Abstract
Primary follicular lymphoma of the gastrointestinal tract (GI-FL) is a rare so far poorly studied entity. We analyzed four FL cases located in the small intestine and duodenum to gain insight in their pathogenesis and to find an explanation for their low tendency to disseminate outside the GI tract. GI-FLs resemble nodal FLs with respect to morphology and expression of typical GC markers such as CD10, CD38, and BCL-6. We established that the high levels of the anti-apoptosis protein BCL-2 in the tumor cells are in all cases due to a t(14;18) involving the immunoglobulin heavy chain and BCL-2 loci. Detailed immunoglobulin gene analyses on microdissected tissue samples further supported the GC-cell derivation: GI-FLs carry extensively mutated variable heavy-chain genes. The mutation patterns indicated that at some time point in development stringent antigen receptor-based selection processes must have occurred. Interestingly, three of four neoplasms expressed surface IgA, an immunoglobulin class typical of the mucosal immune system and seldom found in nodal FL. In contrast to nodal FLs, the GI-FLs expressed the α4β7 integrin, an established mucosa-homing receptor also expressed by normal intestinal B and T lymphocytes and by low-grade mucosa-associated lymphoid tissue lymphomas. However, the chemokine receptor CXCR3, expressed on low-grade mucosa-associated lymphoid tissue lymphomas, was not detected on the GI-FLs or on nodal FLs. The combined data suggests that primary FL of the small intestine is a distinct entity that originates from local antigen-responsive B cells.
Twenty-five to 40% of non-Hodgkin’s lymphomas (NHLs) arise at mucosal sites, most frequently in the gastrointestinal (GI) tract. 1 The most common GI tract lymphomas are the classical low-grade mucosa-associated lymphoid tissue (MALT) B-NHLs. 2,3 Follicular lymphomas (FLs) of the GI tract (GI-FL), by contrast, are rare with an estimated frequency of 1 to 3% among the GI tract B-NHLs. 4-6 They occur most frequently in the small intestine, specifically in the duodenum. 4-11
In the lymph nodes, FL is one of the most common B-NHLs and, by consequence, has been extensively studied. In its classical form, this neoplasm consists of follicular structures that harbor centrocytic and centroblastic tumor cells. These cells proliferate within networks of nonneoplastic follicular dendritic cells, similar to the GC B cells of so-called secondary lymphoid follicles. 12 Like their normal counterparts, the tumor B cells generally express CD10, CD38, and BCL-6 in addition to pan B-cell markers. 12-14 Nodal FLs most often express surface IgM (sIgM) and sIgD, less frequently sIgG and rarely sIgA. At the molecular level, FLs are characterized by the t(14;18)(q32;q21) involving the Ig heavy chain (IGH) and BCL-2 gene loci. 15,16 Because of this translocation, the oncogene BCL-2 is constitutively expressed, preventing cells from apoptosis. 17,18 Molecular analyses of the variable (V) regions of IGH- and IGL- chain genes have further confirmed the germinal center (GC) origin of FLs: the IgVH and IgVL genes of FLs harbor significant numbers of nucleotide substitutions because of somatic hypermutation. 19-21
It is remarkable that in all of the reported cases of primary duodenal FLs, 5,6,8,9 including a large FL that invaded the pancreas, 7 no evidence for distant or systemic disease was found. This low tendency to disseminate outside the GI tract, which clearly contrasts the behavior of nodal FL, 12 may be because of expression of specific adhesion molecules and/or dependence on local stimuli such as antigen or chemokines. 22 Mucosal lymphocytes strongly express the α4β7 integrin whereas its ligand, MAdCAM-1, is selectively expressed on mucosal endothelium. 23,24 Accordingly, mucosa-associated B-NHLs, such as low-grade MALT lymphomas and mantle zone lymphomas presenting as malignant lymphomatous polyposis, have been shown to express the mucosal homing receptor α4β7. 25,26 In addition, it has been reported that intestinal epithelial cells produce a number of chemokines, ie, CLL25 (TECK), 27,28 CCL5 (RANTES), 29 CCL9 (MIG), CCL10 (IP10), and CCL11 (I-TAC). 30,31 The respective receptors for these chemokines, CCR9, CCR5, and CXCR3 are expressed by α4β7+ T lymphocytes present in the lamina propria and in the epithelium. 27,28,30-32 Interestingly, it has recently been shown that CLL25 (TECK) also attracts IgA-secreting cells to the intestine. 33 Furthermore, CXCR3 is expressed by a small subset of peripheral B cells and by distinct types of B-cell malignancies such as low-grade MALT lymphoma, splenic marginal zone lymphoma, and B-cell chronic lymphocytic leukemia (B-CLL). 34-36 However, CXCR3 expression has not been detected in nodal FLs. 36 Thus, expression of adhesion molecules and chemokine receptors determine homing and dissemination of normal and malignant B cells.
To explore to what extent FLs of the small intestine resemble their nodal counterparts and on the other hand to explain their localized nature, we performed a detailed analysis of the configuration of the expressed IgVH chain genes and the expression of lymphocyte-homing receptors in four cases of GI-FL. The results of these studies strongly suggest that these lymphomas are the offspring of local antigen-responsive B cells.
Materials and Methods
Patient Material
Fresh tissue material of the four GI-FLs, originating from a small bowel resection in one case and from endoscopically taken biopsies in three other cases, was in part snap-frozen in liquid nitrogen and in part fixed in formalin and paraffin-embedded. Patient 1, a 60-year-old male, was admitted with nausea and vomiting because of an ileus. On laparotomy, a stricturing tumor of 3.7 cm in diameter was found that extended transmurally up to 1 mm from the serosa. Patients 2, 3, and 4 were females of 68, 45, and 35 years of age that underwent endoscopic examinations for nonspecific GI complaints. In patient 2, a lesion was seen in the pars descendens of the duodenum covering an area of 3 cm in diameter with a conspicuously nodular surface. A low-grade MALT B-cell lymphoma was suspected. In patient 3, a polypous tumor with a diameter of 1.5 cm was found in the area of the ampulla of Vater (see Figure 2A ▶ ). In patient 4 a lesion in the duodenum and focally in the ileum was found. In none of the four patients was histological evidence obtained for systemic disease. Patient 4 however, was treated chemotherapeutically based on demonstration of a t(14;18) by polymerase chain reaction (PCR) on bone marrow. All achieved a disease-free status. The clinical data of the patients are summarized in Table 1 ▶ .
Figure 2.
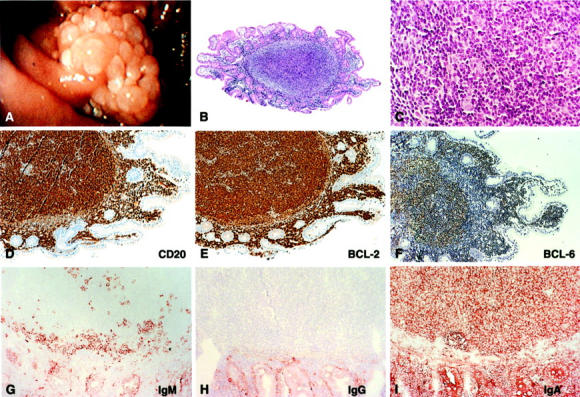
Endoscopy and histology of the duodenal polyp of patient 3. A: Endoscopic picture of the adenoma-like structure found near the ampulla of Vater. B and C: H&E staining of a section through one of the tumor nodules showing a follicle-like lymphocytic infiltrate in the mucosa at ×50 and ×400 magnification, respectively. D: CD20 staining, proving the B-cell origin of the majority of the infiltrating lymphocytes. E: BCL-2 staining showing strong overexpression of this oncogene by the B cells in the follicular infiltrates. F: BCL-6 staining showing expression of this typical GC B-cell marker. G, H, and I: IgM, IgG, and IgA stainings showing that the tumor cells express IgA exclusively.
Table 1.
Clinical Findings and Disease Course of the GI Tract Follicular Lymphomas Analyzed
| Patient | Sex | Age | Localization | Stage* | Therapy | Time† | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | m | 60 | Jejunum | IE | Resection | 41 | Alive, without disease |
| 2 | f | 68 | Duodenum | IE | Radiotherapy | 60 | Alive, without disease |
| 3 | f | 45 | Duodenum | IE | Radiotherapy | 31 | Alive, without disease |
| 4 | f | 35 | Duodenum | IE‡ | Chemotherapy | 24 | Alive, without disease |
*Ann Arbor staging classification.
†Disease-free survival time in months.
‡Positive t(14;18) on bone marrow samples but no histological evidence for bone marrow localization.
Tissue material of nodal FLs, MALT lymphomas, B-cell chronic lymphocytic leukemia, and normal ileum, tonsil, and lymph node were obtained from surgically removed specimens of our hospital.
Immunohistochemistry
The immunohistochemical stainings were performed on acetone-fixed cryostat sections and/or on formalin-fixed paraffin-embedded sections using the highly sensitive Powervision+ detection system (ImmunoVision Technologies, Daly City, CA). Endogenous peroxidase activity of cryostat sections was blocked with 0.1% NaN3 and 0.3% H2O2 in phosphate-buffered saline and of paraffin sections, after deparaffinization and rehydration with 0.3% H2O2 in methanol. Visualization of antibody binding was performed for the cryostat sections with 3-amino-9-ethylcarbazole (Sigma, St. Louis, MO), 0.03% H2O2 in sodium acetate, pH 4.9, and for the paraffin sections with 3,3′-diaminobenzidine (Sigma), 0.03% H2O2 in Tris-HCl, pH 7.6. The sections were counterstained with hematoxylin (Merck, Darmstadt, Germany). Monoclonal antibodies (mAbs) specific for CD10 (CALLA), IgM, κ- and λ-light chains (Becton and Dickinson, Erembodegem-Aalst, Belgium), IgG, IgA, CD20 (B-Ly1), CD21-L (DRC-1, R4/23), BCL-2 (124), and BCL-6 (PG-B6P) (DAKO, Glostrup, Denmark), CD38 (HIT2) (CLB, Amsterdam, the Netherlands), CXCR3 (1C6) (Pharmingen, San Diego, CA), and α4β7 (Act-1) 37 were used. mAbs for IgM, IgG, IgA, κ, λ, CD21-L, α4β7, CD10, and CD38 were only used on cryostat sections, mAbs for CD20, BCL-2, BCL-6, and CXCR3 were used on cryostat and on paraffin sections. For the CXCR3, BCL-2, and BCL-6 mAbs, the paraffin sections were pretreated with citrate buffer (10 mmol/L, pH 6.5) at 100°C for 10 minutes.
Amplification and Analysis of t(14;18)
High-molecular weight DNA was obtained from frozen tissue specimens by lysis in sodium dodecyl sulfate and 100 μg/ml of proteinase K. The samples were digested at 56°C for 16 hours, followed by phenol-chloroform extraction and ethanol precipitation. After washing, the DNA samples were dissolved in distilled water. These genomic DNA samples were tested for the presence of the t(14;18)(q32,q21) using a PCR targeted at the BCL-2/JH breakpoint. The BCL-2 mbr2 primer was used in combination with a reverse JH consensus primer JH18. 38 The PCR products were analyzed on a 1.5% agarose gel and subsequently purified. The purified PCR products were sequenced on both strands using the Big Dye terminator cycle-sequencing kit and an ABI sequencer (Perkin Elmer Corp., Norwalk, CT).
Microdissection and cDNA Synthesis
Microdissection of groups of cells was performed with a PALM laser-microbeam system [Positioning and Ablation with Laser Microbeams (PALM), GmbH, Bernried, Germany]. Frozen tissue sections of 10 μm were mounted on plastic membranes and stained for 1 minute with hematoxylin. For RNA analyses, samples of ∼50 cells were dissected out of tumor follicles and catapulted into a 20-μl cDNA reaction mixture (see below) and kept on ice. Without previous RNA isolation, cDNA was synthesized using 2 nmol of Pd(N)6 primer (Pharmacia Biotech, Roosendaal, The Netherlands) and 160 U of M-MLV reverse transcriptase (Live Technologies, Breda, The Netherlands). The reaction mixture further contained 8 mmol/L dithiothreitol, 1 mmol/L of each dNTP, 1× first strand buffer (50 mmol/L Tris-HCl, pH 8.3, 75 mmol/L KCl, 3 mmol/L MgCl2) and 24 U of RNase inhibitor (Boehringer Mannheim, Almere, The Netherlands). The reaction was performed for 15 minutes at 37°C after which the enzyme was inactivated during 10 minutes at 95°C. After cDNA synthesis, 20 μl of water was added.
Amplification of the VH Gene by PCR
The IGVH locus and the applied primers in the PCR reactions are schematically depicted in Figure 1 ▶ . VH family-specific PCRs were performed using different VH family-specific leader primers 39 in combination with reverse primers specific for either Cα (patients 1 and 3) or Cμ (patient 2). (Cμ1: 5′ CGTATCCGACGGGGAATTCTC 3′; Cα1: 5′ TTCGCTCCAGGTCACACTG 3′). In the first round of amplification 1 μl of cDNA was used in a 25-μl PCR reaction volume. The PCR mixture contained 1× Taq buffer (20 mmol/L Tris HCl, 50 mmol/L KCl, pH 8.4), 0.2 mmol/L of each dNTP, 1.5 mmol/L MgCl2, 1 U of Taq polymerase (Life Technologies, Breda, The Netherlands) and 0.5 μmol/L of each primer. First, 10 PCR cycles were performed in the thermal cycler (PTC-100; MJ Research Inc., Watertown, MA) successively 30 seconds at 95°C, 20 seconds at 57°C, and 20 seconds at 72°C. The next 40 cycles of amplification consisted of 30 seconds at 95°C, 20 seconds at 55°C, and 20 seconds at 72°C. The reaction was completed for 6 minutes at 72°C. Under the same conditions, the complementary determining region 3 (CDR3) was amplified in a nested PCR reaction using 2.5 μl of the first PCR product in a 25-μl reaction volume using a forward primer specific for framework region 3 (FR3) in combination with an appropriate nested reverse Cα or Cμ primers. 39 The PCR products were analyzed on a 3% Methaphor agarose gel (FMC Bioproducts, Rockland, ME).
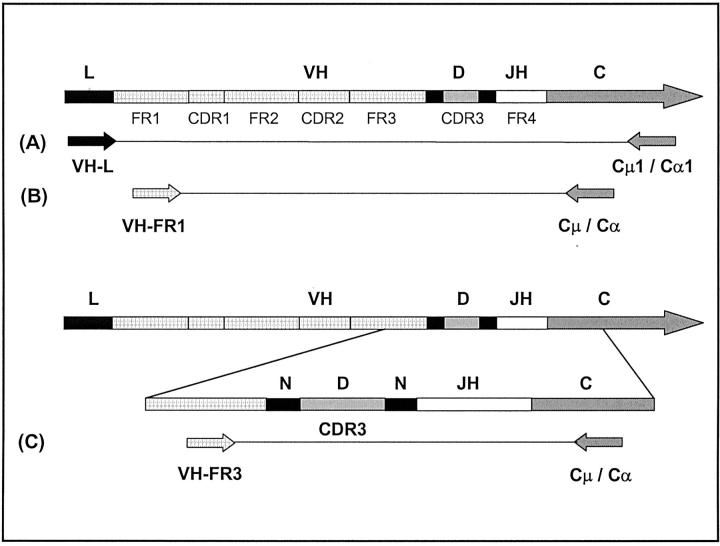
Figure 1.
Schematic representation of the IgH locus and the primers used in the PCRs on the FLs. L, leader sequence; VH, variable gene segment; D, diversity gene segment; JH, joining gene segment; CH, constant gene segment; N, nontemplated nucleotide additions; FR, framework region; CDR, complementarity determining region. A: VH family leader-specific PCR. To amplify the VH gene, VH family-specific primers annealing in the leader regions were combined with downstream primers specific for the constant regions of the immunoglobulin gene, ie, either Cμ1 for IgM-expressing and Cα1 for IgA-expressing lymphomas. B: Nested VH family-FR1 PCR. The VH region was amplified out of the products of PCR-A using nested VH family-specific FR1 primers and Cμ and Cα downstream primers. C: Nested CDR3-specific PCR. The CDR3 regions were amplified out of the products of PCR-A, using a consensus FR3 primer and nested Cμ or Cα downstream primers.
To obtain enough material for sequencing, the tumor-specific IgVH products of first VH family-specific PCR were amplified in a nested PCR using the appropriate VH-FR1-specific primer in combination with nested Cα or Cμ primers. Also 2.5 μl of PCR product of the first VH family-specific PCR was used here in a 25-μl reaction volume under the same PCR conditions (VH3-FR1, 5′-TCCCTGAGACTCTCCTGTG-3′) PCR products were analyzed on a 1% standard agarose gel. The PCR products were sequenced on both strands. The IgVH sequences found were compared with published germline IgVH sequences using the Vbase database 40 and DNAplot 41 on the internet (http://www.mrc-cpe.cam.ac.uk) to identify somatic mutations. The amino acid sequences of the CDR3 regions were analyzed using the National Center for Biotechnolgy Information Protein-BLAST program, option “search for short nearly exact matches” (http://www.ncbi.nlm.nih.gov/BLAST).
Statistical Analysis
To calculate whether there is significant selection against replacement (R) mutations in the framework regions (FR), we used the binomial distribution model as proposed by Chang and Casali 42 and the multinomial distribution model as proposed by Lossos and colleagues 43 Because the framework regions are essential for the overall structure of the IgV region, in normal Ag-selected B cells counterselection for R mutations in these regions occurs. This results in lower replacement/silent ratios in the FR regions (R/S <1.5) than would be expected if mutations would occur by chance only (R/S = 2.9).
Results
Histopathological and Molecular Features of Primary FLs of the Small Intestine
We studied four cases of primary FL of the small intestine (see Materials and Methods). In patient 3, a typically polypous tumor with a diameter of 1.5 cm was found in the area of the ampulla of Vater (Figure 2A) ▶ . Histologically, the tumors of all four patients consisted of dense infiltrates of predominantly small cleaved lymphocytes admixed with variable numbers of centroblasts and a few immunoblasts. The infiltrates displayed a clear nodular growth pattern reminiscent of normal lymph follicles (Figure 2, B and C) ▶ . Starry sky macrophages, which are prominent in reactive germinal centers, were absent. Unlike in classical MALT-type lymphomas, the lymphoid cells did not infiltrate and destruct the gland epithelium, ie, no lymphoepithelial lesions were present. Immunohistochemistry demonstrated that the tumor cells consisted of mature CD20+ B cells, expressing the typical GC B-cell markers CD38, CD10, and BCL-6, which are also expressed by the vast majority of nodal FLs 12-14 (Figure 2, D and F ▶ ; Table 2 ▶ ). Interestingly, the tumor cells of patients 1, 3, and 4 were IgM−, IgG−, and IgA+ (Figure 2, G to I ▶ ; Table 2 ▶ ). CD21-L (DRC-1) stainings demonstrated that the tumor cells expand mainly in networks of follicular dendritic cells (Figure 5 ▶ , Table 2 ▶ ). However, BCL-2+ BCL-6+ IgA+ tumor cells were also found scattered in the lamina propria (Figure 2; E, F, and I) ▶ . Like nodal FLs, all four GI-FLs were found to carry a t(14;18). The translocations in all cases involved the major breakpoint region (mbr), located in the 3′ untranslated region of the BCL-2 gene, adjacent to one of the JH gene segments of the IGH locus (Table 2 ▶ and data not shown). 15,16
Table 2.
Immunohistochemistry and t(14;18) PCR
| Patient no. | Immunohistochemistry | PCR t(14;18) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CD20 | CD21-L | Ig isotype | Light chain | BCL-6 | CD10 | CD38 | BCL-2 | ||
| 1 | + | + | IgA | nc | + | + | + | + | + |
| 2 | + | + | IgM | κ | + | + | + | + | + |
| 3 | + | + | IgA | κ | + | + | + | + | + |
| 4 | + | + | IgA | κ | + | + | + | + | + |
+, The reactivity of the monoclonal antibody with the tumor cells, except for the CD21-L staining in which it indicates the reactivity with the nonmalignant FDCs.
nc, Not clear.
Figure 5.
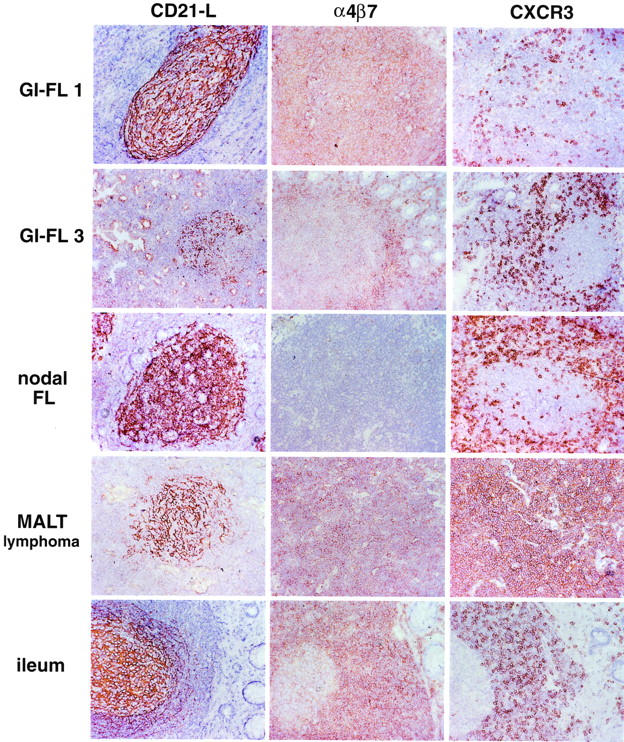
Immunohistochemical detection of CD21-L, α4β7, and CXCR3 of GI-FL, nodal FL, MALT lymphoma. and normal ileum. In contrast to nodal FLs the GI-FLs express α4β7. Neither nodal FLs nor GI-FLs express CXCR3. The T cells surrounding the malignant follicles are CXCR3-positive. Low-grade MALT lymphoma expresses both α4β7 and CXCR3. In normal ileum, all mantle zone lymphocytes strongly express α4β7 and a significant fraction expresses CXCR3. The GC B cells display low, but detectable α4β7 expression but no CXCR3 expression.
Ig VH Gene Analysis on Microdissected Tumor Samples
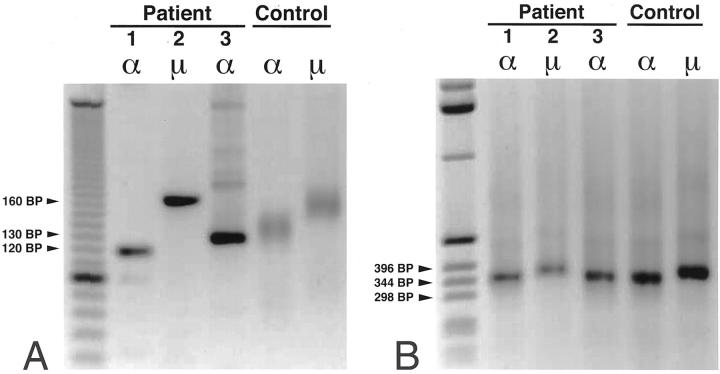
We amplified the IgVH regions out of almost pure samples of tumor cells microdissected from frozen tissue sections of the GI-FL of patients 1, 2, and 3. In these experiments, separate PCRs were performed applying six VH family leader region-specific primers in combination with appropriate reverse constant heavy chain region-specific primers, being either Cα1, in lymphomas 1 and 3 or Cμ1 in lymphoma 2 (Figure 1) ▶ . On the PCR products thus obtained, not visible on agarose gel, a nested CDR3-specific PCR was performed using an FR3 region-specific primer, which anneals just 5′ to the D gene, combined with a nested Cα or Cμ region-specific reverse primer (Figure 1) ▶ . Of each GI-FL, these nested CDR3 PCRs yielded sharp bands on agarose gel only in the condition that a VH3-specific leader primer had been used in the first PCR (Figure 3A) ▶ . As this finding was reproducible in multiple tissue samples of each lymphoma we were confident that we had confirmed clonality and identified the IgVH genes, with their respective CDR3 regions, that were expressed by the lymphomas. To obtain sufficient amounts of PCR products for sequencing, nested PCRs on the primary VH3 PCR products were performed using a 5′ primer annealing in the FR1 region in combination with the nested Cα or Cμ reverse primers (Figures 1 and 3B) ▶ ▶ . To ascertain that these nested IgVH products indeed originated from the tumor clones, we also performed a seminested CDR3 PCR on them. In all three cases, the obtained CDR3 products had sizes identical to the original CDR3 amplimers (not shown). The amplified IgVH genes were sequenced and analyzed. Of lymphomas 1, 2, and 3 we obtained VH sequences out of six, two, and six malignant follicles, respectively. GI-FL 1 used the V3–11 gene in combination with the JH4b gene, GI-FL 2 used the V3-48 and JH4b genes, and GI-FL 3 used the V3-7 and JH6 genes (Table 3) ▶ . Corbett and colleagues 44 proposed stringent criteria for the assignment of D genes: at least 10 constitutive nucleotides of identity are required to confidently assign a D gene segment. According to these criteria, we could not determine a D gene used in any of the three cases.
Figure 3.
Nested CDR3- and VH FR1-specific PCRs. A: The products obtained by the VH leader-specific PCR were subjected to CDR3 PCRs using the FR3 upstream primer and Cα (patients 1 and 3) or Cμ (patient 2) downstream primers. These nested CDR3 PCRs on the VH3 PCR products yielded sharp bands of 120-, 160-, and 130-bp lengths on gel, proving the clonal nature of the proliferating cells of patients 1, 2, and 3, respectively. Control CDR3 PCRs on polyclonal B cells, shown in the last two lanes, yielded smear patterns on gel. B: To be able to sequence the VH products, the VH3-leader PCR products were subjected to nested PCRs using the VH3-FR1 upstream primers in combination with Cα (patients 1 and 3) or Cμ (patient 2) downstream primers. The obtained VH3-FR1 PCR products were purified and sequenced. To give an impression of the lengths of the amplimers, the size of some marker DNA bands are indicated.
Table 3.
Ig Heavy Chain Gene Sequence Analyses of the Small Intestinal Follicular Lymphomas
| Patient no. | Ig isotype | VH family | Closest VH germline gene | No. of mutations (%)* | JH gene |
|---|---|---|---|---|---|
| 1 | IgA | 3 | V3-11 | 40 (17.7%) | 4b |
| 2 | IgM | 3 | V3-48 | 32 (14.2%) | 5b |
| 3 | IgA | 3 | V3-7 | 21 (9.3%) | 6b |
*Counted starting from codon 23 where the FR1 primer ends.
Number of Mutations, Mutation Patterns, and CDR3 Amino Acid Sequence Analysis
The GI-FLs of patients 1, 2, and 3 all expressed extensively mutated IgVH genes with, respectively, 40, 32, and 21 nucleotide differences in their VH genes compared to germline VH3 genes of closest homology (Figure 4 ▶ , Table 3 ▶ ). We assessed the distribution of replacement (R) mutations versus silent (S) mutations over the CDR and FR regions. In all three cases the R/S ratios found in the FR regions were lower than those of the CDR regions (Table 4) ▶ . According to the statistical analysis of Lossos and colleagues, 43 in all cases the number of R mutations in the FR regions were significantly lower (P < 0.05) than would be expected if the mutations had occurred at random and in the absence of selective forces (Table 4) ▶ . These data indicate that despite the high number of somatic mutations the overall structure of the IgVH and thus of the B cell receptor (BCR) was preserved in these lymphomas.
Figure 4.
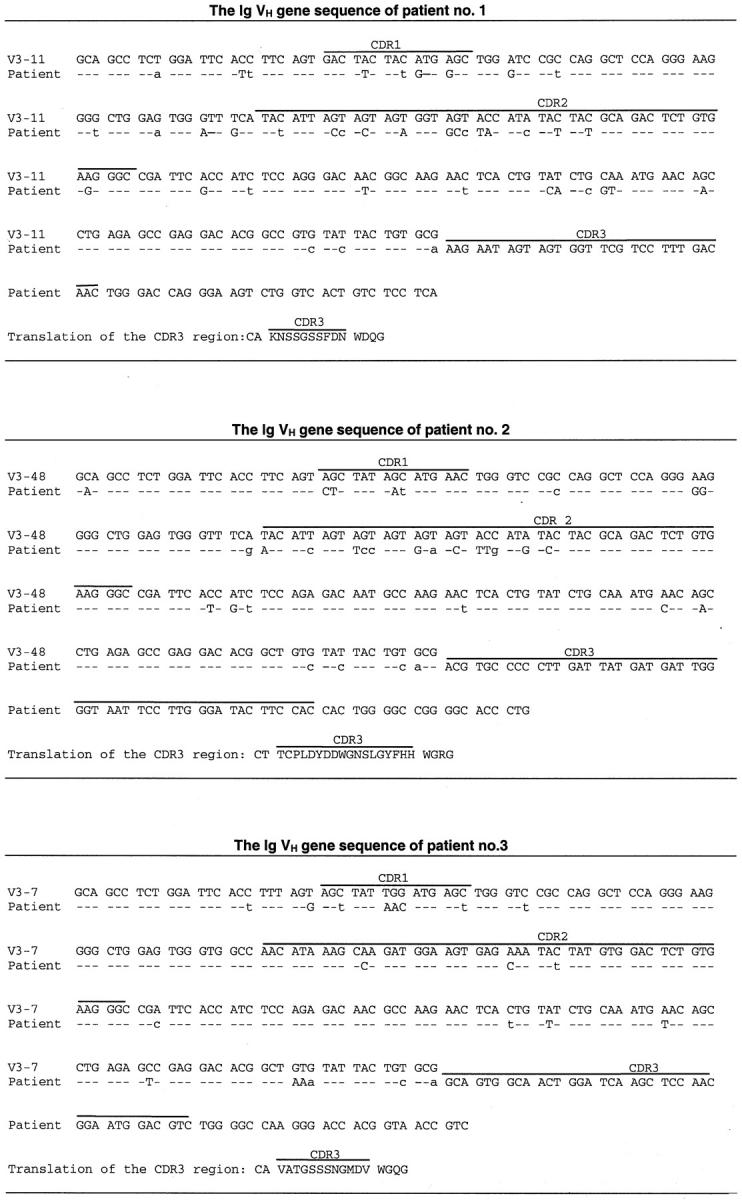
The IgVH sequences of the patients compared to the most homologous germline IgVH sequences. The individual complementarity regions (CDRs) are indicated with lines. Identity with the germline sequence is shown by dashes. Replacement mutations are indicated with uppercase letters and silent mutations are indicated with lowercase letters.
Table 4.
Distribution of Mutations in the IgVH Genes of Three GI FLs
| Patient no. | Total no. of mutations* | Observed mutations in the CDR regions | Observed mutations in the framework regions* | P value†‡ | P value†§ | ||||
|---|---|---|---|---|---|---|---|---|---|
| R | S | R/S | R | S | R/S | ||||
| 1 | 40 | 13 | 5 | 2.6 | 11 | 11 | 1.0 | 0.05 | <0.05 |
| 2 | 32 | 12 | 5 | 2.4 | 8 | 7 | 1.2 | 0.14 | <0.05 |
| 3 | 21 | 5 | 3 | 1.7 | 6 | 7 | 0.9 | <0.05 | <0.05 |
It has been reported that the amino acid sequence of the CDR3 regions of 50% of a panel of 20 gastric MALT lymphomas showed significant homology to previously reported CDR3 sequences. 45 In two of these gastric MALT lymphomas, as well as in the majority of salivary gland MALT lymphomas, 46 the CDR3 regions displayed at least 75% sequence homology with rheumatoid factors. Amino acid sequence analysis of the CDR3 regions of the GI-FLs presented here however did not reveal any resemblance to reported CDR3 regions, suggesting that the GI-FLs express unique CDR3 regions.
GI-FLs but Not Nodal FLs Express the Mucosa Homing Integrin α4β7
Tissue sections of the intestinal and nodal FLs, MALT lymphomas, B-CLL, as well as normal tonsil, lymph node, and distal ileum (Peyer’s patches) were stained with monoclonal antibodies specific for α4β7 and CXCR3, respectively (Figure 5 ▶ , Table 5 ▶ ). Among the lymphomas, exclusively the GI-FLs and the low-grade MALT lymphomas expressed the mucosa-specific homing integrin α4β7. By contrast, and in agreement with our previous results, 26 the vast majority of nodal FLs and the GCs of lymph nodes were α4β7-negative. In the normal ileum, the GCs displayed low expression of α4β7, whereas the mantle zone B lymphocytes and lamina propria T lymphocytes were strongly positive (Figure 5 ▶ , Table 5 ▶ ). However, unlike MALT lymphomas, both the intestinal and nodal FLs expressed CD38 but lacked CXCR3 expression. Thus, the GI-FLs resemble normal GC B cells of tonsil and Peyer’s patches (Figure 5 ▶ , Table 5 ▶ ). In agreement with Jones and colleagues, 36 we found that two of the three tested cases of B-CLL were strongly CXCR3-positive (Table 5) ▶ .
Table 5.
Expression of α4β7, CXCR3, and CD38
| Tissue type | Immunohistochemistry | ||
|---|---|---|---|
| α4β7 | CXCR3 | CD38 | |
| Small intestinal follicular lymphoma | 4/4 | 0/4 | 4/4 |
| Follicular lymphoma* | 2/21 | 0/6 | 3/3 |
| MALT lymphoma* | 14/15 | 6/6 | 0/4 |
| B-cell chronic lymphocytic leukemia* | 0/6 | 2/3 | nd |
| Ileum† | 4/4 | 0/7 | 5/5 |
| Tonsil† | 0/2 | 0/3 | 2/2 |
| Lymph node† | 0/2 | nd | nd |
n/n, number of positive cases/number tested cases; nd, not done.
*The α4β7 stainings include also previously reported cases. (11 FLs, 10 MALT lymphomas, and 6 B-CLLs). 26
†Indicated is the reactivity of the monoclonal antibody with GC B cells. The GCs of the ileum showed a low expression with the anti-α4β7 monoclonal antibody.
Discussion
In this study we present four cases of GI-FL localized in the jejunum and the duodenum, the latter, according to previous reports, 4-6 is the most frequent localization of this entity. In patient 1, the neoplasm had caused obstruction of the small intestine resulting in an ileus. In patients 2, 3, and 4, the lesions were relatively small, located in the duodenum and not evidently responsible for the patients’ complaints. In accordance with other reports, 5-9 the lesions had a conspicuously nodular surface. In patient 3, the appearance was even truly polypous and located near the ampulla of Vater. This localization has also been reported in five of eight GI tract FLs of a previous study and in four other cases. 5,7-9 It is also remarkable that 15 of the 22 duodenal FL patients reported so far 5-9 including our three duodenal FLs (2, 3, and 4), are female. Yoshino and colleagues 5 speculated that this might somehow be related to female predominance of bile duct diseases.
Cytologically and histologically the neoplasms were indistinguishable from their nodal counterparts. This morphological resemblance was supported by the immunohistochemical and molecular analyses. In addition to the pan B-cell surface protein CD20, the tumor cells expressed CD38, CD10, and BCL-6, markers typical of GC stage-derived B-cell malignancies and are generally not expressed by low-grade MALT or mantle cell lymphoma (Table 2) ▶ . 12-14 We established that the constitutive overexpression of BCL-2 is because of a t(14;18) also typical of nodal FLs. 15,16,47 This indicates that with respect to the earliest genetic alterations, the pathogenesis of nodal and GI-FL is similar.
The Ig gene analyses demonstrated that all three analyzed GI-FLs carried heavily mutated IgVH regions proving that the tumor cells indeed had undergone GC stage-specific alterations (Figure 4 ▶ , Tables 3 and 4 ▶ ▶ ). The mutation frequencies were significantly higher than those found in normal (post) GC B cells, 21 compatible with a prolonged stay of the tumor cells in the GC environment. Moreover, we observed discrete nucleotide differences between molecular clones derived of each lymphoma (data not shown). Although this so-called intraclonal V gene diversity must be a reflection of the somatic hypermutation process, it is not certain to what extent this process continues during the tumor stage. 20,48 Detailed analysis of the observed mutation patterns indicated that, at least at some time of development, counterselection for potentially harmful replacement mutations must have occurred in the FRs. These patterns, physiologically found in normal antigen-selected B cells, suggest that expression of an intact B-cell receptor (BCR) is also essential for the tumor cells to survive (Table 4) ▶ .
It is intriguing that in three of our four GI-FLs analyzed, no evidence was obtained for distant or widespread disease and that these patients became disease-free after local therapy only. In patient 4 chemotherapeutical treatment was given, based on the demonstration of a t(14;18) by PCR on bone marrow. The localized nature of these neoplasms was most clearly illustrated in a patient described by Misdraji and colleagues 7 in whom, despite the fact that the duodenal FL had a significant volume and had invaded the pancreas, there were no signs of metastasis. In our study, the localized nature may be because of the fact that in at least two of the four cases (ie, patients 2 and 3) the lesions happened to be diagnosed at a very early stage of disease. Still, this is in clear contrast to nodal FLs that are in majority systemic at the time of diagnosis, ie, Ann Arbor stage III or IV. 12 Conversely, despite the systemic nature of the latter entity, the GI tract is not a frequent localization of primary nodal FLs. Thus supposedly, expansion of these neoplasms at mucosal sites depends on highly specific phenotypic qualities. In this respect, our observation that the GI-FLs differ from their nodal counterparts in that they express α4β7, a well-defined mucosal homing receptor that is specifically expressed by normal mucosal lymphocytes and by low-grade MALT lymphomas, 26 is highly significant (Figure 5 ▶ , Table 5 ▶ ). Also the lack of CXCR3 expression is compatible with their origin, because this chemokine receptor was not found to be expressed by normal GC B cells of the GI tract either. MALT lymphomas, supposed to be derived of post-GC B cells, by contrast do express CXCR3 (Figure 5 ▶ , Table 5 ▶ ).
Another explanation for their low metastasizing potential is invoked by the Ig analyses. The fact that three of the four analyzed GI-FLs express IgA is of note because this isotype is seldom expressed by nodal FLs. In fact, this finding again indicates that these lymphomas may originate from local, antigen-responsive precursor cells, as IgA is the principal Ig class of the mucosal immune system (Table 2) ▶ . This contention, obviously supported by the presence and distribution of somatic mutations in IgVH, may imply that the BCRs expressed by these FLs still have binding capacity for antigens originating from the gut lumen. The localized nature of these GI-FLs may thus also be because of dependence on growth-supporting signals elicited by these BCR ligands potentially presented by follicular dendritic cells in the tumor follicles. The group of primary GI-FLs may therefore be an attractive entity to study the concept of antigen-driven lymphomagenesis in humans.
Acknowledgments
We thank Marjon Clement and Esther Schilder-Tol for performing t(14;18) analyses, Jos Mulder and Folkert Morsink for technical advice and immunohistochemical stainings, and Drs. Christian Brixko and Marianne Lecomte for providing clinical information and follow-up on case 4.
Footnotes
Address reprint requests to C. J. M. van Noesel, M.D., Ph.D., Department of Pathology, Academic Medical Center, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands. E-mail: c.j.vannoesel@amc.uva.nl.
C. J. M. v. N. is a fellow of the Netherlands Royal Academy of Arts and Sciences and L. D. L. is a research associate of the Belgian National Fund for Scientific Research.
References
- 1.Freeman C, Berg JW, Cutler SJ: Occurrence and prognosis of extranodal lymphomas. Cancer 1972, 29:252-260 [DOI] [PubMed] [Google Scholar]
- 2.Isaacson PG: Gastrointestinal lymphoma. Hum Pathol 1994, 25:1020-1029 [DOI] [PubMed] [Google Scholar]
- 3.Isaacson PG, Spencer J: The biology of low-grade MALT lymphomas. J Clin Pathol 1995, 48:395-397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.LeBrun DP, Kamel OW, Cleary ML, Dorfman RF, Warnke RA: Follicular lymphomas of the gastrointestinal tract, pathologic features in 31 cases and bcl-2 oncogenic protein expression. Am J Pathol 1992, 140:1327-1335 [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshino T, Miyake K, Ichimura K, Mannami T, Ohara N, Hamazaki S, Akagi T: Increased incidence of follicular lymphoma in the duodenum. Am J Surg Pathol 2000, 24:688-693 [DOI] [PubMed] [Google Scholar]
- 6.Shia J, Teruya-Feldstein J, Pan D, Hegde A, Klimstra DS, Chaganti RSK, Qin J, Portlock CS, Filippa DA: Primary follicular lymphoma of the gastrointestinal tract: a clinical and pathologic study of 26 cases. Am J Surg Pathol 2002, 26:216-224 [DOI] [PubMed] [Google Scholar]
- 7.Misdraji J, Del Castillo CF, Ferry J: Follicle center lymphoma of the ampulla of Vater presenting with jaundice. Am J Surg Pathol 1997, 21:484-488 [DOI] [PubMed] [Google Scholar]
- 8.Freeman HJ, Anderson ME, Gascoyne RD: Clinical, pathological and molecular genetic findings in small intestinal follicle centre cell lymphoma. Can J Gastroenterol 1997, 11:31-34 [DOI] [PubMed] [Google Scholar]
- 9.Poggi MM, Cong PJ, Coleman CN, Jaffe ES: Low-grade follicular lymphoma of the small intestine. J Clin Gastroenterol 2002, 34:155-159 [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto Y, Nakamura N, Kuze T, Ono N, Abe M: Multiple lymphomatous polyposis of the gastrointestinal tract is a heterogenous group that includes mantle cell lymphoma and follicular lymphoma: analysis of somatic mutation of immunoglobulin heavy chain variable region. Hum Pathol 1999, 30:581-587 [DOI] [PubMed] [Google Scholar]
- 11.Sakata Y, Iwakiri R, Sakata H, Fujisaki J, Mizuguchi M, Fukushima N, Fujimoto K: Primary gastrointestinal follicular center lymphoma resembling multiple lymphomatous polyposis. Dig Dis Sci 2001, 46:567-570 [DOI] [PubMed] [Google Scholar]
- 12.Harris NL, Ferry JA: Follicular lymphoma. Knowles DM eds. Neoplastic Hematopathology. ed 2 2001:pp 823-853 Lippincott Williams and Wilkins, Baltimore
- 13.Dogan A, Bagdi E, Munson P, Isaacson PG: CD10 and BCL6 expression in paraffin sections of normal lymphoid tissue and B-cell lymphomas. Am J Surg Pathol 2000, 24:846-852 [DOI] [PubMed] [Google Scholar]
- 14.Dogan A, Du M-Q, Aiello A, Diss TC, Ye H-T, Pan L-X, Isaacson PG: Follicular lymphomas contain a clonally linked but phenotypically distinct neoplastic B-cell population in the interfollicular zone. Blood 1998, 91:4708-4714 [PubMed] [Google Scholar]
- 15.Tsujimoto Y, Cossman J, Jaffe E, Croce CM: Involvement of the bcl-2 gene in human follicular lymphoma. Science 1985, 228:1440-1443 [DOI] [PubMed] [Google Scholar]
- 16.Cleary ML, Sklar J: Nucleotide sequence of a t(14;18) chromosomal breakpoint in follicular lymphoma and demonstration of a breakpoint-cluster region near a transcriptionally active locus on chromosome 18. Proc Natl Acad Sci USA 1985, 82:7439-7443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hockenbery D, Nuñez G, Milliman C, Schreiber RD, Korsmeyer J: Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature 1990, 348:334-336 [DOI] [PubMed] [Google Scholar]
- 18.Meijerink JJP: T(14;18), a journey to eternity. Leukemia 1997, 11:2175-2187 [DOI] [PubMed] [Google Scholar]
- 19.Bahler DW, Campbell MJ, Hart S, Miller RA, Levy S, Levy R: Ig VH gene expression among human follicular lymphomas. Blood 1991, 78:1561-1568 [PubMed] [Google Scholar]
- 20.Aarts WM, Bende RJ, Steenbergen EJ, Kluin PM, Ooms ECM, Pals ST, van Noesel CJM: Variable heavy chain gene analysis of follicular lymphomas: correlation between heavy chain isotype expression and somatic mutation load. Blood 2000, 95:2922-2929 [PubMed] [Google Scholar]
- 21.Klein U, Goossens T, Fischer M, Kanzler H, Braeuninger A, Rajewsky K, Küppers R: Somatic hypermutation in normal and transformed human B cells. Immunol Rev 1998, 162:261-280 [DOI] [PubMed] [Google Scholar]
- 22.Drillenburg P, Pals ST: Cell adhesion receptors in lymphoma dissemination. Blood 2000, 95:1900-1910 [PubMed] [Google Scholar]
- 23.Holzmann B, McIntyre BW, Weisman IL: Identification of a murine Peyer’s patch-specific lymphocyte homing receptor as an integrin molecule with an α chain homologous to human VLA-4α. Cell 1989, 56:37-46 [DOI] [PubMed] [Google Scholar]
- 24.Berlin C, Berg EL, Briskin M, Andrew DP, Kilshaw PJ, Holzmann B, Weissman IL, Hamann A, Butcher EC: α4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 1993, 74:185-195 [DOI] [PubMed] [Google Scholar]
- 25.Pals ST, Drillenburg P, Dragosics B, Lazarovits AI, Radaszkiewicz T: Expression of the mucosal homing receptor α4β7 in malignant lymphomatous polyposis of the intestine. Gastroenterology 1994, 107:1519-1523 [DOI] [PubMed] [Google Scholar]
- 26.Drillenburg P, van der Voort R, Koopman G, Dragosics B, van Krieken JHJM, Kluin PM, Meenan J, Lazarovits AI, Radaszkiewicz T, Pals ST: Preferential expression of the mucosal homing receptor integrin α4β7 in gastrointestinal non-Hodgkin’s lymphomas. Am J Pathol 1996, 150:919-927 [PMC free article] [PubMed] [Google Scholar]
- 27.Zabel BA, Agace WW, Campbell JJ, Heath HM, Parent D, Roberts AI, Ebert EC, Kassam N, Qin S, Zovko M, LaRosa GJ, Yang L-L, Soler D, Butcher EC, Ponath PD, Parker CM, Andrew DP: Human G protein-coupled receptor GPR-9–6/CC chemokine receptor 9 is selectively expressed on intestinal homing T lymphocytes, mucosal lymphocytes and thymocytes and is required for thymus-expressed chemokine-mediated chemotaxis. J Exp Med 1999, 190:1241-1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kunkel EJ, Campbell JJ, Haraldsen G, Pan J, Boisvert J, Roberts AI, Ebert EC, Vierra MA, Goodman SB, Genovese MC, Wardlaw AJ, Greenberg HB, Parker CM, Butcher EC, Andrew DP, Agace WW: Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J Exp Med 2000, 192:761-768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazzucchelli L, Hauser C, Zgraggen K, Wagner HE, Hess MW, Laissue JA, Mueller C: Differential in situ expression of the genes encoding the chemokines MCP-1 and RANTES in human inflammatory bowel disease. J Pathol 1996, 178:201-206 [DOI] [PubMed] [Google Scholar]
- 30.Shibahara T, Wilcox JN, Couse T, Madara JL: Characterization of epithelial chemoattractants for human intestinal intraepithelial lymphocytes. Gastroenterology 2001, 120:60-70 [DOI] [PubMed] [Google Scholar]
- 31.Dwinell MB, Lügering N, Eckmann L, Kagnoff MF: Regulated production of interferon-inducible T-cell chemoattractants by human intestinal epithelial cells. Gastroenterology 2001, 120:49-59 [DOI] [PubMed] [Google Scholar]
- 32.Agace WW, Roberts AI, Wu L, Greineder C, Ebert EC, Parker CM: Human intestinal lamina propria and intraepithelial lymphocytes express receptors specific for chemokines induced by inflammation. Eur J Immunol 2000, 30:819-826 [DOI] [PubMed] [Google Scholar]
- 33.Bowman EP, Kuklin NA, Youngman KR, Lazarus NH, Kunkel EJ, Greenberg HB, Butcher EC: The intestinal chemokine thymus-expressed chemokine (CCL25) attracts IgA antibody-secreting cells. J Exp Med 2002, 195:269-275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, Koch AE, Moser B, Mackay CR: The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest 1998, 101:746-754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trentin L, Agostini C, Facco M, Piazza F, Perin A, Siviero M, Gurrieri C, Galvan S, Adami F, Zambello R, Semenzato G: The chemokine receptor CXCR3 is expressed on malignant B cells and mediates chemotaxis. J Clin Invest 1999, 104:115-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones D, Benjamin RJ, Shahsafaei A, Dorfman DM: The chemokine receptor CXCR3 is expressed in a subset of B-cell lymphomas and is a marker of B-cell chronic lymphocytic leukemia. Blood 2000, 95:627-632 [PubMed] [Google Scholar]
- 37.Lazarovits AI, Mosciki RA, Kurnick JT, Camerini D, Bhan AK, Baird LG, Erikson M, Colvin RB: Lymphocyte activation antigens: a monoclonal antibody, anti-Act-1 defines a new late lymphocyte activation antigen. J Immunol 1984, 133:1857-1862 [PubMed] [Google Scholar]
- 38.Derksen PWB, Langerak AW, Kerkhof E, Wolvers-Tettero ILM, Boor PP, Mulder AH, Vrints LW, Coebergh JW, van Krieken JH, Schuuring E, Kluin PM, van Dongen JJM: Comparison of different polymerase chain reaction-based approaches for clonality assessment of immunoglobulin heavy-chain gene rearrangements in B-cell neoplasia. Mod Pathol 1999, 12:794-805 [PubMed] [Google Scholar]
- 39.Aarts WM, Willemze R, Bende RJ, Meijer CJLM, Pals ST, van Noesel CJM: VH gene analysis of primary cutaneous B-cell lymphomas: evidence for ongoing somatic hypermutation and isotype switching. Blood 1998, 92:3857-3864 [PubMed] [Google Scholar]
- 40.Cook GP, Tomlinson IM: The human immunoglobulin VH repertoire. Immunol Today 1995, 16:237-242 [DOI] [PubMed] [Google Scholar]
- 41.Giudicelli V, Chaume D, Bodmer J, Müller W, Busin C, Marsh S, Bontrop R, Marc L, Malik A, Lefranc M-P: IMGT, the international ImMunoGeneTics database. Nucl Acid Res 1997, 25:206-211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang B, Casali P: The CDR1 sequences of a major proportion of human germline Ig VH genes are inherently susceptible to amino acid replacement. Immunol Today 1994, 15:367-373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lossos IS, Tibshirani R, Narasimhan B, Levy R: The inference of antigen selection on Ig genes. J Immunol 2000, 165:5122-5126 [DOI] [PubMed] [Google Scholar]
- 44.Corbett SJ, Tomlinson IM, Sonnhammer ELL, Buck D, Winter G: Sequence of the human immunoglobulin diversity (D) segment locus: a systematic analysis provides no evidence for the use of DIR segments, inverted D segments, “minor” D segments or D-D recombination. J Mol Biol 1997, 270:587-597 [DOI] [PubMed] [Google Scholar]
- 45.Zucca E, Bertoni F, Roggero E, Cazzaniga G, Bosshard G, Biondi A, Cavalli F: Autoreactive B cell clones in marginal-zone B cell lymphoma (MALT lymphoma) of the stomach. Leukemia 1998, 12:247-253 [DOI] [PubMed] [Google Scholar]
- 46.Miklos JA, Swerdlow SH, Bahler DW: Salivary gland mucosa-associated lymphoid tissue lymphoma immunoglobulin VH genes show frequent use of V1–69 with distinctive CDR3 features. Blood 2000, 95:3878-3884 [PubMed] [Google Scholar]
- 47.Weiss LM, Warnke RA, Sklar J, Cleary ML: Molecular analysis of the t(14;18) chromosomal translocation in malignant lymphomas. N Engl J Med 1987, 317:1185-1189 [DOI] [PubMed] [Google Scholar]
- 48.Aarts WM, Bende RJ, Bossenbroek JG, Pals ST, van Noesel CJM: Variable heavy chain gene analysis of follicular lymphomas: subclone selection rather than clonal evolution over time. Blood 2001, 98:238-240 [DOI] [PubMed] [Google Scholar]