Abstract
Onset of the maternal-placental circulation was studied by Doppler ultrasonography in 65 pairs of age-matched normal and abnormal pregnancies. In normal pregnancies intervillous blood flow increased with gestational age, being detected in 9 of 25 cases at 8 to 9 weeks but in 18 of 20 at 12 to 13 weeks (P = 0.001). By contrast, in abnormal pregnancies flow was detected in nearly all cases (22 of 25) at 8 to 9 weeks (P < 0.001). In addition, regional differences were observed between the groups. Early flow was restricted to the peripheral regions of most normal placentas (P < 0.001), whereas in missed miscarriages it was most common in central regions or throughout the placenta (P < 0.05 and P < 0.001, respectively). Immunoreactivity for heat shock protein 70 and nitrotyrosine residues was greater in samples from peripheral than from central regions of normal placentas (P = 0.028 and P = 0.019, respectively), and from missed miscarriages compared to controls (P = 0.005 and P = 0.001, respectively). Our results indicate that oxidative damage to the trophoblast, induced by premature and widespread onset of the maternal placental circulation secondary to shallow trophoblast invasion, is a key factor in early pregnancy loss. High oxygen concentrations in the periphery of normal early placentas may similarly induce local regression of the villi, leading to formation of the chorion laeve.
Placentation in the human is characterized by diffuse infiltration of the endometrium and the inner third of the myometrium by extravillous trophoblast cells. 1,2 These cells breach the endometrial vessels, 3,4 and the human placenta is classified as being of the hemochorial type, with the fetal trophoblast being bathed directly by maternal blood. Originally, it was thought that the placental intervillous circulation is established from 1 week after implantation onwards. 2 However, this well-established dogma was challenged by the studies of Hustin and Schaaps, 5,6 demonstrating that the maternal intraplacental circulation is limited before 12 weeks of gestation.
The most convincing evidence obtained by Hustin and Schaaps 5,6 came from injections of the arterial circulation of pregnant hysterectomy specimens with barium sulfate, followed by fixation, sectioning, radiography, and three-dimensional reconstruction. These data indicated that during the first trimester the intervillous space of the developing placenta is separated from the uterine circulation by plugs of trophoblast cells that occlude the tips of the uteroplacental arteries. 5 At the end of the first trimester these plugs are dislocated, allowing maternal blood to flow freely and continuously in the intervillous space. Thus, according to these findings, in normal pregnancy the human placenta is not truly hemochorial until the end of the first trimester.
Hustin and Schaaps 5,6 also included ultrasound data in their original studies. Using a transvaginal probe they detected few, if any, moving echoes within the intervillous space during the first trimester, suggesting again only limited flow. The advent of Doppler ultrasound has enabled the flow velocity waveforms within the different small branches of the uterine and feto-placental vessels to be evaluated in vivo. When comparing Doppler features of the placental circulation at different gestational ages, we found that a nonpulsatile signal corresponding to maternal intraplacental blood flow could not be identified inside the intervillous space before 10 weeks of gestation. 7 This finding has been confirmed by other authors, 8,9 provoking a vigorous debate involving both ultrasonographers 10,11 and anatomists 12,13 about the status of the maternal circulation during the first trimester. By contrast, in complicated early pregnancies the placenta appears on color flow mapping to be hypervascularized well before the end of the first trimester. 11,14-17 This observation has not been disputed, and the differences between the Doppler signals and the histopathological findings in early pregnancy failure compared to normal pregnancies are so striking that they constitute one of the strongest arguments in favor of the Hustin and Schaaps 5,6 hypothesis. In complicated pregnancies invasion of the endometrium by the extravillous trophoblast is severely restricted compared to the normal situation. 18 Plugging of the spiral arteries is therefore less complete and may predispose to the early onset of the maternal circulation. We have previously shown that placental tissues contain low concentrations and activities of antioxidant enzymes during the first trimester, and so, are highly susceptible to oxygen-mediated damage in vitro. 19,20 Recently, we reported a sharp peak in the expression of markers of oxidative stress in the trophoblast at 8 to 9 weeks associated with onset of the circulation in normal pregnancies, and speculated that excessive placental oxidative stress in early pregnancy could be a factor in the pathogenesis of early pregnancy failure and pre-eclampsia. 21
In view of the potential danger from oxidative stress it is likely that onset of maternal blood flow to the placenta is normally a progressive phenomenon, with communication between the uteroplacental arteries and the intervillous space being established in a small number of vessels at a time from the end of the second month of pregnancy onwards. This is supported by angiographic findings in vivo demonstrating that few entry sites into the intervillous space could be identified at 6.5 weeks of gestation, whereas many were present at 12 weeks. 22 Theoretically, the opening of individual vessels may occur either independently at random sites throughout the placenta, or on a more co-ordinated regional basis. Anatomical studies have shown that trophoblastic migration and morphological changes in the uteroplacental arteries are most extensive in the more central area of the placental bed. 3,4 Hence, by analogy with the findings in complicated pregnancies we hypothesize that the onset of placental blood flow will occur predominately first in the peripheral regions of the placenta and gradually progress toward the center. We also hypothesize that this pattern will be lost in abnormal pregnancies in which trophoblast invasion is uniformly shallow.
The overall aim of this study was to obtain more detailed information on the onset of the maternal intraplacental circulation in normal and abnormal pregnancies. There were three principal objectives. Firstly, to determine whether there are regional differences within the placenta of normal pregnancies in the timing of the onset of the maternal circulation that might reflect differing degrees of extravillous trophoblast invasiveness. Secondly, to determine whether there are regional differences in villous histology that would provide corroborative evidence of blood flow in vivo as evaluated by ultrasound and Doppler imaging. Finally, to evaluate if the trophoblast of early pregnancy failure is subject to excessive and/or premature oxidative stress that correlates with the entry of maternal blood into the intervillous space in vivo. Hence, we sought immunohistochemical and ultrastructural evidence of local differences in placental oxidative stress reflecting onset of maternal blood flow, and compared this with the ultrasound data obtained in vivo. We also examined archival histological material of placentas in situ to determine whether regional differences in maternal blood flow may be instrumental in regression of the villi over the abembryonic pole of the gestational sac and formation of the chorion laeve.
Materials and Methods
Cases
The normal study group consisted of 65 healthy women seen for ultrasound examination between 8 and 13 weeks of gestation before surgical termination of pregnancy for psychological reasons. Gestational age was calculated from the last menstrual period and confirmed by ultrasound measurements of the crown-rump length. The abnormal study group included 65 women with a diagnosis of missed miscarriage, ie, an empty gestational sac on high-resolution transvaginal ultrasound examination (n = 20) or a gestational sac containing embryo remnants (n = 45), matched for gestational age with the normal pregnancies and requesting a surgical evacuation. Clinical details were recorded for each woman, and only patients who were certain of their menstrual dates entered the study group. All women gave their written informed consent to participate in this study which had been approved by the University College London Hospitals Committee on the Ethics of Human Research.
Ultrasound/Doppler Examination
Ultrasound and Doppler examinations of the placental circulations were performed using a 5.0-MHz curvilinear transvaginal probe with pulsed color and power Doppler facilities (Aloka, Tokyo, Japan). In each case, the placental insertion of the umbilical cord was located using color mapping and accordingly the definitive placenta was divided into a central zone (inner one-third portion under the cord insertion) and a peripheral zone (outer one-third portions on each side of the central zone) (Figure 1A) ▶ . Gray-scale ultrasound examination was used to detect moving echoes inside the intervillous space as previously described by Hustin and Schaaps. 5 The presence of intervillous blood flow, ie, a continuous venous-like flow (Figure 1B) ▶ inside the placental tissue was evaluated using color-power Doppler. Low-pulse rate frequencies were used to allow a minimum blood flow velocity detectable of 3.7 cm/second for color Doppler and 0.4 cm/second for power Doppler. Cases were recorded as to whether intervillous blood flow was detectable or not, and if so whether it was present in the center, periphery, or both.
Figure 1.
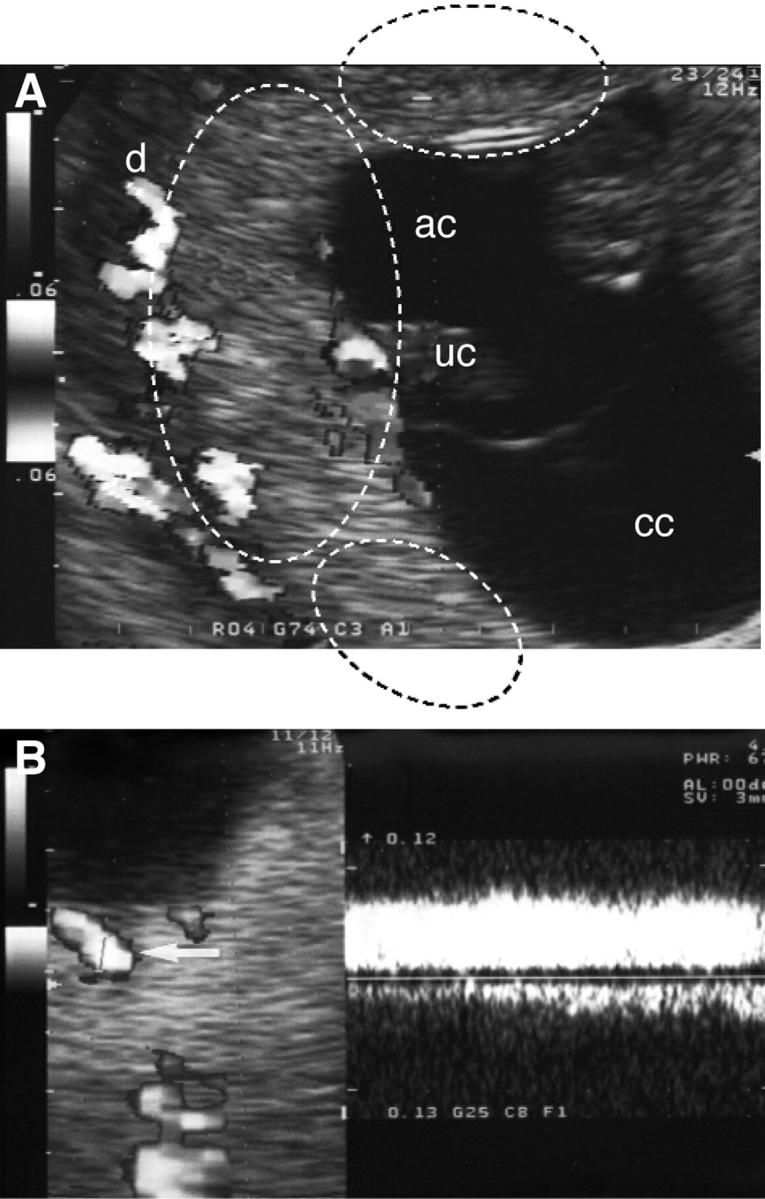
A: Mapping of the placental circulation at 8 weeks of gestation with color Doppler showing the fetal circulation in the umbilical cord (uc) and the maternal circulation within the decidua (d). The dashed lines indicate the boundaries of the regions considered central and peripheral in this example. Note the absence of any signals from within the placenta. cc, chorionic cavity; ac, amniotic cavity. B: Color mapping of the placenta and spectral analysis of the intervillous space at 12 weeks of gestation showing a continuous venous-like flow (arrows) inside the placental tissue.
Collection and Processing of Experimental Tissue
For the regional comparisons within normal placentas villous samples were obtained under ultrasound guidance using a chorionic villous sampling technique under general anesthesia before the termination procedure. This ensured that the normal spatial relationships were preserved. For ethical reasons sampling was limited to two sites; one from the central area, defined as under the cord insertion, and one from the periphery, as far from the cord insertion as possible. The tissues were fixed immediately for either immunohistochemistry or electron microscopy (n = 16 and 6 pairs for immunohistochemistry and transmission electron microscopy, respectively).
For the comparisons between normal placentas and cases of missed miscarriage, villous samples were excised from as close to the central region as could be recognized after delivery of the placenta by low-pressure vacuum aspiration (n = 24 pairs for immunohistochemistry).
Samples for immunohistochemistry were fixed in 4% paraformaldehyde for 2 hours and processed for paraffin wax embedding. Samples for transmission electron microscopy were fixed in 2% glutaraldehyde for 2 hours and processed for embedding in Araldite resin.
Immunohistochemical Staining
All tissues were subjected to immunohistochemical analysis for heat shock protein 70 (inducible form, HSP70i), a marker of cellular stress, and for nitrotyrosine residues (N-Tyr) and hydroxynonenal (HNE), which are markers of protein and lipid oxidative damage, respectively.
For HSP70i two secondary detection systems were used: a fluorescein isothiocyanate-labeled anti-rabbit secondary antibody (1/100 for 2 hours at room temperature; Calbiochem, Nottingham, UK) for confocal microscopy, and the Vectastain Elite ABC kit (Vector Laboratories, Peterborough, UK) and Sigma-Fast DAB (Sigma, Poole, UK) according to the manufacturer’s instructions for light microscopy. For fluorescein isothiocyanate staining the sections were blocked for 20 minutes in 1.5% goat serum/phosphate-buffered saline and incubated overnight at 4°C with rabbit polyclonal anti-HSP70i (1 μg/ml; StressGen, York, UK). Negative controls consisted of sections incubated with 1 μg/ml of nonimmune rabbit serum. For avidin-biotin complex/diaminobenzidine (ABC/DAB) the sections were processed as below.
For N-Tyr and HNE detection sections were incubated with 1% H2O2 for 30 minutes at room temperature to block endogenous peroxidases, and then with the primary antibodies for 1 hour at room temperature. Rabbit polyclonal anti-N-Tyr (Upstate Biotechnology, Lake Placid, NY) was diluted to 1 in 1000 (0.59 μg/ml), and rabbit polyclonal anti-HNE (Calbiochem) was diluted to 1 in 2000. All antibodies were detected using the Vectastain Elite ABC kit and Sigma-Fast DAB according to the manufacturers’ instructions. For each antibody all of the slides were stained in a single batch so that conditions were uniform.
The intensity of staining for HSP70i in the central and peripheral samples from normal placentas was quantified by confocal fluorescence microscopy using our existing protocol. 21 Because of the large number of sections involved and the physical limitations of viewing these on the confocal microscope, the center/periphery samples were stained in groups. The central and peripheral samples from individual placentas were always stained and viewed in the same run, and are therefore directly comparable. However, possible daily variations in laser power and/or staining conditions mean that the samples from different placentas are not directly comparable. To take account of this, the results are expressed as a ratio of peripheral versus central fluorescence intensity rather than absolute values. For the remaining sections, semiquantification of DAB staining was achieved by a 0 to 4+ scoring system by the same observer (JH), after blinding for group, gestational age, and region.
Quantification of Syncytiotrophoblastic Stress
To quantify the relative proportions of stressed and unstressed syncytiotrophoblast between central and peripheral regions of normal placentas, and between central regions of normal placenta and missed miscarriages, 1-μm resin sections were viewed using a ×63 objective. Sections were blinded for group and for region. A pair of diagonal cross-hairs was superimposed at random on the images of villi using the VIDS IV imaging system (Synoptics, Cambridge, UK). When a cross-hair intersected the villous surface the syncytiotrophoblast at that point was classified as either stressed (loss of microvilli, vacuolation, pale staining, flattening of underlying cytotrophoblast cells) or healthy (microvilli present, no vacuolation, dark staining, rounded cytotrophoblast cells) (Figure 2A) ▶ . The number of hits on healthy syncytiotrophoblasts was then expressed as a percentage of the total number of hits with the villous surface. At least 25 fields of view were examined per sample yielding 50 to 100 intersections with the villous surface.
Figure 2.
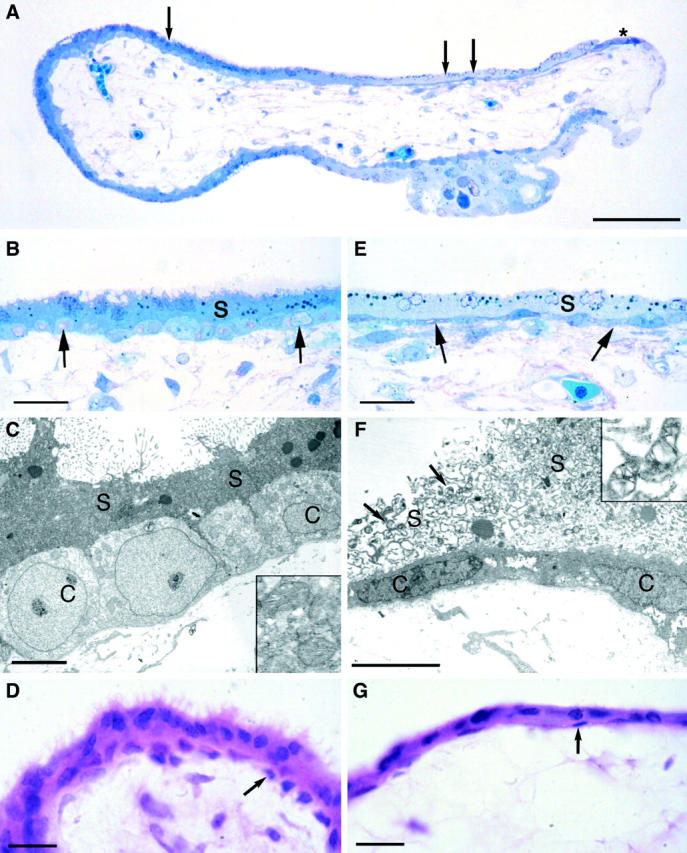
A: Light micrograph of a semithin resin section of a placental villus showing marked differences in the staining characteristics with methylene blue of the trophoblast around its circumference. The darkly staining syncytiotrophoblast on the left (single arrow) is considered healthy, whereas the pale staining area on the right (double arrow) is considered stressed. Note that the cytotrophoblast cells under the stressed syncytium are conspicuously elongated, and that they come to the surface at the extreme right-hand margin of the villus (asterisk) where they are forming a new syncytiotrophoblastic layer. B: Higher power photomicrograph of the healthy syncytiotrophoblast at the point marked by a single arrow in A. The apical border of the syncytiotrophoblast (s) carries numerous microvilli, and the syncytioplasm is uniformly dense. The underlying cytotrophoblast cells (arrows) are rounded and evenly spaced along the basement membrane. Both layers of the trophoblast show a similar staining intensity, indicating equivalent protein composition. C: Transmission electron micrograph of an area equivalent to that illustrated in B. Note the apical microvilli, the dense syncytioplasm (s), and the rounded cytotrophoblast cells (c). The syncytial mitochondria (inset) display a regular cristal arrangement. D: Light micrograph of a paraffin section of an 8 week villus demonstrating a healthy syncytiotrophoblast with numerous microvilli and an underlying complete layer of rounded cytotrophoblast cells (arrows). E: Higher power photomicrograph of the stressed area at the point in A marked by the double arrows. Note the absence of microvilli on the apical surface, the vacuolated and leached appearance of the syncytioplasm (s), and the elongated nature of the underlying cytotrophoblast cells (arrows). F: Transmission electron micrograph of an area equivalent to that illustrated in E. There is a complete absence of microvilli, and often the integrity of the apical membrane is lost. The syncytioplasm (s) is heavily vacuolated, and the underlying cytotrophoblast cells (c) are elongated along the basement membrane. The syncytial mitochondria (arrows and inset) display gross dilatation of the intracristal space, similar to that seen in severe oxidative stress in vitro. 20 G: Light micrograph of a paraffin section of an 8 week villus displaying stressed syncytiotrophoblast and flattening of the underlying cytotrophoblast cells (arrows). Scale bars: 500 μm (A); 50 μm (B, E); 5 μm (C, F); 20 μm (D, G).
Archival Histological Material
Reference was also made to the Boyd collection of histological placental material housed in the Department of Anatomy at the University of Cambridge, assembled by Professor J. D. Boyd in the 1950s. Slides were available from six cases of placenta in situ demonstrating the complete gestational sac, ranging in gestational age from 6 to 12 weeks. A variety of stains had been used in the preparation of the material, but the majority of the sections examined had been stained with either hematoxylin and eosin (H&E) or Masson’s trichrome.
Electron Microscopy
Samples were secondary fixed in 1% osmium tetroxide in Pipes buffer for 1 hour at room temperature and embedded in Araldite epoxy resin. Semithin sections (1 μm) were stained with methylene blue and viewed at the light microscope level. Ultra-thin sections (50 nm) were counterstained with uranyl acetate followed by lead citrate, and viewed using a Philips CM100 microscope (FEI/Philips, Eindhoven, The Netherlands).
Statistics
Standardized kurtosis showed that the samples derived from a normal distribution and gestational age values are thus expressed as mean (±SD). The rates of detection of intervillous flow were compared using Pearson’s chi-square test with Yates’s continuity correction for small sample size. The ratio of peripheral to central fluorescence intensity was correlated with gestational age using Pearson’s correlation coefficient. Immunohistochemical scorings for the various groups were compared using the nonparametric Wilcoxon signed rank test. The results were considered statistically significant at P < 0.05.
Results
Ultrasound and Doppler Findings
Table 1 ▶ presents the detection of intervillous blood flow in both groups at the different gestational age periods. Within the normal cases it can be seen that there was a progressive increase in the rate of detection of blood flow with gestational age (9 of 25 at 8 to 9 weeks, 12 of 20 at 10 to 11 weeks, and 18 of 20 at 12 to 13 weeks; chi-square = 13.50; P = 0.001). By contrast, blood flow was detectable in nearly all of the abnormal cases early in gestation but subsequently reduced (22 of 25 at 8 to 9 weeks, 20 of 20 at 10 to 11 weeks, and 11 of 20 at 12 to 13 weeks; chi-square = 14.58; P < 0.001). Hence, a significant difference in the presence or absence of intervillous blood flow was found between normal and abnormal pregnancies, being more common in the abnormal cases at the 8- to 9-week (chi-square = 14.35, P < 0.001) and 10- to 11-week (chi-square = 10.00, P = 0.002) periods, but less common at the 12- to 13-week period (chi-square = 6.14, P = 0.013).
Table 1.
Distribution and Comparison of Intervillous Blood Flow Detection in 65 Normal and 65 Abnormal Early Pregnancies at 8 to 9 Weeks (25 Pairs), 10 to 11 Weeks (20 Pairs), and 12 to 13 Weeks (20 Pairs)
| Gestational age (weeks) | Normal | Abnormal | ||||
|---|---|---|---|---|---|---|
| C | P | C & P | C | P | C & P | |
| 8 to 9 | 1 | 5 | 3 | 9 | 1 | 12 |
| 10 to 11 | 2 | 7 | 3 | 4 | 2 | 14 |
| 12 to 13 | 3 | 7 | 8 | 2 | 1 | 8 |
C, Center only; P, periphery only.
A significantly different distribution was also found for the location of intervillous blood flow between the two groups. Central flow and generalized flow (center and periphery) were more frequently seen in abnormal than in normal pregnancies (chi-square = 4.60, P < 0.05, and chi-square = 13.21, P < 0.001, respectively), whereas peripheral flow was more commonly observed in normal than in abnormal pregnancies (chi-square = 11.89, P < 0.001).
Regional Differences in Trophoblast Morphology within Normal Placentas
When viewing the 1-μm sections of the resin-embedded tissue from the normal placentas it was notable that striking differences in the morphology of the trophoblast layer existed between villi and even between different areas on the same villus (Figure 2A) ▶ . In the majority of areas the syncytiotrophoblast had been stained slightly darker than the underlying cytotrophoblast cells by the methylene blue. In these regions the apical microvilli were prominent and the cytotrophoblast cells were rounded and closely approximated to each other (Figure 2B) ▶ . Ultrastructural analysis confirmed a normal complement and configuration of organelles at these sites (Figure 2C) ▶ . Hence these were considered to represent healthy unstressed trophoblast. By contrast, in other areas the syncytiotrophoblast was very pale stained, heavily vacuolated, and the apical microvilli were often greatly attenuated or absent. At these sites the underlying cytotrophoblast cells stained intensely and their profiles were characteristically flattened and elongated over the basement membrane (Figure 2E) ▶ . These features were confirmed at the ultrastructural level, when in addition it was evident that the syncytiotrophoblastic mitochondria were severely distorted (Figure 2F) ▶ . There was loss of the normal cristal architecture because of marked swelling of the intracristal space, accompanied by increased electron density of the matrix. Such areas were considered to represent regions of severe syncytial stress. Once identified in this way, these combinations of features could also be visualized at the light microscope level on standard H&E-stained paraffin sections (Figure 2, D and G) ▶ . In all cases there was a greater proportion of unstressed syncytiotrophoblasts in the central region of the placenta (n = 6, mean = 70.2%, SD = 21.4) compared to the peripheral regions (n = 6, mean = 46.4%, SD = 19.6) (paired t statistic = 3.68, P = 0.014).
Immunohistochemistry of Regional Differences in Normal Placentas
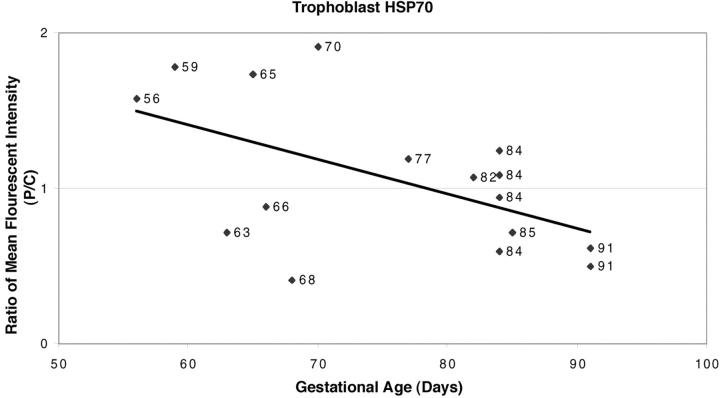
Analysis of the quantitative confocal fluorescent immunohistochemistry data revealed that the relative expression of HSP70i in the different regions of the normal placentas was significantly correlated with gestational age (r = −0.542, n = 16, P = 0.028). Expression was higher in the trophoblast of villi from the peripheral regions compared to their central counterparts early in gestation, but as gestation advanced the difference became less apparent (Figure 3) ▶ . After ∼11 weeks (77 days) staining in the trophoblast was of similar intensity in both the peripheral and central regions, indicating that the maternal circulation is almost fully established throughout the placenta by this age.
Figure 3.
Ratio of fluorescent intensity of HSP70 immunoreactivity in the syncytiotrophoblasts from peripheral and central regions of normal placentas at different gestational ages demonstrating that staining was higher in the peripheral regions early in gestation. Although there is considerable variation, reflecting individual differences in timing of the onset of blood flow, there is a general trend for expression to be higher in the peripheral regions of the placenta early in gestation (r = −0.542, P = 0.028). Later in gestation, when blood flow occurs throughout the placenta, the ratio approaches unity.
For the ABC/DAB markers of oxidative stress, a significant difference in the semiquantitative scores was only detected for the formation of N-Tyr residues (z = −2.344, P = 0.019 and z = −0.296, P = 0.767 for N-Tyr and HNE, respectively). Again, this was greatest in the villi from the peripheral regions.
Histological and Immunohistochemical Comparison of Normal and Abnormal Placentas
Villi sampled from the central region of placentas from missed miscarriages frequently displayed the trophoblastic changes associated with stress as above. Thus the syncytiotrophoblast layer was often thin and devoid of microvilli, while cytotrophoblast cells were infrequent and flattened (Figure 4) ▶ . The stromal core was notably avascular in those cases in which there was a large disparity between fetal age estimated from menstrual dates and from the crown-rump length, indicating early fetal demise. Quantification from the resin sections confirmed that the proportion of healthy syncytiotrophoblast (as depicted in Figure 2 ▶ ) was indeed lower in the villi from the missed miscarriages compared to the normal controls (n = 15, mean = 45.9%, SD = 20.8; n = 6, mean = 70.2%, SD = 21.4; unpaired t statistic, 2.41; P = 0.027).
Figure 4.
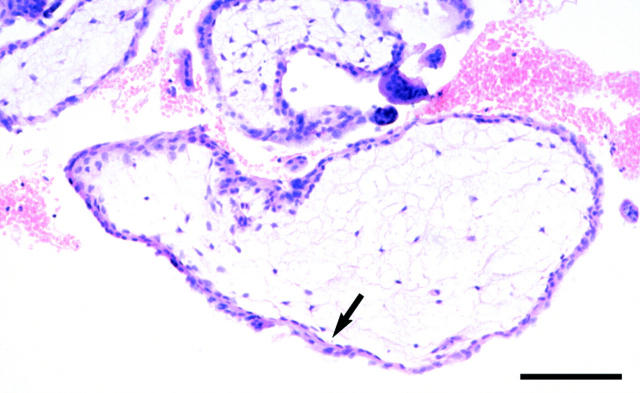
Light micrograph of a paraffin section from a 10-week gestational age missed miscarriage in which there was ultrasound evidence of strong intervillous blood flow. The discrepancy between fetal crown rump length and gestational age was nearly 4 weeks, indicating early fetal demise. The syncytiotrophoblast is thinned, with loss of microvilli, and the underlying cytotrophoblast cells are flattened (arrows). The stromal core is avascular, and displays a reduced cellularity. Scale bar, 100 μm.
The ABC/DAB immunohistochemical findings demonstrated strong reactivity in the syncytiotrophoblast, particularly near the apical surface (Figure 5) ▶ , for both expression of HSP70 and the formation of N-Tyr residues. Analysis of the semiquantitative scores indicated a highly significantly increase in central villi from cases of missed miscarriage compared with age-matched controls (z = 2.83, P = 0.005, and z = 3.29, P = 0.001, respectively). By contrast, staining was variable among the cytotrophoblast cells, with many showing low levels of immunoreactivity. Within the villous core the stromal cells, macrophages, and endothelial cells demonstrated an intermediate level of staining. No difference in immunoreactivity for HNE was detected in the trophoblast between the two groups (z = −1.39, P = 0.166).
Figure 5.
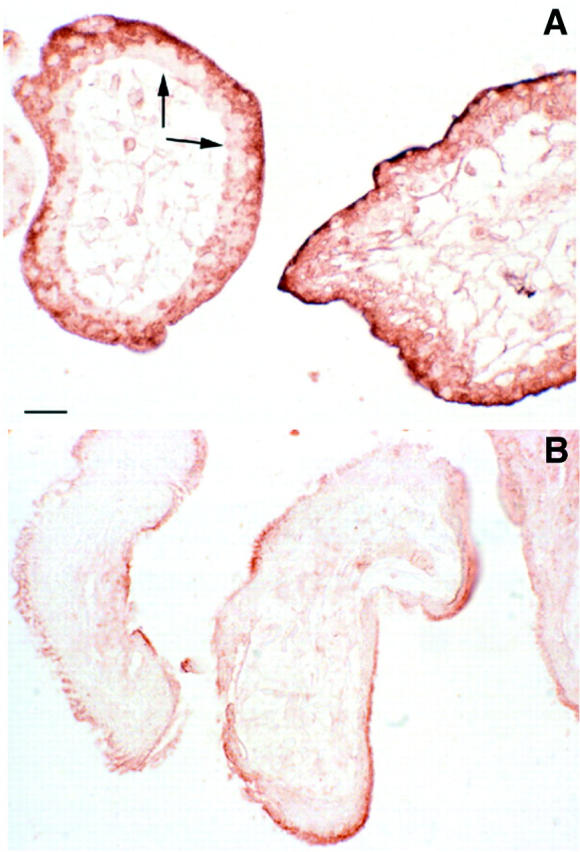
Immunohistochemistry for N-Tyr residues in a missed miscarriage at 8 weeks of gestational age (A) and an age-matched normal pregnancy (B), demonstrating the stronger reaction in the former. Staining is particularly intense in the syncytiotrophoblast, but many of the cytotrophoblast cells (arrows) show little immunoreactivity. This pattern is the inverse of that seen for the antioxidant enzymes. 19 Scale bar, 20 μm.
Placenta in Situ Specimens
The earliest specimen available was associated with a 20-mm crown-rump length embryo, and so was estimated to be of ∼60 days of gestational age. Villi were present over the entire surface of the chorionic sac, although they were notably shorter over the abembryonic pole in contact with the decidua capsularis (Figure 6A) ▶ . Marked differences were observed between the villi around the circumference of the chorionic sac (Figure 6B) ▶ . Those villi around the embryonic pole and over much of the surface were well vascularized by fetal vessels containing nucleated erythrocytes, and the trophoblastic covering was clearly two-layered, with an outer layer of syncytiotrophoblasts and an inner complete layer of cuboidal cytotrophoblast cells (Figure 6C) ▶ . The intervillous space contained a proteinaceous precipitate and maternal erythrocytes were only occasionally observed. As one moved toward the abembryonic pole a striking change was seen. Within a relatively short distance the villi became avascular, and fetal blood vessels were no longer present within the wall of the chorionic sac (Figure 6, B and D) ▶ . The syncytiotrophoblast layer appeared thin, with no microvilli on the apical surface, and the nuclei were rounded and stained darkly. The cytoplasm was strongly eosinophilic, and in places the syncytiotrophoblast appeared to be lifting away from the underlying cytotrophoblast cells. The cytotrophoblast cells were more dispersed, forming an incomplete layer, and appeared somewhat flattened. The stromal core contained fewer cells than in villi from the embryonic pole.
Figure 6.

A: Placenta in situ specimen at 8.5 weeks of gestational age. Villi are present over the entire surface of the chorionic sac, although they are notably shorter over the abembryonic pole in association with the decidua capsularis (asterisk). B: Higher power view of the area indicated by the dashed lines in A illustrating the transition in villous morphology as one moves toward the abembryonic pole. The villi in the lower part, toward the central region of the placenta, are well vascularized, whereas those in the upper part, toward the periphery, contain no blood vessels. Fetal blood vessels can only be traced in the wall of the chorionic sac as far as the arrowhead. C: Higher power view of villi from the lower region of the area illustrated in B showing the presence of fetal vessels in the cellular stromal core. The trophoblast layer is thick, with a well-developed syncytiotrophoblast and a complete layer of underlying cytotrophoblast cells (arrows). There is only a light proteinaceous precipitate in the intervillous space (IVS). D: Higher power view of villi from the upper region of the area illustrated in B confirming the absence of fetal vessels and the reduction in cellularity of the stromal core. The trophoblast covering is thinned, and the syncytiotrophoblast appears to be detaching from the cytotrophoblast cells at certain points (arrows). The cytotrophoblast cells (arrowheads) form an incomplete layer and are flattened. E: Villi from under the decidua capsularis (DC) at the point marked by the asterisk in A. The villi are surrounded by numerous maternal erythrocytes filling the intervillous space (IVS), and display similar trophoblastic changes to those illustrated in D. The villi are equally avascular. F: Resin section of an 11 week villus from the chorion laeve surrounded by maternal erythrocytes. The syncytiotrophoblast (S) is vacuolated, indicating stress, and no cytotrophoblast cells or fetal vessels are present. Scale bars: 1.0 cm (A); 1.0 mm (B); 100 μm (C–E); 20 μm (F).
These changes took place toward the margins of the placenta but before the decidua capsularis was reached (Figure 6A) ▶ . The intervillous space was narrowed under the decidua capsularis, and by contrast to elsewhere around the chorionic sac maternal erythrocytes were a conspicuous feature in this region (Figure 6E) ▶ . The remaining villi were avascular and again the trophoblastic layers were thinned. The syncytiotrophoblast contained dense clumps of pyknotic nuclei, whereas cytotrophoblast cells were only occasionally seen. These findings were confirmed on resin sections of recently collected samples from the chorion laeve, where vacuolation of the syncytiotrophoblast could also be observed (Figure 6F) ▶ .
In the older placenta in situ specimens villous regression was more advanced, although there was considerable individual variation. In three specimens the intervillous space had primarily collapsed in the region of the decidua capsularis and was filled with clot in which villous remnants were enmeshed. In the oldest specimen available, associated with a 60-mm crown-rump fetus and so estimated to be of 14 weeks of gestational age, there was still a covering of villi under the decidua capsularis similar to that shown in Figure 6A ▶ . In all cases, however, the peripheral villi were avascular compared to their central counterparts.
Discussion
Our recent review 23 of the Boyd embryological collection has confirmed and expanded the data of Hustin and Schaaps 5 showing that during the first 10 to 12 weeks of normal human pregnancy the apical portions of the majority of uteroplacental arteries are partially obliterated by plugs of invading trophoblast cells. These plugs prevent a continuous flow of maternal blood into the intervillous space during this period, 5 but at the end of the first trimester they are progressively dislocated. These first trimester uterine hemodynamic changes are linked to a general increase in circulating maternal blood volume and to structural changes in the uteroplacental arteries that transform them into a low-resistance vascular network. 7,24 As a result, a true flow of blood is able to enter the placenta. Before this the intervillous space is filled with an acellular fluid, probably derived from plasma and the endometrial glands. 5,25 Our data therefore support the concept that in normal pregnancies the intervillous circulation is gradually established between the beginning of the third month and the end of the fourth month of gestation.
They also demonstrate that in normal pregnancies onset of maternal blood flow is most often initiated in the peripheral regions of the placenta, and this may be related to regional differences in the extent of plugging of the maternal arteries. Quantitative morphometric analyses of trophoblastic migration through the endometrium of the human placental bed have demonstrated that maximal invasive activity occurs first at the center of the implantation site and subsequently extends centrifugally to produce an annular pattern. 3,4 Invasion of the endometrial arteries is therefore most extensive in the central regions, and these findings are supported by Doppler ultrasound data showing that later, around mid-gestation, the resistance to maternal blood flow through the uteroplacental arteries is lower in the central than in the peripheral areas of the placental bed. 26 Deeper and more extensive endovascular invasion will also lead to more complete plugging, and hence it might be expected that these plugs will be dislocated later than those in the peripheral regions. The evidence available suggests, therefore, that the normal establishment of a continuous intervillous circulation is an incremental phenomenon, starting in the periphery and expanding progressively to the rest of the placenta thereafter.
This concept is supported by the immunohistochemical and morphological evidence of temporospatial differences in the degree of trophoblastic oxidative stress presented here. We have previously demonstrated that during the first trimester the oxygen tension within the intervillous space is ∼20 mmHg, 21 and that the placental tissues display low activities of the principal antioxidant enzymes. 19-21 Concentrations are particularly low in the syncytiotrophoblast, and so this tissue is especially vulnerable to oxidative stress both in vivo and in vitro. Thus, when the oxygen tension rises threefold in the intervillous space with the onset of maternal arterial flow at the start of the second trimester, a burst of oxidative stress is observed in the trophoblast. This is manifested by the formation of N-Tyr residues, the expression of HSP70i, and morphological changes in the mitochondria. 21 More extensive changes are induced when first trimester villi are exposed to ambient oxygen concentrations in vitro. The syncytiotrophoblast undergoes rapid degeneration, with severe vacuolation and loss of apical microvilli. The mitochondria display loss of the transmembrane potential within 1 hour, which is associated with swelling of the intracristal space. 20 If the villi are maintained for 24 to 48 hours in culture then a new syncytiotrophoblast layer is generated from the underlying cytotrophoblast cells. 27 During this process the cytotrophoblast cells become elongated and undergo differentiation, before finally fusing. Remarkably, the various stages of this process can be seen occurring around the circumference of a single villus in Figure 4 ▶ . The finding that both immunohistochemical and morphological indices of oxidative stress are greater in the peripheral regions of normal placentas is therefore entirely consistent with the detection of higher rates of maternal blood flow in these regions in vivo. Indeed, oxygen-mediated events may play a role in the regression of peripheral villi from the chorion frondosum, and the formation of the chorion laeve, as demonstrated by the archival placenta in situ specimens.
Regression of the villi occurs principally during weeks 10 to 14 of gestation (the third month after ovulation), although there is considerable individual variation. 1 This is clearly an important phase of placental remodeling, yet the stimulus and mechanisms by which it occurs are at present unknown. The morphological changes in the syncytiotrophoblast are consistent with increased oxidative stress, and the depletion of the cytotrophoblast cell population may reflect removal of the hypoxic stimulus that normally promotes their proliferation. 28,29 Equally, the regression of the fetal vessels within these villi may be because of local changes in the balance of angiogenic growth factors, such as members of the vascular endothelial growth factor family and the angiopoietins. 30 Many of these factors are oxygen sensitive, and are required both to promote vessel growth and to maintain existing vessels. This is particularly so for immature vessels that lack a pericyte coat, as demonstrated by the finding that pruning of the retinal vasculature induced by hyperoxia can be blocked by administration of vascular endothelial growth factor-A. 31 We have recently shown that the majority of the fetal vessels in the first trimester placenta are not associated with pericytes, and so are in a plastic condition and vulnerable to changes in oxygenation. 32 The early placenta thus displays greater heterogeneity than generally assumed and sampling under ultrasound guidance may be necessary to ensure that truly comparable tissues are obtained.
A very different situation is seen in cases of early pregnancy failure, for the cytotrophoblastic shell is thin and fragmented, and trophoblastic infiltration is reduced or absent in approximately two-thirds of patients. 18 In a study correlating ultrasonographic with pathological features, we found extensive dislocation of the trophoblastic shell and a massive infiltration of the intervillous space by maternal blood in cases presenting with a continuous intervillous blood flow on color mapping before 12 weeks of gestation. 16 The present data show that at 8 to 9 weeks, and at 10 to 11 weeks, an intervillous blood flow is significantly more commonly detected inside the placenta of abnormal pregnancies, in particular in the central area (Table 1) ▶ , compared to normal controls. These findings suggest that in early pregnancy failure the initial central trophoblastic migration and vascular plugging is insufficient, allowing abnormally large quantities of maternal blood to enter the placenta. As a result, oxidative damage to the trophoblast is significantly increased, which will prevent normal development of the villous tree and hence compromise the anchoring of the placenta. 33 The occasional instances of central flow seen here in the supposedly normal pregnancies possibly represent cases that would have miscarried if the pregnancies had been allowed to continue, given the large sample size. By contrast, at 12 to 13 weeks, an intervillous blood flow was less frequently detected on ultrasound/Doppler examination (Table 1) ▶ in the abnormal pregnancies. These were essentially cases of missed miscarriages retained in utero for several weeks after embryonic demise, during which the placenta had become extremely atrophic as the villi regressed. The intervillous space had primarily collapsed, and was filled with clotted maternal blood.
It appears from our data that there are strong parallels between the formation of the chorion laeve in normal pregnancies and the placental changes observed in missed miscarriage (compare Figures 4 and 6E ▶ ▶ ). Both situations are associated with reduced trophoblast invasion and plugging of the endometrial arteries, early onset of maternal blood flow, and trophoblastic stress. Ultimately, they lead to avascularity and regression of the villi, followed by collapse of the intervillous space. The principal difference between the two lies in the physical extent of the placenta involved, and so they may represent the physiological and pathological extremes of a spectrum of villous remodeling induced by oxygen and maternal blood flow. Between these extremes may lie the formation of maternal intervillous blood-lakes. Small blood-lakes are frequently observed by ultrasound toward the periphery of the placenta in normal pregnancies during the second and third trimesters, 34 whereas larger examples are considerably more common in pregnancies complicated by intrauterine growth retardation and pregnancy-induced hypertension. 35 They are often associated with vascular lesions such as intervillous thrombosis and extensive fibrin deposition, and clearly represent hemodynamic disturbances linked to the passage of maternal blood through inadequately transformed uteroplacental arteries. 36 Overall, the peripheral region of the early placenta is subject to the most hemodynamic turbulence, and around 1 to 2% of early pregnancies are complicated by subchorionic bleeding leading to a marginal hematoma between the chorion and decidua. 37 This is a vulnerable region because it connects the definitive placenta with the membranes and a subchorionic hemorrhage in the first trimester increases the risk of miscarriage, stillbirth, abruptio placentae, and preterm labor later in pregnancy. 37
In conclusion, our findings indicate that in normal pregnancies the development of the intervillous circulation is a progressive phenomenon that starts in the periphery of the placenta and is only fully established during the second trimester. During the first week after implantation when the decidual circulation is still sluggish, ie, a menstrual type of circulation, minute quantities of endometrial blood may enter the lacunae between the primitive villi. At that stage, and until the definitive placenta is formed at 8 to 9 weeks, the gestational sac is extremely vulnerable to any imbalance between the development of the uterine circulation and trophoblastic growth both inside and outside the placenta. In early pregnancy failure the premature entry of maternal blood into the intervillous space irreversibly disrupts the materno-embryonic interface. The reduced trophoblastic invasion has greatest impact in the center of the implantation site, with the premature entry of maternal blood displacing the villi and inducing excessive oxidative stress to the villous trophoblast. This hypothesis concurs with the fact that at the end of the first trimester the rate of miscarriage drops to a steady state for the remainder of the pregnancy. 38
Footnotes
Address reprint requests to Dr. G. J. Burton, Department of Anatomy, Downing Street, Cambridge CB2 3DY, UK. E-mail: gjb2@cam.ac.uk.
Supported by (grant 41) from Tommy’s, the Baby Charity, London, United Kingdom.
References
- 1.Boyd JD, Hamilton WJ: The Human Placenta. 1970. Heffer and Sons, Cambridge
- 2.Moore KL, Persaud TVN: The developing human. Clinically Orientated Embryology ed 5 1993. WB Saunders, Philadelphia
- 3.Pijnenborg R, Dixon G, Robertson WB, Brosens I: Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta 1980, 1:3-19 [DOI] [PubMed] [Google Scholar]
- 4.Pijnenborg R, Bland JM, Robertson WB, Dixon G, Brosens I: The pattern of interstitial trophoblastic invasion of the myometrium in early human pregnancy. Placenta 1981, 2:303-316 [DOI] [PubMed] [Google Scholar]
- 5.Hustin J, Schaaps JP: Echographic and anatomic studies of the maternotrophoblastic border during the first trimester of pregnancy. Am J Obstet Gynecol 1987, 157:162-168 [DOI] [PubMed] [Google Scholar]
- 6.Schaaps JP, Hustin J: In vivo aspect of the maternal-trophoblastic border during the first trimester of gestation. Troph Res 1988, 3:39-48 [Google Scholar]
- 7.Jauniaux E, Jurkovic D, Campbell S: In vivo investigations of anatomy and physiology of early human placental circulations. Ultrasound Obstet Gynecol 1991, 1:435-445 [DOI] [PubMed] [Google Scholar]
- 8.Jaffe R, Woods JR: Color Doppler imaging and in vivo assessment of the anatomy and physiology of the early uteroplacental circulation. Fertil Steril 1993, 60:293-297 [DOI] [PubMed] [Google Scholar]
- 9.Coppens M, Loquet P, Kollen F, De Neubourg F, Buytaert P: Longitudinal evaluation of uteroplacental and umbilical blood flow changes in normal early pregnancy. Ultrasound Obstet Gynecol 1996, 7:114-121 [DOI] [PubMed] [Google Scholar]
- 10.Valentin L, Sladkevicius P, Laurini R, Söderberg H, Marsal K: Uteroplacental and luteal circulation in normal first-trimester pregnancies: Doppler ultrasonographic and morphologic study. Am J Obstet Gynecol 1996, 174:768-775 [DOI] [PubMed] [Google Scholar]
- 11.Kurjak A, Kupesic S: Doppler assessment of the intervillous blood flow in normal and abnormal early pregnancy. Obstet Gynecol 1997, :252-256 [DOI] [PubMed] [Google Scholar]
- 12.Carter AM: When is the maternal placental circulation established in man? Placenta 1997, 18:83-87 [DOI] [PubMed] [Google Scholar]
- 13.Craven CM, Ward K: Syncytiotrophoblastic fragments in first-trimester decidual veins: evidence of placental perfusion by the maternal circulation early in pregnancy. Am J Obstet Gynecol 1999, 181:455-459 [DOI] [PubMed] [Google Scholar]
- 14.Kurjak A, Zalud I, Salihagic A, Crvenkovic G, Matijevic R: Transvaginal color Doppler in the assessment of abnormal early pregnancy. J Perinat Med 1991, 19:155-165 [DOI] [PubMed] [Google Scholar]
- 15.Jaffe R, Warsof SL: Color Doppler imaging in the assessment of uteroplacental blood flow in abnormal first trimester intrauterine pregnancies: an attempt to define the etiologic mechanisms. J Ultrasound Med 1992, 11:41-44 [DOI] [PubMed] [Google Scholar]
- 16.Jauniaux E, Zaidi J, Jurkovic D, Campbell S, Hustin J: Comparison of colour Doppler features and pathologic findings in complicated early pregnancy. Hum Reprod 1994, 9:243-247 [DOI] [PubMed] [Google Scholar]
- 17.Schwärzler P, Holden D, Nielsen S, Hahlin M, Sladkevicius P, Bourne TH: The conservative management of first trimester miscarriages and the use of colour Doppler sonography for patient selection. Hum Reprod 1999, 14:1341-1345 [DOI] [PubMed] [Google Scholar]
- 18.Hustin J, Jauniaux E, Schaaps JP: Histological study of the materno-embryonic interface in spontaneous abortion. Placenta 1990, 11:477-486 [DOI] [PubMed] [Google Scholar]
- 19.Watson AL, Palmer ME, Jauniaux E, Burton GJ: Variations in expression of copper/zinc superoxide dismutase in villous trophoblast of the human placenta with gestational age. Placenta 1997, 18:295-299 [DOI] [PubMed] [Google Scholar]
- 20.Watson AL, Skepper JN, Jauniaux E, Burton GJ: Susceptibility of human placental syncytiotrophoblastic mitochondria to oxygen-mediated damage in relation to gestational age. J Clin Endocrinol Metab 1998, 83:1697-1705 [DOI] [PubMed] [Google Scholar]
- 21.Jauniaux E, Watson AL, Hempstock J, Bao Y-P, Skepper JN, Burton GJ: Onset of maternal arterial bloodflow and placental oxidative stress; a possible factor in human early pregnancy failure. Am J Pathol 2000, 157:2111-2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burchell C: Arterial blood flow in the human intervillous space. Am J Obstet Gynecol 1969, 98:303-311 [DOI] [PubMed] [Google Scholar]
- 23.Burton GJ, Jauniaux E, Watson AL: Maternal arterial connections to the placental intervillous space during the first trimester of human pregnancy; the Boyd Collection revisited. Am J Obstet Gynecol 1999, 181:718-724 [DOI] [PubMed] [Google Scholar]
- 24.Jauniaux E, Johnson MR, Jurkovic D, Ramsay B, Campbell S, Meuris S: The role of relaxin in the development of the uteroplacental circulation in early pregnancy. Obstet Gynecol 1994, 84:338-342 [PubMed] [Google Scholar]
- 25.Burton GJ, Watson AL, Hempstock J, Skepper JN, Jauniaux E: Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy. J Clin Endocrinol Metab 2002, 87:2954-2959 [DOI] [PubMed] [Google Scholar]
- 26.Matijevic R, Meekins JW, Walkinshaw SA, Neilson JP, McFadyen IR: Spiral artery blood flow in the central and peripheral areas of the placental bed in the second trimester. Obstet Gynecol 1995, 86:289-292 [DOI] [PubMed] [Google Scholar]
- 27.Palmer ME, Watson AL, Burton GJ: Morphological analysis of degeneration and regeneration of syncytiotrophoblast in first trimester villi during organ culture. Hum Reprod 1997, 12:379-382 [DOI] [PubMed] [Google Scholar]
- 28.Fox H: Effect of hypoxia on trophoblast in organ culture. A morphologic and autoradiographic study. Am J Obstet Gynecol 1970, 107:1058-1064 [DOI] [PubMed] [Google Scholar]
- 29.Genbacev O, Joslin R, Damsky CH, Polliotti BM, Fisher SJ: Hypoxia alters early gestation human cytotrophoblast differentiation/invasion in vitro and models the placental defects that occur in preeclampsia. J Clin Invest 1996, 97:540-550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charnock-Jones DS, Burton GJ: Placental vascular morphogenesis. Baillieres Best Pract Res Clin Obstet Gynaecol 2000, 14:953-968 [DOI] [PubMed] [Google Scholar]
- 31.Benjamin LE, Hemo I, Keshet E: A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development 1998, 125:1591-1598 [DOI] [PubMed] [Google Scholar]
- 32.Zhang EC, Burton GJ, Smith SK, Charnock-Jones DS: Placental vessel adaptation during gestation and to high altitude: changes in diameter and perivascular cell coverage. Placenta 2002, 23:751-762 [DOI] [PubMed] [Google Scholar]
- 33.Genbacev O, Miller RK: Post-implantation differentiation and proliferation of cytotrophoblast cells: in vitro models—a review. Placenta 2000, 21(Suppl A):S45-S49 [DOI] [PubMed] [Google Scholar]
- 34.Jauniaux E, Campbell S: Ultrasonographic assessment of placental abnormalities. Am J Obstet Gynecol 1990, 163:1650-1658 [DOI] [PubMed] [Google Scholar]
- 35.Jauniaux E, Ramsay B, Campbell S: Ultrasonographic investigation of placental morphologic characteristics and size during the second trimester of pregnancy. Am J Obstet Gynecol 1994, 170:130-137 [DOI] [PubMed] [Google Scholar]
- 36.Jauniaux E, Nicolaides KH: Placental lakes, absent umbilical artery diastolic flow and poor fetal growth in early pregnancy. Ultrasound Obstet Gynecol 1996, 7:141-144 [DOI] [PubMed] [Google Scholar]
- 37.Ball RH, Ade CM, Schoenborn JA, Crane JP: The clinical significance of ultransonographically detected subchorionic hemorrhages. Am J Obstet Gynecol 1996, 174:996-1002 [DOI] [PubMed] [Google Scholar]
- 38.Goldstein SR: Embryonic death in early pregnancy: a new look at the first trimester. Obstet Gynecol 1994, 84:294-297 [PubMed] [Google Scholar]