Abstract
Mutations in polycystin-2 (PC2) cause autosomal dominant polycystic kidney disease. A function for PC2 in the heart has not been described. Here, we show that PC2 coimmunoprecipitates with the cardiac ryanodine receptor (RyR2) from mouse heart. Biochemical assays showed that the N terminus of PC2 binds the RyR2, whereas the C terminus only binds to RyR2 in its open state. Lipid bilayer electrophysiological experiments indicated that the C terminus of PC2 functionally inhibited RyR2 channel activity in the presence of calcium (Ca2+). Pkd2−/− cardiomyocytes had a higher frequency of spontaneous Ca2+ oscillations, reduced Ca2+ release from the sarcoplasmic reticulum stores, and reduced Ca2+ content compared with Pkd2+/+ cardiomyocytes. In the presence of caffeine, Pkd2−/− cardiomyocytes exhibited decreased peak fluorescence, a slower rate of rise, and a longer duration of Ca2+ transients compared with Pkd2+/+. These data suggest that PC2 is important for regulation of RyR2 function and that loss of this regulation of RyR2, as occurs when PC2 is mutated, results in altered Ca2+ signaling in the heart.
Keywords: intracellular calcium channel, polycystic kidney disease, lipid bilayer, calcium imaging, cardiac cells
Autosomal dominant polycystic kidney disease (ADPKD) is a genetic disease that affects 1 in 1,000 live births (1). Patients with ADPKD develop bilateral fluid-filled cysts in the kidney and clinical complications such as intracranial aneurysms, hypertension, defective cardiac valve formation, and left ventricular hypertrophy (2–4). ADPKD results in end-stage renal failure in adulthood, and cardiovascular complications are a leading cause of death in patients with ADPKD (3). ADPKD arises as a result of mutations in either of two genes: polycystic kidney disease gene 1 (PKD1) or polycystic kidney disease gene 2 (PKD2) (5). PKD1 encodes gene product polycystin-1 (PC1), which is a 460-kDa plasma membrane protein (6) that is thought to be involved in protein–protein interactions with PC2 (7), E-cadherin (8), α-, β-, and γ-catenin (8), Na+K+-ATPase-α (9), JAK/STAT (10), and STAT-6 (11), among others. The gene product of PKD2, PC2, encodes a 110-kDa protein that has six transmembrane-spanning domains with cytosolic C and N termini (12). The C terminus is involved in protein–protein interactions (7, 13–15), whereas a domain in the N terminus was found recently to be sufficient and necessary for PC2 translocation to the cilia independently of PC1 (16). PC2 shares sequence homology with the transient receptor potential (TRP) channel family (13). Immunolocalization and biochemical studies showed that PC2 resides in the sarcoplasmic reticulum (SR) membrane and the primary cilia (17, 18). Patch clamping of human syncytiotrophoblast or Sf9 insect cells overexpressing PC2 and fusion of PC2 microsomes to planar lipid bilayer membranes have demonstrated that PC2 is a nonselective cation channel regulated by Ca2+ (17, 19).
PC2 interacts with other integral membrane proteins. In the plasma membrane, its C terminus coimmunoprecipitates with TRPC1 (13). Extensive biochemical assays and Ca2+ imaging experiments in HEK-293 cells showed that the C terminus of TRP channels directly associated with the N terminus of the inositol 1,4,5-trisphosphate receptor (InsP3R) type 1 (14). The functional effect of this molecular interaction was the activation TRP channels by the InsP3 signaling cascade and/or depletion of intracellular Ca2+ stores (14). Recently, by using coimmunoprecipitation and patch-clamping techniques, PC2 was shown to interact the InsP3R in Xenopus oocytes overexpressing PC2 (20). The C terminus of PC2 (CPC2) was responsible for modifying the kinetics of Ca2+ transients of the InsP3R in Xenopus oocytes (20). Unlike the InsP3R, the functional relationship between PC2 and the second intracellular Ca2+ channel, the ryanodine receptor (RyR) is unknown. However, the aforementioned evidence that PC2 participates in the regulation of the InsP3R, which shares sequence homology in the fifth and sixth transmembrane domain with the RyR (21), suggests a potential pairing between PC2 and the RyR.
PC2 is expressed in all three muscle types, tissues in which RyRs are highly expressed (22). mRNA for Pkd2 and its homologue, Pkd2L2, were shown to be present in rat left ventricular myocytes, and both polycystins have been hypothesized to underlie the previously characterized large conductance nonselective cation channels present in the heart (23). PC2 is also expressed in skeletal muscle to a lesser extent (22) and in aortic vascular smooth muscle (24), where it was shown to coimmunoprecipitate with PC1 (25). In smooth muscle, PC2 was shown to decrease the activity of store-operated Ca2+ channels (26), and the Ca2+ release from the intracellular stores of heterozygous cells were reduced compared with wild-type smooth muscle cells (25). Interestingly, it was observed that Pkd2−/− mice develop cardiovascular abnormalities (27, 28). Although previous reports suggested that PC2 is present in the heart, its exact function in the heart is still unknown. We investigated the potential molecular and functional interrelationship between PC2 and the cardiac RyR (RyR2) to characterize the function of PC2 in the heart tissue.
Our results show that PC2 associates with the RyR2 coimmunoprecipitation studies, resulting in the functional inhibition of RyR2 by PC2. The N terminus of PC2 (NPC2) was determined to be essential in the binding of PC2 to the RyR2, whereas the CPC2 interacts with the RyR2 only when the RyR2 is in its open state to inhibit RyR2 channel activity. Cardiomyocytes lacking PC2 (Pkd2−/−) exhibited a significantly higher frequency of spontaneous oscillations compared with cells from wild-type (Pkd2+/+) embryos, presumably because of a relief of inhibition of the RyR2 by the lack of PC2. In addition, Pkd2−/− cardiomyocytes had a reduced level of Ca2+ in the SR stores and subsequently a reduced amplitude for the Ca2+ transients compared with cardiomyocytes from Pkd2+/+ embryos. These data demonstrate that Pkd2 is important for the regulation of RyR2 Ca2+ signaling in the heart.
Results
PC2 Coimmunoprecipitates the RyR2.
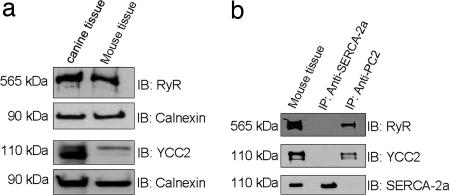
To determine whether there was molecular interaction between RyR2 and PC2 in native heart tissue, we first verified the expression of these proteins. Immunoblotting of heart lysates obtained from SR fractions of cardiac tissue confirmed the presence of RyR2 and PC2 in both canine and mouse heart (Fig. 1a). To determine whether there was an interaction between PC2 and RyR2, a PC2 polyclonal antibody (YCC2) was used to immunoprecipitate PC2 from mouse heart lysate, and then the precipitated sample was probed for RyR2 (Fig. 1b). RyR2 was present in this complex, but another abundant cardiac protein, sarco/endoplasmic reticulum Ca2+-ATPase 2a (SERCA-2a), was not (Fig. 1b). When SERCA-2a monoclonal antibody was used to immunoprecipitate SERCA-2a from mouse heart lysate, neither RyR2 nor PC2 was present in the sample (Fig. 1b). These data show that the RyR2 associates with PC2.
Fig. 1.
Immunoprecipitation of RyR2 by PC2. (a) Expression of RyR2 and PC2 in canine and mouse heart. Lysates from canine and mouse heart were resolved on SDS/PAGE and immunoblotted (IB) with anti-RyR, anti-PC2 (YCC2), or anti-calnexin antibodies. Lysate, in lane 1, is from canine heart. Calnexin was used as the loading control in these experiments. The upper two panels are from the same blot, and the lower two panels are from the same blot. (b) Coimmunoprecipitation of RyR2 by PC2. Lysate isolated from mouse heart was subjected to immunoprecipitation (IP) with YCC2. Samples were blotted with antibodies to RyR2, PC2 (YCC2), and SERCA-2a. In these experiments, SERCA-2a was used as a negative control.
NPC2 Binds to the RyR2.
To determine which region of PC2 is required to precipitate RyR2, GST fusion constructs containing the NPC2 and CPC2 were to used to coprecipitate RyR2 (Fig. 2a). We also tested the ability of L703X, an intact PC2 construct with a C-terminal truncation and a hemagglutinin (HA) tag to bind to the RyR2 (Fig. 2a). Experiments were performed by using the RyR2 SR microsomes in the absence of any agonist to maintain the channel in its closed state. Under these conditions, the NPC2 and L703X associated with the RyR2 (Fig. 2c), whereas the CPC2 failed to bind the receptor (Fig. 2c). These results indicate that the N-terminal region of PC2 is required for interaction with the RyR2. Although CPC2 could not bind the RyR2 in its closed state, it did bind to the RyR2 in the presence of 10 μM ryanodine (Fig. 2d, lane 3). The RyR can be maintained in the open state by the addition of ryanodine in low concentrations (≤10 μM) (29). This finding indicates that CPC2 only interacts with RyR2 when the channel is activated, thereby implying that CPC2 either binds to the receptor within the RyR2 pore region or to regions of the RyR2 that are exposed only when channel is in its open state.
Fig. 2.
In vitro binding experiments. (a) Schematic representation of PC2 constructs used for in vitro binding experiments. (b) NPC2 binds the RyR2. In vitro binding experiments were performed with GST and HA fusion constructs as described in Materials and Methods. Samples were immunoblotted with antibodies to RyR2, GST, and HA. M indicates protein standard. (c) CPC2 binds to RyR2 in its open state. CPC2 was immobilized on glutathione beads and treated as described in Materials and Methods. Samples were immunoblotted (IB) with antibodies to RyR2 and GST. GST alone was used as for negative control. (d) N-terminal amino acid residues of PC2 that bind RyR2. A schematic representation of the N-terminal deletion constructs of PC2-L703X is shown. (e) PC2 N-terminal amino acid residues 130–220 bind the RyR2. Lysates from Madin-Darby canine kidney cells stably expressing mutants were resolved on SDS/PAGE, and the membrane containing proteins were immunoblotted by using anti-RyR and anti-HA antibodies.
To determine which amino acid residues in the NPC2 are responsible for binding to the RyR2, HA-tagged N-terminal deletion mutants recently characterized in the independent targeting of PC2 to the primary cilia were used for in vitro binding experiments (16). The NPC2 consists of ≈224 aa (12). The deletion fragments Δ(5–72) PC2-L703X lacking amino acids 5–72, Δ(72–130) PC2-L703X lacking amino acids 72–130, and Δ(130–220) PC2-L703X lacking amino acids 130–220 were used (Fig. 2b). Cell lysates from Madin-Darby canine kidney cells stably expressing these mutants were incubated with HA beads, and immunoblotting of the protein fragments associated with the beads revealed that the Δ(5–72) PC2-L703X and Δ(72–130) PC2-L703X (Fig. 2e) bound to the RyR2, whereas no binding was observed with Δ(130–220) PC2-L703X (Fig. 2e, lane 4). This finding indicates that amino acids 130–220 are necessary for the association between PC2 and the RyR2.
CPC2 Regulates RyR2 Channel Activity.
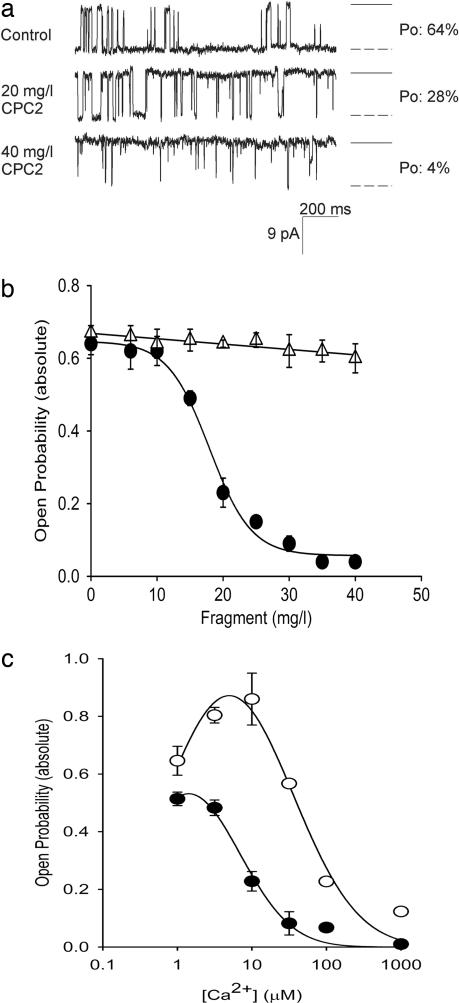
To investigate the functional consequence of the molecular coupling between PC2 and RyR2 in vitro, single-channel recordings of the RyR2 were obtained after incorporating the RyR2 in planar lipid bilayer. Purified N- and C-terminal GST fragments of PC2 were added to the cis or trans side of the lipid bilayer membrane. Representative traces of single-channel recordings of the RyR2 showed that the CPC2 decreased the open probability of the channel (Fig. 3a). The open probability of RyR2 was inhibited by CPC2 in a concentration-dependent manner when added to the cis side (Fig. 3b, circles). In contrast, the NPC2 did not affect the RyR2 open probability when added to the cis side (Fig. 3b, triangles). These results show that PC2 regulates the channel activity of the RyR2. Neither the CPC2 nor NPC2 had an effect when added to the trans side (data not shown). The effect of the CPC2 on the Ca2+ dependence of RyR2 was tested in the presence of increasing Ca2+ concentrations. At low Ca2+ concentrations when the probability of opening of the RyR2 was low, addition of the CPC2 had no measurable effect on RyR2 channel activity (data not shown). However, at higher Ca2+ concentrations, the open probability of the RyR2 was drastically increased, and in this situation the addition of the CPC2 decreased the probability of observing the RyR2 in the open state (Fig. 3d). This Ca2+ dependence of the inhibition of RyR2 by PC2 strongly suggests that CPC2 is more effective when the RyR2 is in the open state.
Fig. 3.
The CPC2 inhibits RyR2 channel activity. (a) Cs+ currents obtained in the absence and presence of increasing concentration of GST-CPC2. Downward deflections represent channel openings. Experiments were performed under symmetrical conditions (250 mM CsCl in both the cis and trans side and 7–10 μM Ca2+ in the cis side). Currents traces were obtained at a holding potential of −25 mV and filtered at 400 Hz. To the right of each trace, the open state is represented as a dashed line, and the solid line represents the closed state. Open probability (Po) of channel at each concentration is shown on the right. (b) The CPC2 decreases the open probability of RyR2. Shown is concentration-dependent inhibition of RyR2 open probability when GST-CPC2 (filled circles; n = 3) and GST-NPC2 (open triangles; n = 3) is added to the cis side. Experiments were performed as described in Materials and Methods. (c) Ca2+-dependent inhibition of RyR2 by CPC2. The CPC2 decreases the open probability of RyR2 in the presence of increasing Ca2+ concentration (n = 3). Experiments were also performed in the absence (open circles) or presence (filled circles) of 17 μg/ml of GST-CPC2 (IC50) in the cis side before Ca2+ additions.
Pkd2−/− Cardiomyocytes Exhibit Altered Ca2+ Signaling.
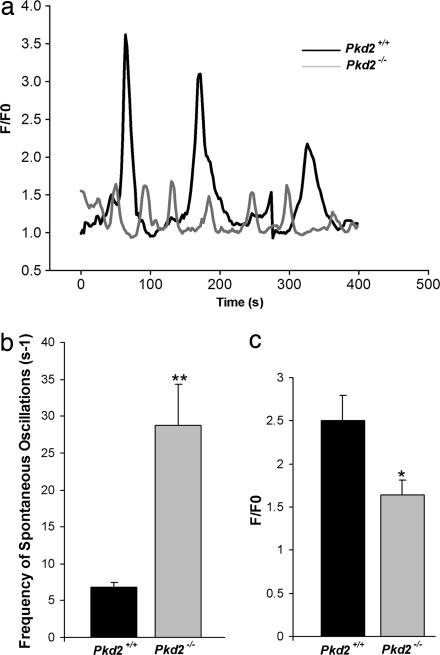
Cardiomyocytes have inherent pacemaker activity and therefore undergo spontaneous Ca2+ oscillations (30). The intracellular Ca2+ channel involved in Ca2+ oscillations in cardiomyocytes is the RyR2 (30). Both Pkd2 and RyR2 are expressed in mouse cardiomyocytes starting at embryonic day 9.5 (28, 30). Although the InsP3R type 2 is present in the cardiomyocytes, its mRNA was shown to be 50 times lower than that of RyR2 in cardiomyocytes (31, 32). To further explore the regulation of RyR2 function by PC2, we examined the frequency of spontaneous Ca2+ oscillations of the Pkd2+/+ and Pkd2−/− cardiomyocytes isolated at embryonic day 17.5. These experiments were performed with Ca2+ present in the extracellular medium to maintain the Ca2+ signaling components of the extracellular space, the cytosol, and the SR stores. A comparison of the spontaneous Ca2+ oscillations in Pkd2+/+ and Pkd2−/− cardiomyocytes show marked differences (Fig. 4a). The cardiomyocytes from Pkd2−/− embryos exhibited a significant increase in the frequency of spontaneous Ca2+ oscillations compared with cardiomyocytes from Pkd2+/+ embryos (29 ± 6 versus 7 ± 1 s−1) (Fig. 4b). In addition, the peak fluorescence of Ca2+ release during each oscillation was significantly smaller in the cardiomyocytes from Pkd2−/− embryos compared with those from Pkd2+/+ embryos (1.6 ± 0.16 versus 2.5 ± 0.96) (Fig. 4c). The decreased amplitude of the Ca2+ transients suggests that cardiomyocytes from Pkd2−/− embryos have a low Ca2+ content in the SR stores.
Fig. 4.
PC2 alters frequency and peak fluorescence of spontaneous oscillations in mouse cardiomyocytes. (a) Spontaneous oscillations of Pkd2+/+ and Pkd2−/− mouse cardiomyocytes. Representative traces of Ca2+ oscillations were monitored in the presence of Ca2+. The peak fluorescence for each genotype was measured, and each oscillation was normalized internally. The graph depicts the changes in fluorescence (F/F0) over time. (b) The frequency of spontaneous Ca2+ oscillations was determined by using a spectral analysis program in MATHLAB software (48). Statistical significance was calculated by using a one-tailed t test: ∗∗, P < 0.001 (n = 6 for Pkd2+/+; n = 12 for Pkd2−/−). (c) The change in peak fluorescence (F/F0) of each genotype was measured as described in Materials and Methods. Statistical significance was calculated by using a one-tailed t test: ∗, P < 0.05 (n = 28 for Pkd2+/+; n = 16 for Pkd2−/−).
PC2 Is Required for Proper Maintenance of Ca2+ Concentration in the SR Stores.
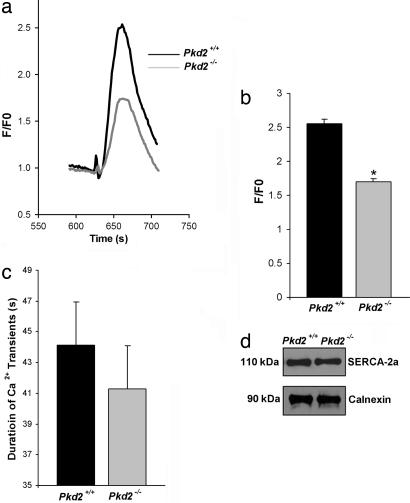
To determine the resting level of Ca2+ in the SR stores, we measured the Ca2+ released from the SR stores of cardiomyocytes from Pkd2+/+ and Pkd2−/− embryos after the addition of thapsigargin. Thapsigargin irreversibly inhibits the SERCA pump, thereby depleting the SR stores of their Ca2+ content (33). These experiments were performed in the presence of 1 mM EGTA to buffer any contaminating Ca2+ in the extracellular solution. The Ca2+ transients in cardiomyocytes elicited by thapsigargin show that the peak fluorescence in cardiomyocytes from Pkd2−/− embryos was decreased compared with that of cardiomyocytes from Pkd2+/+ mice (1.7 ± 0.1 versus 2.6 ± 0.1) (Fig. 5 a and b). This result supports the suggestion that the Ca2+ content present in the SR stores in the cardiomyocytes from Pkd2−/− embryos is reduced compared with the Pkd2+/+ embryos and is consistent with the conclusions from the comparison of the spontaneous oscillations (Fig. 4). Additional consistent findings are that Pkd2+/− aortic vascular smooth muscle cells have a decreased SR Ca2+ concentration when monitored directly using fura-2 (34). To ensure that our result was the result of a functional interaction between RyR2 and PC2 rather than an altered level of expression of SERCA-2a levels in the Pkd2−/− cardiomyocytes, we monitored the amount of SERCA-2a by using Western blot analysis. Cardiomyocytes from Pkd2+/+ and Pkd2−/− embryos have similar levels of SERCA-2a (Fig. 5d).
Fig. 5.
PC2 required for maintenance the Ca2+ content in the SR stores. (a) Representative traces of Ca2+ transients in Pkd2+/+ and Pkd2−/− cardiomyocytes. Ca2+ transients were induced by application of 5 μM thapsigargin to the EGTA-buffered Ca2+-free extracellular solution. (b) Ca2+ content in the SR store. F/F0 was determined as described in Materials and Methods. Pkd2−/− mouse cardiomyocytes have a decreased Ca2+ content in the SR store. Statistical significance was calculated by using a one-tailed t test: ∗∗, P < 0.001 (n = 40 for Pkd2+/+; n = 40 for Pkd2−/−). (c) Duration of Ca2+ transient in Pkd2+/+ and Pkd2−/− cardiomyocytes. There was no difference in the duration of Ca2+ transients (n = 17 for Pkd2+/+ and n = 23 for Pkd2−/− cardiomyocytes). (d) SERCA-2a has similar expression levels in Pkd2+/+ and Pkd2−/− mouse cardiomyocytes. Tissue lysates were prepared from mouse heart at embryonic day 17.5 as described in Materials and Methods.
PC2 Is Required for Normal Ca2+ Signaling Kinetics of RyR2.
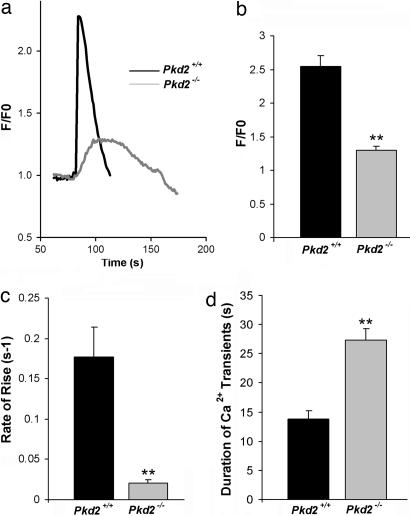
To determine whether PC2 affects the Ca2+ transients generated by activation of the RyR2, the RyR agonist caffeine was used to stimulate the receptor in the cardiomyocytes from Pkd2+/+ and Pkd2−/− embryos. These experiments were performed by using a Ca2+-free extracellular solution containing 1 mM EGTA. Representative responses in both Pkd2+/+ and Pkd2−/− cardiomyocytes show that, in the presence of 20 mM caffeine, the peak fluorescence is 50% smaller in the cardiomyocytes from Pkd2−/− mice as compared with those from Pkd2+/+ embryos (1.3 ± 0.1 versus 2.5 ± 0.2) (Fig. 6a and b). In addition, the rate of rise of the transient was slower (180 ± 30 versus 20 ± 4 ms−1) in the Pkd2−/− cardiomyocytes (Fig. 6c). These results are expected because of the lower Ca2+ content of the SR. The duration of the Ca2+ transient at ≈50% maximum amplitude was greatly prolonged (27 ± 2 versus 14 ± 2 s) (Fig. 6d) for the cardiomyocytes from Pkd2−/− embryos as compared with the Pkd2+/+ embryos. These results show that PC2 plays an important role in shaping the characteristic features of normal RyR Ca2+ signaling.
Fig. 6.
PC2 modulates the kinetics of Ca2+ transient from RyR2. (a) Representative traces of Ca2+ transient in Pkd2+/+ and Pkd2−/− cardiomyocytes. Cardiomyocytes were loaded with fluo-4, and Ca2+ transients were then elicited by 20 mM caffeine in the absence of extracellular Ca2+. (b) Comparison of the Ca2+ transient. Shown are F/F0 in Pkd2+/+ (n = 32) and Pkd2−/− (n = 40) cardiomyocytes. Statistical significance was calculated by using a one-tailed t test: ∗∗, P < 0.001. (c) Rate of rise of Ca2+ transient in Pkd2+/+ and Pkd2−/− cardiomyocytes. Pkd2−/− cardiomyocytes have significantly decreased peak in fluorescence compared with Pkd2+/+ cardiomyocytes: ∗∗, P = 0.001 (one-tailed t test). (d) Duration of Ca2+ transient in Pkd2+/+ and Pkd2−/− cardiomyocytes. Pkd2−/− cardiomyocytes had a significantly longer duration of Ca2+ transients compared with Pkd2+/+. Statistical significance was calculated by using a one-tailed t test: ∗∗, P < 0.001 (number of experiments for each genotype: 35).
Discussion
RyR2-dependent Ca2+ signaling has been shown to be important for regulating various cellular processes such as muscle contraction (35) and is regulated by different cytosolic and luminal proteins (36, 37). We show in this study that RyR2-dependent Ca2+ signaling is modulated by PC2, another ion channel. Our characterization of PC2–RyR2 interaction revealed several important aspects. First, PC2 binds to the RyR2 and interacts with the receptor through its N-terminal domains (Figs. 1 and 2). We determined that both of these cytoplasmic domains of PC2 have designated functions in the interaction with RyR2. The NPC2 has no effect on RyR2 channel activity, suggesting that the NPC2 binds to regions of the RyR2 that do not undergo conformational changes associated with channel gating. The first 15 aa of PC2 that are responsible for PC2 cilia targeting (16) are in one of the N-terminal regions that do not appear to bind the RyR2. In addition, the CPC2 only binds to RyR2 in its open state.
Second, PC2 blocked the single-channel currents of RyR2 through its C-terminal domain (Fig. 3), and this effect is enhanced when the Ca2+ concentration is increased to concentrations that open the RyR2 channel. This result implies that the C terminus targets regions of RyR2 that are made accessible after channel activation and that the functional consequence of PC2–RyR2 interaction depends on the conformation of the receptor. However, it is possible that the CPC2 increases the inhibitory effect of Ca2+ on the RyR2. We also found that the PC2 fragments had no effect when added to the trans side of the RyR2, suggesting that PC2 interacts with the RyR2 from the cytoplasmic side rather than from the lumen of the SR. It seems plausible, therefore, that PC2 is required for the inhibition of Ca2+ release through the RyR2 only when the channel is in the open state.
Third, the spontaneous Ca2+ oscillations of cardiomyocytes from Pkd2−/− mice exhibited an altered frequency and peak fluorescence compared with the cardiomyocytes from the Pkd2+/+ mice (Fig. 4), which strongly suggests that PC2 is important for maintaining the kinetics of spontaneous Ca2+ oscillations in developing cardiomyocytes. The nature of the altered oscillations in Pkd2−/− cardiomyocytes can be attributed to at least two factors: the absence of PC2 and the lowered Ca2+ content in the SR stores. The absence of PC2 leads to an increase in the frequency of spontaneous Ca2+ oscillations in cardiomyocytes, which is caused by the relief of inhibition of RyR2. The Ca2+ content in the SR has a vital role in shaping the normal pattern of Ca2+ release during Ca2+ signaling. Abnormal SR Ca2+ content has been shown to have devastating cellular consequences and implications in many cell systems (38, 39). In Pkd2+/− aortic vascular smooth muscle cells, the decreased amount of Ca2+ in the SR resulted in an increase in cellular proliferation and apoptosis and was hypothesized to be the basis for the cardiovascular abnormalities encountered in patients with ADPKD (34).
Consistent with results observed in the vascular smooth muscle, we found that the Ca2+ content in the SR of the Pkd2−/− cardiomyocytes was significantly reduced compared with Pkd2+/+ (Fig. 5), which accounts for the decreased Ca2+ release during Ca2+ oscillations (see Fig. 4). These results are consistent with the suggestion that PC2 is important for maintaining the normal Ca2+ content in the SR (26). The reduced SR Ca2+ content is likely a consequence of the relief of inhibition of the RyR2, a mechanism that would allow an increased Ca2+ leak from the SR stores, thereby reducing the total Ca2+ present in the SR. These results also have implications in muscle contractility; Drosophila PC2 has been hypothesized to participate in vascular smooth muscle contractility through intracellular Ca2+ homeostasis with the help of RyR (40).
Fourth, the absence of PC2 led to altered kinetics of Ca2+ transients from the RyR2 (Fig. 6). The Ca2+ transients elicited by caffeine, a RyR agonist, in Pkd2−/− cardiomyocytes had a significantly slower rate of rise and a longer duration than those measured in Pkd2+/+ cardiomyocytes (Fig. 6). It is likely that the RyR2 remains open longer because the amount of Ca2+ released is reduced, leading to less Ca2+-induced inhibition of this receptor. In addition, it is likely that the alteration in the existing network between PC2 and other members of the Ca2+ signaling pathway such as TRPC1 and InsP3R leads to a delayed cytosolic Ca2+ clearance (26). On the basis of the measured differences in the duration of the caffeine-induced Ca2+ transient, it is clear that the rate at which Ca2+ is pumped back into the SR by SERCA and the rate of Ca2+ removal from cytosol to extracellular space by plasma membrane channels are slower in the cardiomyocytes from Pkd2−/− embryos as compared with those from Pkd2+/+ cardiomyocytes.
This study begins to address the effect of PC2 on RyR2 function. In summary, we show a protein–protein interaction between two intracellular Ca2+ channels and provide support for the suggestion that there is a functional component for this interaction as well. The fact that loss of PC2 altered RyR2 function in vivo suggests that RyR2 is also important in the PC2 signaling pathway in the heart. An understanding of the possible downstream consequences for this functional interaction will lead to further understanding of PC2 and the cardiovascular phenotypes presented by patients with ADPKD. We hypothesize that the modulation of the kinetic properties of RyR2 by PC2 leads to a decreased amount of Ca2+ release from the SR stores. This decrease in SR Ca2+ load of and altered RyR2 function could, in turn, activate a series of molecular events resulting in the development of cardiovascular abnormalities observed in patients with ADPKD.
Materials and Methods
Antibodies.
Rabbit anti-PC2 polyclonal antibody (YCC2) generated against the CPC2 (41) was generated by S.S. Other antibodies used are from commercial sources: monoclonal anti-RyR (clone MA3–916; Affinity BioReagents, Neshanic Station, NJ), mouse anti-SERCA-2a (clone MA3–919; Affinity BioReagents), polyclonal anti-calnexin (SPA-860; Stressgen Biotechnologies, Victoria, Canada), polyclonal anti-GST antibody (Amersham Biosciences), and monoclonal anti-HA (clone SG77; Zymed, Carlsbad, CA).
Mouse Heart Tissue Isolation, Immunoblotting, and Coimmunoprecipitation.
Mouse hearts were isolated from adult mice as described (42). For immunoblotting experiments, mouse heart lysates were solubilized with 1% CHAPS for 1 h under constant agitation. Lysates were collected and centrifuged at 7,000 × g for 15 min at 4°C. Twenty micrograms of lysate was resolved on a 4–20% SDS/PAGE. Membrane was incubated with primary antibodies generated for RyR2 at 1:1,000, YCC2 at 1:10,000, or calnexin at 1:2,000. Samples were detected with chemiluminescence detection (ECL) reagents (Amersham Pharmacia Biotech). For all immunoblotting experiments, the protein concentration was measured by using the Bradford Assay (Pierce, Rockford, IL). Immunoprecipitation and immunoblotting experiments were performed by using mouse heart tissue lysates; 200 μg of tissue lysate was incubated with either YCC2 (1:1,000) or 4 μg of anti-SERCA-2a antibody overnight at 4°C under constant agitation. Coimmunoprecipitation was performed as described (43). The membrane was then incubated with primary antibodies: anti-SERCA-2a (1:1,000), anti-RyR2 (1:1,000), and YCC2 (1:10,000).
In Vitro Binding Experiments.
A GST fusion protein that encodes either the entire CPC2 or amino acids 103–203 of the NPC2 was used in the binding experiments. GST-CPC2 and GST-NPC2 DNA constructs were transformed into BL21-Gold cells (Stratagene, La Jolla, CA) according to manufacturer protocol. These constructs were purified by using standard glutathione agarose affinity procedures as described (9). Madin–Darby canine kidney cells stably expressing HA-tagged proteins [Δ(5–72) PC2-L703X, Δ(72–130) PC2-L703X, and Δ(130–220) PC2-L703X] were lysed on ice in lysis buffer (150 mM NaCl/50 mM Tris/2 mM DTT/1% CHAPS/1 μl/ml protease inhibitor). The in vitro binding assays using SR microsomes (44) that were solubilized with 1% CHAPS and these constructs were performed as described (16). The PVDF membrane was incubated with either of the following antibodies: anti-RyR (1:1,000), anti-HA (1:1,000), and anti-GST (1:1,000). Representative results calculated from at least three independent experiments are shown.
Single-Channel Recordings.
SR vesicles were isolated from canine heart and incorporated into planar lipid bilayer as described (29, 42). Single-channel activity of RyR2 was measured in the presence or absence of GST-CPC2 or GST-NPC2. Constructs were added to either the cis or trans side. In the experiments to monitor the Ca2+ dependence, channels were recorded with a holding potential of 0 mV by using 50 mM Ba2+ dissolved in 250 mM Hepes, pH 7.35, in the trans side as a charge carrier. The cis side contained 110 mM Tris dissolved in 250 mM Hepes, pH 7.35. The Ca2+ concentration was calculated and presented as −log[Ca2+] (45). Channel data were acquired as described (42). Open probability was determined by using current recordings from at least 2 s of continuously recorded data. All representative current traces shown in the figures were filtered at 500 Hz and are at least 500 ms long.
Isolation of Mouse Cardiomyocytes and Ca2+ Imaging Experiments.
Pkd2−/− mice were generated from mating Pkd2+/− mice. Cardiomyocytes were isolated from Pkd2+/+ and Pkd2−/− embryos at embryonic day 17.5 and cultured by using a method as described with a few modifications (46). Ca2+ imaging experiments were performed by using fluo-4-loaded cells, which then were observed with a Zeiss LSM510 confocal microscope (47). Drugs applied to the bath solution were thapsigargin (5 μM; Calbiochem-Novabiochem, San Diego) and caffeine (20 mM; Sigma). From the Ca2+ transients, we were able to determine the rate of rise and the duration of Ca2+ transients (47).
Statistical Analysis.
Statistical analysis was performed by using a one-tailed Student's t test. In all figures, error bars represent the SEM.
Acknowledgments
We thank Drs. Yiqiang Cai and Lin Geng for providing the constructs used in the in vitro binding experiments. This work is supported by National Institutes of Health Fellowship DK062635 (to G.I.A.) and Yale Center for the Study of Polycystic Kidney Disease Grant P50 DK57328 (to S.S. and B.E.E.).
Abbreviations
- PC
polycystin
- ADPKD
autosomal dominant polycystic kidney disease
- TRP
transient receptor potential
- SR
sarcoplasmic reticulum
- InsP3
inositol 1,4,5-trisphosphate
- InsP3R
InsP3 receptor
- CPC2
C terminus of PC2
- RyR
ryanodine receptor
- RyR2
cardiac ryanodine receptor
- NPC2
N terminus of PC2
- SERCA-2a
sarco/endoplasmic reticulum Ca2+-ATPase 2a.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Gabow PA, Grantham JJ. In: Diseases of the Kidney. Schrier RW, Gottschalk CW, editors. Boston: Little, Brown; 1997. pp. 535–569. [Google Scholar]
- 2.Belz MM, Fick-Brosnahan GM, Hughes RL, Rubinstein D, Chapman AB, Johnson AM, McFann KK, Kaehny WD, Gabow PA. Kidney Int. 2003;63:1824–1830. doi: 10.1046/j.1523-1755.2003.00918.x. [DOI] [PubMed] [Google Scholar]
- 3.Chapman AB, Johnson AM, Rainguet S, Hossack K, Gabow P, Schrier RW. J Am Soc Nephrol. 1997;8:1292–1297. doi: 10.1681/ASN.V881292. [DOI] [PubMed] [Google Scholar]
- 4.Igarashi P, Somlo S. J Am Soc Nephrol. 2002;13:2384–2398. doi: 10.1097/01.asn.0000028643.17901.42. [DOI] [PubMed] [Google Scholar]
- 5.Calvet JP. J Nephrol. 1998;11:24–34. [PubMed] [Google Scholar]
- 6.Hughes J, Ward CJ, Peral B, Aspinwall R, Clark K, San Millan JL, Gamble V, Harris PC. Nat Genet. 1995;10:151–160. doi: 10.1038/ng0695-151. [DOI] [PubMed] [Google Scholar]
- 7.Tsiokas L, Kim E, Arnould T, Sukhatme VP, Walz G. Proc Natl Acad Sci USA. 1997;94:6965–6970. doi: 10.1073/pnas.94.13.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Adelsberg J. Nephrol Dial Transplant. 2000;15:1–2. doi: 10.1093/ndt/15.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Zatti A, Chauvet V, Rajendran V, Kimura T, Pagel P, Caplan MJ. Mol Biol Cell. 2005;16:5087–5093. doi: 10.1091/mbc.E05-03-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhunia AK, Piontek K, Boletta A, Liu L, Qian F, Xu PN, Germino FJ, Germino GG. Cell. 2002;109:157–168. doi: 10.1016/s0092-8674(02)00716-x. [DOI] [PubMed] [Google Scholar]
- 11.Low SH, Vasanth S, Larson CH, Mukherjee S, Sharma N, Kinter MT, Kane ME, Obara T, Weimbs T. Dev Cell. 2006;10:57–69. doi: 10.1016/j.devcel.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, Reynolds DM, Cai Y, Gabow PA, Pierides A, et al. Science. 1996;272:1339–1342. doi: 10.1126/science.272.5266.1339. [DOI] [PubMed] [Google Scholar]
- 13.Tsiokas L, Arnould T, Zhu C, Kim E, Walz G, Sukhatme VP. Proc Natl Acad Sci USA. 1999;96:3934–3939. doi: 10.1073/pnas.96.7.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boulay G, Brown DM, Qin N, Jiang M, Dietrich A, Zhu MX, Chen Z, Birnbaumer M, Mikoshiba K, Birnbaumer L. Proc Natl Acad Sci USA. 1999;96:14955–14960. doi: 10.1073/pnas.96.26.14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallagher AR, Cedzich A, Gretz N, Somlo S, Witzgall R. Proc Natl Acad Sci USA. 2000;97:4017–4022. doi: 10.1073/pnas.97.8.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geng L, Okuhara D, Yu Z, Tian X, Cai Y, Shibazaki S, Somlo S. J Cell Sci. 2006;119:1383–1395. doi: 10.1242/jcs.02818. [DOI] [PubMed] [Google Scholar]
- 17.Koulen P, Cai Y, Geng L, Maeda Y, Nishimura S, Witzgall R, Ehrlich BE, Somlo S. Nat Cell Biol. 2002;4:191–197. doi: 10.1038/ncb754. [DOI] [PubMed] [Google Scholar]
- 18.Yoder BK, Hou X, Guay-Woodford LM. J Am Soc Nephrol. 2002;13:2508–2516. doi: 10.1097/01.asn.0000029587.47950.25. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Perrett S, Kim K, Ibarra C, Damiano AE, Zotta E, Batelli M, Harris PC, Reisin IL, Arnaout MA, Cantiello HF. Proc Natl Acad Sci USA. 2001;98:1182–1187. doi: 10.1073/pnas.98.3.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Wright JM, Qian F, Germino GG, Guggino WB. J Biol Chem. 2005;280:41298–41306. doi: 10.1074/jbc.M510082200. [DOI] [PubMed] [Google Scholar]
- 21.Furuichi T, Yoshikawa S, Miyawaki A, Wada K, Maeda N, Mikoshiba K. Nature. 1989;342:32–38. doi: 10.1038/342032a0. [DOI] [PubMed] [Google Scholar]
- 22.Markowitz GS, Cai Y, Li L, Wu G, Ward LC, Somlo S, D'Agati VD. Am J Physiol. 1999;277:F17–F25. doi: 10.1152/ajprenal.1999.277.1.F17. [DOI] [PubMed] [Google Scholar]
- 23.Volk T, Schwoerer AP, Thiessen S, Schultz JH, Ehmke H. Cardiovasc Res. 2003;58:76–88. doi: 10.1016/s0008-6363(02)00858-1. [DOI] [PubMed] [Google Scholar]
- 24.Torres VE, Cai Y, Chen X, Wu GQ, Geng L, Cleghorn KA, Johnson CM, Somlo S. J Am Soc Nephrol. 2001;12:1–9. doi: 10.1681/ASN.V1211. [DOI] [PubMed] [Google Scholar]
- 25.Qian F, Germino FJ, Cai Y, Zhang X, Somlo S, Germino GG. Nat Genet. 1997;16:179–183. doi: 10.1038/ng0697-179. [DOI] [PubMed] [Google Scholar]
- 26.Qian Q, Hunter LW, Li M, Marin-Padilla M, Prakash YS, Somlo S, Harris PC, Torres VE, Sieck GC. Hum Mol Genet. 2003;12:1875–1880. doi: 10.1093/hmg/ddg190. [DOI] [PubMed] [Google Scholar]
- 27.Wu G, Somlo S. Mol Genet Metab. 2000;69:1–15. doi: 10.1006/mgme.1999.2943. [DOI] [PubMed] [Google Scholar]
- 28.Wu G, Markowitz GS, Li L, D'Agati VD, Factor SM, Geng L, Tibara S, Tuchman J, Cai Y, Park JH, et al. Nat Genet. 2000;24:75–78. doi: 10.1038/71724. [DOI] [PubMed] [Google Scholar]
- 29.Buck ED, Lachnit WG, Pessah IN. J Pharmacol Exp Ther. 1999;289:477–485. [PubMed] [Google Scholar]
- 30.Takeshima H, Komazaki S, Hirose K, Nishi M, Noda T, Iino M. EMBO J. 1998;17:3309–3316. doi: 10.1093/emboj/17.12.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moschella MC, Marks AR. J Cell Biol. 1993;120:1137–1146. doi: 10.1083/jcb.120.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perez PJ, Ramos-Franco J, Fill M, Mignery GA. J Biol Chem. 1997;272:23961–23969. doi: 10.1074/jbc.272.38.23961. [DOI] [PubMed] [Google Scholar]
- 33.Berridge MJ, Bootman MD, Roderick HL. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 34.Kip SN, Hunter LW, Ren Q, Harris PC, Somlo S, Torres VE, Sieck GC, Qian Q. Circ Res. 2005;96:873–880. doi: 10.1161/01.RES.0000163278.68142.8a. [DOI] [PubMed] [Google Scholar]
- 35.Marks AR. Am J Physiol. 1997;272:H597–H605. doi: 10.1152/ajpheart.1997.272.2.H597. [DOI] [PubMed] [Google Scholar]
- 36.Marx SO, Reiken S, Hisamatsu Y, Gaburjakova M, Gaburjakova J, Yang YM, Rosemblit N, Marks AR. J Cell Biol. 2001;153:699–708. doi: 10.1083/jcb.153.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meissner G. Annu Rev Physiol. 1994;56:485–508. doi: 10.1146/annurev.ph.56.030194.002413. [DOI] [PubMed] [Google Scholar]
- 38.Marks AR. J Clin Invest. 2003;111:597–600. doi: 10.1172/JCI18067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Missiaen L, Robberecht W, van den Bosch L, Callewaert G, Parys JB, Wuytack F, Raeymaekers L, Nilius B, Eggermont J, De Smedt H. Cell Calcium. 2000;28:1–21. doi: 10.1054/ceca.2000.0131. [DOI] [PubMed] [Google Scholar]
- 40.Gao Z, Joseph E, Ruden DM, Lu X. J Biol Chem. 2004;279:14225–14231. doi: 10.1074/jbc.M312223200. [DOI] [PubMed] [Google Scholar]
- 41.Cai Y, Maeda Y, Cedzich A, Torres VE, Wu G, Hayashi T, Mochizuki T, Park JH, Witzgall R, Somlo S. J Biol Chem. 1999;274:28557–28565. doi: 10.1074/jbc.274.40.28557. [DOI] [PubMed] [Google Scholar]
- 42.Anyatonwu GI, Buck ED, Ehrlich BE. J Biol Chem. 2003;278:45528–45538. doi: 10.1074/jbc.M307863200. [DOI] [PubMed] [Google Scholar]
- 43.Schlecker C, Boehmerle W, Jeromin A, DeGray B, Varshney A, Sharma Y, Szigeti-Buck K, Ehrlich BE. J Clin Invest. 2006;116:1668–1674. doi: 10.1172/JCI22466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anyatonwu GI, Ehrlich BE. J Biol Chem. 2005;280:29488–29493. doi: 10.1074/jbc.M504359200. [DOI] [PubMed] [Google Scholar]
- 45.Bezprozvanny I, Watras J, Ehrlich BE. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- 46.Ibarra C, Estrada M, Carrasco L, Chiong M, Liberona JL, Cardenas C, Diaz-Araya G, Jaimovich E, Lavandero S. J Biol Chem. 2004;279:7554–7565. doi: 10.1074/jbc.M311604200. [DOI] [PubMed] [Google Scholar]
- 47.Johenning FW, Zochowski M, Conway SJ, Holmes AB, Koulen P, Ehrlich BE. J Neurosci. 2002;22:5344–5353. doi: 10.1523/JNEUROSCI.22-13-05344.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uhlen P. Sci STKE. 2004;2004:pl15. doi: 10.1126/stke.2582004pl15. [DOI] [PubMed] [Google Scholar]