Abstract
Circadian rhythms and the genes that make up the molecular clock have long been implicated in bipolar disorder. Genetic evidence in bipolar patients suggests that the central transcriptional activator of molecular rhythms, CLOCK, may be particularly important. However, the exact role of this gene in the development of this disorder remains unclear. Here we show that mice carrying a mutation in the Clock gene display an overall behavioral profile that is strikingly similar to human mania, including hyperactivity, decreased sleep, lowered depression-like behavior, lower anxiety, and an increase in the reward value for cocaine, sucrose, and medial forebrain bundle stimulation. Chronic administration of the mood stabilizer lithium returns many of these behavioral responses to wild-type levels. In addition, the Clock mutant mice have an increase in dopaminergic activity in the ventral tegmental area, and their behavioral abnormalities are rescued by expressing a functional CLOCK protein via viral-mediated gene transfer specifically in the ventral tegmental area. These findings establish the Clock mutant mice as a previously unrecognized model of human mania and reveal an important role for CLOCK in the dopaminergic system in regulating behavior and mood.
Keywords: bipolar disorder, circadian rhythms, dopamine
It has been hypothesized for quite some time that bipolar disorder is associated with abnormalities in the circadian system (1, 2). Virtually all individuals with bipolar disorder have major alterations in circadian functions including sleep, activity, hormonal secretions, and appetite. Appearance of mania can cycle with a regular, even seasonal, pattern, further suggesting a circadian component to its pathology. In addition, normalization of both sleep/wake cycles and social zeitgebers often is essential for mood stabilization, while disruptions in these rhythms can trigger manic episodes (3). Some successful treatments for mood disorders rely on altering the circadian cycle (4). Depression symptoms are also diurnal, being more prevalent during the winter months (termed seasonal affective disorder) and in parts of the world that receive little sunlight for extended periods of time (5).
Molecular components of the circadian clock in mammals have been identified. These proteins form a feedback loop of transcriptional activation and repression by the CLOCK/BMAL1 and Period (Per)/Cryptochrome (Cry) protein complexes in the suprachiasmatic nucleus (6). The orphan nuclear receptor, REV-ERBα, and the transcriptional regulators, DBP and E4BP4, participate in adjoining feedback loops, while casein kinase-1 ε and δ phosphorylate various circadian proteins leading to changes in their stability, activity, binding partners, and subcellular localization (7). Recently, studies have implicated glycogen synthase kinase-3 β (GSK3β) as a central regulator of the circadian clock (8, 9), and this enzyme is a known target of the mood-stabilizing drug, lithium (10). Lithium lengthens the circadian period in several organisms, including Drosophila, rodents, and humans, and this effect may be important for its therapeutic efficacy (11). Furthermore, another mood stabilizer, valproate, alters the expression of several circadian genes in the amygdala (12), and chronic treatment with the antidepressant fluoxetine increases expression of Clock and Bmal1 in the hippocampus (13). Together, these findings support the view that circadian genes in multiple regions of the brain are important in the development and treatment of mood disorders.
Studies in human populations have begun to identify polymorphisms in certain circadian genes that associate with mood disorders and, in particular, bipolar disorder. Two studies have identified a polymorphism in the 3′ flanking region of Clock that associates with a greater lifetime number of manic episodes, as well as greater insomnia, early morning waking, and decreased need for sleep in bipolar patients (14, 15). Polymorphisms in CLOCK's binding partner, BMAL1, have also been associated with bipolar disorder (16, 17). These results suggest that CLOCK/BMAL1 may be centrally involved in the manifestation, frequency, and severity of bipolar episodes, yet the molecular mechanisms involved remain unknown.
Results
Clock Mutant Mice Show a Greater Preference for Rewarding Stimuli Similar to Bipolar Patients in the Manic State.
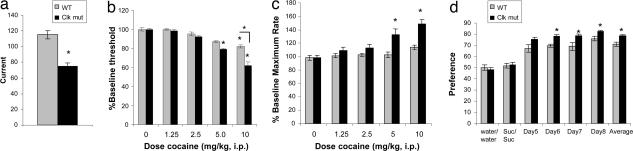
Given these links between Clock and bipolar disorder, we determined whether mice carrying a mutation in the Clock gene exhibit mood-related behavioral abnormalities. The mutation in these mice was created through N-ethyl-N-nitrosourea mutagenesis and results in a dominant-negative protein that cannot activate transcription (18). Previously, we and others reported that the Clock mutant mice are extremely hyperactive both in response to novelty and over the entire light/dark cycle (19, 20). In addition, these mice show enhanced behavioral responses to cocaine (20). These results suggest that the Clock mutant mice are more sensitive to the rewarding effects of psychostimulants. Manic patients, too, exhibit an increase in psychomotor activation and in rewarding responses. Therefore, we determined whether the Clock mutant mice exhibit a general derangement in reward mechanisms as seen in human mania. To do this, we first examined the level of intracranial self-stimulation (ICSS), a test in which rodents self-administer rewarding electrical stimulation through electrodes implanted in the medial forebrain bundle (MFB) [supporting information (SI) Fig. 5]. MFB stimulation is very rewarding to animals and enables highly sensitive assessments of hedonic state (21, 22). Furthermore, during ICSS testing, rodents fulfill several key diagnostic criteria used for mania in people, including increased goal-directed activities and excessive involvement in this rewarding activity even under conditions where there is a high potential for painful (or lethal) consequences (22). Moreover, drugs that cause symptoms like those of mania in humans (e.g., cocaine, amphetamine) facilitate ICSS behavior in rats, indicating hyperfunction of brain reward systems (23). We found that the Clock mutant mice require lower currents to sustain ICSS responding (Fig. 1a), suggesting that this mutation made the stimulation more rewarding. To the best of our knowledge, this is the first demonstration of a mutation that increases sensitivity to the rewarding effects of electrical brain stimulation itself. When the mice were subsequently tested with cocaine, the drug dose dependently decreased ICSS thresholds in both wild-type and Clock mutant mice (Fig. 1b). However, the decreases in ICSS threshold first became evident at a lower dose in the Clock mutant mice, and the decreases were significantly larger in the Clock mutant mice than in wild-type mice at 10 mg/kg cocaine. Interestingly, cocaine also dramatically increased maximum rates of responding in the Clock mutant mice (Fig. 1c), suggesting enhanced performance capabilities. Our method of analysis (curve-shift) can differentiate drug-induced alterations in reward function from drug-induced alterations in performance (20, 21). Indeed, cocaine-induced shifts in the rate frequency functions are parallel (SI Fig. 6), indicating that disruption of Clock function increases both the reward-related and performance-enhancing effects of cocaine.
Fig. 1.
Effect of Clock disruption on rewarding electrical stimulation of the MFB and sucrose preference. (a) Clock mice required a lower minimal current (mean ± SEM) to sustain reliable ICSS (∗, P < 0.01, Student's t test, n = 7–9 per group). (b) Cocaine decreased ICSS thresholds (mean ± SEM) at a lower dose in Clock mutant mice (5.0 mg/kg) than in controls (10 mg/kg). Furthermore, the threshold-lowering effects of 10 mg/kg of cocaine were significantly larger in Clock mutant mice. (c) Cocaine significantly increased ICSS rates (mean ± SEM) in Clock mutant mice. ∗, P < 0.01 for within-group comparisons with saline (SAL; 0.0 dose); ∗, P < 0.01 for between-genotype comparisons, Scheffé tests, n = 6–7 mice per group. (d) Clock mutant mice display a greater preference for 1% sucrose (Suc) than WT mice over several days of testing when given a choice between sucrose and water. ∗, P < 0.05, Student's t test, n = 8–10.
To explore the response of the Clock mutants to a natural reward, we tested their sucrose preference, which is also a sensitive measure of hedonic state because it is reduced by chronic stress and restored by antidepressant treatments (24). Consistent with our ICSS and cocaine preference results, Clock mutant mice displayed an increase in their preference for sucrose (Fig. 1d), with no differences in total fluid intake (data not shown). Taken together, the Clock mutant mice show a greater preference for a range of rewarding stimuli, indicative of a hyperhedonic state, which resembles the feelings of euphoria and increased substance abuse characteristic of human mania.
The Clock Mutant Mice Display Other Behavioral Responses Associated with Mania Including Less Depression-Like Behavior and Lowered Anxiety.
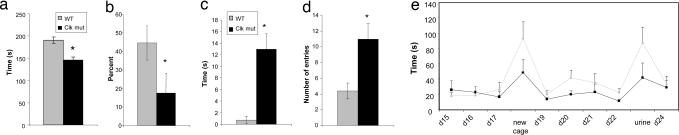
Other models of mood-related behavior in rodents involve the measurement of helplessness or “despair.” These tests are relevant to human depression because they are highly predictive of the therapeutic efficacy of antidepressant drugs (25). We, therefore, performed the Porsolt forced swim and learned helplessness tests on Clock mutant mice and their wild-type littermates. In both tests, we found a reduction in helpless behavior as measured by immobility time in the forced swim test and the percent of escape failures in the learned helplessness test (Fig. 2a and b). These results suggest that the Clock mutant mice have lower levels of depression-like behavior, which correlates with their increase in preference for rewarding stimuli.
Fig. 2.
Effect of the Clock mutation in models of depression and anxiety. (a) Clock mutants (clk mut) spend less time immobile in the forced swim test (∗, P < 0.05, Student's t test, n = 5–9). (b) Clock mutants had fewer escape failures in the learned helplessness paradigm (∗, P < 0.05, Student's t test, n = 5–6). (c) Clock mutants spend more time in the center of an open field (∗, P < 0.05, Student's t test, n = 9–12). (d) Clock mutants enter the open arms of the elevated plus maze more frequently (∗, P < 0.05, Student's t test, n = 9–12). (e) Clock mutants have a shorter latency to approach and eat a cracker in the presence of aversive stimuli (gray line, wt; black line, clk mut; differences in the combined response to aversive stimuli are significant, P < 0.05, Student's t test, n = 9–10).
To further explore their behavioral phenotype, we measured levels of anxiety-like behavior. Bipolar patients in the manic state often display low levels of anxiety, greater risk-taking, and greater impulsivity (26). For these measures, we used the open field and elevated plus maze paradigms. Both tests measure the amount of time spent in an anxiety-provoking space, such as the middle of an open field or unprotected arm of a raised platform, and both are sensitive to treatment with anxiolytic drugs (27, 28). In both paradigms, the Clock mutants showed lower levels of anxiety-like behavior (Fig. 2 c and d). To control for the possible effects of increased locomotor activity on these tests, we also performed a test in which we measured the latency to approach and eat a cracker under stressful conditions. The latency to approach and eat the cracker in the normal home cage environment was similar for both Clock mutant and wild-type mice (Fig. 2e, days 15–17), indicating that the increased motor activity in the mutants does not influence this test. However, when presented with a novel environment or an aversive stimulus (bobcat urine), the Clock mutant mice were much less affected than wild-type mice (Fig. 2e). These results confirm that the Clock mutants are less anxious or fearful than their wild-type littermates.
In addition to our results, other groups have reported increased exploratory behavior in the Clock mutant mice and a decrease in the amount of time that they spend in all stages of sleep (19, 29). Thus, as summarized in Table 1, the overall behavioral profile of the Clock mutant mice is strikingly similar to human bipolar patients when in the manic state.
Table 1.
A comparison between common symptoms of bipolar patients in the manic state and the behavioral responses of the Clock mutant mice
| Symptoms of mania | Clock mutant mice |
|---|---|
| Disrupted circadian rhythms | Disrupted circadian rhythms |
| Hyperactivity | Hyperactivity |
| Decreased sleep | Decreased sleep |
| Feelings of extreme euphoria | Hyperhedonia/less helplessness |
| Increased risk-taking | Reduced anxiety |
| Propensity toward drug abuse | Increased preference for cocaine |
The Manic-Like Behavior of the Clock Mutant Mice Can Be Reversed with Lithium Treatment.
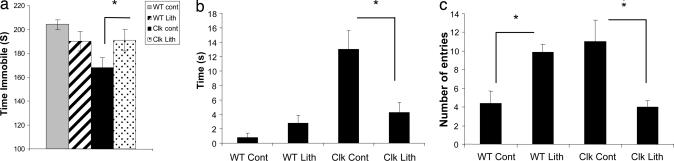
Lithium is a commonly prescribed mood stabilizer that is particularly effective in treating mania (30). To determine whether the behavioral abnormalities of the Clock mutants can be reduced by lithium treatment, we gave LiCl in the drinking water at 600 mg/liter for 10 days as described (31). We find that this paradigm produces a stable serum Li+ concentration of 0.41 ± 0.06 mmol/liter, which is at the low end of the therapeutic range for human patients (32). Such a concentration is preferable in the current study, because it does not significantly impact the health of the animals (i.e., no decrease in weight or signs of dehydration) or reduce their locomotor activity as seen with higher concentrations. When measured in the forced swim test, we found that chronic lithium treatment increased the immobility time of the Clock mutant mice to near wild-type levels (Fig. 3a). In addition, their responses in the open field and elevated plus maze tests also returned to near wild-type levels after lithium treatment (Fig. 3 b and c). These results suggest that chronic lithium treatment returns levels of anxiety- and mood-related behavior to normal levels. Because the locomotor activity in the Clock mutant and wild-type mice is not affected by this concentration of lithium, this validates that these behavioral effects are specific to helplessness and anxiety and not due to general changes in locomotor activity. Larger acute or chronic doses of lithium result in a decrease in locomotor activity in the Clock mutants and wild-type mice to a similar degree (data not shown). Behavior of the wild-type mice was not significantly affected by this concentration of LiCl in the forced swim test or open field. However, there was a change in their time spent in the open arms of the elevated plus maze. Previous studies that examined the effect of lithium on behavioral measures in wild-type mice have led to mixed results. A recent study by Bersudsky et al. (33) found that lithium treatments that produced serum levels of 1.3–1.4 mmol/liter decreased the immobility time of wild-type mice in the forced swim test; however, levels of 0.8 mMol/liter or lower had no effect. Our mice have lithium serum levels that are much lower (∼0.4 mmol/liter), thus it is consistent that the wild-type mice would not show many differences in behavioral measures, although there may be an occasional effect if a measure is particularly sensitive.
Fig. 3.
Effect of lithium treatment on the Clock mutants in behavioral measures. (a) LiCl (600 mg/liter, 10 days) leads to an increase in the time immobile of the Clock mutants in the forced swim test. LiCl treatment decreases the time spent by the Clock mutants in the left of an open field (b) and the number of entries into the open arms of the elevated plus maze (c). (In all tests, ∗, P < 0.05, Student's t test, n = 8.)
Restoration of Functional CLOCK in the Ventral Tegmental Area (VTA) of the Clock Mutants Rescues Their Behavioral Abnormalities.
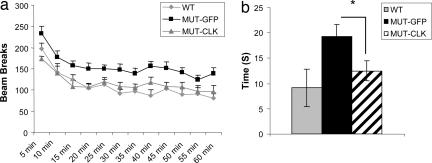
Previously we reported that the Clock mutant animals display an increase in dopamine cell firing and bursting in the VTA (20), a critical component of the brain's reward pathways. There is a wealth of literature that links this midbrain dopaminergic system with mania (34), and chronic lithium treatment in rats attenuates VTA dopaminergic function (35). We, therefore, hypothesized that, although CLOCK is expressed throughout the brain, its loss from the VTA per se contributes to the manic-like state seen in the Clock mutant mice. To test this possibility, we used viral-mediated gene transfer to deliver a functional CLOCK protein directly to the VTA of adult Clock mutant mice and determined whether we can rescue their behavioral abnormalities. This approach has been used successfully to deliver other transcription factors to specific brain regions of adult mice and determine their effects on behavior (36). Unlike the Period and Cryptochrome proteins, CLOCK expression in the brain does not cycle with any significant amplitude (37), thus our viral expression does not need to cycle to mimic endogenous expression. We injected the CLOCK-expressing virus or a GFP control virus directly into the VTA of the Clock mutant mice using stereotaxic surgery (SI Fig. 7). We estimate that ≈35% of the dopaminergic neurons are infected with the viral vectors using our delivery methods and viral concentrations (SI Fig. 7). The animals recovered for 2 weeks to allow full expression of the viral transgenes. We then tested these mice in measures of locomotor activity and anxiety. We found that functional CLOCK expression in the VTA of Clock mutant animals was sufficient to normalize the hyperactivity in the Clock mutant mice (Fig. 4a). In addition, levels of anxiety as measured in the open field returned to near wild-type levels (Fig. 4b). These results show that CLOCK function in the VTA is important in regulating these behavioral responses.
Fig. 4.
Effect of CLOCK viral expression in the VTA of Clock mutant mice. (a) Locomotor hyperactivity in response to novelty and time spent in the left of an open field (b) is reduced after 2 weeks of functional CLOCK overexpression in the VTA of Clock mutant mice. In a, total locomotor activity levels are significantly different (∗, P < 0.05, Student's t test, n = 9–12).
Discussion
Taken together, our results indicate that the Clock mutant mice represent a bona fide model of human mania. Their behavioral profile is strikingly similar in several behavioral dimensions to bipolar patients when in the manic state, including their treatment by lithium. The lack of availability of suitable animal models of mania has been one of the greatest impediments in the field. Furthermore, these mice may be particularly useful for studying the pathophysiology of both mania and addiction and help to explain why these debilitating conditions are often comorbid. Thus far, there have been no indications that the Clock mutant mice cycle between mania and depression. However, future studies are needed to determine whether depression-like symptoms occur in these mice after periods of stress, activity, or sleep deprivation.
Few studies have examined the importance of CLOCK function outside of the suprachiasmatic nucleus. Our results indicate that CLOCK has an important role in the VTA in regulating dopaminergic activity, locomotor activity, and anxiety. It will be interesting in future studies to determine the full extent of CLOCK's function in the VTA in a range of behavioral measures. Our previous studies found that several genes involved in dopaminergic signaling are differentially regulated in the VTA of the Clock mutant mice, suggesting that CLOCK affects the transcription of these genes through its actions in this brain region (20). Future experiments will determine which genes are direct transcriptional targets of CLOCK in the VTA and how they are involved in regulating dopaminergic activity and manic-like behavior.
It is also likely that CLOCK expression in other brain regions is important in regulating mood-related behaviors. Indeed antidepressant treatment has been shown to increase CLOCK expression in the hippocampus, a region of the brain that has long been associated with depression (13, 38). Furthermore, CLOCK's regulation of circadian rhythms in the suprachiasmatic nucleus could also be involved in regulating mood. Thus our analysis of CLOCK's role in these behaviors is just beginning. Future studies should explore the function of CLOCK in these other regions.
Materials and Methods
Mice.
Clock mutant mice were created by N-ethyl-N-nitrosourea mutagenesis and produce a dominant-negative CLOCK protein as described (18). Male Clock mutant (ClkΔ19/ClkΔ19) and wild-type (+/+) littermate controls, 6–10 weeks old, were used in all studies. Mice were group housed on a 12/12 light/dark cycle (lights on 7 a.m., lights off 7 p.m.) with food and water ad libitum unless otherwise specified. All behavioral assays were performed between ZT3 and ZT7. All animal use was approved by our institutional animal care and use committee.
ICSS.
Self-stimulation surgery and training.
Mice were anesthetized with i.p. injections of ketamine (100 mg/kg) plus xylazine (15 mg/kg) (Sigma–Aldrich, St. Louis, MO). Monopolar electrodes (0.250-mm diameter; Plastics One, Roanoke, VA) were implanted in the MFB at the level of the lateral hypothalamus (1.9 mm posterior to bregma, 0.8 mm lateral to midline, and 4.8 mm below dura; ref. 39). Electrodes were insulated except at the flattened tip, and the anode was a stainless steel wire (0.125-mm diameter) wrapped around a stainless steel screw threaded into the skull. Electrodes were secured to the skull with dental cement.
After 1 week of recovery, mice were trained on a fixed-ratio schedule (FR1) to respond for brain stimulation as described (40). Each operant conditioning chamber (Med Associates, St. Albans, VT) was equipped with a 2 × 5-cm wheel manipulandum. Each quarter turn of the wheel earned a 0.5-sec train of square-wave cathodal pulses (0.1-msec pulse duration) at a set frequency of 158 Hz. The stimulation current (50–150 uA) was adjusted to the lowest value that would sustain a reliable rate of responding (>40 rewards per min) for 3 consecutive days. This was considered the “Minimal Current,” reflecting sensitivity to the rewarding effects of the stimulation. Once the minimal current was identified for each mouse, it was held constant.
Each mouse was then adapted to brief tests with each of a descending series of 15 stimulation frequencies. Each series (or rate–frequency “curve”) comprised 1-min test trials at each frequency. For each frequency, there was a 5-sec “priming” phase during which noncontingent stimulation was given, a 50-sec test phase during which the number of responses was counted, and a 5-sec time-out period during which no stimulation was available. The stimulation frequency was then lowered by 10% (0.05 log units), and another trial was started. After responding had been evaluated at each of the 15 frequencies, the procedure was repeated such that each mouse was given six such series per day (90 min of training). During the training procedure, the range of frequencies was adjusted for each mouse so that the highest 5–6 frequencies would sustain responding.
To quantify ICSS thresholds (the frequency at which the stimulation becomes rewarding), a least-squares line of best fit was plotted across the frequencies that sustained responding at 20, 30, 40, 50, and 60% of the maximum rate. Threshold was defined as the frequency at which the line intersected the x axis (θ-0; ref. 40). Drug testing started when mean thresholds varied by <10% over 3 consecutive days.
Drug testing.
Cocaine HCl (Sigma–Aldrich) was dissolved in 0.9% saline and administered in a single i.p. injection at a volume of 10 ml/kg. On each test day, three rate–frequency curves were determined for each mouse immediately before drug treatment. The first curve served as a warm-up period; the second and third curves were averaged to obtain the baseline (threshold and maximal response rate) parameters. Each mouse then received an i.p. injection of cocaine or vehicle, and six more 15-min rate–frequency curves were obtained. Mice received cocaine (0.625–10.0 mg/kg) twice; doses were given in ascending and then descending order. There were no differences between the first and second treatment at any dose; therefore, the data were combined into single means. Each drug treatment followed a test with vehicle on the preceding day to ensure that the mouse had recovered from prior treatments and to minimize the possibility of conditioned drug effects. Group differences were analyzed by using ANOVAs; significant effects were analyzed further by using post hoc (Scheffé) tests. At the end of the experiments, electrode placements were confirmed by sectioning fixed brains.
Sucrose Preference.
An 8-day sucrose preference protocol was used in which mice remained individually housed 3 days before the start day (day 1) and during the course of the remaining 8 days. On day 1, their normal water bottles were replaced with two 50-ml tubes (bottle “A” and bottle “B”) fitted with bottle stoppers containing two-balled sipper tubes. The position of bottles A and B were switched daily so as to avoid a side bias, and the fluid consumed from each bottle was measured daily. During days 1 and 2, bottles A and B were filled with normal drinking water (wt/wt). During days 3 and 4, both bottles were filled with a solution of 1% sucrose dissolved in drinking water (s/s). On days 5–8, bottle A contained 1% sucrose, and bottle B contained drinking water (s/w). Preference on each day for each mouse was calculated as 100·(VolA/[VolA+VolB]) and averaged across the days for a given condition (wt/wt, s/s, or s/w). Total fluid consumed was calculated as [VolA + VolB] and averaged similarly.
Open Field Activity.
Mice were placed in the periphery of a novel open field environment (44 × 44 cm, walls 30-cm high) in a dimly lit room and allowed to explore for 5 min. The animals were monitored from above by a video camera connected to a computer running video tracking software (Ethovision 3.0; Noldus, Leesburg, VA) to determine the time, distance moved, and number of entries into two areas: the periphery (5 cm from the walls) and the left (14 × 14 cm). The open field arenas were wiped and allowed to dry between mice. Data were analyzed with Student's t test.
Elevated Plus Maze.
Mice were placed in the left of a black Plexiglas elevated plus maze (each arm 30-cm long and 5-cm wide with two opposite arms closed by 25-cm high walls) elevated 31 cm in a dimly lit room and allowed to explore for 5 min. The animals were monitored from above by a video camera connected to a computer running video tracking software (Ethovision 3.0) to determine time spent in the open and closed arms, time spent in the middle, and the number of entries into the open and closed arms. The apparatus was wiped and allowed to dry between mice. Data were analyzed with Student's t test.
Forced Swim (Porsolt) Test.
Mice were placed in a beaker (16.5-cm diameter) of water (21–25°C) to a depth of 7 inches. The mice remained in the water for 6 min and were then removed and allowed to dry in a clean dry cage before returning to their home cage. The water was changed between each subject. The mice were monitored from the side by a video camera, and data were stored on a videotape for later analysis. Only the last 4 min of the test were scored for latency to the first immobility and total time spent immobile. The experimenter scoring the behavior was blind to the experimental treatment. Immobility was defined as no volitional body or limb movement. Data were analyzed with Student's t test.
Locomotor Activity.
Mice were placed individually into clean standard plastic mouse cages for 1 h. The cages were placed in a Photobeam Activity System (San Diego Instruments, San Diego, CA) that monitors animal activity in one direction by using five photobeams. The number of beam breaks were recorded every 5 min. Overall locomotor differences were analyzed by using Student's t test.
Learned Helplessness Following Inescapable Shock.
Mice were placed in a chamber in which they cannot escape (Med Associates) and received two shocks (5-sec duration at 0.3 mA) per min for 1 h and then returned to their home cage. This is repeated on day 2. On day 3, the automatic guillotine door opened concurrent with presentation of each foot shock, allowing the mice to escape (one 0.3-mA, 30-sec shock per min for 30 min), and the latency to escape is measured. Data were analyzed with Student's t test.
Reward/Aversion Test.
The test used was modified from the procedure described in Merali et al. (41). Briefly, the animals were isolated for 10 days before the start of testing. On the 11th day, a piece of graham cracker (Honey Maid Graham Cracker crumbs; Nabisco, East Hanover, NJ) was placed in the cage, and the latency to approach and begin to eat the cracker was measured. This was repeated on a daily basis, at approximately the same time in the light cycle, until the animals achieved a baseline criterion of <20% variation over 3 consecutive days. At this point, the first manipulation was carried out, which consisted of placing the animal into a new cage before the introduction of the cracker. Again the latency was measured. Subsequent to this, the animals were rebaselined for 3 days before the next manipulation. This involved the introduction of a small amount of ultra-filtered bobcat urine (Elliots Hardware, Dallas, TX), absorbed onto vermiculite, before the introduction of the cracker. Again the latency was measured. After the test the animals were placed in clean cages. Finally, the baseline condition was repeated. The test continued under normal conditions for 1 additional day. Significance was measured for responses in the presence of stressful stimuli by Student's t test.
Lithium Treatment.
Lithium chloride was mixed into the drinking water at 600 mg/liter and given for 10 days. This procedure was chosen based on animal health concerns, drug effectiveness in preliminary studies, and results from Dehpour et al. (31) by using similar procedures. Controls were given normal water.
Serum Lithium Measurements.
Trunk blood was removed into siliconized tubes and immediately centrifuged for 10 min. Serum was removed and frozen. Samples were analyzed by spectrophotometry by using the Roche Hitachi (Roche Applied Science, Indianapolis, IN) P800 Modular system at 505-nm (primary) and 480-nm (secondary) wavelengths and verified by using BioRad (Hercules, CA) controls.
Viral Preparation.
CLOCK cDNA was subcloned from a pcDNA3-CLOCK vector (Invitrogen, Carlsbad, CA) described by King et al. (18) via a BamHI restriction site within the multiple cloning region into the pAAV-MCS vector (Stratagene, La Jolla, CA), which carries the ITR sequences necessary for AAV packaging. Activity of AAV-CLOCK was assessed by demonstrating CLOCK expression in Hek293 cells by immunoblotting. In addition, when transformed into PC-12 with a luc-reporter plasmid containing several E-box sites, pAAV-CLOCK generated robust luciferase expression.
Viral Packaging.
Viral production was performed by using a triple-transfection, helper-free method as described in ref. 42. Briefly, HEK293 cells were cultured in 10 150 × 25-mm cell culture dishes and transfected with pAAV-shRNA, pHelper, and pAAV-RC plasmids (Stratagene) by using a standard calcium phosphate method. Cells were collected, pelleted, and resuspended in freezing buffer (0.15 M NaCl and 50 mM Tris, pH 8.0) 66–70 h after transfection. After two freeze–thaw cycles to lyse the cells, benzoase was added (50 units per ml, final), and the mixture was incubated at 37°C for 30 min. The lysate was added to a centrifuge tube containing a 15, 25, 40, and 60% iodixanol step gradient. The gradient was spun at 350,000 × g for 60 min at 18°C, and the 40% fraction was collected. This fraction was added to a heparin affinity column, washed with 0.1 M NaCl, and eluted with 0.4 M NaCl. Elution buffer was exchanged with 1× PBS using Amicon (Beverly, MA) BioMax 100K NMWL concentrators. The final purified virus was stored at −80°C. The virus was then titered by using an AAV ELISA kit (Progen, Heidelberg, Germany) and used for infection of HT1080 cells.
Stereotaxic VTA Surgeries.
Mice were anesthetized with a ketamine (60 mg/kg, i.p.)-Xylazine (10 mg/ml) mix and given bilateral microinjections (0.5 μl over 5 min) of AAV vectors encoding GFP (as a control) or CLOCK into the dopamine-rich caudal VTA (anteroposterior, −3.2; lateral, +1.0; dorsoventral, 4.6 mm below dura according to Paxinos and Watson using a 33-gauge Hamilton syringe angled at 7.5° from the midline to avoid piercing the sinus system).
Immunohistochemistry.
Animals were placed under anesthesia [ketamine (60 mg/kg, i.p.)-Xylazine (10 mg/ml)], and the heart was exposed. PBS was circulated through the body for 5 min. Paraformaldehyde (4%) was circulated through the body for 15 min. Brains were removed at the end of the procedure and placed in PBS + 20% glycerol. Slices (35 μm) were taken and rinsed in PBS. Nonspecific binding was blocked using 3% donkey serum (Jackson ImmunoResearch, West Grove, PA). Antibodies for tyrosine hydroxylase (Sigma–Aldrich; 1:5,000) and CLOCK (Santa Cruz Biotechnology, Santa Cruz, CA; 1:200) or GFP (Invitrogen; 1:1,000) were added to the slices and incubated overnight. The following day, the slices were washed in PBS and incubated with secondary antibodies conjugated to Cy2 and Cy3 (Jackson ImmunoResearch; 1:200). After washing, the sections were mounted on slides, dehydrated, and coverslipped using DPX mounting media (Electron Microscopy Sciences, Hatfield, PA). Images were obtained by using confocal microscopy.
Supplementary Material
Acknowledgments
We thank Cathy Steffen for animal care and breeding; Arash Khatami and Gayatri Shrikhande for assistance with the behavioral measures; Kalisa Myers, Lance Adkins, Yousuf Parupia, Barbara Duke, and Tamara West for assistance with the serum lithium measurements; Daniel Yang, Alice Ann Spurgin, and Laurent Coque for assistance with the construction and confirmation of viral injection sites; and Steve McKnight (University of Texas Southwestern, Dallas, TX) for the E-box luciferase construct. This work was supported by the National Institute on Drug Abuse and the National Institute of Mental Health/National Institutes of Health.
Abbreviations
- ICSS
intracranial self-stimulation
- MFB
medial forebrain bundle
- VTA
ventral tegmental area.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 6097.
This article contains supporting information online at www.pnas.org/cgi/content/full/0609625104/DC1.
References
- 1.Jones SH. Clin Psychol Rev. 2001;21:1193–1209. doi: 10.1016/s0272-7358(01)00111-8. [DOI] [PubMed] [Google Scholar]
- 2.Mansour HA, Monk TH, Nimgaonkar VL. Ann Med. 2005;37:196–205. doi: 10.1080/07853890510007377. [DOI] [PubMed] [Google Scholar]
- 3.Boivin DB. J Psychiatry Neurosci. 2000;25:446–458. [PMC free article] [PubMed] [Google Scholar]
- 4.Bunney WE, Bunney BG. Neuropsychopharmacology. 2000;22:335–345. doi: 10.1016/S0893-133X(99)00145-1. [DOI] [PubMed] [Google Scholar]
- 5.Saeed SA, Bruce TJ. Am Fam Physician. 1998;57:1340–1346. 1351–1352. [PubMed] [Google Scholar]
- 6.King DP, Takahashi JS. Annu Rev Neurosci. 2000;23:713–742. doi: 10.1146/annurev.neuro.23.1.713. [DOI] [PubMed] [Google Scholar]
- 7.Ueda HR, Hayashi S, Chen W, Sano M, Machida M, Shigeyoshi Y, Iino M, Hashimoto S. Nat Genet. 2005;37:187–192. doi: 10.1038/ng1504. [DOI] [PubMed] [Google Scholar]
- 8.Harada Y, Sakai M, Kurabayashi N, Hirota T, Fukada Y. J Biol Chem. 2005;280:31714–31721. doi: 10.1074/jbc.M506225200. [DOI] [PubMed] [Google Scholar]
- 9.Iitaka C, Miyazaki K, Akaike T, Ishida N. J Biol Chem. 2005;280:29397–29402. doi: 10.1074/jbc.M503526200. [DOI] [PubMed] [Google Scholar]
- 10.Gould TD, Manji HK. Neuropsychopharmacology. 2005;30:1223–1237. doi: 10.1038/sj.npp.1300731. [DOI] [PubMed] [Google Scholar]
- 11.Klemfuss H. Pharmacol Ther. 1992;56:53–78. doi: 10.1016/0163-7258(92)90037-z. [DOI] [PubMed] [Google Scholar]
- 12.Ogden CA, Rich ME, Schork NJ, Paulus MP, Geyer MA, Lohr JB, Kuczenski R, Niculescu AB. Mol Psychiatry. 2004;9:1007–1029. doi: 10.1038/sj.mp.4001547. [DOI] [PubMed] [Google Scholar]
- 13.Manev H, Uz T. Trends Pharmacol Sci. 2006;27:186–189. doi: 10.1016/j.tips.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Benedetti F, Serretti A, Colombo C, Barbini B, Lorenzi C, Campori E, Smeraldi E. Am J Med Genet B Neuropsychiatr Genet. 2003;123:23–26. doi: 10.1002/ajmg.b.20038. [DOI] [PubMed] [Google Scholar]
- 15.Serretti A, Benedetti F, Mandelli L, Lorenzi C, Pirovano A, Colombo C, Smeraldi E. Am J Med Genet B Neuropsychiatr Genet. 2003;121:35–38. doi: 10.1002/ajmg.b.20053. [DOI] [PubMed] [Google Scholar]
- 16.Nievergelt CM, Kripke DF, Barrett TB, Burg E, Remick RA, Sadovnick AD, McElroy SL, Keck PE, Jr, Schork NJ, Kelsoe JR. Am J Med Genet B Neuropsychiatr Genet. 2006;141:234–241. doi: 10.1002/ajmg.b.30252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mansour HA, Wood J, Logue T, Chowdari KV, Dayal M, Kupfer DJ, Monk TH, Devlin B, Nimgaonkar VL. Genes Brain Behav. 2006;5:150–157. doi: 10.1111/j.1601-183X.2005.00147.x. [DOI] [PubMed] [Google Scholar]
- 18.King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, Steeves TD, Vitaterna MH, Kornhauser JM, Lowrey PL, et al. Cell. 1997;89:641–653. doi: 10.1016/s0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Easton A, Arbuzova J, Turek FW. Genes Brain Behav. 2003;2:11–19. doi: 10.1034/j.1601-183x.2003.00002.x. [DOI] [PubMed] [Google Scholar]
- 20.McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, Nestler EJ. Proc Natl Acad Sci USA. 2005;102:9377–9381. doi: 10.1073/pnas.0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Markou A, Koob GF. Neuropsychopharmacology. 1991;4:17–26. [PubMed] [Google Scholar]
- 22.Tomasiewicz HC, Mague SD, Cohen BM, Carlezon WA., Jr Brain Res. 2006;1093:83–94. doi: 10.1016/j.brainres.2006.03.102. [DOI] [PubMed] [Google Scholar]
- 23.Wise RA. Annu Rev Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- 24.Matthews K, Forbes N, Reid IC. Physiol Behav. 1995;57:241–248. doi: 10.1016/0031-9384(94)00286-e. [DOI] [PubMed] [Google Scholar]
- 25.Cryan JF, Mombereau C. Mol Psychiatry. 2004;9:326–357. doi: 10.1038/sj.mp.4001457. [DOI] [PubMed] [Google Scholar]
- 26.Steiner J. Br J Med Psychol. 1972;45:365–374. doi: 10.1111/j.2044-8341.1972.tb02219.x. [DOI] [PubMed] [Google Scholar]
- 27.Mi XJ, Chen SW, Wang WJ, Wang R, Zhang YJ, Li WJ, Li YL. Pharmacol Biochem Behav. 2005;81:683–687. doi: 10.1016/j.pbb.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 28.Suaudeau C, Rinaldi D, Lepicard E, Venault P, Crusio WE, Costentin J, Chapouthier G. Physiol Behav. 2000;71:517–523. doi: 10.1016/s0031-9384(00)00383-8. [DOI] [PubMed] [Google Scholar]
- 29.Naylor E, Bergmann BM, Krauski K, Zee PC, Takahashi JS, Vitaterna MH, Turek FW. J Neurosci. 2000;20:8138–8143. doi: 10.1523/JNEUROSCI.20-21-08138.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shastry BS. Bioessays. 1997;19:199–200. doi: 10.1002/bies.950190304. [DOI] [PubMed] [Google Scholar]
- 31.Dehpour AR, Sadr SS, Azizi MR, Namiranian K, Farahani M, Javidan AN. Pharmacol Toxicol. 2002;90:89–93. doi: 10.1034/j.1600-0773.2002.900206.x. [DOI] [PubMed] [Google Scholar]
- 32.Gelenberg AJ, Kane JM, Keller MB, Lavori P, Rosenbaum JF, Cole K, Lavelle J. N Engl J Med. 1989;321:1489–1493. doi: 10.1056/NEJM198911303212201. [DOI] [PubMed] [Google Scholar]
- 33.Bersudsky Y, Shaldubina A, Belmaker RH. Behav Pharmacol. 2007;18:77–80. doi: 10.1097/FBP.0b013e32801416ed. [DOI] [PubMed] [Google Scholar]
- 34.Diehl DJ, Gershon S. Compr Psychiatry. 1992;33:115–120. doi: 10.1016/0010-440x(92)90007-d. [DOI] [PubMed] [Google Scholar]
- 35.Ferrie L, Young AH, McQuade R. J Psychopharmacol. 2005;19:229–234. doi: 10.1177/0269881105051525. [DOI] [PubMed] [Google Scholar]
- 36.Carlezon WA, Jr, Nestler EJ, Neve RL. Crit Rev Neurobiol. 2000;14:47–67. doi: 10.1080/08913810008443546. [DOI] [PubMed] [Google Scholar]
- 37.Ko CH, Takahashi JS. Hum Mol Genet. 2006;15(Suppl 2):R271–R277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- 38.Uz T, Ahmed R, Akhisaroglu M, Kurtuncu M, Imbesi M, Dirim Arslan A, Manev H. Neuroscience. 2005;134:1309–1316. doi: 10.1016/j.neuroscience.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic; 2001. [Google Scholar]
- 40.Gilliss B, Malanga CJ, Pieper JO, Carlezon WA., Jr Psychopharmacology (Berlin) 2002;163:238–248. doi: 10.1007/s00213-002-1153-8. [DOI] [PubMed] [Google Scholar]
- 41.Merali Z, Levac C, Anisman H. Biol Psychiatry. 2003;54:552–565. doi: 10.1016/s0006-3223(02)01827-9. [DOI] [PubMed] [Google Scholar]
- 42.Hommel JD, Sears RM, Georgescu D, Simmons DL, DiLeone RJ. Nat Med. 2003;9 doi: 10.1038/nm964. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.