Abstract
Natural killer (NK) cells are components of the innate immune system that recognize and kill tumor or virus-infected target cells through specific NK activating receptor/ligand interactions. Lymphocyte function-associated antigen (LFA)-1 and its ligand ICAM-1 are also required to initiate conjugation and actin cytoskeletal remodeling. The NK activating receptors, many of which are expressed on a single NK cell, signal the polarization of the microtubule organizing center (MTOC) together with cytolytic granules to the synapse with target cells. After ligation of any one of these receptors, Src family kinases initiate activation of two signal pathways, the phosphoinositide-3 kinase → ERK2 and the phospholipase Cγ → JNK1 pathways. Both are required for polarization of the MTOC and cytolytic granules, a prerequisite for killing the targets. Crosslinking of CD28, NKG2D, NKp30, NKp46, NKG2C/CD94, or 2B4 leads to the phosphorylation of both ERK2 and JNK1, although they use different proximal signaling modules. Thus, many, if not all, activating receptors stimulate these two distal pathways, independent of the proximal signaling module used. By contrast, CD2, DNAM-1, and β1-integrin crosslinking do not activate either pathway; they may be costimulatory molecules or have another function in the synapse.
Keywords: costimulatory molecules, conjugation, cytoskeletal remodeling, immune synapse
Natural killer (NK) cells are a primary innate defense against virus infections and tumors (1). Many human NK cell surface receptors that activate killing have been identified. Although some of their ligands have been discovered, many, particularly viral ligands, remain to be found. The NK cell C-type lectin-like receptors (NKG family receptors) include NKG2C/CD94 and NKG2D that stimulate activating signals for cytotoxicity by binding to HLA-E and MICA/B or ULBP1/2/3/4 respectively on target cells (2). The NK cell cytotoxicity receptors (NCR family) are composed of NKp30, NKp44 and NKp46 (3). All of these activate NK cell cytotoxicity, although their ligands are not completely known. CD16 (FcγRIII) is an unusual NK cell activating receptor that mediates antibody-dependent cellular cytotoxicity through recognizing a pathogenic antigen in complex with its specific antibody. Many additional NK surface receptors exist, e.g., CD28 that binds CD80 (B7.1) and CD86 (B7.2) (4); 2B4 (CD244) that interacts with CD48 (5); CD2 [lymphocyte function-associated antigen (LFA)-2] that recognizes both CD48 and CD58 (LFA-3); LFA-1 (αLβ2 integrin, CD11a/CD18) that binds ICAM-1 (CD54), ICAM-2 (CD102), and ICAM-3 (CD50); DNAM-1 that interacts with poliovirus receptor (PVR); and β1 integrins that recognize both fibronectin and laminin 1. These NK receptors trigger signal transduction when cross-linked by the corresponding ligands on target cells and either elicit cytotoxicity directly or modulate NK cell cytotoxic activity (costimulatory molecules) (3, 6, 7). It is unclear to which group some of these receptors belong.
Different NK receptors employ distinct signaling modules to stimulate the signaling networks. For instance, the YxxM motif within the cytoplasmic tail of DAP10 that associates with NKG2D, as well as the same motif in the cytoplasmic tail of CD28, is phosphorylated at the tyrosine residue to initiate positive signals (8). The DAP12 and CD3ζ modules, both of which contain the classic immunoreceptor tyrosine-based activation motif, associate with NKG2C/CD94 and NCR respectively (9). 2B4 can mediate either activating or inhibitory signals through the phosphorylation of its immunoreceptor tyrosine-based switch motif within its cytoplasmic domain (10). CD28, NKG2D, and NKp46 dimerize (11, 12). All accessory signaling adaptors (DAP10, DAP12, CD3ζ, and FcγRIII) are disulfide linked dimers, and in the case of NKG2D at least, each receptor homodimer associates with two signaling homodimers (11). No report of dimerization of 2B4 or its ligand CD48 has appeared.
Despite variation of the proximal signaling modules, tyrosine phosphorylation in the various receptor motifs mediated by Src family kinases (SFKs) has been suggested to play a common role in initiating the more distal activating signals, i.e., Lck and Fyn with NCR (13), Lck with NKG2D (14), and Fyn with 2B4 (10). Then, signaling pathways that lead from tyrosine phosphorylation to two MAPK family members, ERK2 and JNK1, play pivotal roles (15, 16). Phosphorylation of each of these at the TxY motifs was found in the human NK cell lines YTS, NKL, and NK92, as well as in peripheral human NK cells (refs. 4 and 17; B.G., C. L. Li, H. Kopcow, X.C., J.L.S., unpublished data). Both the phosphoinositide-3 kinase (PI3K)-mediated ERK2 phosphorylation pathway and the phospholipase Cγ-dependent JNK1 phosphorylation pathway have been extensively studied (4, 7, 17, 18). Suppressing ERK2 or JNK1 phosphorylation with specific chemical inhibitors or using small RNA interference confirmed the requirement of both MAPKs for NK cell cytotoxicity. Moreover, both were shown to be essential for the polarization of the microtubule organizing center (MTOC) along with cytolytic granules to the NK immunological synapse (NKIS) in pathways initiated by the receptors CD28 and NKG2D. However, whether activation of all NK surface receptors elicits cytotoxic signals by using both ERK2 and JNK1 phosphorylation to trigger MTOC and granule polarization remains unknown. This question is addressed in the present article.
Results
Cytotoxicity of NK Cell Lines.
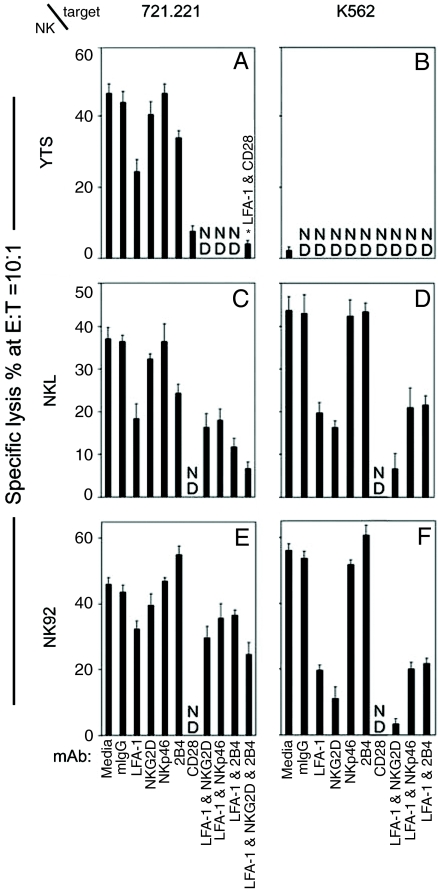
Different NK cell lines use distinct activating receptors to mediate cytotoxicity. In this study, three NK tumor cell lines have been used, NK92, NKL, and YTS, together with two target cell lines that activate these cells, 721.221 (an MHC class 1-negative mutant B lymphoblastoid cell line) and K562 (an erythroleukemia cell line that also is MHC class I-negative). Initially, the expression of four activating receptors, CD28, NKG2D, NKp46, and 2B4, on these lines was assessed. The activating receptors expressed by YTS include CD28 and a much lower level of 2B4. Both NK92 and NKL expressed NKG2D, NKp46, and 2B4 (data not shown). All of the NK lines also expressed LFA-1, which is required for conjugate formation and actin polymerization. Monoclonal antibody blocking experiments were carried out to assess the importance of each of these “adhesion” and activating receptors in cytotoxicity against the two target cells (Fig. 1). Both target cells expressed high levels of ICAM-1 and -2, the ligands for LFA-1. The mAb for LFA-1 substantially blocked killing in four of the six systems used (Fig. 1). However, the killing of 721.221 cells by NK92 was only slightly affected and YTS cells did not kill K562 (4).
Fig. 1.
Distinct pairs of NK receptors are required for killing different target cells by the NK cell lines. YTS, NKL, and NK92 tumor cell lines were used to kill 721.221 and K562 targets in a 51Cr-release cytotoxicity assay. Specific mAb (10 μg/ml) were incubated with the NK cells before mixing with the targets. The means and SDs of the percent lysis at effector:target ratio (E:T) = 10:1 are shown after three or four independent experiments. ND, not determined; ∗, the mAb used in this experiment is specified separately.
Killing by YTS Cells.
With regard to the activating receptors, as expected, YTS cell killing of 721.221 cells (Fig. 1A) was only blocked by anti-CD28 mAb. YTS cells do not kill K562 (Fig. 1B), because neither of the CD28 ligands (CD80, CD86) is expressed on K562.
Killing by NKL Cells.
None of the activating receptor mAb blocked NKL cell killing of 721.221 cells substantially by itself, although some blocking was observed with anti-2B4 mAb (Fig. 1C). LFA-1 mAb also only partially blocked killing. However, the combination of anti-LFA-1, 2B4, and NKG2D mAb was very effective (Fig. 1C). NKL cell killing of K562 cells was dominated by NKG2D and was particularly inhibited by the combination of anti-NKG2D plus anti-LFA-1 mAb (Fig. 1D). Anti-2B4 and anti-NKp46 mAb had little effect.
Killing by NK92 Cells.
None of the activating receptor mAb had any significant effect on NK92 cell killing of 721.221 cells (Fig. 1E). A modest inhibition was observed with anti-LFA-1 mAb that was not increased by anti-NKG2D, 2B4, and NKp46 mAb. The activating receptor that is functional in this system remains to be identified. On the other hand, the killing of K562 by NK92 cells was effectively blocked by anti-NKG2D mAb and by anti-LFA-1 mAb (Fig. 1F). The combination of these two mAb essentially eliminated killing. Again, however, anti-2B4 mAb and anti-NKp46 mAb had little effect. In this system, the killing is also dominated by NKG2D. Clearly, the combination of receptor/ligand pairs effective for killing is different for each of the NK cell lines and also for different target cell lines.
Differential Effects of Anti-LFA 1 mAb and Anti-Activating Receptor mAb on Cytoskeletal Remodeling, MTOC, and Granule Polarization.
Two systems were used to study this phenomenon, using confocal microscopy: YTS as effector cell with 721.221 as its target, and NK92 as effector cell with K562 as its target. Both require LFA-1 for conjugation and cytoskeletal remodeling. However, in the YTS/721.221 pair, killing was dominated by CD28 and its interaction with CD80 on the target (Fig. 2 A and B), whereas in the case of the NK92/K562 pair, killing was dominated by the interaction of NKG2D with MICA/B or ULBP4 as its ligand on K562 cells (Fig. 2 C and D). In both cases, the conjugate formation was blocked by anti-LFA-1 mAb but not by anti-CD28 mAb nor anti-NKG2D mAb, whereas MTOC and granule polarization were blocked by anti-CD28 mAb or anti-NKG2D mAb. In these cases, the umbrella of microtubules with the MTOC and granules at its center remained unpolarized.
Fig. 2.
NK activating receptors are required for the polarization of the MTOC and cytolytic granules, whereas LFA-1 mediates conjugate formation. (A) YTS cells were conjugated with 721.221 cells in the presence of control mIgG or anti-CD28 mAb. The conjugates were fixed and permeabilized for intracellular staining of F-actin (green), microtubules (MT, red), and granules (blue) simultaneously. (B) The percentage of conjugates showing F-actin polymerization in the NKIS and MTOC and granule polarization to the NKIS (means and SDs). At least 50 conjugates images were examined in each case. The FACS-based conjugation assay was used with YTS-GFP cells and 721.221-RFP cells preincubated with control mIgG, anti-LFA-1 mAb, or anti-CD28 mAb. Results from six independent conjugation assays were averaged. (C) Similar imaging experiments were done with NK92 cells conjugated with K562 targets in the presence of either control mIgG, anti-LFA-1 mAb, or anti-NKG2D mAb. (D) The results of observing >50 images are shown with SDs. ∗, Percent F-actin polymerization and MTOC and granule polarization in the presence of anti-LFA-1 mAb are the values obtained in examining the small number of conjugates formed under these conditions.
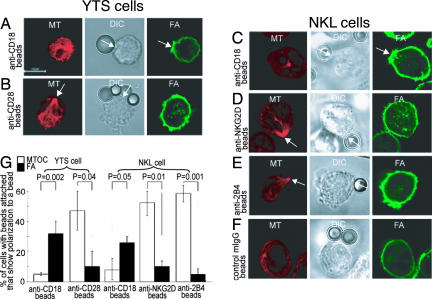
Independent Actin Polymerization and MTOC Polarization Induced by mAb-Coated Beads.
Next, NK cells were coated with biotinylated anti-CD18 mAb, anti-CD28 mAb, or anti-NKG2D mAb (see Materials and Methods). Then streptavidin-coated polysytrene beads were each mixed with YTS cells (anti-CD18 or anti-CD28 mAb-coated) or NKL cells (anti-NKG2D mAb-coated) to cross-link the receptors (Fig. 3). After 30 min of incubation at 37°C, cells were fixed and permeabilized, then stained with Alexa488-phalloidin to visualize F-actin or with Cy3-anti-β-tubulin mAb to visualize microtubules. Confocal microscopy revealed that the anti-CD18 mAb-coated beads polarized F-actin to a site of bead contact while leaving microtubules at various places in the cells unpolarized (Fig. 3 A and C). By contrast, when anti-CD28 mAb-coated beads were used with YTS cells (Fig. 3B) or anti-NKG2D mAb-coated beads were used with NKL cells (Fig. 3D), F-actin remained unpolarized, whereas the MTOC polarized to the site of the mAb-coated beads. When more than one bead was attached to the cell, then only one of the beads served as the focal point of polarization. Control mIgG-coated beads failed to polarize either the MTOC or actin filaments (Fig. 3F). Quantitation and statistical analysis is shown in Fig. 3G.
Fig. 3.
LFA-1 cross-linking initiates F-actin polymerization, whereas CD28, NKG2D, or 2B4 cross-linking polarize the MTOC. (A–F) YTS cells (A and B) and NKL cells (C–F) were coated with biotinylated anti-CD18 (the β2 subunit of LFA-1) mAb (A and C), anti-CD28 mAb (B), anti-NKG2D mAb (D), anti-2B4 mAb (E), or anti-class I ME1 mAb as control (F) and then mixed with streptavidin-coated polystyrene beads. After 0.5–2 h at 37°C, the cells were fixed and permeabilized for the intracellular staining of F-actin (green) and MT (red). (D) The percentage of conjugates showing F-actin polymerization at the bead-cell contact site and of MTOC polarization toward the bead-cell contact site (arrows) are shown. P values were calculated after examining >50 images for each experiment. (G) Quantitation and statistical analysis.
Together, the experiments in Fig. 2 and Fig. 3 show that LFA-1 and the activating receptors are required (Fig. 2) and that they are sufficient by themselves (Fig. 3) for polarization of either F-actin or the MTOC. Moreover, these experiments reinforce the conclusion that two separate pathways, actin cytoskeletal rearrangement and microtubule and granule polarization, regulate the formation of a NK cell synapse and thus the cytotoxic function of a NK cell.
Many Activating Receptors Are Coupled to the ERK2 and JNK1 Phosphorylation Pathways Despite Their Use of Different Signaling Modules.
Ten NK cell receptors that use a variety of proximal signaling molecules were studied: CD28, NKG2D, NKp46, NKp30, NKG2C/CD94, 2B4, LFA-1, β1 integrin, CD2, and DNAM-1. Each of these receptors on either YTS, NK92, or NKL cells was cross-linked by the addition of a specific mAb followed by rabbit-anti-mouse F(ab′)2 antibodies and incubated for times varying from 0 to 15 min. SDS gels were run, and phospho-ERK, ERK, JNK, and phospho-JNK were detected with specific antibodies. Cross-linking of 6 of the 10 NK receptors above (CD28, NKG2D, NKG2C/CD94, NKp46, NKp30, and 2B4) resulted in phosphorylation of both ERK2 and JNK1 (Fig. 4). Using the streptavidin polystyrene bead technique (Fig. 3), cross-linking of biotinylated anti-2B4 mAb on NK cells was also shown to polarize the MTOC and granules to the point of bead contact. (Biotinylated mAb for NKp30, NKp46, and NKG2C were not available). LFA-1 cross-linking resulted in weak and transient phosphorylation of ERK2 and JNK1 (see also ref. 4), whereas no significant phosphorylation of either was seen on cross-linking DNAM-1, CD2, or β1-integrin.
Fig. 4.
Many NK activating receptors are coupled to the ERK2 and JNK1 phosphorylation pathways. LFA-1 and CD28 on YTS cells (A); NKG2D, NKp30, and NKp46 on NK92 cells (B); and DNAM-1, 2B4, CD2, NKG2D, NKG2C/CD94, and β1 integrin on NKL cells (C) were cross-linked (×) for the indicated times. Cells treated with control mIgG were used as negative controls, and phorbol 12-myristate 13-acetate/ionomycin (PMA/ION) stimulation was used as a positive control. The total cell lysate was loaded into SDS/PAGE gels for Western blot analysis. Anti-phosphERK, anti-phosphoJNK, anti-panERK, and anti-panJNK rabbit polyclonal antibodies were used to detect the pattern of phosphorylation as a function of time. The second batch of polyclonal anti-phosphoJNK antibody obtained from Cell Signaling used in C did not detect phosphoJNK as strongly as that used in A and B. The results are representative of at least three independent experiments.
Distinct Pathways Lead to Phospho-ERK and Phospho-JNK Formation.
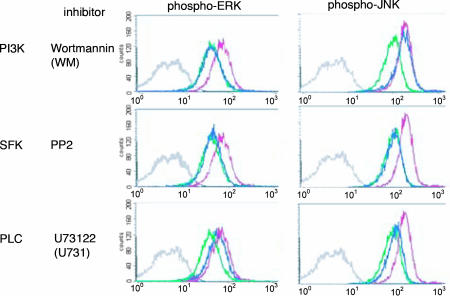
To further confirm and extend these experiments, phospho-ERK and phospho-JNK were measured directly by intracellular staining in NKL cells cross-linked with anti-NKG2D mAb in the presence of various inhibitors (Fig. 5). Wortmannin, an inhibitor of PI3K; PP2, an inhibitor of SKFs; and U73122, an inhibitor of PLC-γ, were used. PP2 inhibited the phosphorylation of both ERK2 and JNK1, whereas wortmannin inhibited only the formation of phospho-ERK and U731 only the phosphorylation of JNK.
Fig. 5.
NKG2D cross-linking on NKL cells activates a bifurcated signaling pathway to phosphorylate ERK and JNK. Biotinylated anti-NKG2D mAb was used to cross-link the activating receptor on NKL cells by adding streptavidin for 10 min. Experiments were done in the presence of DMSO as control, 200 nM Wortmannin (WM, inhibitor of PI3K), 2 μM PP2 (inhibitor of SFK), and 1 μM U73122 (U731, inhibitor of phospholipase Cγ), all prepared in DMSO. After cross-linking, the NKL cells were immediately fixed and permeabilized for intracellular staining of phospho-ERK or phospho-JNK with Alexa647 nm conjugated-specific mAb. One representative result from three independent flow cytometry analyses is shown. The gray line indicates control mIgG staining, the green line shows the specific mAb staining in resting cells, the red line represents the specific staining after NKG2D cross-linking in the presence of DMSO, and the blue line is the result of NKG2D cross-linking in the presence of the indicated inhibitor.
Discussion
These findings, together with data published previously by us and others (4, 16, 17), lead to the scheme shown in Fig. 6. To induce apoptosis of target cells, NK cells recognize specific ligands on target cells, trigger activating signals by phosphorylating molecular regulators, remodel the cytoskeletal elements by polymerizing F-actin in the NKIS and polarizing the MTOC and cytolytic granules to the NKIS, and finally release granule components by delivering perforin and granzymes across the NKIS (19). It should also be noted that NK cells have a second mode of signaling, dominant inhibitory signaling mediated by the NK inhibitory receptors [killer cell Ig-like receptors (e.g., KIR2DL1) and lectin-like receptor (NKG2A/CD94)]. The inhibitory receptors function, at least in part, by directly or indirectly preventing formation of a multiprotein complex at the activating NKIS (20).
Fig. 6.
Schematic representation showing that many NK activating receptors employ the same SFK-dependent PI3K → ERK2 pathway and phospholipase Cγ → JNK1 pathway to polarize the MTOC and cytolytic granules during NK cell cytotoxicity, independent of the proximal signaling modules.
How do the multiple NK activating receptors stimulate the killing of a specific target cell? The virus-infected or tumor targets differ from their healthy counterparts by up-regulating or expressing distinct ligands, the pattern of which is recognized by the corresponding receptors on the surface of the NK cells. Although multiple activating receptors exist, their expression profiles on human peripheral blood NK cells are heterogeneous. Some NK cells may have high expression of a certain set of activating receptors and thus be capable of killing specific virus-infected or tumor target cells whose ligands they match. In addition, each of these receptors may recognize several different ligands as in the case of other components of innate immunity, Toll-like receptors that recognize pathogen-associated molecular patterns. Only one example of this has been reported. NKp46 recognizes both the hemagglutinin (HA) of influenza virus and the haemagglutinin-neuraminidase of parainfluenza virus (21).
In all of the cases examined LFA-1 is also required for efficient killing because NK activating receptors signal polarization of the MTOC and cytolytic granules but not actin polymerization. After blocking (Fig. 2) or in the absence of activating receptors on the NK cell, an NK/target conjugate could still form with synaptic F-actin polymerization induced by the LFA-1/ICAM interaction. The “target” cell would remain intact because of the failure of MTOC and granule polarization in the NK cell after conjugation. Here, F-actin polymerization was triggered by LFA-1 signaling and served to strengthen NK/target conjugation. When actin inhibitors were used in the experiment, conjugate formation was severely impaired (data not shown). A recent report suggested that the MTOC might itself physically transport the granules onto the synaptic plasma membrane (22).
The molecular mechanisms through which ERK2 and JNK1 regulate MTOC polarization are not known. The colocalization of ERK2 to microtubules in NK cells and the presence of JNK1 in the NKIS have been previously observed (ref. 4 and B.G., C. L. Li, H. Kopcow, X.C., and J.L.S., unpublished data). Paxillin, a microtubule-associated scaffold protein, was shown to be phosphorylated as the result of the both the phospholipase Cγ →JNK1 (16) and PI3K→ERK2 pathways (23). A functional connection between kinesin, a microtubule plus-end directed motor, and JNK activity has been reported (24). Moreover, dynein has been shown to be involved in MTOC polarization (25). ERK2 and JNK1 signals may synergize to modulate the motor activities of kinesin and dynein molecules through phosphorylation, or they may work in parallel, each targeting some critical step during MTOC polarization. Finally, the ability to phosphorylate ERK2 and JNK1 on ligation may provide a useful definition of a NK activating receptor.
Materials and Methods
Cell Culture and Transfection.
The human EBV-transformed B cell line 721.221, the human erythroleukemia line K562, the human NK tumor line YTS, and GFP-transfected YTS were maintained in RPMI medium 1640 supplemented with 10% FCS, l-glutamine. In case of DsRed transfected 721.221 cells, medium was supplemented with Geneticin (1.6 mg/ml) (Gibco, Carlsbad, CA). NKL and NK92 cell lines were cultured in MyeloCult liquid media (StemCell Technologies, Vancouver, BC, Canada) supplemented with 100 units/ml recombinant human IL-2. Peripheral human NK cells were isolated by Rosette Sep Human NK Cell (StemCell Technologies) from the leukopak provided by Massachussets General Hospital. The purified NK cells (>95% pure, as determined by CD56+CD3− cells in flow cytometry) were cultured overnight in MyeloCult supplemented with 100 units/ml IL-2.
pEGFP-N1 and pDsRed2-C1 constructs were electrotransfected (Amaxa, Cologne, Germany) into YTS or NK92 and 721.221 or K562 cells, respectively. Positively transfected cells were sorted by using a MoFlo high performance cell sorter. Cell sorting was performed repeatedly to maintain stable cell lines.
Antibodies and Reagents.
The following antibodies were used: anti-LFA-1 mAb (clone TS1/18; gift from T. Springer, Boston, MA), anti-β1 integrin mAb (gift from M. Hemler, Boston, MA), anti-CD28 mAb (clone 28.2; eBioscience, San Diego, CA), anti-NKp30 mAb (R&D Systems, Minneapolis, MN), anti-human MHC I (clone ME1, Biotin-conjugated; our lab), anti-pan ERK1/2 (rabbit polyclonal; Upstate Biotechnology, Lake Placid, NY), anti-pan ERK1/2 (clone 16; BD Bioscience, San Jose, CA), anti-phosphoERK [rabbit polyclonal antibody for Western blot (Cell Signaling Technology, Danvers, MA) and Alexa647 nm-conjugated-mAb for intracellular staining, clone 20A (BD Bioscience)], anti-phosphoJNK (rabbit polyclonal antibody for Western blot and the Alexa647 nm-conjugated mAb for intracellular staining, Cell Signaling), anti-β-tubulin(clone tub2.1, Cy3-conjugated, Sigma–Aldrich, St. Louis, MO), anti-granzyme B (clone GB11, Alexa647 nm-conjugated, BD Bioscience), rabbit-anti-mouse IgG Fc F(ab′)2 (Jackson ImmunoResearch Laboratories, West Grove, PA), anti-rabbit IgG (clone RG-96, HRP-conjugated, Sigma–Aldrich), and anti-mouse IgG (HRP-conjugated; Sigma–Aldrich). All other mAb (NKG2D, NKp46, 2B4, DNAM-1, and CD2) were from BD Bioscience. Phalloidin (Alexa488 nm-conjugated) was obtained from Molecular Probes (Eugene, OR). The kinase inhibitors wortmannin, PP2, and U73122 were from Calbiochem (San Diego, CA). All other chemicals were from Sigma–Aldrich.
51Cr-Release Killing Assay.
NK cell cytotoxicity was evaluated by the 51Cr-release assay as described in ref. 26. NK cells were coincubated with 10 μg/ml blocking mAbs for 30 min before mixing with the targets.
Confocal Microscopy.
The imaging work was done as described in ref. 4. To visualize cytoskeletal remodeling after cross-linking a particular receptor, NK cells adhering to poly(l-lysine)-coated slides were precoated with 5 μg/ml biotinylated anti-Class I (ME1, as control), anti-CD28, anti-NKG2D, anti-2B4, or anti-LFA-1 mAb for 15 min at 37°C. The cells were then briefly washed with PBS before streptavidin-coated polystyrene beads (6.7–8.0-μm diameter; Spherotech, Lake Forest, IL) were added onto the slides. The cells, after mixing with the beads for 0.5–2 h at 37°C, were fixed, permeabilized, and stained.
Cell Stimulation, Western Blot, and Intracellular Staining.
Cell stimulation, Western blot, and intracellular staining were performed as described in ref. 4. Briefly, 106 NK cells were incubated with 10 μg/ml mAbs on ice for 30 min, then 20 μg/ml rabbit-anti-mouse F(ab′)2 or 10 μg/ml streptavidin was added, and the cells were incubated for the indicated times at 37°C in PBS supplemented with 2% FCS. For negative controls, NK cells were incubated with 10 μg/ml either control mIgG or Biotin-conjugated ME1 mAb, and incubated with F(ab′)2 or streptavidin for the same time course. After stimulation, cells were either mixed with an equal volume of ice cold 1× lysis buffer (0.5% Nonidet P-40/100 mM Tris·HCl, pH 7.4/150 mM NaCl/2 mM Na3VO4) and lysed for 30 min at 4°C or fixed with an equal volume of 3.2% formaldehyde and permeabilized with ice cold methanol for 30 min at 4°C. Minute 0 corresponded to samples that were fixed or lysed before cross-linking. Western blot analysis was performed as described in ref. 21. For FACS analysis, the fixed and permeablized cells were stained with Alexa647 nm-conjugated anti-phospho-ERK or anti-phospho-JNK mAb in the presence of 1% BSA by using Alexa647 nm-conjugated mIgG as the negative control.
FACS-Based Conjugation Assay.
YTS-GFP (5 × 105) and 721.221-RFP (5 × 105) cells were mixed and centrifuged at 100 × g for 1 min, in 100 μl of 10 mM Hepes buffer. After incubation for 20 min at 37°C, the cells were resuspended by gently pipetting, fixed in 1% paraformaldehyde, and immediately analyzed by FACS. The conjugation ratio was computed as the portion of GFP/RFP double-positive events within the GFP positive events.
Acknowledgments
This work was supported by National Institutes of Health Grants AI050207 and AI053330.
Abbreviations
- LFA
lymphocyte function-associated antigen
- MTOC
microtubule organizing center
- NK
natural killer
- NKIS
NK immunological synapse
- PI3K
phosphoinositide-3 kinase
- SFK
Src family kinase.
Footnotes
The authors declare no conflict of interest.
References
- 1.Cerwenka A, Lanier LL. Nat Rev Immunol. 2001;1:41–49. doi: 10.1038/35095564. [DOI] [PubMed] [Google Scholar]
- 2.Jamieson AM, Diefenbach A, McMahon CW, Xiong N, Carlyle JR, Raulet DH. Immunity. 2002;17:19–29. doi: 10.1016/s1074-7613(02)00333-3. [DOI] [PubMed] [Google Scholar]
- 3.Chiesa S, Tomasello E, Vivier E, Vely F. Mol Immunol. 2005;42:477–484. doi: 10.1016/j.molimm.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 4.Chen X, Allan DS, Krzewski K, Ge B, Kopcow H, Strominger JL. Proc Natl Acad Sci USA. 2006;103:10346–10351. doi: 10.1073/pnas.0604236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eissmann P, Beauchamp L, Wooters J, Tilton JC, Long EO, Watzl C. Blood. 2005;105:4722–4729. doi: 10.1182/blood-2004-09-3796. [DOI] [PubMed] [Google Scholar]
- 6.Bryceson YT, March ME, Ljunggren HG, Long EO. Blood. 2006;107:159–166. doi: 10.1182/blood-2005-04-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vivier E, Nunes JA, Vely F. Science. 2004;306:1517–1519. doi: 10.1126/science.1103478. [DOI] [PubMed] [Google Scholar]
- 8.Markiewicz MA, Carayannopoulos LN, Naidenko OV, Matsui K, Burack WR, Wise EL, Fremont DH, Allen PM, Yokoyama WM, Colonna M, Shaw AS. J Immunol. 2005;175:2825–2833. doi: 10.4049/jimmunol.175.5.2825. [DOI] [PubMed] [Google Scholar]
- 9.Chiesa S, Mingueneau M, Fuseri N, Malissen B, Raulet DH, Malissen M, Vivier E, Tomasello E. Blood. 2006;107:2364–2372. doi: 10.1182/blood-2005-08-3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colonna M. Nat Immunol. 2005;6:961–962. doi: 10.1038/ni1005-961. [DOI] [PubMed] [Google Scholar]
- 11.Garrity D, Call ME, Feng J, Wucherpfennig KW. Proc Natl Acad Sci USA. 2005;102:7641–7646. doi: 10.1073/pnas.0502439102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yokoyama WM, Koning F, Kehn PJ, Pereira GM, Stingl G, Coligan JE, Shevach EM. J Immunol. 1988;141:369–376. [PubMed] [Google Scholar]
- 13.Augugliaro R, Parolini S, Castriconi R, Marcenaro E, Cantoni C, Nanni M, Moretta L, Moretta A, Bottino C. Eur J Immunol. 2003;33:1235–1241. doi: 10.1002/eji.200323896. [DOI] [PubMed] [Google Scholar]
- 14.Billadeau DD, Upshaw JL, Schoon RA, Dick CJ, Leibson PJ. Nat Immunol. 2003;4:557–564. doi: 10.1038/ni929. [DOI] [PubMed] [Google Scholar]
- 15.Wei S, Gamero AM, Liu JH, Daulton AA, Valkov NI, Trapani JA, Larner AC, Weber MJ, Djeu JY. J Exp Med. 1998;187:1753–1765. doi: 10.1084/jem.187.11.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meresse B, Chen Z, Ciszewski C, Tretiakova M, Bhagat G, Krausz TN, Raulet DH, Lanier LL, Groh V, Spies T, et al. Immunity. 2004;21:357–366. doi: 10.1016/j.immuni.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 17.Jiang K, Zhong B, Gilvary DL, Corliss BC, Hong-Geller E, Wei S, Djeu JY. Nat Immunol. 2000;1:419–425. doi: 10.1038/80859. [DOI] [PubMed] [Google Scholar]
- 18.Upshaw JL, Arneson LN, Schoon RA, Dick CJ, Billadeau DD, Leibson PJ. Nat Immunol. 2006;7:524–532. doi: 10.1038/ni1325. [DOI] [PubMed] [Google Scholar]
- 19.Vyas YM, Maniar H, Dupont B. Immunol Rev. 2002;189:161–178. doi: 10.1034/j.1600-065x.2002.18914.x. [DOI] [PubMed] [Google Scholar]
- 20.Krzewski K, Chen X, Orange JS, Strominger JL. J Cell Biol. 2006;173:121–132. doi: 10.1083/jcb.200509076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mandelboim O, Lieberman N, Lev M, Paul L, Arnon TI, Bushkin Y, Davis DM, Strominger JL, Yewdell JW, Porgador A. Nature. 2001;409:1055–1060. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 22.Stinchcombe JC, Majorovits E, Bossi G, Fuller S, Griffiths GM. Nature. 2006;444:236. doi: 10.1038/nature05071. [DOI] [PubMed] [Google Scholar]
- 23.Robertson LK, Mireau LR, Ostergaard HL. J Immunol. 2005;175:8138–8145. doi: 10.4049/jimmunol.175.12.8138. [DOI] [PubMed] [Google Scholar]
- 24.Inomata H, Nakamura Y, Hayakawa A, Takata H, Suzuki T, Miyazawa K, Kitamura N. J Biol Chem. 2003;278:22946–22955. doi: 10.1074/jbc.M212160200. [DOI] [PubMed] [Google Scholar]
- 25.Combs J, Kim SJ, Tan S, Ligon LA, Holzbaur EL, Kuhn J, Poenie M. Proc Natl Acad Sci USA. 2006;103:14883–14888. doi: 10.1073/pnas.0600914103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orange JS, Harris KE, Andzelm MM, Valter MM, Geha RS, Strominger JL. Proc Natl Acad Sci USA. 2003;100:14151–14156. doi: 10.1073/pnas.1835830100. [DOI] [PMC free article] [PubMed] [Google Scholar]