Medulloblastomas, embryonal neoplasms arising in the cerebellum, are the most common malignant brain tumors in children. They are composed of primitive cells with the potential to differentiate along neuronal and glial lines. Similar lesions, known as primitive neuroectodermal tumors (PNETs), arise outside the posterior fossa, albeit rarely. Several molecular pathways important in cerebellar development have been implicated in medulloblastoma pathogenesis (reviewed in 1-3 ). A better understanding of the cells and signaling events involved in medulloblastoma tumorigenesis will be critical to the development of targeted therapies for these aggressive neoplasms, as evidenced by the inhibition of medulloblastoma growth by drugs blocking Hedgehog pathway activity. 4 In this issue of The American Journal of Pathology, Tong and colleagues 5 describe a new medulloblastoma model in which tumors arise from the cerebellar external granule cell layer (EGL) of mice lacking p53 and PARP. The up-regulation of the Hedgehog pathway effector Gli in all of their tumors suggests a more general role for Hedgehog signaling than was previously appreciated. In medulloblastomas, some cellular pathways may be more equal than others.
Cerebellar Development and Medulloblastoma
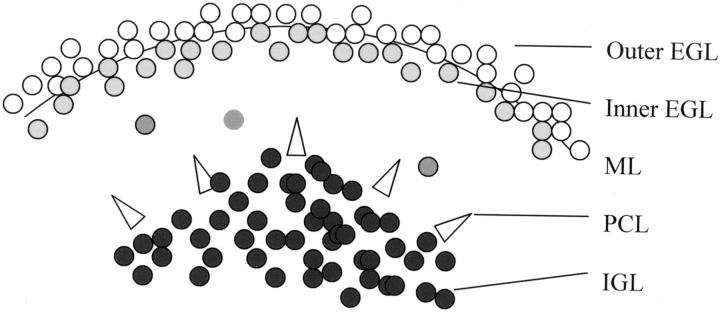
The analysis of cerebellar development has shed considerable light on medulloblastoma pathogenesis, as several genetic pathways seem to be critical for the development of both normal cerebellar structures and central nervous system (CNS) embryonal tumors. Unlike cerebral cortex, which derives from a single subventricular matrix, the cerebellum develops from two germinal matrix regions (reviewed in 6 ). In cerebellum, subventricular matrix cells give rise to neurons of the deep nuclei, Purkinje cells, Golgi neurons, and glial cells. A second matrix region, the EGL, is formed by neuroblasts from the rhombic lip that migrate over the cerebellar surface. These neuroblasts first proliferate in the outer EGL, then exit the cell cycle and move to the inner EGL (Figure 1) ▶ . Immature granule neurons migrate inwards from the EGL along Bergmann glia, transiting through the molecular layer and past the Purkinje cells to take up residence in the internal granule cell layer (IGL).
Figure 1.
The developing cerebellum. EGL, external granule cell layer; ML, molecular layer; PCL, Purkinje cell layer; IGL, internal granule cell layer.
The Hedgehog pathway is the best-characterized regulator of EGL proliferation and cerebellar size. The ligand Sonic Hedgehog is secreted by Purkinje cells, and promotes proliferation of granule cell precursors in the EGL by binding to its receptor PTCH. 7-9 Several markers can be used to track the exit of EGL neuroblasts from the cell cycle and their progressive differentiation. Proliferating neuroblasts in the outer EGL express Math-1, p53, and NeuN. 10-12 As cells move to the inner portion of the EGL, they down-regulate proliferation markers and begin expressing promoters of neuronal differentiation such as the cell cycle-dependent kinase inhibitor p27Kip1 and the bHLH transcription factors NeuroD and NeuroD2. 13-16 Markers of neuronal differentiation such as class III β-tubulin, MAP-2, synaptophysin, and nestin are also expressed in post-mitotic inner EGL cells or in differentiated neurons of the IGL. 17-20
The histogenesis of medulloblastomas has been controversial for many years. Some feel they arise primarily from primitive neuroectodermal cells in the germinal matrix surrounding the ventricle. 21,22 Others have argued that proliferating neuroblasts of the cerebellar EGL are the most likely progenitors. 23,24 It is also possible that cells from both of these locations give rise to medulloblastomas. 25 Whatever their origin, it is clear that many human medulloblastomas express the cerebellar developmental markers discussed above.
Medulloblastoma Genetics
In man, three inherited syndromes associated with medulloblastomas have been described: Turcot’s, Gorlin’s, and Li Fraumeni (reviewed in 3 ). Gorlin’s syndrome results from inherited mutations in the Hedgehog pathway gene PTCH. Mutations in the Hedgehog pathway members PTCH, PTCH 2, Smo, or Sufu have been identified in approximately 25% of sporadic medulloblastomas as well. 26-30 In addition, mutation of the PTCH gene in mice causes medulloblastoma-like tumors to form in 10 to 15% of heterozygotes by 6 months of age. 31,32 These murine tumors derive from the EGL, providing further support for the histogenetic importance of this neuroblast layer in medulloblastomas.
Turcot’s syndrome is caused by germline mutations in the gene APC, a member of the Wnt signaling pathway. The pathway contains several proteins (APC, Axin, GSK3) acting in concert to promote the proteosomal degradation of β-catenin. 33 Mutations in APC, β-catenin, or Axin have been identified in approximately 25% of sporadic medulloblastomas. 34-37
Li Fraumeni syndrome is caused by inherited mutations in the p53 tumor suppressor gene (reviewed in 38 ). Affected individuals develop a large spectrum of CNS and extra-CNS tumors, including medulloblastomas. 39 Interestingly, alterations in p53 are relatively rare in sporadic medulloblastomas, with an incidence of approximately 5%. 40-43 MDM2 amplification can inhibit p53 function in many tumor types, but no such amplification has been detected in medulloblastomas. 40,44
Mouse Medulloblastoma Models
Despite the paucity of human medulloblastomas with p53 mutations, a growing number of investigators have reported that lack of p53 function plays an important role in the formation of medulloblastomas in rodent models. The first experiments to suggest this were performed in Syrian golden hamsters. Perinatal infection of EGL cells by JC virus resulted in medulloblastomas, presumably via the inactivation of p53 and Rb by virus-encoded T antigen. 45,46 Subsequent experiments using retrovirus-mediated transfer of SV40 T antigen or transgenic expression of JC virus T antigen in mice and rats confirmed the medulloblastoma-promoting effects of this protein. 47,48 Targeted deletion of both p53 and Rb in the cerebellum also results in medulloblastoma. 49
Loss of p53 can enhance the medulloblastoma-promoting effects of PTCH mutation. Wetmore and colleagues 50 have demonstrated that p53 inactivation markedly increases the number of medulloblastomas forming in PTCH heterozygous animals. Ionizing radiation also seems to strongly promote medulloblastoma development in PTCH heterozygotes when applied to newborn mice in which the EGL is still proliferating. 51 In all of these models, tumors developed months after the initial genetic insults, suggesting additional mutational events were required.
It is unclear to what extent the murine medulloblastomas with loss of p53 or Rb function accurately model human tumors. While these neoplasms appear similar to human medulloblastomas, many have viewed them with skepticism because human cases largely lack mutations in these genes. The data presented above suggest that inactivation of p53, Rb, and other genes controlling DNA repair and apoptosis may promote medulloblastoma formation in mice by fostering the accumulation of genetic defects in other cellular pathways. If genetic instability during a defined developmental window is responsible for tumor formation, it is the additional mutational events in the tumors that will best define the genetic similarity between murine and human medulloblastomas. In their paper, Tong and colleagues 5 show that increased genetic instability caused by abrogation of p53 and PARP function results in murine medulloblastomas with activation of the Hedgehog pathway. This represents the first examination of Hedghog function in murine medulloblastomas without underlying PTCH mutations. The selection for molecular events activating Hedgehog signaling suggests the medulloblastomas arising in mice with genomic instability may indeed accurately model human medulloblastomas.
Mice Lacking PARP and p53 Develop Medulloblastomas
Poly(ADP-ribose) polymerase (PARP) binds DNA breaks and facilitates their repair. In earlier work, Tong and colleagues 52 showed that p53 and PARP interact to maintain genome integrity. Others have demonstrated that loss of PARP in neurons causes a resistance to cell death. 53 Deletion of both PARP and p53 in transgenic mice results in embryonal brain tumors not seen with p53 loss alone, suggesting that cooperation of DNA end-processing and cell cycle checkpoint molecules is required to suppress malignant transformation of neuronal cells. 52
The paper in this issue characterizes the embryonal tumors arising in p53, PARP null mice more closely. 5 CNS tumors developed in approximately half of the animals with a median age of onset of 16 weeks. Interestingly, more than twice as many males developed tumors as females, a ratio similar to that observed in humans. The increased frequency and somewhat more aggressive biology of medulloblastomas in boys have never been explained, and this new mouse model may prove useful in examining the phenomenon. All but one of the tumors were centered in the cerebellum, with the final lesion detected in the cerebral cortex. The tumors appeared highly similar to human medulloblastomas, with sheets of embryonal cells and “neuroblastic” rosettes. In eight animals early lesions were observed in the EGL.
Immunohistochemical analysis supported the similarity to human medulloblastoma. Tumors were positive for the neuronal markers NeuN, MAP-2, and synaptophysin. GFAP-positive tumor cells were also occasionally seen. MATH-1, a neuron-specific basic helix-loop-helix transcription factor required for the proliferation of granule cells in the cerebellum, was expressed in the tumors. As is the case in human medulloblastomas, numerous chromosomal aberrations were detected. Most intriguingly, the Hedgehog pathway appeared to be activated in all tumors examined, with markedly increased expression of the Hedgehog effector Gli, possibly resulting from PTCH deficiency.
This work raises several questions. First, is PARP mutated in human medulloblastomas? It is clear that many genes involved in medulloblastoma pathogenesis remain to be discovered, and PARP may be one of these. The tumor aneuploidy caused by PARP deficiency in mice is similar to that seen in human medulloblastomas, supporting a possible causal association. Alternatively, loss of PARP could facilitate mutagenesis in mice by promoting additional DNA damage and chromosomal aberrations, but not be involved in human lesions. Loss of heterozygosity and sequence analysis of the PARP gene in human tumors will be required to further evaluate these issues.
A second question is whether the single tumor arising in the cerebral cortex was generated through the same genetic mechanism as the cerebellar medulloblastomas. This cortical lesion seems similar to the supratentorial PNET found in humans. As discussed above, no EGL exists during cerebral cortical development, thus the tumor must have developed from different precursors. Human supratentorial PNET are considerably rarer than medulloblastoma, making them difficult to study. By examining similar lesions in mice, we may be able to better define the precursor cells and genetic pathways involved in their formation.
Finally, it remains to be seen how PTCH expression is lost in the tumors reported by Tong and colleagues. While it was initially proposed that loss of only one PTCH allele was sufficient for medulloblastoma formation in mice, recent reports suggest that the second allele is also inactivated by methylation or mutation. 4,51,54,55 PTCH could be inactivated by one of these methods in the p53, PARP null tumors, or its expression might be down-regulated by other means. The loss of PTCH expression and activation of Gli in all of the tumors examined is particularly interesting in light of the recent finding that 100% of human medulloblastomas tested respond to Hedghog inhibitors in vitro, while only a quarter of the cases should have mutations in the pathway. 4 Taken together, these data suggest that Hedgehog activity could be critical in most, if not all, medulloblastomas. The analysis of additional cellular pathways in this new murine tumor model may identify other genes commonly mutated on the road to medulloblastoma formation.
Acknowledgments
I thank Dr. David M. Berman and Dr. Peter C. Burger for helpful discussions and critical reading of the manuscript. Charles Eberhart is a recipient of a Burroughs Wellcome Career Award in the Biomedical Sciences.
Footnotes
Address reprint requests to Charles G. Eberhart, Johns Hopkins School of Medicine, Department of Pathology, 558 Ross Research Building, 720 Rutland Avenue, Baltimore, MD 21205-2196. E-mail: ceberha@jhmi.edu.
References
- 1.Gilbertson R: Paediatric embryonic brain tumours: biological and clinical relevance of molecular genetic abnormalities. Eur J Cancer 2002, 38:675-685 [DOI] [PubMed] [Google Scholar]
- 2.Ellison D: Classifying the medulloblastoma: insights from morphology and molecular genetics. Neuropathol Appl Neurobiol 2002, 28:257-282 [DOI] [PubMed] [Google Scholar]
- 3.Taylor MD, Mainprize TG, Rutka JT: Molecular insight into medulloblastoma and central nervous system primitive neuroectodermal tumor biology from hereditary syndromes: a review. Neurosurgery 2000, 47:888-901 [DOI] [PubMed] [Google Scholar]
- 4.Berman DM, Karhadkar SS, Hallahan AR, Pritchard JI, Eberhart CG, Watkins DN, Chen JK, Cooper MK, Taipale J, Olson JM, Beachy PA: Medulloblastoma growth inhibition by hedgehog pathway blockade. Science 2002, 297:1559-1561 [DOI] [PubMed] [Google Scholar]
- 5.Tong W, Ohgaki H, Huang H, Granier C, Kleihues P, Wang Z: Null mutation of DNA strand break-binding molecule poly (ADP-ribose) polymerase causes medulloblastomas in p53−/− mice. Am J Pathol 2003, 162:343-352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldowitz D, Hamre K: The cells and molecules that make a cerebellum. Trends Neurosci 1998, 21:375-382 [DOI] [PubMed] [Google Scholar]
- 7.Dahmane N, Ruiz-i-Altaba A: Sonic hedgehog regulates the growth and patterning of the cerebellum. Development 1999, 126:3089-3100 [DOI] [PubMed] [Google Scholar]
- 8.Wechsler-Reya RJ, Scott MP: Control of neuronal precursor proliferation in the cerebellum by Sonic hedgehog. Neuron 1999, 22:103-114 [DOI] [PubMed] [Google Scholar]
- 9.Kenney AM, Rowitch DH: Sonic hedgehog promotes G(1) cyclin expression and sustained cell cycle progression in mammalian neuronal precursors. Mol Cell Biol 2000, 20:9055-9067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helms AW, Johnson JE: Progenitors of dorsal commissural interneurons are defined by MATH1 expression. Development 1998, 125:919-928 [DOI] [PubMed] [Google Scholar]
- 11.van Lookeren Campagne M, Gill R: Tumor-suppressor p53 is expressed in proliferating and newly formed neurons of the embryonic and postnatal rat brain: comparison with expression of the cell cycle regulators p21Waf1/Cip1, p27Kip1, p57Kip2, p16Ink4a, cyclin G1, and the proto-oncogene Bax. J Comp Neurol 1998, 397:181-198 [DOI] [PubMed] [Google Scholar]
- 12.Sarnat HB, Nochlin D, Born DE: Neuronal nuclear antigen (NeuN): a marker of neuronal maturation in early human fetal nervous system. Brain Dev 1998, 20:88-94 [DOI] [PubMed] [Google Scholar]
- 13.Miyazawa K, Himi T, Garcia V, Yamagishi H, Sato S, Ishizaki Y: A role for p27/Kip1 in the control of cerebellar granule cell precursor proliferation. J Neurosci 2000, 20:5756-5763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JE, Hollenberg SM, Snider L, Turner DL, Lipnick N, Weintraub H: Conversion of Xenopus ectoderm into neurons by NeuroD, a basic helix-loop-helix protein. Science 1995, 268:836-844 [DOI] [PubMed] [Google Scholar]
- 15.Miyata T, Maeda T, Lee JE: NeuroD is required for differentiation of the granule cells in the cerebellum and hippocampus. Genes Dev 1999, 13:1647-1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olson JM, Asakura A, Snider L, Hawkes R, Strand A, Stoeck J, Hallahan A, Pritchard J, Tapscott SJ: NeuroD2 is necessary for development and survival of central nervous system neurons. Dev Biol 2001, 234:174-187 [DOI] [PubMed] [Google Scholar]
- 17.Helms AW, Gowan K, Abney A, Savage T, Johnson JE: Overexpression of MATH1 disrupts the coordination of neural differentiation in cerebellum development. Mol Cell Neurosci 2001, 17:671-682 [DOI] [PubMed] [Google Scholar]
- 18.Leclerc N, Vallee A, Nabi IR: Expression of the AMF/neuroleukin receptor in developing and adult brain cerebellum. J Neurosci Res 2000, 60:602-612 [DOI] [PubMed] [Google Scholar]
- 19.Stottmann RW, Rivas RJ: Distribution of TAG-1 and synaptophysin in the developing cerebellar cortex: relationship to Purkinje cell dendritic development. J Comp Neurol 1998, 395:121-135 [PubMed] [Google Scholar]
- 20.Andrae J, Hansson I, Afink GB, Nister M: Platelet-derived growth factor receptor-α in ventricular zone cells and in developing neurons. Mol Cell Neurosci 2001, 17:1001-1013 [DOI] [PubMed] [Google Scholar]
- 21.Rorke LB: The cerebellar medulloblastoma and its relationship to primitive neuroectodermal tumors. J Neuropathol Exp Neurol 1983, 42:1-15 [PubMed] [Google Scholar]
- 22.Hart MN, Earle KM: Primitive neuroectodermal tumors of the brain in children. Cancer 1973, 32:890-897 [DOI] [PubMed] [Google Scholar]
- 23.Kadin ME, Rubinstein LJ, Nelson JS: Neonatal cerebellar medulloblastoma originating from the fetal external granular layer. J Neuropathol Exp Neurol 1970, 29:583-600 [DOI] [PubMed] [Google Scholar]
- 24.Rubinstein LJ: Embryonal central neuroepithelial tumors and their differentiating potential: a cytogenetic view of a complex neuro-oncological problem. J Neurosurg 1985, 62:795-805 [DOI] [PubMed] [Google Scholar]
- 25.Katsetos CD, Herman MM, Krishna L, Vender JR, Vinores SA, Agamanolis DP, Schiffer D, Burger PC, Urich H: Calbindin-D28k in subsets of medulloblastomas and in the human medulloblastoma cell line D283 Med. Arch Pathol Lab Med 1995, 119:734-743 [PubMed] [Google Scholar]
- 26.Smyth I, Narang MA, Evans T, Heimann C, Nakamura Y, Chenevix-Trench G, Pietsch T, Wicking C, Wainwright BJ: Isolation and characterization of human patched 2 (PTCH2), a putative tumour suppressor gene in basal cell carcinoma and medulloblastoma on chromosome 1p32. Hum Mol Genet 1999, 8:291-297 [DOI] [PubMed] [Google Scholar]
- 27.Reifenberger J, Wolter M, Weber RG, Megahed M, Ruzicka T, Lichter P, Reifenberger G: Missense mutations in SMOH in sporadic basal cell carcinomas of the skin and primitive neuroectodermal tumors of the central nervous system. Cancer Res 1998, 58:1798-1803 [PubMed] [Google Scholar]
- 28.Pietsch T, Waha A, Koch A, Kraus J, Albrecht S, Tonn J, Sorensen N, Berthold F, Henk B, Schmandt N, Wolf HK, von Deimling A, Wainwright B, Chenevix-Trench G, Wiestler OD, Wicking C: Medulloblastomas of the desmoplastic variant carry mutations of the human homologue of Drosophila patched. Cancer Res 1997, 57:2085-2088 [PubMed] [Google Scholar]
- 29.Lam CW, Xie J, To KF, Ng HK, Lee KC, Yuen NW, Lim PL, Chan LY, Tong SF, McCormick F: A frequent activated smoothened mutation in sporadic basal cell carcinomas. Oncogene 1999, 18:833-836 [DOI] [PubMed] [Google Scholar]
- 30.Taylor MD, Liu L, Raffel C, Hui CC, Mainprize TG, Zhang X, Agatep R, Chiappa S, Gao L, Lowrance A, Hao A, Goldstein AM, Stavrou T, Scherer SW, Dura WT, Wainwright B, Squire JA, Rutka JT, Hogg D: Mutations in SUFU predispose to medulloblastoma. Nat Genet 2002, 31:306-310 [DOI] [PubMed] [Google Scholar]
- 31.Goodrich LV, Milenkovic L, Higgins KM, Scott MP: Altered neural cell fates and medulloblastoma in mouse patched mutants. Science 1997, 277:1109-1113 [DOI] [PubMed] [Google Scholar]
- 32.Hahn H, Wojnowski L, Zimmer AM, Hall J, Miller G, Zimmer A: Rhabdomyosarcomas and radiation hypersensitivity in a mouse model of Gorlin syndrome. Nat Med 1998, 4:619-622 [DOI] [PubMed] [Google Scholar]
- 33.Peifer M: Signal transduction: neither straight nor narrow. Nature 1999, 400:213-215 [DOI] [PubMed] [Google Scholar]
- 34.Huang H, Mahler-Araujo BM, Sankila A, Chimelli L, Yonekawa Y, Kleihues P, Ohgaki H: APC mutations in sporadic medulloblastomas. Am J Pathol 2000, 156:433-437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eberhart CG, Tihan T, Burger PC: Nuclear localization and mutation of β-catenin in medulloblastomas. J Neuropathol Exp Neurol 2000, 59:333-337 [DOI] [PubMed] [Google Scholar]
- 36.Zurawel RH, Chiappa SA, Allen C, Raffel C: Sporadic medulloblastomas contain oncogenic β-catenin mutations. Cancer Res 1998, 58:896-899 [PubMed] [Google Scholar]
- 37.Dahmen RP, Koch A, Denkhaus D, Tonn JC, Sorensen N, Berthold F, Behrens J, Birchmeier W, Wiestler OD, Pietsch T: Deletions of AXIN1, a component of the WNT/wingless pathway, in sporadic medulloblastomas. Cancer Res 2001, 61:7039-7043 [PubMed] [Google Scholar]
- 38.Varley JM, Evans DG, Birch JM: Li-Fraumeni syndrome: a molecular and clinical review. Br J Cancer 1997, 76:1-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barel D, Avigad S, Mor C, Fogel M, Cohen IJ, Zaizov R: A novel germ-line mutation in the noncoding region of the p53 gene in a Li-Fraumeni family. Cancer Genet Cytogenet 1998, 103:1-6 [DOI] [PubMed] [Google Scholar]
- 40.Adesina AM, Nalbantoglu J, Cavenee WK: p53 gene mutation and mdm2 gene amplification are uncommon in medulloblastoma. Cancer Res 1994, 54:5649-5651 [PubMed] [Google Scholar]
- 41.Raffel C, Thomas GA, Tishler DM, Lassoff S, Allen JC: Absence of p53 mutations in childhood central nervous system primitive neuroectodermal tumors. Neurosurgery 1993, 33:301-305discussion 305–306 [DOI] [PubMed] [Google Scholar]
- 42.Badiali M, Iolascon A, Loda M, Scheithauer BW, Basso G, Trentini GP, Giangaspero F: p53 gene mutations in medulloblastoma: immunohistochemistry, gel shift analysis, and sequencing. Diagn Mol Pathol 1993, 2:23-28 [PubMed] [Google Scholar]
- 43.Wang W, Kumar P, Whalley J, Schwarz M, Malone G, Haworth A, Kumar S: The mutation status of PAX3 and p53 genes in medulloblastoma. Anticancer Res 1998, 18:849-853 [PubMed] [Google Scholar]
- 44.Giordana MT, Duo D, Gasverde S, Trevisan E, Boghi A, Morra I, Pradotto L, Mauro A, Chio A: MDM2 overexpression is associated with short survival in adults with medulloblastoma. Neuro-oncol 2002, 4:115-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsuda M, Yasui K, Nagashima K, Mori W: Origin of the medulloblastoma experimentally induced by human polyomavirus JC. J Natl Cancer Inst 1987, 79:585-591 [PubMed] [Google Scholar]
- 46.Zu Rhein GM, Varakis JN: Perinatal induction of medulloblastomas in Syrian golden hamsters by a human polyoma virus (JC). Natl Cancer Inst Monogr 1979, 51:205-208 [PubMed] [Google Scholar]
- 47.Eibl RH, Kleihues P, Jat PS, Wiestler OD: A model for primitive neuroectodermal tumors in transgenic neural transplants harboring the SV40 large T antigen. Am J Pathol 1994, 144:556-564 [PMC free article] [PubMed] [Google Scholar]
- 48.Krynska B, Otte J, Franks R, Khalili K, Croul S: Human ubiquitous JCV(CY) T-antigen gene induces brain tumors in experimental animals. Oncogene 1999, 18:39-46 [DOI] [PubMed] [Google Scholar]
- 49.Marino S, Vooijs M, van Der Gulden H, Jonkers J, Berns A: Induction of medulloblastomas in p53-null mutant mice by somatic inactivation of Rb in the external granular layer cells of the cerebellum. Genes Dev 2000, 14:994-1004 [PMC free article] [PubMed] [Google Scholar]
- 50.Wetmore C, Eberhart DE, Curran T: Loss of p53 but not ARF accelerates medulloblastoma in mice heterozygous for patched. Cancer Res 2001, 61:513-516 [PubMed] [Google Scholar]
- 51.Pazzaglia S, Mancuso M, Atkinson MJ, Tanori M, Rebessi S, Majo VD, Covelli V, Hahn H, Saran A: High incidence of medulloblastoma following x-ray-irradiation of newborn Ptc1 heterozygous mice. Oncogene 2002, 21:7580-7584 [DOI] [PubMed] [Google Scholar]
- 52.Tong WM, Hande MP, Lansdorp PM, Wang ZQ: DNA strand break-sensing molecule poly(ADP-ribose) polymerase cooperates with p53 in telomere function, chromosome stability, and tumor suppression. Mol Cell Biol 2001, 21:4046-4054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ha HC, Snyder SH: Poly(ADP-ribose) polymerase-1 in the nervous system. Neurobiol Dis 2000, 7:225-239 [DOI] [PubMed] [Google Scholar]
- 54.Wetmore C, Eberhart DE, Curran T: The normal patched allele is expressed in medulloblastomas from mice with heterozygous germ-line mutation of patched. Cancer Res 2000, 60:2239-2246 [PubMed] [Google Scholar]
- 55.Zurawel RH, Allen C, Wechsler-Reya R, Scott MP, Raffel C: Evidence that haploinsufficiency of Ptch leads to medulloblastoma in mice. Genes Chromosomes Cancer 2000, 28:77-81 [PubMed] [Google Scholar]