addition of CaMKII, both unphosphorylated (Fig. 2a) and phosphorylated (data not shown), did not largely change the actin polymerization kinetics. We also tested depolymerization of F-actin to G-actin (Fig. 2b). We induced depolymerization of preformed pyrene-labeled F-actin by diluting 10-fold in the presence or absence of CaMKIIβ. The addition of CaMKII did not change the rate of depolymerization significantly. We used the actin polymerizing drug phalloidin as a control for these experiments (data not shown). These results indicate that despite clear bundling of F-actin, the kinetics of polymerization and depolymerization are largely unaltered by CaMKIIβ.
Fig. 2.
Kinetics of polymerization and depolymerization of actin is largely unchanged by CaMKII. Effect of CaMKII on the kinetics of polymerization (a) and depolymerization (b) was examined by using pyrene-labeled actin. (a) G-actin (2 μM, 5% pyrene-labeled) was polymerized with the addition of actin polymerization buffer in the presence (gray line) or absence (black line) of CaMKIIβ (2 μM as monomer). (b) Actin filament disassembly was induced in the absence (black line) or presence of 2 μM CaMKIIβ (gray line) by dilution of preformed 5% pyrene-labeled actin filaments to a final concentration of 0.2 μM. Error bars indicate SEM of three experiments, shown every 1 min.
CaMKIIβ but Not -α Is Necessary for Maintaining Synaptic Structure.
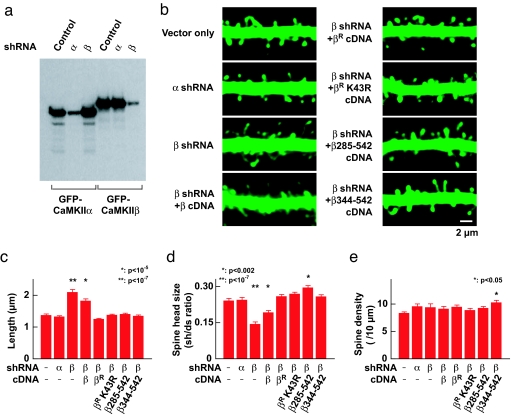
F-actin composes the primary cytoskeleton in the dendritic spine, where it is responsible for structural maintenance and plasticity (12, 23–25). Because CaMKII bundles F-actin in vitro, it is likely that CaMKII also plays a structural role in dendritic spines by bundling F-actin. To test whether endogenous CaMKII in dendritic spines has such a role, we first tested the effects of specific reduction of CaMKIIα and -β proteins by using RNAi and then observing the resulting dendritic spine structure. We constructed plasmid-based short hairpin RNA (shRNA) expression vectors, which specifically down-regulate CaMKIIα or -β respectively (Fig. 3a). They were biolistically transfected in CA1 pyramidal cells in hippocampal organotypic slice culture along with GFP to visualize the structure by using two-photon microscopy. shRNA against CaMKIIα did not have any effect on the number and structure of dendritic spines compared with vector alone (Fig. 3 b–e). In contrast, the shRNA against CaMKIIβ significantly reduced the number of mature dendritic spines and increased the number of long filopodial structures often seen in immature neurons (Fig. 3 b–d). Importantly, the total number of protrusions was unchanged with these manipulations, indicating that the effect of CaMKIIβ shRNA was not to generate new filopodial structures (Fig. 3e). This effect was completely rescued by cotransfecting an expression vector of CaMKIIβ cDNA with silent mutations at the shRNA target sequence (indicated as βR in Fig. 3) but to a lesser extent by using the expression vector carrying the original CaMKIIβ cDNA, confirming the specificity of the effect of shRNA. Thus, we show that CaMKII, specifically the β subunit, is necessary for the maintenance of mature spine structure.
Fig. 3.
Reduction of CaMKIIβ reduces mature dendritic spines and increases filopodial protrusions. (a) The effectiveness and specificity of RNAi. GFP-tagged CaMKIIα and -β were transfected in the Cos7 cells with and without shRNA expression vectors and then blotted with anti-GFP antibody. (b) Two-photon microscopic images of dendritic segment in CA1 pyramidal neurons transfected with control shRNA vector or expression vectors for shRNA against CaMKIIα or -β. To test the specificity of the effect, the expression vectors for CaMKIIβ cDNA with (indicated by βR) or without silent mutations at the shRNA target sequence or deletion mutants were used to rescue the observed phenotype. CaMKIIβR K43R and two deletion mutants lack the kinase activity. See Fig. 4d for schematic drawing of deletion mutants. The neuronal structure was visualized by coexpressing with GFP. (c–e) Plots of the average of the spine length (c), the size of spine head (d), and the density of spines including filopodial protrusions (e). The size of spine head was measured by ratio of fluorescent intensity at the spine head (sh) and the dendritic shaft (ds). Number of observations was as follows (number of cells/number of spines); RNAi vector only, 18/172; CaMKIIα shRNA, 14/261; CaMKIIβ shRNA, 15/163; CaMKIIβ shRNA plus CaMKIIβ cDNA, 24/174; CaMKIIβ shRNA plus CaMKIIβR cDNA, 17/210; CaMKIIβ shRNA plus CaMKIIβR K43R cDNA, 20/253, CaMKIIβ shRNA plus CaMKIIβ 285–542, 20/297; CaMKIIβ shRNA plus CaMKIIβ 344–542, 20/197. Statistical significance compared with vector only is indicated. There was a slight increase in the head volume in cells expressing CaMKIIβ shRNA plus CaMKIIβ 285–542 (d) and in the density in cells expressing CaMKIIβ shRNA plus CaMKIIβ 344–542 (e). The exact mechanism is currently unknown.
Maintenance of Dendritic Spine Structure Is Independent of Kinase Activity or Domain Itself.
We next tested whether kinase activity is necessary for maintaining dendritic spine structure by CaMKIIβ. This hypothesis was tested by rescuing the effect of shRNA using CaMKIIβR K43R, a mutant lacking kinase activity combined with silent mutations that make it resistant to RNAi. This mutant had an actin-bundling activity comparable with wild type CaMKIIβ in a biochemical experiment (see below). If the kinase function is not necessary for spine structure maintenance, this mutant should be sufficient to rescue the spine structure phenotype observed with CaMKIIβ shRNA. As predicted, we found that CaMKIIβR K43R fully rescued the phenotypes observed with shRNA against CaMKIIβ (Fig. 3 b–e).
It is still possible that the kinase domain itself has a structural role independent of the kinase activity. For example, a synaptic scaffolding protein CASK/mLIN-2 has a domain similar to the kinase domain of CaMKII, which serves as an interaction interface with Mint-1/mLIN-10 but has no kinase activity (26). Therefore, it is possible the K43R mutant may still have such function. To rule out this possibility, we tested two deletion mutants CaMKIIβ285–542 and β344–542 lacking kinase domain but still having the F-actin binding and association domains. These deletion mutants also fully rescued the effect of CaMKIIβ shRNA (Fig. 3 b–e). This result indicates that the F-actin binding domain oligomerized together through the associating domain is sufficient for the rescue. These results are most compatible with the idea that the major role of CaMKII in unstimulated synapse is to bundle F-actin and such activity is important for the maintenance of mature dendritic spine structure.
CaMKIIβ but Not -α Affects the Turnover of Actin in Dendritic Spine.
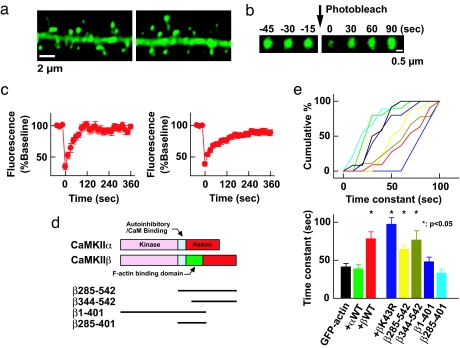
Because we have shown that CaMKIIβ is an F-actin-bundling protein in vitro, we next attempted to observe evidence of the bundling of F-actin by CaMKII in the postsynaptic structure. However, likely because of high density of proteins including actin itself and possible instability during processing, it has been difficult to reveal discrete structure of F-actin in dendritic spine both under light and electron microscopy (12, 24, 25). Also, these techniques do not necessarily give insight into the chemical and functional properties of actin. It has been demonstrated that the actin molecules in dendritic spines undergo constant turnover at a rate regulated by neuronal activity (12, 25). Therefore, we used fluorescent recovery after photobleaching (FRAP) assay to monitor the turnover of actin molecules in dendritic spines. We overexpressed untagged CaMKIIβ along with GFP-actin. We assumed that such an overexpression would result in the formation of an oligomer dominant in the β subunit, having a greater number of F-actin binding sites and therefore more efficient stabilization of F-actin at dendritic spines compared with endogenous CaMKII, which is composed of both α and β subunits. GFP-actin in a single dendritic spine was photobleached and thereafter the recovery of its fluorescence was monitored (Fig. 4). The GFP signal recovered to its original level within 2 min, consistent with a previous report (25). In the presence of overexpressed CaMKIIβ, the turnover was significantly slower, whereas CaMKIIα did not have such an effect (Fig. 4). We could not compare actin turnover in shRNA expressing neurons because the structure of spines were altered dramatically by reduction of CaMKIIβ (Fig. 3).
Fig. 4.
Interaction between actin and CaMKII slows down the turnover of actin in the dendritic spine. (a) Images of dendritic segments expressing GFP-actin with (Right) or without (Left) untagged CaMKIIβ. (b) An example of FRAP assay. Images shown are time-lapse of single dendritic spine head expressing GFP-actin before and after photobleaching. (c) Averaged FRAP of GFP-actin alone (Left) and GFP-actin coexpressed with untagged CaMKIIβ (Right). The first time point after photobleaching was taken as time 0. (d) CaMKIIβ deletion mutants used in FRAP assay. (e) Effect of CaMKIIα, CaMKIIβ, βK43R, and deletion mutants of CaMKIIβ to the time constant of GFP-actin FRAP. The time course of FRAP in individual spine was obtained by fitting with a single exponential curve and plotted. (Upper) Plots of the same data. (Lower) Cumulative plot of the time constant (sec). Bin size is 10 s. The same color code is used in both graphs. The number of spines were as follows: GFP-actin only, 13; CaMKIIα, 11; CaMKIIβ, 12; CaMKIIβ K43R, 9; β1–401, 11; β285–401, 10; β285–542, 11; β344–542, 10. ∗, Statistical significance compared with GFP-actin only (P < 0.05).
The effect of CaMKIIβ on actin turnover did not require kinase activity or the kinase domain itself. CaMKIIβ K43R mutant had the same effect on actin turnover as wild-type CaMKIIβ (Fig. 4 d and e). Also, with deletion mutants lacking the kinase domain but containing actin binding and association domains (CaMKIIβ285–542 and β344–542), we observed the same effect as full length CaMKIIβ (Fig. 4 d and e). Rather it requires oligomerization of actin-binding domain of CaMKIIβ, because a deletion mutant of CaMKIIβ1–401 without the association domain and also β285–401 without the kinase and association domains failed to show the effect on actin turnover (Fig. 4 d and e).
Presently, it is not clear how CaMKIIβ slows down FRAP of GFP-actin. Whereas CaMKII did not have a direct impact on polymerization and depolymerization kinetics of actin (Fig. 2), in dendrites, CaMKII bundling of F-actin may cause inhibition of diffusion or access to actin depolymerizing/severing proteins that affect F-actin dynamics. Given this, however, the most important finding here is that CaMKII affects the actin turnover in a way independent of kinase activity or domain and rather requires an intact actin binding domain associated with each other. This is not expected if CaMKII is purely a signal transduction protein or if the binding with actin is a mechanism to deliver CaMKII to the synapse. Rather, it is most reasonably explained by a structural function of CaMKII to bundle F-actin.
Activation of CaMKII Leads to a Loss of Bundling Activity.
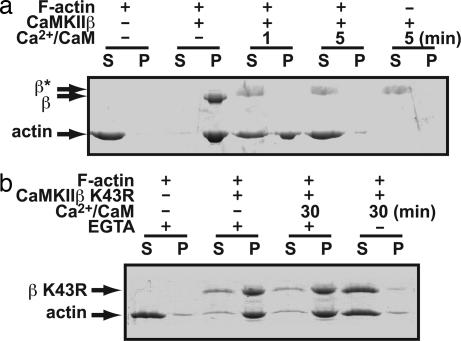
We finally wanted to see whether the kinase activity is involved in the regulation of actin bundling. To test this, we preformed bundled F-actin with CaMKIIβ and subsequently activated the enzyme by addition of Ca2+/calmodulin. To reveal a specific effect of autophosphorylation, EGTA was added at indicated time to remove the Ca2+/calmodulin complex from CaMKII and the coincubation was continued. We observed a significant reversal of bundling of F-actin after CaMKII was activated by Ca2+/calmodulin (Fig. 5a). The reversal of bundling after activation by Ca2+/calmodulin increased with longer exposure of CaMKII to Ca2+/calmodulin. This was not observed with a K43R mutant, confirming the importance of autophosphorylation (Fig. 5b). However, the F-actin-bundling activity of K43R was also blocked by Ca2+/calmodulin if EGTA was not added and Ca2+/calmodulin remained attached to the kinase throughout the bundling and sedimentation steps (Fig. 5b), confirming previous results that calmodulin binding impairs F-actin binding (18, 19). Taken together, both Ca2+/calmodulin binding and the subsequent autophosphorylation work in concert to reverse CaMKII-mediated bundling of F-actin. Consistent with this finding, the actin binding region of CaMKII contains multiple serines/threonines, many of which are actually autophosphorylated rapidly upon activation (M. K. Hayashi, R. Narayanan, and Y. Hayashi, unpublished).
Fig. 5.
Inhibition of F-actin-bundling activity of CaMKIIβ after activation by Ca2+/calmodulin and resultant autophosphorylation. (a) CaMKIIβ was preincubated with polymerized F-actin for 5 min and then stimulated by the addition of Ca2+/calmodulin. At the indicated time points, 1 mM EGTA was added, which was then followed by F-actin bundling and sedimentation steps. β*, Gel-shifted population because of autophosphorylation. These bands were not stained well with CBB likely because of heavy autophosphorylation and resulting incorporation of negative charges. (b) A similar experiment to a using K43R (kinase-null) mutant of CaMKIIβ. EGTA –, A sample in which EGTA was not added and Ca2+/calmodulin persists throughout the reaction. This result reveals the effect of Ca2+/calmodulin binding independent of autophosphorylation.
Discussion
We describe here a role for CaMKII as a structural protein bundling F-actin. This requires the presence of the β subunit in the CaMKII oligomer but not kinase activity itself. This feature of CaMKII is necessary for maintaining the dendritic spine structure. Not only that, when kinase is activated, CaMKII looses the ability to bundle F-actin. Both binding with Ca2+/calmodulin and autophosphorylation of CaMKII contribute to this event. It has been speculated that CaMKII might have a structural role because of its abundance in the postsynaptic density (2, 14); our report demonstrates a specific structural role for CaMKII as an F-actin-bundling protein necessary for maintenance of postsynaptic structure.
Previous reports indicate that the overexpression of CaMKIIβ but not α in neurons in dissociated culture increases filopodia motility, dendritic arborization, and spine density (27, 28). These effects were abolished by blocking kinase activity with a point mutation or a kinase inhibitor. Whereas it is possible that the precise location of kinase activity is important for these effects (27), it is also possible that the bundling of F-actin and its dynamic regulation by a mechanism involving kinase activity may be important.
CaMKII accumulates on the dendritic spine upon neuronal stimulation (19, 20, 29–31). Because CaMKIIα does not bundle F-actin but still shows activity dependent accumulation, the accumulation is independent of F-actin binding (19). Instead, self-association of CaMKII (20, 30) or binding with the NR2B subunit of NMDA receptors (31), densin-180 (32, 33), and/or α-actinin (32–34) may play a role in such activity-dependent synaptic delivery of CaMKII.
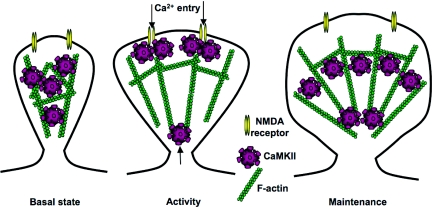
In view of these results, we suggest the following model for the role of CaMKII in structural plasticity (Fig. 6). At resting synapses, F-actin is bundled by CaMKII, thereby maintaining a stable structure. When CaMKII is activated by neuronal activity and the resultant Ca2+ influx, it detaches from F-actin and allows its reorganization by other signal transduction machineries, such as small G proteins. Meanwhile, more CaMKII are recruited to the synapse by self-association or interaction of NR2B to play a role as a signal transduction molecule. Such mechanism may also set a new level for the amount of CaMKII at dendritic spines, which may in turn affect the amount of bundled F-actin in the spines, and consequently the structure of the dendritic spines. Upon returning to the unphosphorylated state, CaMKII bundles the newly reorganized F-actin and maintains the remodeled spine structure. In this way, CaMKII may have a serve as a “gating” mechanism that keeps dendritic spine structure constant at resting Ca2+ levels, allowing modification when the Ca2+ level increases, and subsequently preserving the plastic change. Duration of activated CaMKII gives a time-window during which actin can be remodeled. Consistent with this view, the structural plasticity of dendritic spines requires CaMKII activation (13). In this way, CaMKII plays a dual role in excitatory synaptic plasticity: a signaling role during neuronal activity, and an actin-bundling role during the basal state in dendritic spines, thereby affecting both functional and structural plasticity of dendritic spines
Fig. 6.
A model for the “gating” mechanism of synaptic structure by CaMKII. (Left) At resting synapses, actin is bundled by CaMKII, thereby maintaining a stable structure. (Center) When CaMKII is activated by neuronal activity and the resultant Ca2+ influx, it detaches from F-actin and allows its reorganization by other signal transduction machineries, such as small G proteins. Meanwhile, more CaMKII are recruited to the synapse by self-association or interaction with NR2B and plays a role as a signal transduction molecule. Such mechanism may set a new level for the amount of CaMKII at dendritic spines. (Right) Upon returning to the unphosphorylated state, CaMKII bundles the newly reorganized F-actin and maintains the remodeled spine structure. The new level of CaMKII may affect the amount of bundled F-actin in the spines, and consequently the structure of the dendritic spines.
Methods
Expression Vectors.
Baculoviral expression vectors for rat CaMKIIα and -β have been reported (35, 36). Human β-actin fused with GFP is from Clontech (Mountain View, CA). Untagged rat CaMKIIα and β were expressed by using Clontech's pEGFP-C1 vector by replacing the coding region of EGFP with respective cDNAs. Deletion mutants were constructed by PCR-mediated method and expressed by using pCMV-myc expression vector (Clontech). For CaMKIIα and β RNAi constructs, the following sequences were chosen as target sequence: for CaMKIIα; 5′-CCACTACCTTATCTTCGAT-3′; for CaMKIIβ; 5′-GAGTATGCAGCTAAGATCA-3′. The shRNAs were expressed by using a plasmid-based expression vector, pSuper, and their effectiveness and specificity were confirmed by cotransfecting them with GFP-tagged CaMKII subunits in Cos7 cells (12) and immunoblotting with anti-GFP antibody (Fig. 3a). To rescue the phenotype observed with RNAi experiments, CaMKIIβ cDNA was made resistant to RNAi by incorporating five mismatched silent mutations at the shRNA target region (indicated as CaMKIIβR). The resistance of CaMKIIβR cDNA to RNAi was confirmed by Western blotting.
Actin-Bundling Assay.
Non-muscle actin (Cytoskeleton) was polymerized in F-actin buffer containing 10 mM Tris·HCl, 0.2 mM DTT, 0.2 mM CaCl2, 2 mM MgCl2, 50 mM KCl, and 0.5 mM ATP, pH 7.5. The purified CaMKII [for additional information, see supporting information (SI) Methods], precentrifuged to remove large aggregates, or elution buffer without 2 mM EGTA was added. After incubation at room temperature (homomeric CaMKIIα or -β) or on ice (heteromeric CaMKIIα and β) for 30 min, the mixture was centrifuged at 10,000 × g for 10 min to sediment bundled F-actin but not linear, unbundled F-actin (21). The pellet and supernatant were subjected to SDS/PAGE and the gel was stained with Coomassie Brilliant Blue (CBB). To estimate binding affinity, we added varying concentrations of CaMKII to 3 μM F-actin. The CBB-stained gels were scanned, and the band intensities were measured by using Metamorph (Molecular Devices).
Electron Microscopy.
Actin and CaMKII were allowed to react for 30 min as described in F-actin-bundling assay. The reaction mixture was adsorbed onto freshly glow-discharged, carbon-coated copper grids for 30 s and the excess sample was removed by wicking with filter paper. The bound particles were stained with 2% uranyl acetate for 30 s, and the excess stain was removed with filter paper. Images were recorded on a slow-scan CCD camera with a JEOL electron microscope operating at 200 kV at a magnification of 15,000.
Actin Assembly and Disassembly.
In vitro actin assembly and disassembly were performed as in Sagot et al. (22). Actin polymerization was monitored by the increase in fluorescence of 5% pyrenyl-labeled nonskeletal muscle actin (Cytoskeleton). Nonskeletal muscle actin (50 μM) in G-buffer (10 mM Tris·HCl/0.2 mM CaCl2/0.2 mM DTT/0.2 mM ATP, pH 7.5) was thawed overnight at 4°C, diluted to 22.5 μM in G-buffer and precleared by centrifugation at 200,000 × g for 60 min at 4°C. Actin (2 μM) was mixed with 2 μM CaMKIIβ and the reaction was initiated by transfer to a quartz fluorometer cuvette (3-mm light path) containing 20× initiation mix (40 mM MgCl2/1 M KCl/10 mM ATP). Pyrene fluorescence was recorded at 407 nm with an excitation at 365 nm at room temperature. The amounts of sedimentable F-actin at 100,000 × g were similar in the presence or absence of CaMKIIβ (data not shown). For actin disassembly assays, 2 μM CaMKIIβ was incubated for 5 min in the presence of preassembled actin filaments (2 μM). Baseline was adjusted to zero and the increase in fluorescence was plotted. Disassembly was induced by dilution in F-buffer (G-buffer containing 1× initiation mix) to a final concentration of 0.2 μM. The initial F-actin fluorescence at the beginning of the reaction was used as 100% to plot the disassembly reaction.
CaMKII Activation.
CaMKII was stimulated in a buffer containing 40 mM Hepes, 1 mM Mg-acetate, 0.1 mM EGTA, 0.2 mM Ca2+, 50 μM ATP, and 10 μM (for Fig. 5b) or 1 μM (others) calmodulin (35). The reaction was started by adding calmodulin and stopped by adding 1 mM EGTA at the indicated time after start. Phosphorylation of CaMKII in the presence or absence of F-actin during kinase reaction did not change the sedimentation characteristics of CaMKII.
Neuronal Imaging.
Organotypic slice cultures of hippocampus were prepared from postnatal day 6–8 rats as described (5, 7, 12). They were biolistically transfected at days in vitro (DIV)7 days (for experiment described in Fig. 3) or DIV 4 days (for Fig. 4), and the imaging experiments were carried out from CA1 pyramidal neurons 4 days (for Fig. 3) or 3–5 days after the transfection (for Fig. 4). For RNAi experiment, the expression vectors of GFP and shRNA were transfected at 1:5 ratio; for rescue experiments, the expression vectors for GFP, RNAi, and the original or resistant cDNA of CaMKIIβ were transfected at 1:5:5 ratio; for FRAP experiments, GFP-actin and untagged CaMKII were transfected at a ratio of 1:5. Neurons were imaged with a custom-made two-photon microscope 3–5 days after transfection in a solution containing 119 mM NaCl, 2.5 mM KCl, 4 mM CaCl2, 4 mM MgCl2, 26.2 mM NaHCO3, 1 mM NaH2PO4, and 11 mM glucose, pH 7.4, and gassed with 95% O2 and 5% CO2 (12). GFP was imaged at 860 nm (excitation) and 570 nm short-pass (emission). For analyses, image stacks typically composed of 15–20 sections taken at 0.5-μm intervals were z-projected (summation), median-filtered, and background-subtracted by using Metamorph. For FRAP experiments, photobleaching was achieved by scanning a spine of interest at maximal excitation power (≈1.4 W of Ti-sapphire laser output) 10 times. The FRAP data were analyzed as described (12). For morphological analyses, dendritic spines separated well from dendritic shaft by at least 0.75 μm from the edge of the dendritic shaft were chosen. To measure the size of spine heads, the fluorescence profile across a line drawn on spines, and adjacent dendritic shafts were measured in ×10 zoomed images; the ratio of fluorescent peaks of spine head and dendritic shaft were obtained as an index of spine head size. To normalize the thickness of dendritic shafts, the images were obtained at a consistent distance from the soma. Spine length was defined as the distance from the edge of the dendritic shaft to the tip of the spine. Spine density was obtained from 5 x zoomed images. All analyses were done blind to the DNA construct used. Results are reported as mean± SEM. Statistical significance was determined by using Dunnett's test (37) and defined at P < 0.05.
Supplementary Material
Acknowledgments
We thank Drs. Mariko Hayashi, Avital Rodal, Atsuhiko Ishida, Bernardo Sabatini, J. Troy Littleton, Morgan Sheng, Susumu Tonegawa, Neal Waxham, Paul Matsudaira, and Hitoshi Fujisawa for valuable advice and sharing of resources; Mr. Travis Emery for editing; and Ms. Cortina McCurry for participating in the early part of this study. This work was supported by grants from RIKEN and the Ellison Medical Foundation and by National Institutes of Health Grant RO1 DA017310 (to Y.H.).
Abbreviations
- LTP
long-term potentiation
- CaMKII
Ca2+-calmodulin-dependent protein kinase II
- FRAP
fluorescent recovery after photobleaching
- shRNA
short hairpin RNA
- CBB
Coomassie Brilliant Blue.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0701656104/DC1.
References
- 1.Bliss TV, Collingridge GL. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 2.Lisman J, Schulman H, Cline H. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- 3.McGlade-McCulloh E, Yamamoto H, Tan SE, Brickey DA, Soderling TR. Nature. 1993;362:640–642. doi: 10.1038/362640a0. [DOI] [PubMed] [Google Scholar]
- 4.Benke TA, Lüthi A, Isaac JT, Collingridge GL. Nature. 1998;393:793–797. doi: 10.1038/31709. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- 6.Malinow R, Malenka RC. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- 7.Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- 8.Derkach V, Barria A, Soderling TR. Proc Natl Acad Sci USA. 1999;96:3269–3274. doi: 10.1073/pnas.96.6.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Science. 1997;276:2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- 10.Lamprecht R, LeDoux J. Nat Rev Neurosci. 2004;5:45–54. doi: 10.1038/nrn1301. [DOI] [PubMed] [Google Scholar]
- 11.Yuste R, Bonhoeffer T. Annu Rev Neurosci. 2001;24:1071–1089. doi: 10.1146/annurev.neuro.24.1.1071. [DOI] [PubMed] [Google Scholar]
- 12.Okamoto K, Nagai T, Miyawaki A, Hayashi Y. Nat Neurosci. 2004;7:1104–1112. doi: 10.1038/nn1311. [DOI] [PubMed] [Google Scholar]
- 13.Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erondu NE, Kennedy MB. J Neurosci. 1985;5:3270–3277. doi: 10.1523/JNEUROSCI.05-12-03270.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng J, Kim MJ, Cheng D, Duong DM, Gygi SP, Sheng M. J Biol Chem. 2004;279:21003–21011. doi: 10.1074/jbc.M400103200. [DOI] [PubMed] [Google Scholar]
- 16.Shen K, Teruel MN, Subramanian K, Meyer T. Neuron. 1998;21:593–606. doi: 10.1016/s0896-6273(00)80569-3. [DOI] [PubMed] [Google Scholar]
- 17.Urushihara M, Yamauchi T. Eur J Biochem. 2001;268:4802–4808. doi: 10.1046/j.1432-1327.2001.02410.x. [DOI] [PubMed] [Google Scholar]
- 18.Ohta Y, Nishida E, Sakai H. FEBS Lett. 1986;208:423–426. doi: 10.1016/0014-5793(86)81061-4. [DOI] [PubMed] [Google Scholar]
- 19.Shen K, Meyer T. Science. 1999;284:162–166. doi: 10.1126/science.284.5411.162. [DOI] [PubMed] [Google Scholar]
- 20.O'Leary H, Lasda E, Bayer KU. Mol Biol Cell. 2006;17:4656–4665. doi: 10.1091/mbc.E06-03-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bähler M, Greengard P. Nature. 1987;326:704–707. doi: 10.1038/326704a0. [DOI] [PubMed] [Google Scholar]
- 22.Sagot I, Rodal AA, Moseley J, Goode BL, Pellman D. Nat Cell Biol. 2002;4:626–631. doi: 10.1038/ncb834. [DOI] [PubMed] [Google Scholar]
- 23.Hayashi Y, Majewska AK. Neuron. 2005;46:529–532. doi: 10.1016/j.neuron.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Capani F, Martone ME, Deerinck TJ, Ellisman MH. J Comp Neurol. 2001;435:156–170. doi: 10.1002/cne.1199. [DOI] [PubMed] [Google Scholar]
- 25.Star EN, Kwiatkowski DJ, Murthy VN. Nat Neurosci. 2002;5:239–246. doi: 10.1038/nn811. [DOI] [PubMed] [Google Scholar]
- 26.Butz S, Okamoto M, Südhof TC. Cell. 1998;94:773–782. doi: 10.1016/s0092-8674(00)81736-5. [DOI] [PubMed] [Google Scholar]
- 27.Fink CC, Bayer KU, Myers JW, Ferrell JE, Jr, Schulman H, Meyer T. Neuron. 2003;39:283–297. doi: 10.1016/s0896-6273(03)00428-8. [DOI] [PubMed] [Google Scholar]
- 28.Thiagarajan TC, Piedras-Renteria ES, Tsien RW. Neuron. 2002;36:1103–1114. doi: 10.1016/s0896-6273(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 29.Otmakhov N, Tao-Cheng JH, Carpenter S, Asrican B, Dosemeci A, Reese TS, Lisman J. J Neurosci. 2004;24:9324–9331. doi: 10.1523/JNEUROSCI.2350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hudmon A, Lebel E, Roy H, Sik A, Schulman H, Waxham MN, De Koninck P. J Neurosci. 2005;25:6971–6983. doi: 10.1523/JNEUROSCI.4698-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bayer KU, De Koninck P, Leonard AS, Hell JW, Schulman H. Nature. 2001;411:801–805. doi: 10.1038/35081080. [DOI] [PubMed] [Google Scholar]
- 32.Dhavan R, Greer PL, Morabito MA, Orlando LR, Tsai LH. J Neurosci. 2002;22:7879–7891. doi: 10.1523/JNEUROSCI.22-18-07879.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walikonis RS, Oguni A, Khorosheva EM, Jeng CJ, Asuncion FJ, Kennedy MB. J Neurosci. 2001;21:423–433. doi: 10.1523/JNEUROSCI.21-02-00423.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robison AJ, Bass MA, Jiao Y, MacMillan LB, Carmody LC, Bartlett RK, Colbran RJ. J Biol Chem. 2005;280:35329–35336. doi: 10.1074/jbc.M502191200. [DOI] [PubMed] [Google Scholar]
- 35.Takao K, Okamoto K, Nakagawa T, Neve RL, Nagai T, Miyawaki A, Hashikawa T, Kobayashi S, Hayashi Y. J Neurosci. 2005;25:3107–3112. doi: 10.1523/JNEUROSCI.0085-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waxham MN, Tsai AL, Putkey JA. J Biol Chem. 1998;273:17579–17584. doi: 10.1074/jbc.273.28.17579. [DOI] [PubMed] [Google Scholar]
- 37.Dunnett CW. J Am Stat Assoc. 1955;50:1096–1121. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.