Abstract
Brain angiogenesis inhibitors (BAI) are putative transmembrane proteins containing an extracellular domain with thrombospondin type-1 repeats which can exhibit anti-angiogenic activity. BAI1 mRNA is expressed mainly in the brain, while BAI2 and BAI3 mRNAs are more widely expressed. We hypothesized that the BAI family might have anti-tumoral properties and studied the expression of BAI1 protein in normal human brain and in glioblastoma multiforme. We generated an anti-BAI1 antibody and showed that BAI1 was widely expressed in normal brain but was absent in 28 glioma cell lines and in the majority of human glioblastoma investigated. BAI1 expression did not correlate with TP53 status and we did not confirm previous findings that p53 regulates BAI1 mRNA expression in glioma cells. The finding that expression of BAI proteins may be lost during tumor formation is of special interest as restoration of their function in tumors may be of therapeutic benefit.
The brain angiogenesis inhibitor 1 (BAI1) gene was cloned in a screen for genes containing consensus binding sites for the p53 transcription factor. 1 The predicted BAI1 protein sequence includes a putative seven transmembrane-spanning region, an intracellular TEV domain that can interact with PDZ domain-containing proteins, an extracellular RGD integrin-recognition motif, an extracellular domain with five thrombospondin (TSP) type-1 repeats 2,3 and a putative GPS (G-protein-coupled receptor proteolytic site) domain (Kaur B and Van Meir EG, unpublished results). Recombinant peptides corresponding to three TSP type 1 repeats demonstrate anti-angiogenic activity. Two close homologues of BAI1, BAI2 and BAI3, have also been identified. Both of these genes share significant sequence identity to BAI1 but have a broader tissue expression range suggesting 4 that they have homologous functions in different organs. In normal (non-neoplastic) tissues, BAI1 mRNA was found to be predominantly expressed in the brain, colon, and intestine suggesting a potential role in the maintenance of tumor angiostasis. 1,5-7 Given the putative anti-angiogenic function of the BAI1 protein family, we hypothesized that tumor formation may require loss of its expression. Here, we examined the validity of this hypothesis for brain tumors. We chose to examine the potential significance of BAI1 protein in the development of human glioblastoma multiforme (GBM) because it is the most common and malignant form of brain tumor. It is also characterized by extensive vascular proliferation suggesting that it had to overcome anti-angiogenic obstacles during its development. 8 To study the relevance of BAI1 in human gliomagenesis, we investigated BAI1 protein and mRNA expression and their relation to TP53 status in glioma cell lines and in GBM resection specimens.
Materials and Methods
Glioblastoma Cell Lines and Culture Conditions
Description and culture conditions of the 28 human glioma cell lines used to investigate BAI1 expression were as previously described. 9 Three p53-inducible systems were used to test for induction of BAI1 mRNA by p53. In the dexamethasone-inducible system (GM47–23 cells) 10 a TP53 cDNA under the control of a glucocorticoid response element was cloned in T98G human glioma cells, and p53 expression induced by 1 μmol/L final concentration of dexamethasone (Sigma Chemicals, St. Louis, MO) for 18 hours. GM47–23 cells that carry endogenous mutated TP53 alleles at codon 237, were maintained in supplemented Eagle’s medium containing 1X HAT medium supplement (Sigma) and 0.25 mg/ml of xanthine and 0.025 mg/ml of mycophenolic acid. 10 In the doxycycline-inducible system (2024 cells), 11 a TP53 cDNA regulated by a tet-on promoter was cloned in C16 human glioblastoma cells. C16 is a clone derived from LNZ308 cells (TP53-null) 11,12 after stable transfections of rtTA transactivator cDNA and is grown in DMEM + 10% fetal bovine serum + 1 μg/ml puromycin. 2024 cells were grown in the same medium with the addition of 300 μg/ml of neomycin. p53 induction is obtained by treating the 2024 cells with a 2 μg/ml final concentration of doxycycline (Sigma) for 24 hours. The third system used was glioblastoma cell line (LN-382) which expresses an endogenous temperature-sensitive mutant of p53. p53 has the biochemical behavior of a mutant protein when the cells are grown in supplemented DMEM at 37°C, and that of a wild-type protein at 34°C. 13
BAI1 Antibody Production
A peptide (CQFDSFLESTRTYLGVE) corresponding to amino acids 103 to 118 in the N-terminal region of the BAI1 protein was synthesized, coupled to keyhole limpet hemocyanin (KLH) (Imject Malemide KLH, Pierce, Rockford, IL) as per manufacturer’s instructions to increase its antigenicity, and subsequently injected into a rabbit with complete Freund’s adjuvant (CFA). The rabbit’s pre-immune sera had been previously tested for lack of cross-reactivity with BAI1 (one initial injection with 0.4 mg peptide followed by four boost injections with 0.2 mg and eight subsequent injections with 0.1 mg every week; Pocono Rabbit Farm and Laboratory Inc., Canadenis, PA). An N-terminal cysteine was added to the synthetic peptide to permit coupling to a sulfalink column for subsequent affinity purification (Sulfolink Coupling gel, Pierce). The peptide was selected from a region of the protein that had very little homology to BAI2 and BAI3, the other two members of the family. The ability of the generated antiserum (no. 399) to recognize BAI1 was tested by immunoblot analysis.
Western Blot Analysis
Immunoblots were performed on cell lysates (lysed in 8 mol/L urea, 4% SDS in 10 mmol/L Tris (pH 7.4)) from indicated cell lines or tumor tissue obtained from resected biopsies of patients, all of whom were diagnosed with GBM. Normal brain (tumor bank numbers in parentheses) was obtained from temporal lobectomy specimens from three patients with epilepsy (01–86, 01–87, 01–88) (frozen within 15 minutes of surgery) and from autopsies of two patients who had suffered from cardiac arrest (99–96) and Alzheimer’s disease (01–89) (frozen between 6 to 8 hours after demise). Equal amounts of protein (40 μg) were resolved on a 7% SDS-PAGE and transferred to nitrocellulose membranes. BAI1 blots were probed with affinity-purified rabbit polyclonal anti-BAI1 antibody (1:1500 dilution), incubated with HRP conjugated to goat anti-rabbit antibody (1:2000, Cat. no. PO448, DAKO Co., Carpinteria, CA), and developed by enhanced chemiluminescence reagents (Pierce). Actin blots were developed by goat anti-human actin antibody (1:500, Cat. no. SC16–16, Santa Cruz Biotechnology Inc., Santa Cruz, CA), followed by HRP-conjugated swine anti-goat antibody (1:2000, Cat. no. 605275, Roche Molecular Biochemicals, Indianapolis, IN), and visualized by enhanced chemiluminescence.
Immunohistochemistry Analysis
Immunohistochemistry was performed on pellets of cultured glioma cells, human GBM, and non-tumoral resection specimens and tissue-arrays [purchased from the National Cancer Institute Tissue Array Research Project (NCI TARP), National Institutes of Health, Bethesda, MD]. In all cases, tissues were fixed in formalin and paraffin-embedded and sections were cut at 6 μm for histology and immunohistochemistry. To generate cell pellets, U251MG cells untransfected or stably transfected with BAI1 cDNA were grown to confluence and pelleted by centrifugation in culture media. Media was replaced by 10% buffered formalin and pellets were fixed overnight followed by routine processing. Archived human glioblastoma resection specimens were retrieved from the Department of Pathology at Emory University Hospital. Tissue sections used for routine histological examination and for immunohistochemistry contained both regions that were diagnostic of GBM as well as adjacent non-neoplastic brain. For immunohistochemical studies, sections were deparaffinized and subjected to antigen retrieval by steaming (20 minutes at 80°C). Slides were then incubated at room temperature with primary antibodies directed toward p53 (mouse monoclonal, 1:20; DO-7 DAKO Co., Carpenteria, CA), CD31 (mouse monoclonal, 1:80; DAKO), or BAI1 (rabbit polyclonal no. 399, 1:4000). Tumors stained for p53 were regarded as positive if strong, nuclear immunoreactivity was seen in over 10% of neoplastic cells. Negative controls for BAI1 immunohistochemistry included pre-immune serum. For all studies, negative controls included normal saline and irrelevant IgG substitution for the primary antibody. Antibodies were detected using the avidin-biotin complex (ABC) method, using diaminobenzidine as the chromogen. Quantification of vascular density in GBM resection specimens was performed by manually counting CD31-positive vascular profiles in 200X fields three times for each of 18 GBM specimens. Vascular profiles were defined as solitary lumens with or without branching lined by endothelial layer that stained with CD-31. For each tumor one CD-31 stained slide was examined in three randomly chosen fields that contained only high-grade astrocytoma. Vascular density was quantified without knowledge of BAI1 status for the tumors.
Transfections and Cloning
All transfections were carried out by plating 1 × 105 cells/well of a six-well plate 24 hours before transfection. One ug of plasmid DNA and 5 μl of GenePORTER reagent (Gene Therapy System, San Diego, CA) were used for each reaction. BAI1, BAI2, and BAI3 transiently transfected 293 cells were generated using expression plasmids previously described. 1,4 Clones stably expressing BAI1 were generated by transfecting U251MG cells with BAI1pcDNA3.1 cDNA followed by selection of neomycin-resistant clones. Isolated clones were tested for BAI1 expression by immunoblot analysis.
Reverse Transcription-Polymerase Chain Reaction Analysis
Total RNA from tumor tissue and glioma cell lines was prepared using Trizol (Invitrogen Life Technologies., Carlsbad, CA) as per the manufacturer’s instructions. Reverse transcription-polymerase chain reaction (RT-PCR) for BAI1, GAPDH, and p21/CDKN1A mRNA expression was performed as described. 1 The RT-PCR was performed using 0.5 μg total RNA from each sample using the Titan One Tube RT-PCR system (Roche Molecular Biochemicals). All reactions involved an initial incubation at 50°C for 30 minutes to allow for the reverse transcription reaction followed by an initial denaturation step at 92°C followed by 30 cycles for GAPDH (annealing temperature, 55°C), or 25 cycles for BAI1 (52°C) and CDKN1A (55°C). The PCR products were separated by gel electrophoresis on a 2% agarose TAE (40 mmol/L Tris-acetate, 1 mmol/L EDTA) gel.
Results
Characterization of an Anti-BAI1 Antibody
We generated a polyclonal anti-human BAI1 antibody (see Materials and Methods). The ability of the affinity-purified antiserum (no. 399) to recognize BAI1 was tested by immunoblot analysis on cell lysates from normal human brain (cerebellar and frontal) obtained from autopsy of a patient who had cardiac arrest (Figure 1A) ▶ . U251MG glioma cells stably transfected with BAI1 cDNA and untransfected cells were used as positive and negative controls. A 170-kd band corresponding to the predicted size of BAI1 was observed in brain lysates and in BAI1 transfected U251MG cells but not in the negative control (Figure 1A) ▶ . Since BAI2 and BAI3 are homologues of BAI1 and would give predicted proteins of about the same size we tested for antibody cross-reaction. 4 Western blot analysis of human embryonic kidney 293 cells transiently transfected with either BAI1, BAI2, or BAI3 cDNAs demonstrated that the antibody is specific for BAI1 (Figure 1B) ▶ .
Figure 1.
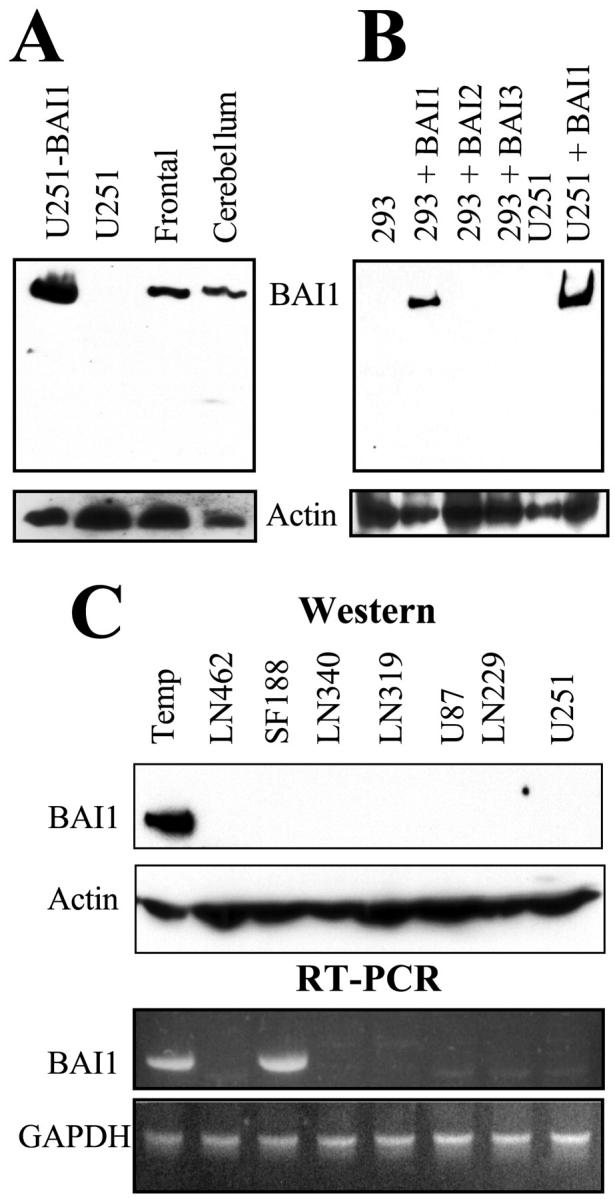
Characterization of anti-BAI1 antibody and absence of expression of BAI1 in glioma cell lines. A: Expression of BAI1 in normal human brain (from autopsy, 01–89). Equal amounts of lysates (lysed in 8 mol/L urea and 4% SDS) from BAI1 transfected or untransfected U251MG (lanes 1 and 2, respectively), frontal (lane 3), and cerebellar (lane 4) portions of human brain tissue were resolved on a 7% SDS-PAGE and transferred on a nitrocellulose membrane and Western blot analysis was performed with polyclonal-affinity purified anti-BAI1 antibody (upper panel) and with anti-actin (lower panel) antibody. The BAI1-specific 170-kd band observed in both the normal tissue and in the BAI1 transfected cells is indicated. B: The anti-BAI1 antibody does not cross-react with BAI2 and BAI3. BAI1, BAI2, and BAI3 transient expression in 293 cells was achieved by transient transfection using expression plasmids previously described. 1,4 Equal amounts (40 μg) of lysates from human embryonic kidney 293 cells either untransfected (lane 1) or transiently transfected with cDNAs encoding BAI1 (lane 2), BAI2 (lane 3), or BAI3 (lane 4) were resolved on a 7% SDS-PAGE and transferred on a nitrocellulose membrane. Blots were probed for BAI1 expression (upper panel) and actin (lower panel). U251MG cells either untransfected (lane 5) or stably transfected with BAI1 cDNA (lane 6) were used as negative and positive controls, respectively. C: Equal amounts of cell lysates from the indicated cell lines or from normal brain obtained from a temporal (temp) lobectomy of a patient with epilepsy (01–87) were examined for BAI1 (panel 1) or for actin (panel 2) expression by immunoblot analysis. BAI1 mRNA (panel 3) expression was evaluated by RT-PCR analysis using 0.5 of total μg RNA prepared from the indicated human glioma cell lines. Primers and PCR conditions were as described. 1 GAPDH was used as a control for gel loading and mRNA integrity.
Immunohistochemical Staining for BAI1 in Normal Human Brain
To establish the specificity of BAI1 IHC on paraffin sections we first performed immunohistochemistry on cell pellets derived from untransfected and BAI1 cDNA-transfected U251MG cells embedded in paraffin. Strong cytoplasmic immunoreactivity for BAI1 in transfected but not in untransfected cells was observed (Figure 2,A1 and A2) ▶ . Cells treated with normal saline or pre-immune serum in place of primary antibody showed no staining (not shown). Immunohistochemistry for BAI1 on the normal human brain (a total of five patient samples) showed diffuse expression in cortex, white matter, and deep gray structures (Figure 2, A3 and A4) ▶ . Both glial and neuronal staining were evident, leading to a homogeneous pattern in the brain parenchyma that spared endothelial cells and perivascular cells (Figure 2, A3 and A4 ▶ , arrow).
Figure 2.
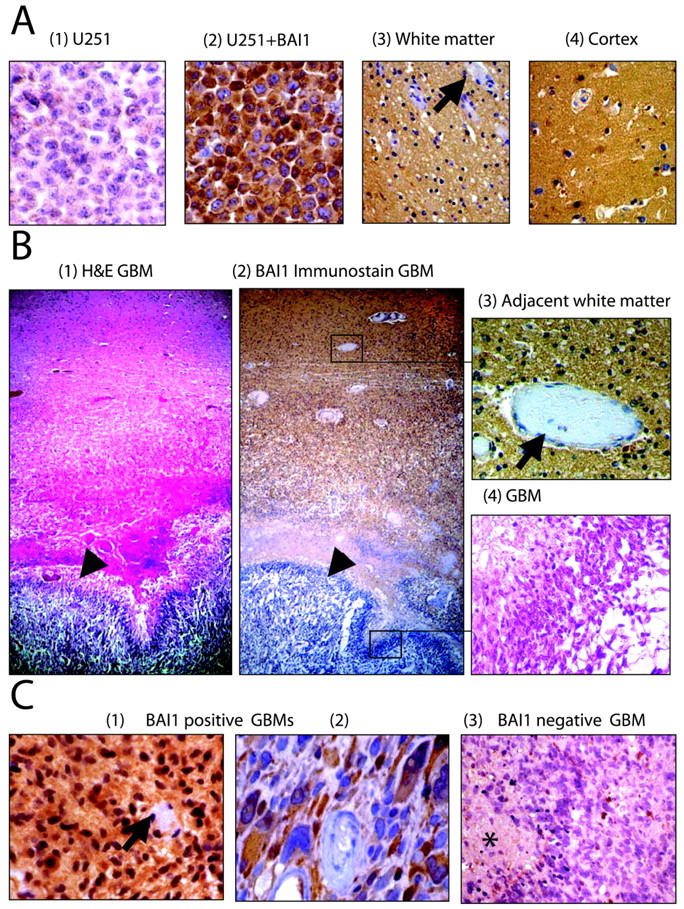
Immunohistochemistry of human glioblastoma and non-neoplastic brain specimens. A: Formalin-fixed pellets of U251MG cells untransfected (1) or stably transfected with BAI1 cDNA (2) were used as negative and positive controls for immunohistochemistry. Immunohistochemistry on non-neoplastic human brain tissue from surgically resected specimens shows diffuse expression of BAI1 in the brain parenchyma of both the white matter (3) and cortex (4). Staining is absent in vascular endothelial cells and perivascular stromal cells (arrows). B: Autopsy specimen containing GBM with adjacent non-neoplastic white matter stained by hematoxylin and eosin (1) and by immunohistochemistry for BAI1 (2). Diffuse staining of the brain parenchyma is observed in non-neoplastic brain but not in the adjacent region of GBM (arrowheads). Higher magnifications of the adjacent brain and neoplastic tissue (in boxes in 2) are shown in 3 and 4. C: Examples of GBM resection specimens showing presence (1 and 2) or absence (3) of cytoplasmic BAI1 expression in neoplastic cells by immunohistochemistry. Arrow in 1 points to vessels showing absence of expression of BAI1, and asterisk in 3 points to necrotic areas within the GBM.
Analysis of BAI1 Expression in Glioblastoma and Normal Brain
To analyze the expression status of BAI1 in human glioblastoma, 18 samples were analyzed by immunohistochemistry. The tumors were scored as positive if they demonstrated specific cytoplasmic staining within the tumor cells of the high-grade component of the neoplasm. BAI1 staining of non-neoplastic brain tissue within the same sections was required as an internal positive control and an absence of staining of endothelial cells and perivascular cells was required as an internal negative control. Thirteen of 18 GBMs (72%) had no BAI1 expression within their high-grade component, whereas immunoreactivity was present in the adjacent non-neoplastic brain (Figure 2B) ▶ . Representative examples of GBM sections staining negative for BAI1 are shown in Figure 2B2 ▶ and Figure 2C3 ▶ . Of particular interest is the section derived from the autopsied brain of an 86-year-old male who, at the time of presentation, was suffering from glioblastoma multiforme and refused any radiation or chemotherapy (Figure 2B) ▶ . BAI1-specific staining is absent in the tumor while the adjacent normal brain shows a diffuse parenchymal staining (Figure 2, B2–B4) ▶ . Specific staining of individual non-neoplastic astrocytes was much more evident when they were reactive, as in the regions surrounding the glial neoplasm. In these instances, distinct cytoplasmic staining of BAI1 was noted (not shown). BAI1 expression was clearly absent in the tumor microvascular cells (Figure 2B3 ▶ and Figure 2C1 ▶ , arrows). In 5 of 18 (28%), distinct cytoplasmic BAI-1 staining of tumor cells within the high-grade component was observed (Figure 2, C1 and C2) ▶ . Interestingly, in the BAI1-positive glioblastomas, there appeared to be a loss of expression in regions of tumor cells pseudopalisading around the foci of necrosis.
To examine relative BAI1 expression in normal brain and GBM at the mRNA level we first performed a search on the serial analysis of gene expression (SAGE) libraries (http://www.ncbi.nlm.nih.gov/SAGE). All six normal brain libraries present in the SAGE database were positive for BAI1 expression, including four with multiple tags confirming the gene expression of BAI1 mRNA in the brain. In contrast, BAI1 expression was seen in 0 of 2 World Health Organization grade II, 0 of 1 grade III, and 1 of 3 grade IV astrocytoma libraries. The only GBM library positive for BAI1 was from a sample that pooled GBMs from five different patients, raising the possibility of normal brain contamination. Results from the SAGE analysis suggest that BAI1 mRNA is also differentially expressed between normal brain and astrocytic tumors including glioblastoma.
BAI1 Expression and Microvascular Density
Given the predicted role of BAI1 in angiogenesis, we examined whether there was a correlation between BAI1 expression and microvascular density in the tumors. Vascular density in the resected specimens was quantified as detailed in Materials and Methods. The densities of CD31-positive vascular profiles in the five BAI1-positive GBMs was 34 ± 10 profiles/200X field and 35 ± 6 profiles/200X field in the thirteen BAI1-negative tumors. Thus, no significant correlation between BAI1 expression and vessel density was observed.
BAI1 Expression and p53 Expression
We examined the relationship of BAI1 protein expression and p53 protein expression by immunohistochemistry in a larger series of human glioblastoma (n = 37) specimens, including the 18 from above and 19 present on a tissue array (NCI TARP tissue array). In this combined series, we could detect expression of BAI1 in only 35% of the glioblastoma samples whereas all of the normal brain samples (a total of five) evaluated were found to be positive for BAI1 expression (P < 0.01, Fisher’s test). Of the BAI1-positive tumors, 46% showed p53 nuclear expression. Of the BAI1-negative tumors, 54% showed nuclear p53 expression. Also, 43% of GBMs with nuclear p53 immunoreactivity were BAI1 positive, while 30% of p53-negative tumors were BAI1 positive. Thus we found that similar percentages of BAI1-positive and -negative tumors showed nuclear expression of p53 protein and similar percentages of p53+ and p53− tumors were found to express BAI1 (not shown).
Analysis of BAI1 Expression in Glioma Cell Lines
BAI1 mRNA and protein expression in glioblastoma cell lines were examined by RT-PCR and immunoblot analysis. BAI1 mRNA was detected in only 8 of 28 human glioma cell lines confirming the preliminary observation by Nishimori et al. 1 Expression was independent of TP53 genetic status (Figure 1C ▶ , Table 1 ▶ ). Unexpectedly, we found by Western blot that BAI1 protein expression was absent in all of the 28 cell lines including those expressing the mRNA. It is possible that the BAI1 expression levels are below the detection limit of Western blotting in the eight cell lines that showed mRNA expression. We are also examining whether the BAI1 protein is post-translationally modified to a form not recognized by our antibody or whether the epitope-containing extracellular domain might have been cleaved off. Alternatively BAI1 gene expression may be controlled at both the transcriptional and translational levels.
Table 1.
BAI1 Expression and Genetic Status of Glioma Cell Lines 9
| Cell line | BAI1 mRNA (RT-PCR) | BAI1 Protein Western | TP53 Status codon (a.a change) | p14 Status codon (a.a change) | p16 Status codon (a.a change) |
|---|---|---|---|---|---|
| Normal brain (n = 5) | + | + | WT/WT | WT | WT |
| SF767 | + | − | WT/WT | WT | WT |
| LN-443 | + | − | WT/WT | del | del |
| LN-444 | + | − | WT/WT | del | del |
| LN-464 | + | − | 113/113 (F→S) | WT | WT |
| SF188 | + | − | 266/266 (G→E) | WT | WT |
| LN-Z308 | + | − | Null/null | WT | WT |
| U138MG | + | − | 213/213 (R→Q) | del | del |
| LN-401 | + | − | 215/215 (S→R) | del | del |
| LN-827 | − | − | WT/E56 stop | del | del |
| D247MG | − | − | WT/WT | del | del |
| U87MG | − | − | WT/WT | del | del |
| LN-319 | − | − | 175/175 | WT | WT |
| LN-235 | − | − | 176/176 (C→S) | WT | WT |
| U373MG | − | − | 273/273 (R→H) | WT | WT |
| LN-405 | − | − | 282/282 (R→W) | WT | WT |
| LN-229 | − | − | 98/98 (P→K) | del | del |
| LN-71 | − | − | 146/146 (W→stop) | del | del |
| LN-428 | − | − | 173 (V→M)/ | del | del |
| 282(R→W) | |||||
| LN-427 | − | − | 176/176 (C→S) | del | del |
| LN-340 | − | − | 181/181 (R→P) | del | del |
| U118MG | − | − | 213/213 (R→Q) | del | del |
| T98G | − | − | 237/237 (M→I) | del | del |
| HS683 | − | − | 248/248 (R→Q) | del | del |
| U251MG | − | − | 273/273 (R→H) | del | del |
| LN-432 | − | − | n.d. | n.d. | n.d. |
| LN-462 | − | − | n.d. | n.d. | n.d. |
| LN-702 | − | − | n.d. | n.d. | n.d. |
| LN-963 | − | − | n.d. | n.d. | n.d. |
wt, wild type; del, deleted; n.d., not determined.
Absence of BAI1 mRNA Regulation by p53 in Human Glioma Cells
To verify p53 regulation of BAI1 as initially suggested by Nishimori et al, 1 we examined the p53 status of the glioma cell lines for which we studied the BAI1 expression (Table 1) ▶ . The mutational status of TP53 (and p14ARF and p16) for most of the glioma cell lines used in this study is known. 9 However, no apparent correlation between the genetic status for these tumor suppressor genes and the expression of BAI1 mRNA in these cell lines was evident (Table 1) ▶ . Among the eight cell lines expressing BAI1 mRNA, three harbor a wild-type TP53 gene, four have mutant TP53, and one is TP53-null (Table 1 ▶ , Figure 1C ▶ ). The cell lines with mutant p53 were as likely to be BAI1 mRNA- expressing as non-expressing. Interestingly, even the LNZ308 cell line, which is null for p53, showed strong BAI1 gene expression.
To test if expression of BAI1 mRNA could be induced by transfecting glioma cells with wt p53 we first tried to reproduce the data of Nishimori et al, 1 and used transient transfection of a wt p53 expression vector in T98G glioma cells. While mRNA for p21/CDKN1A, a known p53 transcriptional target gene was induced, we could not detect any BAI1 mRNA expression in response to p53 (Figure 3A) ▶ . We surmised that perhaps our transfection efficiencies were lower than theirs and therefore tried to overcome this by investigating the induction of BAI1 in GM47–23, a clone of T98G cells containing a stably transfected wt TP53 cDNA inducible by dexamethasone. 10 The presence of transcriptionally active wt p53 on dexamethasone induction in GM47–23 cells was confirmed by the induction of CDKN1A mRNA expression. Dexamethasone had no effect on mRNA expression in parental T98G cells. Again, no BAI1 mRNA induction was observed in this system (Figure 3B) ▶ . To account for glioma heterogeneity and examine p53 effects on BAI1 in a cell line which does not contain endogenous p53 we used clone 2024. This clone derives from the p53-null human glioblastoma cell line LN-Z308 and contains a doxycycline (dox)-inducible wt TP53 cDNA. 11,14 Dox treatment induced CDKN1A mRNA but not BAI1 mRNA in 2024 cells, while dox had no effect on CDKN1A mRNA expression in parental cells and the p53-negative rtTA expressing control clone C16. Interestingly, derivation of C16 and 2024 clones from LN-Z308 cells was accompanied by a loss of constitutive BAI1 mRNA expression (Figure 3C) ▶ . Finally, to rule out effects related to transfection of exogenous gene constructs we examined p53 regulation of BAI1 mRNA in LN-382 cells, a human glioblastoma cell line harboring an endogenous temperature-sensitive mutant of p53. 13 The CDKN1A mRNA but not BAI1 mRNA was induced when the cells were grown at the permissive temperature (34°C) when p53 is active (Figure 3D) ▶ . Our results in three different human glioma cell lines using four different p53 expression systems do not support p53 regulation of BAI1 mRNA expression.
Figure 3.
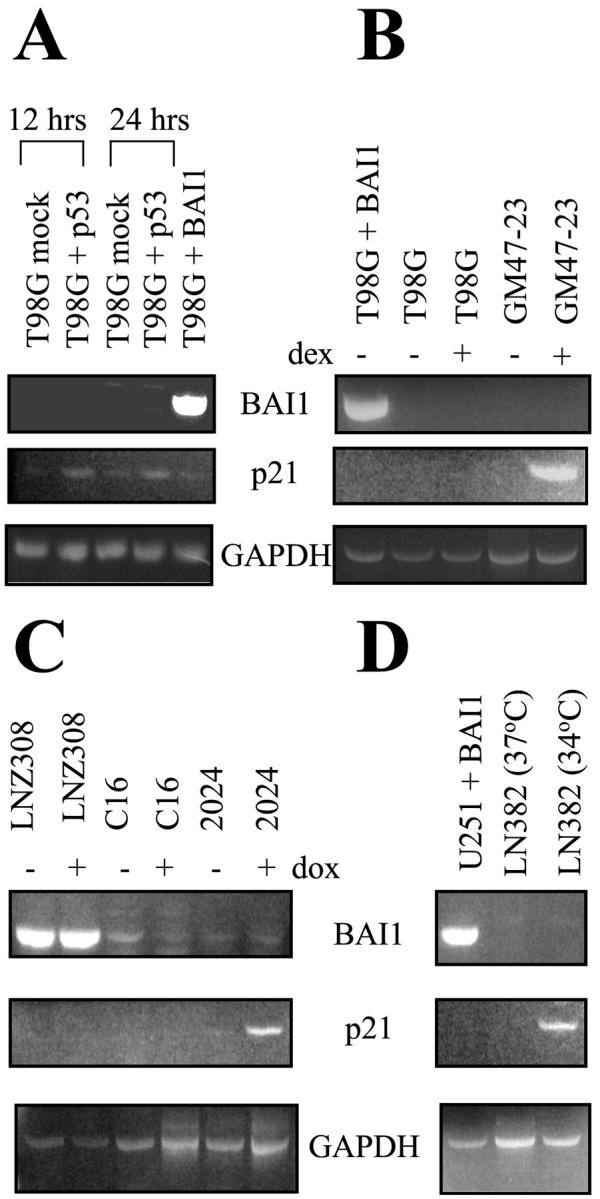
BAI1 mRNA is not induced by p53 in glioma cell lines. Induction of BAI1 mRNA by p53 was evaluated by RT-PCR as detailed in the Materials and Methods section. Induction of p21/CDKN1A mRNA by p53 was used as a positive control. Expression of GAPDH mRNA was used as a control for RNA amounts and integrity. A: T98G cells were transiently transfected with wild-type p53 cDNA. The cells were harvested at 12 hours and 24 hours post-transfection and analyzed for BAI1 mRNA induction of expression by p53 using RT-PCR. T98G cells transfected with BAI1 cDNA or mock-transfected cells were used as positive and negative controls, respectively. B: Activation of wild-type p53 activity in GM 47–23 cells by dexamethasone (1 μmol/L for 18 hours). GM 47–23 is a clone derived from T98G glioma cells and contains a wild-type p53 cDNA under a dexamethasone-responsive promoter. T98G parental cells were used as a negative control and T98G transiently transfected with BAI1 cDNA as a positive control. C: Activation of wild-type p53 in 2024 cells by doxycycline 2024 cells contain a wt TP53 gene regulated by doxycycline-responsive promoter and are derived from C16 cells (which are a clone of LNZ308 cells expressing the rtTA transactivator). WT p53 was induced by treating the cells with doxycycline (2 μg/ml) for 24 hours and the cells were then evaluated for BAI1 mRNA induction by RT-PCR. D: LN382 cells harboring a temperature-sensitive p53 mutant were grown at either 37°C or at 34°C (permissive temperature) for 24 hours and analyzed for BAI1 mRNA induction. U251MG cells stably transfected with BAI1 cDNA were used as a positive control.
Discussion
BAI1 is a putative angiostatic factor in the brain that could have tumor-suppressive properties. We have examined the expression of BAI1 in normal and tumoral brain, and also evaluated whether it related to p53 tumor suppressor expression. We further examined whether p53 transactivates BAI1 mRNA expression in a panel of cell lines representing the genetic and biological heterogeneity of human glioblastoma. To examine BAI1 expression in tissues we designed a polyclonal antibody that specifically recognizes BAI1 and does not cross-react with its close homologues BAI2 and BAI3. In non-neoplastic brain tissue adjacent to neoplasms, BAI1 was found diffusely expressed in cortex, white matter, and deep gray structures. Expression was seen in both glial cells and neurons giving a homogeneous pattern in the brain parenchyma that spared endothelial cells and perivascular cells. Specific staining of non-neoplastic astrocytes was much more evident when they were reactive, as in the regions surrounding the glial neoplasm. In these instances, distinct cytoplasmic staining of BAI1 was noted. Despite its widespread presence in normal brain, expression of BAI1 protein was detected in only 35% of glioblastomas and was entirely absent in 28 glioma cell lines. The fact that about 29% of cell lines did express BAI1 mRNA, but not protein, suggests that BAI1 is expressed at low levels in these cells or is subject to post-transcriptional regulation. Brain tumor development may require a reduction in expression of endogenous inhibitors of angiogenesis as has been previously observed for TSP-1. 15 Further studies will have to establish whether the glioblastomas maintaining BAI1 expression constitute a particular genetic subset. Interestingly, in the BAI1-positive glioblastomas, there appeared to be a loss of expression in regions of tumor cells pseudopalisading around foci of necrosis. Since these cells are believed to be hypoxic, this may reflect a loss of expression of BAI1 in response to decreased partial oxygen pressure. 16 Down-regulation of TSP-1 in response to hypoxia was previously described. 17 We did not find a difference in vessel densities between BAI1-positive and -negative tumors. This is not unexpected as tumors can disrupt the balance between angiogenic and angiostatic factors in different ways. While BAI1 may control vessel growth and vessel density in the brain, the fact that BAI1-positive cells were able to form glioblastoma suggests that the angio-inhibitory function of BAI1 must have been overcome by other factors in these tumors.
Results of the immunohistochemistry for BAI1 and p53 on 37 brain tumor biopsy specimens revealed that similar percentages of BAI1 positive and negative tumors showed nuclear expression of p53 protein. In addition, similar percentages of p53+ and p53− tumors were found to express BAI1. We also found that BAI mRNA did not correlate with TP53 gene status in a series of 28 glioma cell lines. Because of the lack of correlation found between p53 status and BAI1 expression we used four separate expression systems in three glioma cell lines to examine whether p53 can regulate BAI-1 gene transcription as has been previously described. 1 In all four of these systems, expression of wt p53 did not affect the expression of BAI1 mRNA. The reasons for this discrepancy are unclear. One putative p53 binding site (tGGCT-GCCT GGACATGTTC) has been localized to intron 9 of the BAI1 gene. 1 However, the ability of this element to bind and mediate p53-induced target gene expression has not been studied. It is also possible that BAI1 is regulated mainly by p73 or some other member of the p53 family with virtually identical DNA recognition motif. 18 The regulation by p53 may further require additional cooperating transcriptional factors as has been shown for the MSH2 gene which is stimulated by wt p53 in conjunction with c-jun. 19
GD-AIF is an inhibitor of angiogenesis that is released by glioblastoma cells on induction of wt p53 expression. 20 This raised the interesting possibility that BAI1 or a fragment thereof may correspond to GD-AIF. 1 Our finding that BAI1 mRNA levels were not affected by p53 in glioma cell lines indicates that the BAI1 gene is not a general transcriptional target for p53. This does not entirely exclude a relationship between GD-AIF and BAI1, since GD-AIF might still be a p53-regulated cleavage product of BAI1. It is conceivable that p53 might regulate a protease that could cleave off the extracellular domain of BAI1 which would produce GD-AIF. The extracellular portion of BAI1 contains the anti-angiogenic TSP-1 type-1 repeats, and we found that it has a consensus GPS (G-protein coupled receptor proteolytic site) domain at the membrane junction, and its size of about 100 kd is similar to that of GD-AIF. Further studies are warranted to examine this hypothesis.
In conclusion, this study establishes that human glioblastoma genesis is generally accompanied by the absence of BAI1 expression, a putative brain-specific angiogenesis inhibitor. This contrasts with the diffuse expression of BAI1 observed in most cells of the adult human brain. While BAI1 expression is predominantly found in the brain, BAI2 and BAI3 mRNAs are more widely expressed in a series of normal tissues. Given the close homology between BAI1 family members, it will be of interest to examine whether loss of expression is also associated with cancer formation in other organs and whether this is linked to the anti-angiogenic properties of the TSP type-1 repeats found in their extracellular domains.
Note Added in Proof:
BAI1 was recently shown to be expressed in cerebral neuronal cells 21 and infection of human Panc-1 pancreatic adenocarcinoma cells with a BAI1 expressing adenovirus suppressed tumor growth and angiogenesis. 22
Acknowledgments
We thank Dr. Y. Nakamura (University of Tokyo, Japan) for the BAI1, BAI2, and BAI3 expression plasmids and Dr. D.E. Post for reading the manuscript.
Footnotes
Address reprint requests to Erwin G. Van Meir, Laboratory of Molecular-Neuro-Oncology, Winship Cancer Institute, Emory University, 1365-B Clifton Road N.E., Room B5103, Atlanta, GA 30322. E-mail: evanmei@emory.edu.
Supported by National Institutes of Health grants CA86335 (to E.G.V.M.) and NS92180 (to D.J.B.) and the University Research Committee of Emory University (to E.G.V.M.).
Cathárine C. Calkins’ current address is the Department of Dermatology, Emory University, Atlanta, GA 30322.
References
- 1.Nishimori H, Shiratsuchi T, Urano T, Kimura Y, Kiyono K, Tatsumi K, Yoshida S, Ono M, Kuwano M, Nakamura Y, Tokino T: A novel brain-specific p53-target gene, BAI1, containing thrombospondin type-1 repeats inhibits experimental angiogenesis. Oncogene 1997, 15:2145-2150 [DOI] [PubMed] [Google Scholar]
- 2.Shiratsuchi T, Oda K, Nishimori H, Suzuki M, Takahashi E, Tokino T, Nakamura Y: Cloning and characterization of BAP3 (BAI-associated protein 3), a C2 domain-containing protein that interacts with BAI1. Biochem Biophys Res Commun 1998, 251:158-165 [DOI] [PubMed] [Google Scholar]
- 3.Shiratsuchi T, Futamura M, Oda K, Nishimori H, Nakamura Y, Tokino T: Cloning and characterization of BAI-associated protein 1: a PDZ domain-containing protein that interacts with BAI1. Biochem Biophys Res Commun 1998, 247:597-604 [DOI] [PubMed] [Google Scholar]
- 4.Shiratsuchi T, Nishimori H, Ichise H, Nakamura Y, Tokino T: Cloning and characterization of BAI2 and BAI3, novel genes homologous to brain-specific angiogenesis inhibitor 1 (BAI1). Cytogenet Cell Genet 1997, 79:103-108 [DOI] [PubMed] [Google Scholar]
- 5.Lee JH, Koh JT, Shin BA, Ahn KY, Roh JH, Kim YJ, Kim KK: Comparative study of angiostatic and anti-invasive gene expressions as prognostic factors in gastric cancer. Int J Oncol 2001, 18:355-361 [PubMed] [Google Scholar]
- 6.Yoshida Y, Oshika Y, Fukushima Y, Tokunaga T, Hatanaka H, Kijima H, Yamazaki H, Ueyama Y, Tamaoki N, Miura S, Nakamura M: Expression of angiostatic factors in colorectal cancer. Int J Oncol 1999, 15:1221-1225 [DOI] [PubMed] [Google Scholar]
- 7.Hatanaka H, Oshika Y, Abe Y, Yoshida Y, Hashimoto T, Handa A, Kijima H, Yamazaki H, Inoue H, Ueyama Y, Nakamura M: Vascularization is decreased in pulmonary adenocarcinoma expressing brain-specific angiogenesis inhibitor 1 (BAI1). Int J Mol Med 2000, 5:181-183 [DOI] [PubMed] [Google Scholar]
- 8.Holland EC: Glioblastoma multiforme: the terminator. Proc Natl Acad Sci USA 2000, 97:6242-6244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishii N, Maier D, Merlo A, Tada M, Sawamura Y, Diserens AC, Van Meir EG: Frequent co-alterations of TP53, p16/CDKN2A, p14ARF, PTEN tumor suppressor genes in human glioma cell lines. Brain Pathol 1999, 9:469-479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mercer WE, Shields MT, Amin M, Sauve GJ, Appella E, Romano JW, Ullrich SJ: Negative growth regulation in a glioblastoma tumor cell line that conditionally expresses human wild-type p53. Proc Natl Acad Sci USA 1990, 87:6166-6170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albertoni M, Shaw PH, Nozaki M, Godard S, Tenan M, Hamou MF, Fairlie DW, Breit SN, Paralkar VM, de Tribolet N, Van Meir EG, Hegi ME: Anoxia induces macrophage inhibitory cytokine-1 (MIC-1) in glioblastoma cells independently of p53 and HIF-1. Oncogene 2002, 21:4212-4219 [DOI] [PubMed] [Google Scholar]
- 12.Albertoni M, Daub DM, Arden KC, Viars CS, Powell C, Van Meir EG: Genetic instability leads to loss of both p53 alleles in a human glioblastoma. Oncogene 1998, 16:321-326 [DOI] [PubMed] [Google Scholar]
- 13.Ikeda J, Tada M, Ishii N, Saya H, Tsuchiya K, Okaichi K, Mishima K, Sawamura Y, Fulci G, Liu TJ, Van Meir EG: Restoration of endogenous wild-type p53 activity in a glioblastoma cell line with intrinsic temperature-sensitive p53 induces growth arrest but not apoptosis. Int J Cancer 2001, 94:35-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kessler R, Hamou MF, Albertoni M, de Tribolet N, Arand M, Van Meir EG: Identification of the putative brain tumor antigen BF7/GE2 as the (de)toxifying enzyme microsomal epoxide hydrolase. Cancer Res 2000, 60:1403-1409 [PubMed] [Google Scholar]
- 15.de Fraipont F, Nicholson AC, Feige J, Van Meir EG: Thrombospondins and tumor angiogenesis. Trends Mol Med 2001, 7:401-407 [DOI] [PubMed] [Google Scholar]
- 16.Brat DJ, Van Meir EG: Glomeruloid microvascular proliferation orchestrated by VPF/VEGF: a new world of angiogenesis research. Am J Pathol 2001, 158:789-796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tenan M, Fulci G, Albertoni M, Diserens AC, Hamou MF, El Atifi-Borel M, Feige JJ, Pepper MS, Van Meir EG: Thrombospondin-1 is down-regulated by anoxia and suppresses tumorigenicity of human glioblastoma cells. J Exp Med 2000, 191:1789-1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Laurenzi V, Melino G: Evolution of functions within the p53/p63/p73 family. Ann NY Acad Sci 2000, 926:90-100 [DOI] [PubMed] [Google Scholar]
- 19.Scherer SJ, Maier SM, Seifert M, Hanselmann RG, Zang KD, Muller-Hermelink HK, Angel P, Welter C, Schartl M: p53 and c-Jun functionally synergize in the regulation of the DNA repair gene hMSH2 in response to UV. J Biol Chem 2000, 275:37469-37473 [DOI] [PubMed] [Google Scholar]
- 20.Van Meir EG, Polverini PJ, Chazin VR, Su Huang HJ, de Tribolet N, Cavenee WK: Release of an inhibitor of angiogenesis upon induction of wild-type p53 expression in glioblastoma cells. Nat Genet 1994, 8:171-176 [DOI] [PubMed] [Google Scholar]
- 21.Mori K, Kanemura Y, Fujikawa H, Nakano A, Ikemoto M, Ozaki I, Matsumoto T, Tamura K, Yokota M, Arita N: Brain-specific angiogenesis inhibitor 1 (BAI1) is expressed in human cerebral neuronal cells. Neurosci Res 2002, 43:69-74 [DOI] [PubMed] [Google Scholar]
- 22.Duda DG, Sunamura M, Lozonschi L, Yokoyama T, Yatsuoka T, Motoi F, Morii A, Tani K, Asano S, Nakamura Y, Matsuno S: Overexpression of p53-inducible brain specific angiogenesis inhibitor 1 suppresses efficiently tumor angiogenesis. Br J Cancer 2002, 86:490-496 [DOI] [PMC free article] [PubMed] [Google Scholar]


