Abstract
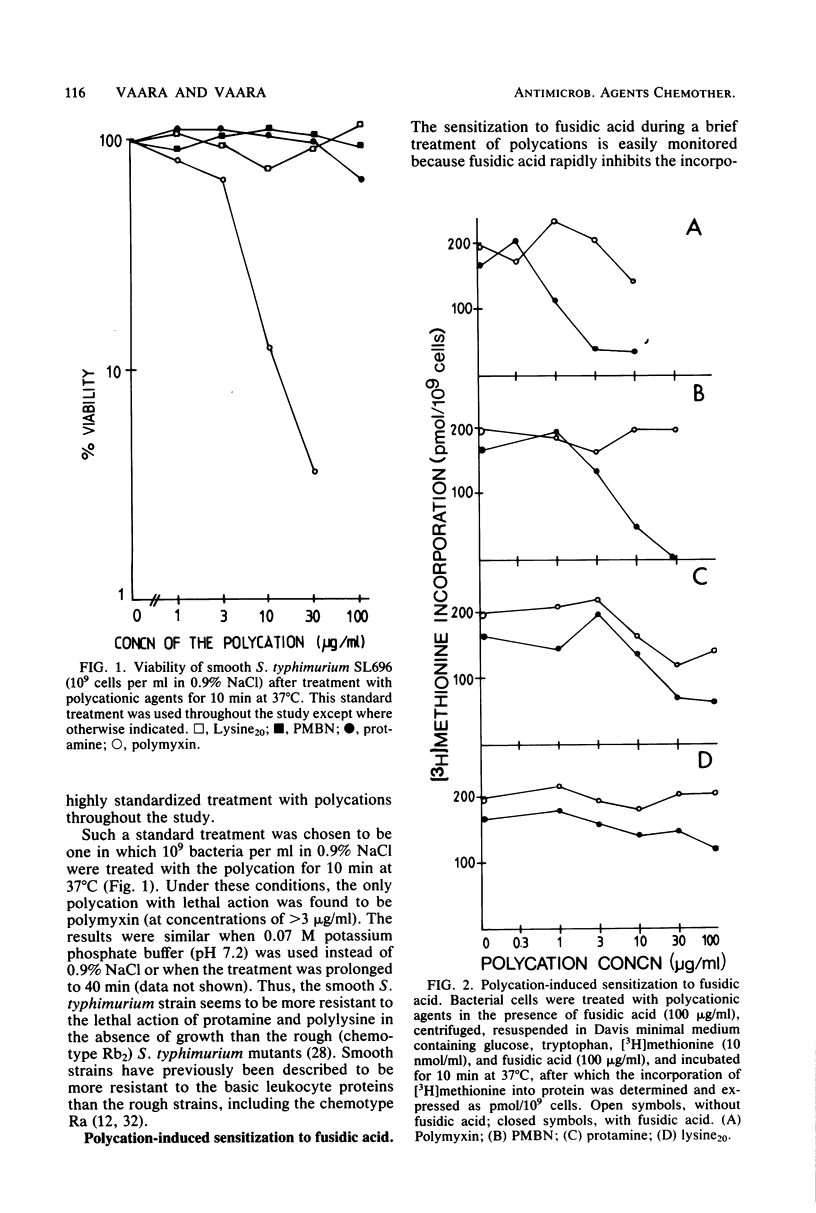
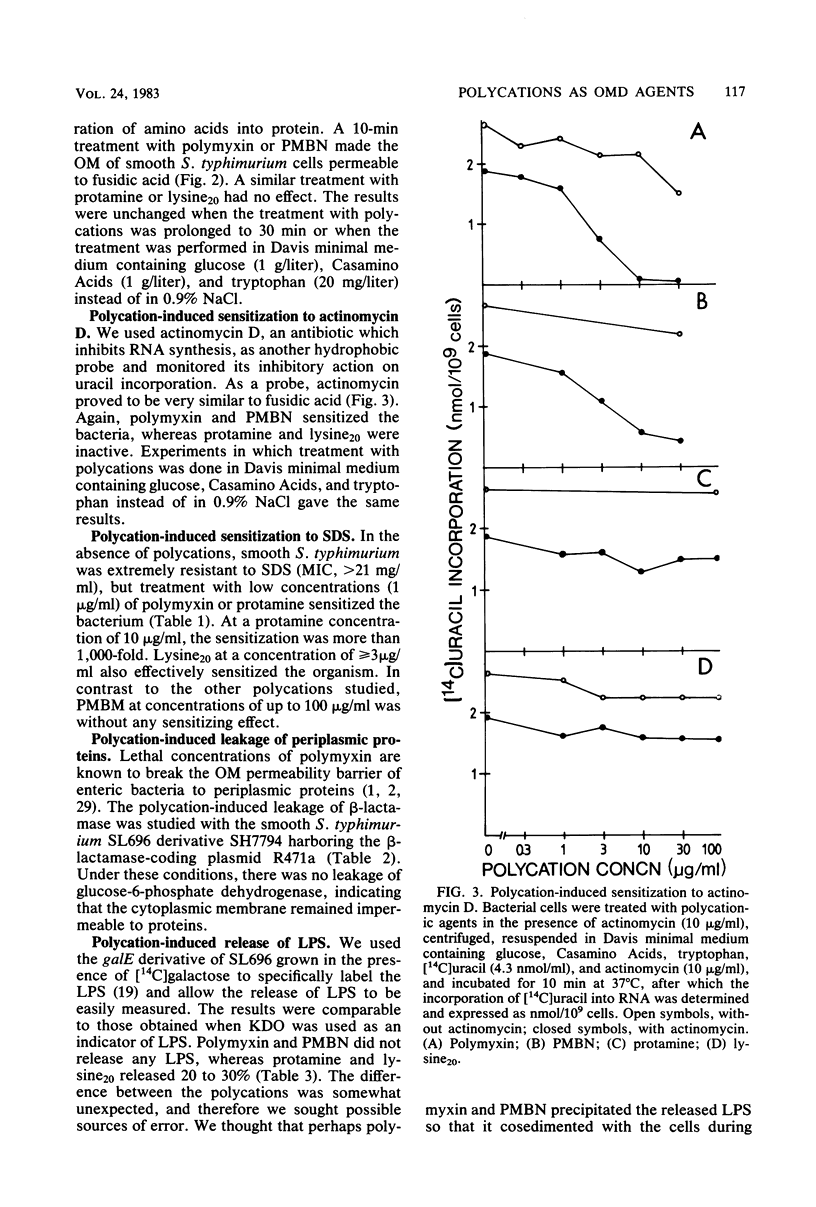
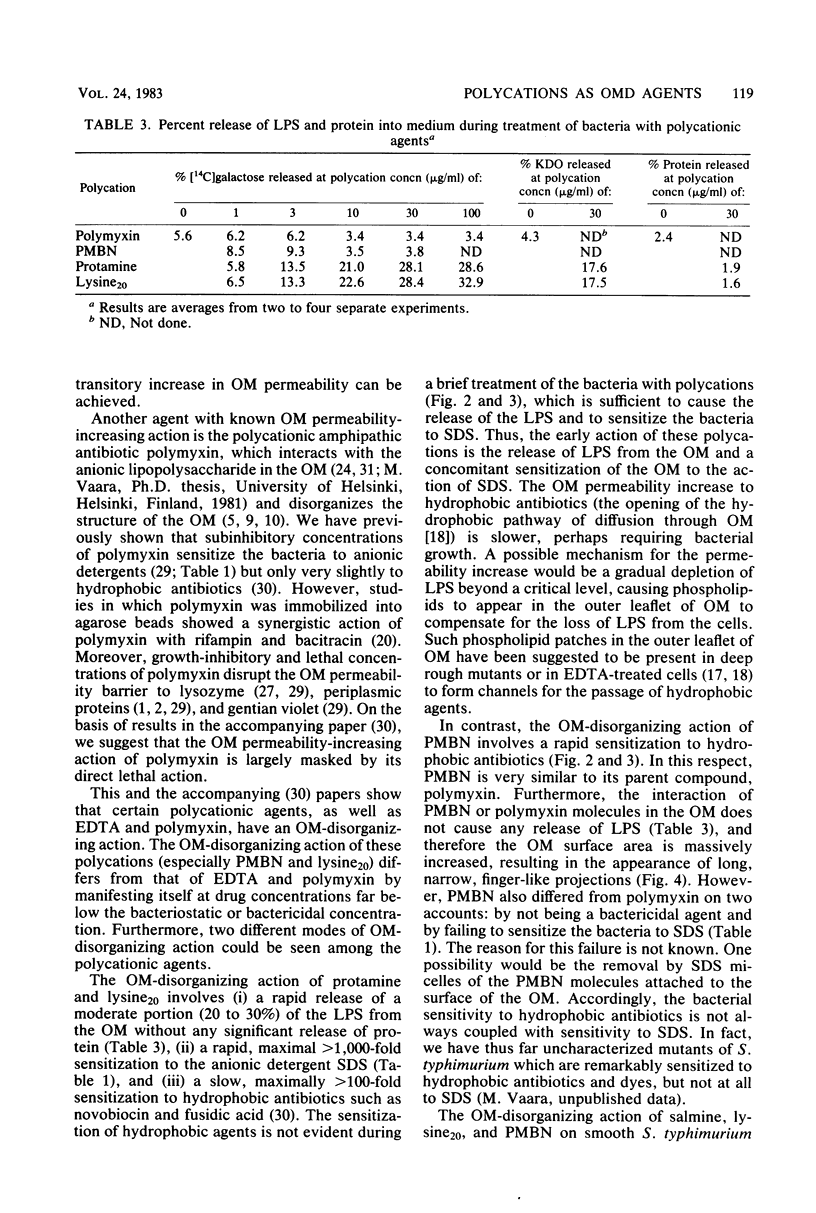
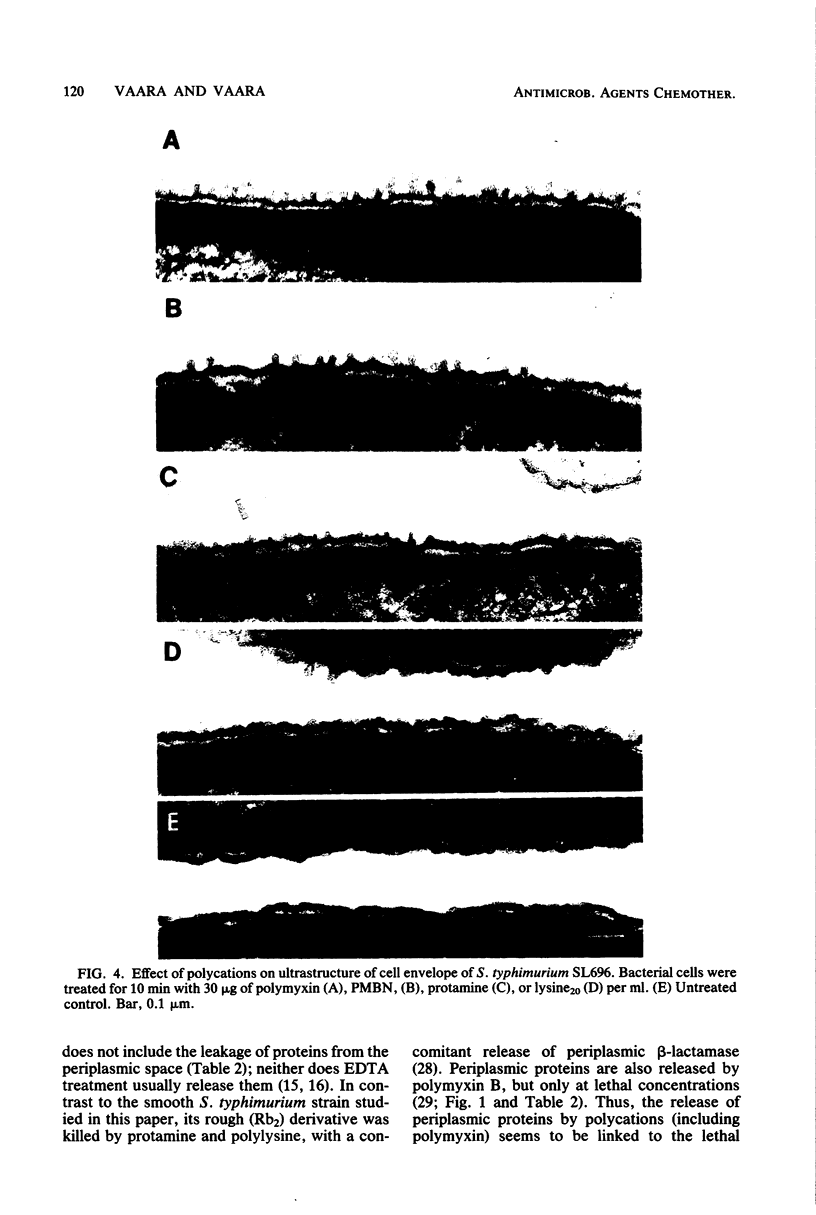
The outer membrane-disorganizing effect of a short (10-min) treatment with polycationic agents was studied with smooth Salmonella typhimurium used as a test organism. The polycationic agents were the protamine salmine, a lysine polymer with 20 lysine residues (lysine20), and the deacylated polymyxin B derivative polymyxin B nonapeptide. Two different types of outer membrane-disorganizing were found. Protamine and lysine20 released 20 to 30% of the lipopolysaccharide from the outer membrane and sensitized the bacteria to the anionic detergent sodium dodecyl sulfate but did not (under these conditions) make the bacteria permeable to the hydrophobic probes fusidic acid and actinomycin D. In contrast, polymyxin B nonapeptide did not release lipopolysaccharide or sensitize the bacteria to sodium dodecyl sulfate but made the outer membrane permeable to the hydrophobic probes. None of the agents was bactericidal under the conditions used or caused any leakage of periplasmic beta-lactamase. Polymyxin B was used as a reference and showed characteristic outer membrane-disorganizing action. In thin-section electron microscopy, polymyxin B nonapeptide caused the appearance of long, narrow, finger-like projections on the outer membrane. Protamine and lysine20 caused a distinctly wrinkled appearance of the outer membrane but no projections.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cerny G., Teuber M. Comparative polyacrylamide electrophoresis of periplasmic proteins released from gram-negative bacteria by polymyxin B. Arch Mikrobiol. 1972;82(4):361–370. doi: 10.1007/BF00424939. [DOI] [PubMed] [Google Scholar]
- Cerny G., Teuber M. Differential release of periplasmic versus cytoplasmic enzymes from Escherichia coli B by polymixin B. Arch Mikrobiol. 1971;78(2):166–179. doi: 10.1007/BF00424873. [DOI] [PubMed] [Google Scholar]
- Irvin R. T., Chatterjee A. K., Sanderson K. E., Costerton J. W. Comparison of the cell envelope structure of a lipopolysaccharide-defective (heptose-deficient) strain and a smooth strain of Salmonella typhimurium. J Bacteriol. 1975 Nov;124(2):930–941. doi: 10.1128/jb.124.2.930-941.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike M., Iida K., Matsuo T. Electron microscopic studies on mode of action of polymyxin. J Bacteriol. 1969 Jan;97(1):448–452. doi: 10.1128/jb.97.1.448-452.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leive L., Kollin V. Controlling EDTA treatment to produce permeable Escherichia coli with normal metabolic processes. Biochem Biophys Res Commun. 1967 Jul 21;28(2):229–236. doi: 10.1016/0006-291x(67)90434-2. [DOI] [PubMed] [Google Scholar]
- Leive L. The barrier function of the gram-negative envelope. Ann N Y Acad Sci. 1974 May 10;235(0):109–129. doi: 10.1111/j.1749-6632.1974.tb43261.x. [DOI] [PubMed] [Google Scholar]
- Lounatmaa K., Mäkelä P. H., Sarvas M. Effect of polymyxin on the ultrastructure of the outer membrane of wild-type and polymyxin-resistant strain of Salmonella. J Bacteriol. 1976 Sep;127(3):1400–1407. doi: 10.1128/jb.127.3.1400-1407.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounatmaa K., Nanninga N. Effect of polymyxin on the outer membrane of Salmonella typhimurium: freeze-fracture studies. J Bacteriol. 1976 Nov;128(2):665–667. doi: 10.1128/jb.128.2.665-667.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrzakowski M. C., Spitznagel J. K. Bactericidal activity of fractionated granule contents from human polymorphonuclear leukocytes: antagonism of granule cationic proteins by lipopolysaccharide. Infect Immun. 1979 Aug;25(2):597–602. doi: 10.1128/iai.25.2.597-602.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NETER E., GORZYNSKI E. A., WESTPHAL O., LUDERITZ O., KLUMPP D. J. The effects of protamine and histone on enterobacterial lipopolysaccharides and hemolysis. Can J Microbiol. 1958 Aug;4(4):371–383. doi: 10.1139/m58-039. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Chou J. Release of surface enzymes in Enterobacteriaceae by osmotic shock. J Bacteriol. 1967 Dec;94(6):1934–1945. doi: 10.1128/jb.94.6.1934-1945.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Nikaido H., Nakae T. The outer membrane of Gram-negative bacteria. Adv Microb Physiol. 1979;20:163–250. doi: 10.1016/s0065-2911(08)60208-8. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Outer membrane of Salmonella typhimurium. Transmembrane diffusion of some hydrophobic substances. Biochim Biophys Acta. 1976 Apr 16;433(1):118–132. doi: 10.1016/0005-2736(76)90182-6. [DOI] [PubMed] [Google Scholar]
- Palva E. T., Mäkelä P. H. Lipopolysaccharide heterogeneity in Salmonella typhimurium analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Eur J Biochem. 1980;107(1):137–143. doi: 10.1111/j.1432-1033.1980.tb04634.x. [DOI] [PubMed] [Google Scholar]
- Rosenthal K. S., Storm D. R. Disruption of the Escherichia coli outer membrane permeability barrier by immobilized polymyxin B. J Antibiot (Tokyo) 1977 Dec;30(12):1087–1092. doi: 10.7164/antibiotics.30.1087. [DOI] [PubMed] [Google Scholar]
- Rosenthal K. S., Swanson P. E., Storm D. R. Disruption of Escherichia coli outer membranes by EM 49. A new membrane active peptide. Biochemistry. 1976 Dec 28;15(26):5783–5792. doi: 10.1021/bi00671a015. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E., Ross H., Ziegler L., Mäkelä P. H. F + , Hfr, and F' strains of Salmonella typhimurium and Salmonella abony. Bacteriol Rev. 1972 Dec;36(4):608–637. doi: 10.1128/br.36.4.608-637.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler M., Osborn M. J. Interaction of divalent cations and polymyxin B with lipopolysaccharide. Biochemistry. 1979 Oct 2;18(20):4425–4430. doi: 10.1021/bi00587a024. [DOI] [PubMed] [Google Scholar]
- Schindler P. R., Teuber M. Action of polymyxin B on bacterial membranes: morphological changes in the cytoplasm and in the outer membrane of Salmonella typhimurium and Escherichia coli B. Antimicrob Agents Chemother. 1975 Jul;8(1):95–104. doi: 10.1128/aac.8.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm D. R., Rosenthal K. S., Swanson P. E. Polymyxin and related peptide antibiotics. Annu Rev Biochem. 1977;46:723–763. doi: 10.1146/annurev.bi.46.070177.003451. [DOI] [PubMed] [Google Scholar]
- Teuber M. Lysozyme-dependent production of spheroplast-like bodies from polymyxin B-treated Salmonella typhimurium. Arch Mikrobiol. 1970;70(2):139–146. doi: 10.1007/BF00412204. [DOI] [PubMed] [Google Scholar]
- Vaara M. Increased outer membrane resistance to ethylenediaminetetraacetate and cations in novel lipid A mutants. J Bacteriol. 1981 Nov;148(2):426–434. doi: 10.1128/jb.148.2.426-434.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M., Vaara T., Jensen M., Helander I., Nurminen M., Rietschel E. T., Mäkelä P. H. Characterization of the lipopolysaccharide from the polymyxin-resistant pmrA mutants of Salmonella typhimurium. FEBS Lett. 1981 Jun 29;129(1):145–149. doi: 10.1016/0014-5793(81)80777-6. [DOI] [PubMed] [Google Scholar]
- Vaara M., Vaara T. Outer membrane permeability barrier disruption by polymyxin in polymyxin-susceptible and -resistant Salmonella typhimurium. Antimicrob Agents Chemother. 1981 Apr;19(4):578–583. doi: 10.1128/aac.19.4.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M., Vaara T. Polycations sensitize enteric bacteria to antibiotics. Antimicrob Agents Chemother. 1983 Jul;24(1):107–113. doi: 10.1128/aac.24.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Elsbach P., Olsson I., Odeberg H. Purification and characterization of a potent bactericidal and membrane active protein from the granules of human polymorphonuclear leukocytes. J Biol Chem. 1978 Apr 25;253(8):2664–2672. [PubMed] [Google Scholar]
- Wilkinson R. G., Gemski P., Jr, Stocker B. A. Non-smooth mutants of Salmonella typhimurium: differentiation by phage sensitivity and genetic mapping. J Gen Microbiol. 1972 May;70(3):527–554. doi: 10.1099/00221287-70-3-527. [DOI] [PubMed] [Google Scholar]




