Abstract
Background
Late endosomes, the last sorting station in the endocytic pathway before lysosomes, are pleiomorphic organelles composed of tubular elements as well as vesicular regions with a characteristic multivesicular appearance, which play a crucial role in intracellular trafficking. Here, we have investigated whether, in addition to these morphologically distinguishable regions, late endosomal membranes are additionally sub-compartmentalized into membrane microdomains.
Methodology/Principal Findings
Using sub-organellar fractionation techniques, both with and without detergents, combined with electron microscopy, we found that both the limiting membrane of the organel and the intraluminal vesicles contain raft-type membrane domains. Interestingly, these differentially localized domains vary in protein composition and physico-chemical properties.
Conclusions/Significance
In addition to the multivesicular organization, we find that late endosomes contain cholesterol rich microdomains both on their limiting membrane and their intraluminal vesicles that differ in composition and properties. Implications of these findings for late endosomal functions are discussed.
Introduction
The general view of the cellular plasma membrane has evolved, over the last 20 years, from that of a homogeneous arrangement of lipids with embedded proteins towards that of a mosaic of microdomains, each having a specific lipid and protein composition [1]. Some are morphologically distinguishable, such as clathrin coated pits and caveolae [2], whereas others, such as lipid rafts are apparently featureless regions of the plasma membrane [3], [4]. Assembly of lipid rafts involves not only lateral aggregation of long and saturated acyl chains (glycosphingolipids, phospholipids) in combination with cholesterol [1], [4], [5] but also protein-protein interactions [6] and protein-lipid interactions. This specific lipid environment would then attract certain proteins with high lipid raft partitioning coefficient, such as doubly acylated src like kinases or some palmitoylated transmembrane proteins [7], [8], [9], [10]. In addition, modification of lipid raft composition can occur either by changes in the environment or the physiological state of the cell [11] or by the binding of ligands to receptors [12], [13]. Importantly, although a single name is used, rafts are likely to represent a heterogeneous group of domains [1], [14].
Lipid rafts have mostly been studied at the plasma membrane due to their accessibility from the outside of the cell– for microscopy and biophysical studies [6], [15], [16]– and to their role in signaling [12], [13], [17] and endocytosis [18], [19], [20]. Characterization of rafts has also been extensively based on their resistance to detergent solubilization, although this widely used biochemical readout has inherent limitations [21], [22], [23], [24], [25]. Nevertheless, the analysis of detergent resistant membranes (DRMs) remains a useful tool [23] in particular in comparative studies.
In addition to the plasma membrane, many intracellular organelles appear to contain raft-like domains [26], [27], [28], [29], [30]. The endoplasmic reticulum was initially thought to be devoid of cholesterol dependent DRMs because of its low cholesterol content. Several recent studies have however reported their existence [31], [32], [33]. Due to the increase in cholesterol and sphingolipids along the secretory pathway, raft-like domains are thought to become more abundant in the Golgi and more specifically the trans-Golgi network [30], [34], [35]. Raft-like domains are also present in the endocytic pathway, as highlighted by studies on the trafficking of GPI-anchored proteins [18], [29], flotillins [36], toxins and viruses [37]. Occurrence of rafts in the endocytic pathway is probably the combined result of de novo assembly and engulfment from the plasma membrane. Endocytosis of raft-like domains can indeed occur both via clathrin-dependent [38], [39] and independent-pathways [18], [19], [36], [40].
Having previously documented the occurrence of DRMs in late endosomes [29], we have characterized these raft-like domains in more detail using morphological approaches and subcellular fractionation followed by sub-organellar fractionation. We show that limiting and internal membranes of this multivesicular compartment [41], [42] both contain raft-like membranes but that these domains differ in their physico-chemical properties and protein composition.
Materials and Methods
Cell culture and reagents
Monolayer of baby hamster kidney (BHK), and C2C12 cells were grown and maintained as described by [43], [44], [45]. Aerolysin was purified and labeled as previously described [29], [46]. Our rabbit anti-flotllin-1 polyclonal antibody was previously described [29], anti-NPC1 was from Dr. E. Ikonen (National Public Health Institute, Helsinki, Finland) and anti-MLN64 from Dr J. F. Strauss (University of Pennsylvania, School of Medicine, Philadelphia, USA).
Subcellular fractionation
Late endosomal fraction was prepared as described [29], [47]. Briefly, BHK cells were harvested and homogenized, a post-nuclear supernatant was prepared and adjusted to 40.6% sucrose, 3 mM imidazole, pH 7.4, loaded at the bottom of an SW41 tube, and overlaid sequentially with 35 and 25% sucrose solutions in 3 mM imidazole, pH 7.4, and then homogenization buffer (HB ; 8.5% sucrose, 3 mM imidazole, pH 7.4). The gradient was centrifuged for 90 min at 35 000 rpm. Early and late endosomal fractions were collected at the 35/25% and 25%/HB interfaces respectively.
Isolation of DRMs from late endosomal fractions
DRMs were prepared from late endosomes as described [29]. Briefly late endosomes were diluted four times, sedimented by centrifugation (TLS.55 Beckman rotor, 30 min, 55 000 r.p.m.) and resuspended in 200 μl of lysis buffer (25 mM Tris-HCl pH 7.4, 150 mM NaCl, 5 mM EDTA) in the presence of Complete, a cocktail of protease inhibitors (Roche) and 1% Triton X-100. After 20 min of incubation at 4°C, the lysat was adjusted to 40% OptiPrep (Nycodenz), overlaid with a 30% and 0% OptiPrep cushions and centrifuged for 2 h centrifugation at 55 000 rpm (4°C) using a TLS.55 rotor. Six fractions were collected from the top and precipitated with 6% trichloroacetic acid in the presence of sodium deoxycholate as a carrier.
Sub-fractionation of late endosomes
Late endosomes were described as above and submitted to 5 sequential freezing in liquid nitrogen and thawing at 37°C cycles in order to mechanically disrupt the compartment. Suspension containing broken late endosomes was then centrifuged for 40 minutes at 70000 rpm in a TLA 100.3 rotor. The pellet was resuspended in 500 µl of 40% sucrose in 3 mM imidazole, pH 7.4 loaded at the bottom of a SW40 tube and overlaid with a linear 8–40% sucrose gradient in the same buffer, and centrifuged at 4°C in the SW40 rotor for 16 h at 35000 rpm. Fractions (1 ml each) were collected from the top of the gradient.
Phospholipid and cholesterol analysis
Lipids were extracted form membrane fractions using CHCl3/MeOH and then separated by two-dimensional thin layer chromatography (TLC) for phospholipids analysis [48], [49]. The first dimension was run with chloroform, methanol, 32% ammonia (65∶35∶5, v/v) and the second with chloroform/acetone/methanol/acetic acid/water (50∶20∶10∶12.5∶5), v/v). Phospholipids were revealed by burning the TLC plate at 160°C after immersion in 1.5 mM cupric acetate-8% H3PO4 solution. For cholesterol analysis, lipids were extracted as above, analyzed on a one-dimensional TLC in heptane/ethylether/acetic acid (18∶6∶2, v/v) and stained with copper. Both cholesterol and phospholipids were quantified by densitometry using the ScanAnalysis software.
Immunoblotting, aerolysin overlays and protein quantification
Proteins were separated by SDS-PAGE using 12.5% acrylamide gels unless stated otherwise and transferred onto a nitrocellulose membrane. Western blots were revealed with SuperSignal Chemiluminescence (Pierce). Aerolysin overlays were performed as described [50]. Protein contents of cellular fractions were determined using bicinchoninic acid (BCA, Pierce).
Electron microscopy
C2C12 cells were fixed in 8% paraformaldehyde and processed for frozen sectioning. Sections were labeled with affinity purified antibodies to flotillin-1 [28] followed by protein A-gold and then with aerolysin-biotin and anti-biotin-gold, as described previously [29].
Results
Lipid composition of late endosomal DRMs
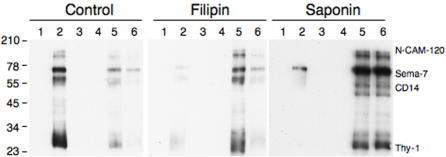
We have previously shown that detergent resistant membranes (DRMs) can be isolated from late endosomes [29] purified from baby hamster kidney (BHK) cells using a well-established subcellular fractionation protocol [29], [47]. These DRMs were found to contain well-characterized raft marker proteins such as GPI-anchored proteins and flotillin-1 (for references concerning these markers see [8], [20], [51], [52]) but were devoid of the transmembrane glycoprotein lamp1 and the lipid anchored GTPase Rab7 [29]. It is important to note that since detergent solubilization was performed on a purified organelle obtained in a relatively low abundance, the detergent to protein ratio used was five to ten times higher, for technical reasons, than that routinely used by us and other on whole cell extracts. Thus the obtained membranes are highly detergent resistant. To test whether late endosomal DRMs are sensitive to cholesterol affecting drugs, an important criterion for being a raft-like domain [4], we treated late endosomes with either the cholesterol clustering agent saponin [53], [54] or the cholesterol binding compound filipin [38]. We did not perform cholesterol extractions using ß-methyl-cyclodextrin, a drug commonly used to disrupt rafts [55], since we have previously shown that on BHK cells this treatment does not to lead the release of GPI-anchored proteins from DRMs [54]. BHK cells contain 4 major GPI-anchored proteins, N-CAM-140, semaphorin-7, CD14 and Thy-1, which can be detected by overlay using the GPI-specific bacterial toxin aerolysin [29]. As shown in Fig. 1, whereas GPI-anchored proteins are abundant in the DRM fraction (fraction 2) of untreated late endosomes, treatment of the purified organelle with either saponin or filipin prior to Triton X-100 solubilization, led to the redistribution of these proteins to the high density detergent soluble fractions on these Optiprep gradients.
Figure 1. Late endosomal DRMs are sensitive to cholesterol affecting reagents.
Late endosomes were prepared from BHK cells using a sucrose step gradient, treated or not with either filipin (1μg/ml for 1 h at 37°C) or saponin (0.4% for 1 h at 4°C) and then submitted to solubilization in 1% Triton X-100 at 4°C. The lysat was submitted to an OptiPrep flotation gradient and 6 fractions of 400 µl were collected. Each fraction was analyzed by SDS-PAGE followed by an aerolysin overlay to identify the GPI-anchored proteins of BKH cells: N-CAM-120, semaphorin-7 (Sema-7), CD14 and Thy-1.
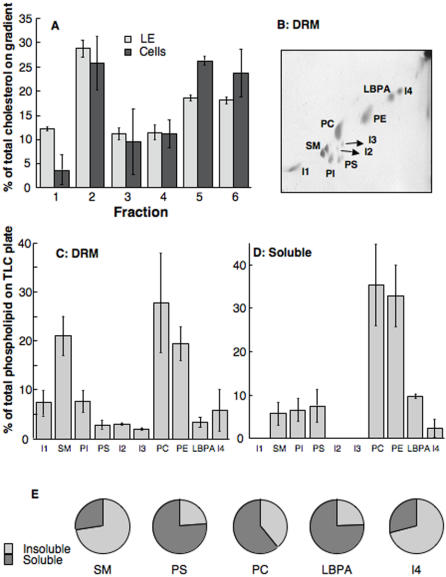
These observations indicate that late endosomal DRMs fulfill the criterion of being cholesterol dependent. We next investigated the distribution of cholesterol itself in these DRM fractions. As shown in Fig. 2A, DRMs from late endosomes contain approximately 40% of the total cholesterol content of compartment (Fractions 1 and 2) as determined by thin layer chromatography (TLC), with<40% in soluble membranes (fractions 5 and 6, note that only fraction 6 is fully soluble since fraction 5 already contains the first interface of the step density gradient between 40 and 35% Optiprep). The percentage of detergent resistant cholesterol was somewhat higher in late endosomes than in whole cells (≈30% in DRMs) (Fig. 2A). This observation is all the more significant considering that the high detergent to protein ratio used to isolate late endosomal DRMs when compared to the one used for the isolation of DRMs from total cells.
Figure 2. Lipid analysis of late endosomal Triton X-100 resistant membranes.
Late endosomes were prepared from BHK cells, submitted to Triton X-100 solubilization and Optiprep gradient analysis as in Fig. 1. Lipids were extracted and analyzed by 1D (A) or 2D TLC (B–E). A: The cholesterol content of each fraction was determined by 1D TLC followed by densitometric analysis. For comparison, total BHK cells were also submitted to Triton solubilization, Optiprep flotation gradients and cholesterol analysis. Error bars represent the standard deviation (n = 3). B: After pooling fractions 1 and 2 from the top of the Optiprep gradient (corresponding to the DRMs), lipids were extracted and analyzed by 2D TLC. I: unidentified lipid, PI: phosphatidylinositol, PS: phosphatidylserine, SM: sphingomyelins, PE: phosphatidylethanolamine, PC: phosphatidylcholine, LBPA: Lysobiphosphatidic acid. C–D: The amount of each phospholipid in fractions 1+2 (C) and fraction 6 (D) were quantified by densitometry. Each phospholipid is expressed as percentage of the total amount of phospholipids on the TLC plate. Error bars represent the standard deviation (n = 3). E: In order to evaluate the distribution of SM, PS, PC, LBPA and I4 through out the Optiprep gradient, the content of these lipids in fractions 1+2 and in fraction 5+6 was determined. For each lipid, the distribution between these two pools was plotted.
We next analyzed the lipid composition of the DRMs fractions (fractions 1+2, Fig. 2B and C) in comparison to that of the detergent soluble fraction 6 (Fig. 2D). Lipids were extracted and the relative proportions of phosphatidyl choline (PC), phosphatidyl ethanolamine (PE), phosphatidyl inositol (PI), phosphatidyl serine (PS), sphingomyelin species (SM), lysobisphosphatidic acid (LBPA) were analyzed by 2 dimensional TLC (Fig. 2B). Each spot on the 2D TLC plate was quantified by densitometry and expressed as a percentage of the total intensity on the plate. The three major known phospholipids in DRMs were SM, PC and PE, but, interestingly, 3 unknown lipids (termed I1 to I3) were detected almost exclusively in the DRMs and one was significantly DRM-enriched (I4). By contrast, the unusual late endosomal lipid LBPA [48], although detected in DRMs, was significantly enriched in the soluble fraction. When plotting relative amounts of several phospholipid species in DRM fractions 1+2 vs. fractions 5+6, SM and I4 were mainly present in the insoluble fractions as opposed to LBPA and PS (Fig. 2E). Other lipids such as PC (Fig. 2E) and PI (not shown) were more evenly distributed.
Taken together, these observations show that DRMs from late endosomes share important properties with plasma membrane raft domains: they are rich in cholesterol and sphingomyelin and are sensitive to cholesterol affecting drugs, they contain raft marker proteins such as GPI-anchored proteins and flotillin-1. In addition they are enriched in 4 intriguing yet uncharacterized phospholipids.
Differential solubilization of DRM associated proteins
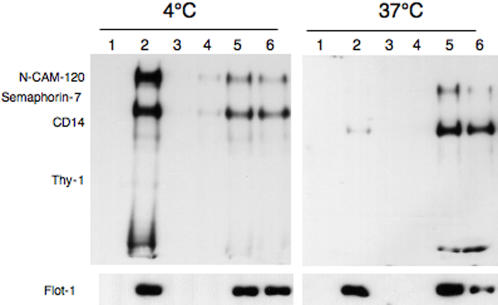
Despite the rather harsh solubilization step (higher detergent to protein ratio than for the preparation of DRMs from whole cells), DRMs isolated from late endosomes contained 40% of the total organellar cholesterol. We therefore wondered whether these domains were particularly resistant to solubilization and therefore performed the solubilization at 37°C. This treatment led to the solubilization of GPI-anchored proteins, but interestingly not to that of flotillin-1 (Fig. 3), suggestive of a differential distribution. Whereas, GPI-anchored proteins and flotillin-1 could be part of the same domain, one being on the periphery and the other in the center, as proposed for prion protein and thy-1 [56], [57], they could also reside on spatially segregated domains. Since late endosomes contain internal vesicles, one attractive possibility is that GPI-anchored proteins and flotillin-1 differentially distribute to the internal and limiting membranes of the organelle.
Figure 3. Detection of two types of late endosomal DRMs.
Late endosomes from BHK cells were submitted to solubilization in 1% Triton X-100 either at 4°C or at 37°C. The lysat was subsequently analyzed on an Optiprep gradient and 6 fractions were collected from the top. The total of each fraction was submitted to SDS-PAGE and Western blotting to detect flotillin-1 or to an aerolysin overlay to reveal GPI-anchored proteins.
Distribution of raft-marker proteins to internal and limiting membranes of late endosomes
We have previously shown that the internal membranes of late endosomes, which contain high amounts of LBPA [48], can be separated from the limiting membrane, after gentle mechanical disruption (by freeze thawing) of the organelle, followed by a continuous sucrose gradient [49]. Using this sub-organellar fractionation protocol, we analyzed the distribution of flotillin-1 and GPI-anchored proteins as well as that of three other proteins involved in cholesterol metabolism: ApoA1– an LDL apoprotein, MLN64 – a late endosomal steroidogenic acute regulatory protein (StAR) domain containing protein involved in sterol trafficking [58] and NPC1 – the Niemann Pick type C 1 protein involved in lipid trafficking [59]. GPI-anchored proteins co-fractionated with LBPA (Fig 4A), which was quantified by ELISA [49], and were mainly found in fractions 4 and 5 (Fig. 4B), indicating that these contained predominantly the intralumenal membranes of late endosomes. As expected, ApoA1, originating from internalized LDL particles, was also concentrated in fraction 4. In contrast, flotillin-1, MLN64 and NPC1 were all found in fractions 6 to 9 which also contain the limiting membrane marker Lamp1 [49]. These findings are in good agreement with electron microscopy studies in which MLN64 was found to be restricted to the limiting membrane of late endosomes [60]. These data altogether indicate that MLN64 and NPC1, which are both involved in sterol trafficking, localize to the limiting membrane.
Figure 4. Distribution of lipid metabolism-related proteins and raft markers in late endosomes.
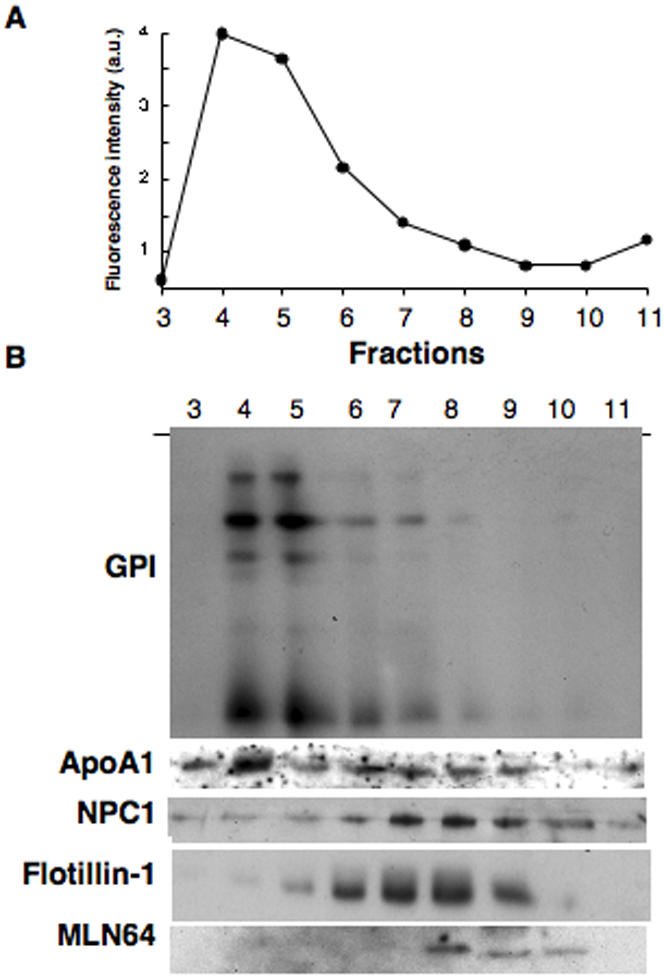
Late endosomes were purified from BHK cells and submitted to sub-organellar fractionation after breaking the organelle by cycles of freezing and thawing followed by sucrose density gradients. 12 fractions were collected from the top and analyzed for the presence of LBPA using an ELISA assay (A) or by SDS-PAGE followed by Western blotting for the presence NPC1, MLN64, flotillin-1 and ApoE (B). GPI-anchored proteins were detected by aerolysin overlay (B).
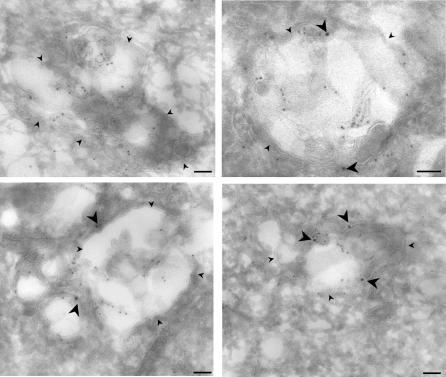
The segregation between GPI-anchored proteins and flotillin-1 was confirmed by electron microscopy using C2C12 cells (Fig. 5, the flotillin-1 antibodies showed negligible labeling by immunoelectron microscopy on BHK cells). For quantifications, frozen sections were double labeled for GPI-anchored proteins (using aerolysin) and flotillin-1. Well-preserved multivesicular late endosomes were examined at random and gold particles (n = 450) were assigned to the limiting membrane or to internal membranes. For 85% of late endosomes, flotillin-1 labeling was higher on limiting membranes with a ratio of 4.8 to 1. On 15% of late endosomes, flotillin-1 was however more abundant on internal membranes leading to an over all ratio of labeling on limiting vs. internal membranes of 3.1 .to 1. The distribution of GPI-anchored proteins was the reverse with a ratio of limiting to internal membranes of 0.3 to 1 for 80% of late endosomes. Again 20% of late endosomes behaved differently showing a higher GPI labeling on the limiting membrane leading to an over all labeling ratio of 0.44 to 1 of limiting to internal membranes.
Figure 5. Immunoelectron microscopic localization of flotillin-1 and GPI-anchored proteins on multivesicular endosomes.
Cultured C2C12 cells were fixed in paraformaldehyde and processed for frozen sectioning. Sections were labeled with antibodies to flotillin-1 and 15 nm protein A-gold and then overlaid with aerolysin-biotin followed by 10 nm anti-biotin-gold. Aerolysin labeling for GPI-anchored proteins is mainly within the internal membranes of the late endosomes. In contrast, flotillin-1 labeling (large arrowheads) is predominantly associated with the limiting membrane (small arrowheads). Bars, 100 nm.
Altogether these observations indicate that detergent resistant membrane domains, with different biochemical properties and different protein composition, are found on the limiting and internal membranes of late endosomes, the former being more resistant and containing flotillin-1, and the latter containing GPI-anchored proteins.
Discussion
It has long been known that late endosomes have a complex morphology with tubular and vesicular regions, which in turn can be multivesicular or multilamellar [41], [42]. These morphological distinct areas, which by themselves define different membrane domains, are likely to be further divided into macro or microdomains. Consistently, Rab9 and Rab7, two late endosomal Rab proteins, occupy distinct domains within late endosomal membranes [61]. Here we have studied the existence of lipid raft-like domains in late endosomes. We used multiple assays, the first of which was the isolation of detergent resistant membranes from the purified organelle. Although this method should be used with care and has its drawbacks [4], [21], [62], it remains powerful in combination with other methods and in comparative studies on the same cell type, complementary approaches which we carried out here.
Our data indicate that late endosomal DRMs are rich in cholesterol (40% of the cholesterol present in the compartment) and in sphingomyelin (70% of that in the organelle), sensitive to cholesterol affecting drugs and contain well-characterized raft-marker proteins, altogether supporting that these DRMs contain raft-like domains [4], [23], [55]. These DRMs also contained some LBPA, a lipid that is confined to late endosomes and abundant in intralumenal membranes. While spurious association of LBPA to DRMs cannot be excluded at this point, it is possible that the unusual nature of this non-hydrolysable lipid confers special properties to these domains, including fusogenic properties [49]. Interestingly, late endosomal DRMs also contained 3 unidentified lipids, I2, I3, and I4, which were not present in the detergent soluble membranes and will be of interest for future studies.
Solubilization of late endosomes at different temperatures revealed differential behavior hinting towards the existence of different raft-like domains within this complex compartment. This hypothesis was supported by sub-organellar fractionation and electron microscopy. More specifically, we found that flotillin-1-positive domains reside on the limiting membrane of late endosomes and are very resistant to detergent solubilization whereas GPI-domains reside on intraluminal vesicles and are more detergent sensitive. Our finding that such raft-like membranes, containing GPI-anchored proteins, are present within intralumenal membranes of these multivesicular endosomes fits nicely with electron microscopy observations using a cholesterol-binding toxin showing that cholesterol is abundant within these lumenal membranes [63]. It has recently been shown that GPI-anchored proteins can be endocytosed from the plasma membrane via a flotillin-1 dependent pathway [36]. Understanding how GPI-anchored proteins and flotillin-1 segregate from one another at later stages of the endocytic pathway will be of great interest. Importantly, both limiting and luminal membranes also contain fluid membranes as illustrated by the detergent sensitivity of lamp1 and LBPA respectively. Thus both limiting and luminal membranes are composed of diverse lipid domains.
It is now well-accepted that the sorting of down regulated signaling receptors into intralumenal membranes mediates their lysosomal targeting and degradation [64]. By contrast, some proteins, like the major glycoprotein Lamp1 [49], [65], the sterol traffic regulator MLN64 [60] and flotillin-1 (this study) remain preferentially on the limiting membrane. In addition, some proteins and receptors can also be sorted into late endosomes, but then recycle back to the limiting membrane, presumably via back-fusion of intralumenal vesicles with the limiting membranes [66] — a process hijacked by some toxins and viruses [67]. It is tempting to speculate that protein and lipid sorting into and out of endosomes may be controlled, at least in part, by differential partitioning into different raft-like membrane domains. In addition, such differences in the protein composition and physico-chemical properties of these two pools of raft domains likely affect their function, which could be altered in lipid storage diseases which have been shown to lead to cholesterol accumulation in late endosomes.
Acknowledgments
We would like to thank Dr. E. Ikonen and Dr J. F. Strauss fpr sharing antibodies and members of the van der Goot and Gruenberg labs for fruitful discussions and critical reading of the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by a grant from the Swiss National Science Foundation to J.G and to G.v.d.G. and a grant from the National Health and Medical Research Council of Australia to R.P. G.v.d.G is an international Fellow of the Howard Hughes Medical Institute.
References
- 1.Hancock JF. Lipid rafts: contentious only from simplistic standpoints. Nat Rev Mol Cell Biol. 2006;7:456–462. doi: 10.1038/nrm1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 3.Harder T, Simons K. Caveolae, DIGs, and the dynamics of shingolipid-cholesterol microdomains. Current Opinion in Cell Biology. 1997;9:534–542. doi: 10.1016/s0955-0674(97)80030-0. [DOI] [PubMed] [Google Scholar]
- 4.Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 5.Simons K, Ikonen E. Functiomal rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 6.Douglass AD, Vale RD. Single-molecule microscopy reveals plasma membrane microdomains created by protein-protein networks that exclude or trap signaling molecules in T cells. Cell. 2005;121:937–950. doi: 10.1016/j.cell.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parton RG, Hancock JF. Lipid rafts and plasma membrane microorganization: insights from Ras. Trends Cell Biol. 2004;14:141–147. doi: 10.1016/j.tcb.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Morris R, Cox H, Mombelli E, Quinn PJ. Rafts, little caves and large potholes: how lipid structure interacts with membrane proteins to create functionally diverse membrane environments. Subcell Biochem. 2004;37:35–118. doi: 10.1007/978-1-4757-5806-1_2. [DOI] [PubMed] [Google Scholar]
- 9.Abrami L, Leppla SH, van der Goot FG. Receptor palmitoylation and ubiquitination regulate anthrax toxin endocytosis. J Cell Biol. 2006;172:309–320. doi: 10.1083/jcb.200507067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linder ME, Deschenes RJ. Palmitoylation: policing protein stability and traffic. Nat Rev Mol Cell Biol. 2007;8:74–84. doi: 10.1038/nrm2084. [DOI] [PubMed] [Google Scholar]
- 11.de Mello Coelho V, Nguyen D, Giri B, Bunbury A, Schaffer E, et al. Quantitative differences in lipid raft components between murine CD4+ and CD8+ T cells. BMC Immunol. 2004;5:2. doi: 10.1186/1471-2172-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magee T, Pirinen N, Adler J, Pagakis SN, Parmryd I. Lipid rafts: cell surface platforms for T cell signaling. Biol Res. 2002;35:127–131. doi: 10.4067/s0716-97602002000200003. [DOI] [PubMed] [Google Scholar]
- 13.Pierce SK. Lipid rafts and B-cell activation. Nat Rev Immunol. 2002;2:96–105. doi: 10.1038/nri726. [DOI] [PubMed] [Google Scholar]
- 14.Pike LJ. Lipid rafts: heterogeneity on the high seas. Biochem J. 2004;378:281–292. doi: 10.1042/BJ20031672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harder T, Scheiffele P, Verkade P, Simons K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J Cell Biol. 1998;141:929–942. doi: 10.1083/jcb.141.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ritchie K, Iino R, Fujiwara T, Murase K, Kusumi A. The fence and picket structure of the plasma membrane of live cells as revealed by single molecule techniques (Review). Mol Membr Biol. 2003;20:13–18. doi: 10.1080/0968768021000055698. [DOI] [PubMed] [Google Scholar]
- 17.Gaus K, Chklovskaia E, Fazekas de St Groth B, Jessup W, Harder T. Condensation of the plasma membrane at the site of T lymphocyte activation. J Cell Biol. 2005;171:121–131. doi: 10.1083/jcb.200505047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma P, Sabharanjak S, Mayor S. Endocytosis of lipid rafts: an identity crisis. Semin Cell Dev Biol. 2002;13:205–214. doi: 10.1016/s1084-9521(02)00049-6. [DOI] [PubMed] [Google Scholar]
- 19.Nabi IR, Le PU. Caveolae/raft-dependent endocytosis. J Cell Biol. 2003;161:673–677. doi: 10.1083/jcb.200302028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parton RG, Richards AA. Lipid rafts and caveolae as portals for endocytosis: new insights and common mechanisms. Traffic. 2003;4:724–738. doi: 10.1034/j.1600-0854.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- 21.Heerklotz H. Triton promotes domain formation in lipid raft mixtures. Biophys J. 2002;83:2693–2701. doi: 10.1016/S0006-3495(02)75278-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lichtenberg D, Goni FM, Heerklotz H. Detergent-resistant membranes should not be identified with membrane rafts. Trends Biochem Sci. 2005;30:430–436. doi: 10.1016/j.tibs.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Brown DA. Lipid rafts, detergent-resistant membranes, and raft targeting signals. Physiology (Bethesda) 2006;21:430–439. doi: 10.1152/physiol.00032.2006. [DOI] [PubMed] [Google Scholar]
- 24.Babiychuk EB, Draeger A. Biochemical characterization of detergent-resistant membranes: a systematic approach. Biochem J. 2006;397:407–416. doi: 10.1042/BJ20060056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gallegos AM, Storey SM, Kier AB, Schroeder F, Ball JM. Structure and cholesterol dynamics of caveolae/raft and nonraft plasma membrane domains. Biochemistry. 2006;45:12100–12116. doi: 10.1021/bi0602720. [DOI] [PubMed] [Google Scholar]
- 26.Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- 27.Gagescu R, Demaurex N, Parton RG, Hunziker W, Huber LA, et al. The recycling endosome of Madin-Darby canine kidney cells is a mildly acidic compartment rich in raft components. Mol Biol Cell. 2000;11:2775–2791. doi: 10.1091/mbc.11.8.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dermine JF, Duclos S, Garin J, St-Louis F, Rea S, et al. Flotillin-1-enriched lipid raft domains accumulate on maturing phagosomes. J Biol Chem. 2001;276:18507–18512. doi: 10.1074/jbc.M101113200. [DOI] [PubMed] [Google Scholar]
- 29.Fivaz M, Vilbois F, Thurnheer S, Pasquali C, Abrami L, et al. Differential sorting and fate of endocytosed GPI-anchored proteins. EMBO J. 2002;21:3989–4000. doi: 10.1093/emboj/cdf398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fullekrug J, Simons K. Lipid rafts and apical membrane traffic. Ann N Y Acad Sci. 2004;1014:164–169. doi: 10.1196/annals.1294.017. [DOI] [PubMed] [Google Scholar]
- 31.Browman DT, Resek ME, Zajchowski LD, Robbins SM. Erlin-1 and erlin-2 are novel members of the prohibitin family of proteins that define lipid-raft-like domains of the ER. J Cell Sci. 2006;119:3149–3160. doi: 10.1242/jcs.03060. [DOI] [PubMed] [Google Scholar]
- 32.Pielsticker LK, Mann KJ, Lin WL, Sevlever D. Raft-like membrane domains contain enzymatic activities involved in the synthesis of mammalian glycosylphosphatidylinositol anchor intermediates. Biochem Biophys Res Commun. 2005;330:163–171. doi: 10.1016/j.bbrc.2005.02.136. [DOI] [PubMed] [Google Scholar]
- 33.Campana V, Sarnataro D, Fasano C, Casanova P, Paladino S, et al. Detergent-resistant membrane domains but not the proteasome are involved in the misfolding of a PrP mutant retained in the endoplasmic reticulum. J Cell Sci. 2006;119:433–442. doi: 10.1242/jcs.02768. [DOI] [PubMed] [Google Scholar]
- 34.Eberle HB, Serrano RL, Fullekrug J, Schlosser A, Lehmann WD, et al. Identification and characterization of a novel human plant pathogenesis-related protein that localizes to lipid-enriched microdomains in the Golgi complex. J Cell Sci. 2002;115:827–838. doi: 10.1242/jcs.115.4.827. [DOI] [PubMed] [Google Scholar]
- 35.Li X, Kaloyanova D, van Eijk M, Eerland R, van der Goot FG, et al. Involvement of a Golgi-resident GPI-anchored Protein in Maintenance of the Golgi Structure. Mol Biol Cell. 2007;(in press) doi: 10.1091/mbc.E06-03-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glebov OO, Bright NA, Nichols BJ. Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nat Cell Biol. 2006;8:46–54. doi: 10.1038/ncb1342. [DOI] [PubMed] [Google Scholar]
- 37.Pelkmans L. Secrets of caveolae- and lipid raft-mediated endocytosis revealed by mammalian viruses. Biochim Biophys Acta. 2005;1746:295–304. doi: 10.1016/j.bbamcr.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 38.Abrami L, Liu S, Cosson P, Leppla SH, van der Goot FG. Anthrax toxin triggers endocytosis of its receptor via a lipid raft-mediated clathrin-dependent process. J Cell Biol. 2003;160:321–328. doi: 10.1083/jcb.200211018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deinhardt K, Berninghausen O, Willison HJ, Hopkins CR, Schiavo G. Tetanus toxin is internalized by a sequential clathrin-dependent mechanism initiated within lipid microdomains and independent of epsin1. J Cell Biol. 2006;174:459–471. doi: 10.1083/jcb.200508170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirkham M, Parton RG. Clathrin-independent endocytosis: new insights into caveolae and non-caveolar lipid raft carriers. Biochim Biophys Acta. 2005;1746:349–363. doi: 10.1016/j.bbamcr.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 41.Gruenberg J. The endocytic pathway: a mosaic of domains. Nat Rev Mol Cell Biol. 2001;2:721–730. doi: 10.1038/35096054. [DOI] [PubMed] [Google Scholar]
- 42.Gruenberg J, Stenmark H. The biogenesis of multivesicular endosomes. Nat Rev Mol Cell Biol. 2004;5:317–323. doi: 10.1038/nrm1360. [DOI] [PubMed] [Google Scholar]
- 43.Gruenberg J, Howell KE. Membrane traffic in endocytosis: insights from cell-free assays. Annu Rev cell Biol. 1989;5:453–481. doi: 10.1146/annurev.cb.05.110189.002321. [DOI] [PubMed] [Google Scholar]
- 44.Abrami L, Fivaz M, Decroly E, Seidah NG, François J, et al. The pore-forming toxin proaerolysin is processed by furin. J Biol Chem. 1998;273:32656–32661. doi: 10.1074/jbc.273.49.32656. [DOI] [PubMed] [Google Scholar]
- 45.Carozzi AJ, Ikonen E, Lindsay MR, Parton RG. Role of cholesterol in developing T-tubules: analogous mechanisms for T-tubule and caveolae biogenesis. Traffic. 2000;1:326–341. doi: 10.1034/j.1600-0854.2000.010406.x. [DOI] [PubMed] [Google Scholar]
- 46.Buckley JT, Halasa LN, Lund KD, MacIntyre S. Purification and some properties of the hemolytic toxin aerolysin. Can J Biochem. 1981;59:430–435. doi: 10.1139/o81-059. [DOI] [PubMed] [Google Scholar]
- 47.Aniento F, Emans N, Griffiths G, Gruenberg J. Cytoplasmic dynein-dependent vesicular transport from early to late endosomes. J Cell Biol. 1993;123:1373–1387. doi: 10.1083/jcb.123.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kobayashi T, Stang E, Fang KS, de Moerloose P, Parton RG, et al. A lipid associated with the antiphospholipid syndrome regulates endosome structure and function. Nature. 1998;392:193–197. doi: 10.1038/32440. [DOI] [PubMed] [Google Scholar]
- 49.Kobayashi T, Beuchat MH, Chevallier J, Makino A, Mayran N, et al. Separation and characterization of late endosomal membrane domains. J Biol Chem. 2002;277:32157–32164. doi: 10.1074/jbc.M202838200. [DOI] [PubMed] [Google Scholar]
- 50.Abrami L, Fivaz M, Glauser P-E, Parton RG, van der Goot FG. A pore-forming toxin interact with a GPI-anchored protein and causes vacuolation of the endoplasmic reticulum. J Cell Biol. 1998;140:525–540. doi: 10.1083/jcb.140.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharma P, Varma R, Sarasij RC, Ira, Gousset K, et al. Nanoscale organization of multiple GPI-anchored proteins in living cell membranes. Cell. 2004;116:577–589. doi: 10.1016/s0092-8674(04)00167-9. [DOI] [PubMed] [Google Scholar]
- 52.Pimpinelli F, Lehmann S, Maridonneau-Parini I. The scrapie prion protein is present in flotillin-1-positive vesicles in central- but not peripheral-derived neuronal cell lines. Eur J Neurosci. 2005;21:2063–2072. doi: 10.1111/j.1460-9568.2005.04049.x. [DOI] [PubMed] [Google Scholar]
- 53.Schroeder RJ, Ahmed SN, Zhu Y, London E, Brown DA. Cholesterol and sphingolipid enhance the Triton X-100 insolubility of glycosylphosphatidylinositol-anchored proteins by promoting the formation of detergent-insoluble ordered membrane domains. J Biol Chem. 1998;273:1150–1157. doi: 10.1074/jbc.273.2.1150. [DOI] [PubMed] [Google Scholar]
- 54.Abrami L, van der Goot FG. Plasma membrane microdomains act as concentration platforms to facilitate intoxication by aerolysin. J Cell Biol. 1999;147:175–184. doi: 10.1083/jcb.147.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simons K, Toomre D. Lipid rafts and signal transduction. Nature Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 56.Madore N, Smith KL, Graham CH, Jen A, Brady K, et al. Functionally different GPI proteins are organized in different domains on the neuronal surface. Embo J. 1999;18:6917–6926. doi: 10.1093/emboj/18.24.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brugger B, Graham C, Leibrecht I, Mombelli E, Jen A, et al. The membrane domains occupied by glycosylphosphatidylinositol-anchored prion protein and Thy-1 differ in lipid composition. J Biol Chem. 2004;279:7530–7536. doi: 10.1074/jbc.M310207200. [DOI] [PubMed] [Google Scholar]
- 58.Strauss JF, 3rd, Liu P, Christenson LK, Watari H. Sterols and intracellular vesicular trafficking: lessons from the study of NPC1. Steroids. 2002;67:947–951. doi: 10.1016/s0039-128x(02)00042-9. [DOI] [PubMed] [Google Scholar]
- 59.Ikonen E, Holtta-Vuori M. Cellular pathology of Niemann-Pick type C disease. Semin Cell Dev Biol. 2004;15:445–454. doi: 10.1016/j.semcdb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 60.Alpy F, Stoeckel ME, Dierich A, Escola JM, Wendling C, et al. The steroidogenic acute regulatory protein homolog MLN64, a late endosomal cholesterol-binding protein. J Biol Chem. 2001;276:4261–4269. doi: 10.1074/jbc.M006279200. [DOI] [PubMed] [Google Scholar]
- 61.Barbero P, Bittova L, Pfeffer SR. Visualization of Rab9-mediated vesicle transport from endosomes to the trans-Golgi in living cells. J Cell Biol. 2002;156:511–518. doi: 10.1083/jcb.200109030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schuck S, Honsho M, Ekroos K, Shevchenko A, Simons K. Resistance of cell membranes to different detergents. Proc Natl Acad Sci U S A. 2003;100:5795–5800. doi: 10.1073/pnas.0631579100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mobius W, van Donselaar E, Ohno-Iwashita Y, Shimada Y, Heijnen HF, et al. Recycling compartments and the internal vesicles of multivesicular bodies harbor most of the cholesterol found in the endocytic pathway. Traffic. 2003;4:222–231. doi: 10.1034/j.1600-0854.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 64.Hurley JH, Emr SD. The ESCRT complexes: structure and mechanism of a membrane-trafficking network. Annu Rev Biophys Biomol Struct. 2006;35:277–298. doi: 10.1146/annurev.biophys.35.040405.102126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Griffiths G, Hoflack B, Simons K, Mellman I, Kornfeld S. The mannose 6-phosphate receptor and the biogenesis of lysosomes. Cell. 1988;52:329–341. doi: 10.1016/s0092-8674(88)80026-6. [DOI] [PubMed] [Google Scholar]
- 66.van der Goot FG, Gruenberg J. Intra-endosomal membrane traffic. Trends Cell Biol. 2006;16:514–521. doi: 10.1016/j.tcb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 67.Gruenberg J, van der Goot FG. Mechanisms of pathogen entry through the endosomal compartments. Nat Rev Mol Cell Biol. 2006;7:495–504. doi: 10.1038/nrm1959. [DOI] [PubMed] [Google Scholar]