Abstract
Glioblastoma multiforme (GBM) is the most malignant variant of human glial tumors. A prominent feature of this tumor is the occurrence of necrosis and vascular proliferation. The regulation of glial neovascularization is still poorly understood and the characterization of factors involved in this process is of major clinical interest. Macrophage migration inhibitory factor (MIF) is a pleiotropic cytokine released by leukocytes and by a variety of cells outside of the immune system. Recent work has shown that MIF may function to regulate cellular differentiation and proliferation in normal and tumor-derived cell lines, and may also contribute to the neovascularization of tumors. Our immunohistological analysis of MIF distribution in GBM tissues revealed the strong MIF protein accumulation in close association with necrotic areas and in tumor cells surrounding blood vessels. In addition, MIF expression was frequently associated with the presence of the tumor-suppressor gene p53. To substantiate the concept that MIF might be involved in the regulation of angiogenesis in GBM, we analyzed the MIF gene and protein expression under hypoxic and hypoglycemic stress conditions in vitro. Northern blot analysis showed a clear increase of MIF mRNA after hypoxia and hypoglycemia. We could also demonstrate that the increase of MIF transcripts on hypoxic stress can be explained by a profound transcriptional activation of the MIF gene. In parallel to the increase of MIF transcripts, we observed a significant rise in extracellular MIF protein on angiogenic stimulation. The data of our preliminary study suggest that the up-regulation of MIF expression during hypoxic and hypoglycemic stress might play a critical role for the neovascularization of glial tumors.
Glioblastoma multiforme (GBM; World Health Organization grade IV) is the most common and also the most malignant variant of human glial tumors. 1 The pathological hallmark of the tumor is its histological heterogeneity and its most prominent feature is the occurrence of necrosis and abundant vascular proliferation. The regulation of angiogenesis in human GBM is poorly understood. The role of the vascular endothelial growth factor and its receptor in glial neovascularization has been investigated in detail. 2 However, complex interactions of additional factors seem also to be important in vascular proliferation. The identification and characterization of factors involved in these processes is of major clinical interest because interference with glial tumor angiogenesis might open novel approaches to rational, targeted tumor therapies.
Macrophage migration inhibitory factor (MIF) is a pleiotropic cytokine released by macrophages, T cells, and by a variety of cells outside of the immune system. 3-8 It has been shown to act as a proinflammatory cytokine, playing a major role in endotoxin shock and counterregulating the effects of glucocorticoids. 9,10 In addition, antibodies to MIF diminish the manifestation of autoimmunity in certain animal models. 11,12
Recent studies have shown that MIF may function to regulate cellular differentiation and proliferation in normal and tumor-derived cell lines, and may also contribute to the neovascularization of murine tumors. 13-16 Accordingly, in an approach to identify candidate genes for the hypoxic tumor phenotype, MIF mRNA was found to be induced by hypoxia in human carcinoma cells, 17 and one investigation detected enhanced levels of MIF transcripts in GBM tissues using microarrays. 18
Mutations of the tumor-suppressor gene p53 were found to be associated with the progression of individual gliomas. Interestingly, two recent reports have shown the ability of MIF to inhibit p53-dependent apoptosis. 19,20 To provide a possible link between MIF and p53 in GBM, we have compared the expression pattern of MIF and p53. In addition, we evaluated the regulation of MIF gene and protein expression after hypoxic and hypoglycemic stress.
Materials and Methods
Tumor Samples
We have constructed a tissue array containing 49 individual GBM samples. Serial sections of these tissues have been stained for MIF and p53. Formalin-fixed and paraffin-embedded samples of surgically removed gliomas were classified as GBM according to the guidelines published by the World Health Organization. Normal brain tissue from frontal cortex and subcortical white matter of an autopsy case without pathological findings served as control.
Immunohistochemistry
Immunolabel was performed using the labeled streptavidin biotin (LSAB) kit according to the manufacturer’s instructions (DAKO, Hamburg, Germany). Briefly, sections (3 μm) were dewaxed in graded alcohols and xylene, thereafter the endogenous peroxidase activity was blocked with 3% hydrogen peroxide solution. For antigen retrieval, the sections were incubated with 0.1 mol/L of sodium citrate buffer in a microwave oven (3 × 5 minutes/600W). The slides were then treated with 0.001% trypsin at 37°C for 15 minutes and washed (3 × 5 minutes) with phosphate-buffered saline (PBS)/Tween. The sections were then incubated with polyclonal antibodies against human MIF, or a monoclonal antibody against human p53 (clone DO-7, DAKO), at a dilution of 1:1000 for 1 hour at 37°C, washed (3 × 5 minutes) in PBS/Tween, and incubated with biotinylated polyclonal antibodies (DAKO), for 30 minutes, washed again (3 × 5 minutes) in PBS/Tween, and subsequently incubated with a streptavidin/peroxidase-conjugated antibody for 30 minutes. Sections were then developed with 3,3-diaminobenzidine, giving a brown product.
For preabsorption the polyclonal MIF antibody was incubated overnight at 4°C, at a dilution of 1:1000 with 100 μg of recombinant MIF. Immune complexes were removed by centrifugation at 12,000 rpm for 10 minutes. Incubation of the sections with preabsorbed MIF antibody did not result in a specifically positive immunohistochemical reaction.
Evaluation of MIF and p53 Immunoreactivity
The scoring was performed under very stringent condition. Only those samples were scored positive in which the majority of the tumor samples showed a positive immunolabel. In a few cases, in which only discrete immunostain was detected, these samples were scored negative.
Northern Blot Analysis
For Northern blot analysis, the cells were harvested at different time points as indicated, and the total RNA was isolated with Trizol (Gibco, Karlsruhe, Germany). RNA (5 μg) was electrophoresed through 1% agarose-formaldehyde gels, transferred onto positively charged nylon membranes (Boehringer, Mannheim, Germany), and hybridized with a human MIF riboprobe. Probes were labeled with digoxigenin using the DIG RNA labeling kit (Boehringer). Chemiluminescence detection was achieved according to the instructions for the use of the CDP-Star (Tropix, Bedford, MA) chemiluminescent substrate for alkaline phosphatase. Blots were exposed to Hyperfilm MP autoradiographic film (Amersham Pharmacia Biotech, Freiburg, Germany) and the films were processed in an Optimax automatic developing machine (MS Laborgeräte, Heidelberg, Germany). To confirm equal loading of the RNA samples, equal amounts of total RNA were blotted onto nitrocellulose membranes and stained with methylene blue as described. 21
Transfection Studies
RGL-3 cells were cultured at 0.75 × 106/ml in Dulbecco’s modified Eagle’s medium in the presence of 5% fetal calf serum, 1% glutamine, and antibiotics for 24 hours before transient transfection. All constructs including the pGL3 basic were transfected into RGL-3 cells using LipofectAMINE reagent as recommended by the supplier (Gibco, Freiburg, Germany). Plasmid DNA (1.5 μl), 15 μl of LipofectAMINE reagent, and medium without antibiotics were used for transient transfection of 1.5 × 106 RGL3 cells. After 5 hours the medium containing DNA and LipofectAMINE was replaced with fresh, complete medium. Cells were cultured for another 8 hours under either normoxic or hypoxic conditions. The cells were harvested with M-PER lysis buffer (Pierce, Rockford, IL), the cellular debris was removed by centrifugation and the supernatants were collected. Protein concentrations were determined using the BCA Protein Assay Kit (Pierce, Rockford, IL). Each set of experiments was repeated 5 to 10 times in duplicates. Luciferase activity was measured with 10 to 30 μg protein cell extracts in a luminometer analyzer (Lumistar; BMG Lab Technologies, Offenburg, Germany) using the Promega Luciferase Assay Kit as recommended by the manufacturer. One hundred μl of substrate was automatically added to each probe.
Western Blot Analysis
For the determination of the extracellular MIF protein content, supernatants of the glioma cell cultures (1 × 107 cells/Petri dish) were concentrated using Centricon 10 concentrators (Amicon, Beverly, MA) according to the operating manual. Samples were run under reducing conditions on 4 to 12% Nu PAGE gels (Novex, San Diego, CA) and blotted onto Optitran nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany). Western blotting was performed according to the instructions of the Super Signal Ultra Western blot kit (Pierce). Briefly, the blots were blocked for 1 hour in Roti-Block (Roth, Karlsruhe, Germany) blocking buffer and then incubated with anti-murine MIF polyclonal antibodies (dilution, 1:12,000). The membranes were washed four times with PBS containing 0.05% Tween 20, and incubated for 1 hour with horseradish peroxidase-conjugated secondary antibody [goat anti-rabbit IgG (Pierce) at a 1:250,000 dilution]. The blots were washed four times for 10 minutes, incubated for 5 minutes in Super Signal Ultra substrate working solution (Pierce) and exposed to autoradiographic film (Hyperfilm MP, Amersham Pharmacia Biotech). The films were processed in an Optimax automatic developing machine.
MIF-Enzyme-Linked Immunosorbent Assay (ELISA)
For measurement of MIF protein, supernatants from normal, hypoxic, or hypoglycemic LN-18 cell cultures were analyzed by sandwich ELISA, using a monoclonal anti-human MIF capture antibody, and biotinylated goat anti-human MIF IgG detection antibody (R&D Systems, Wiesbaden, Germany), and purified human rMIF as standard.
Cell Culture
Human glioblastoma cells (LN18) were cultured in Dulbecco’s modified Eagle’s medium (Gibco) with 5% fetal calf serum (Biochrom, Berlin, Germany) supplemented with Pen/Strep and l-Glut (Gibco). They were subcultured twice weekly and plated out for the experiments 24 hours before start in semiconfluent density in Dulbecco’s modified Eagle’s medium with 1% fetal calf serum. Before the start of the kinetics, medium had been exchanged.
Hypoxia/Hypoglycemia
Anaerobic atmospheres were generated by the use of the GasPak Plus system (Becton Dickinson, Heidelberg, Germany) according to the manufacturer’s instructions. Hypoglycemic conditions have been created by using glucose-free Dulbecco’s modified Eagle’s medium (Gibco) supplemented with 1% fetal calf serum as a culture medium.
Results
MIF and p53 Expression in Human GBM
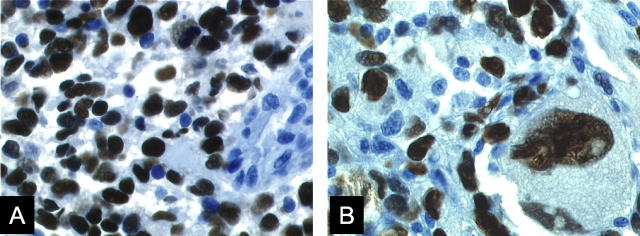
Immunohistochemistry using polyclonal antibodies directed against human MIF demonstrated immunoreactivity in 17 (35%) of 49 malignant gliomas examined (Figure 1; A, B, and C ▶ , and Table 1 ▶ .). MIF immunoreactivity could be detected in the cytoplasm of the tumor cells (Figure 1; A, B, and C) ▶ . In 16 samples (32%), we observed p53 protein label within the nuclei of tumor cells (Figure 2 ▶ , Table 1 ▶ ). Within the group of MIF-positive tumor samples, we obtained in 11 samples (65%) a clear co-localization with p53 (Table 1) ▶ .
Figure 1.
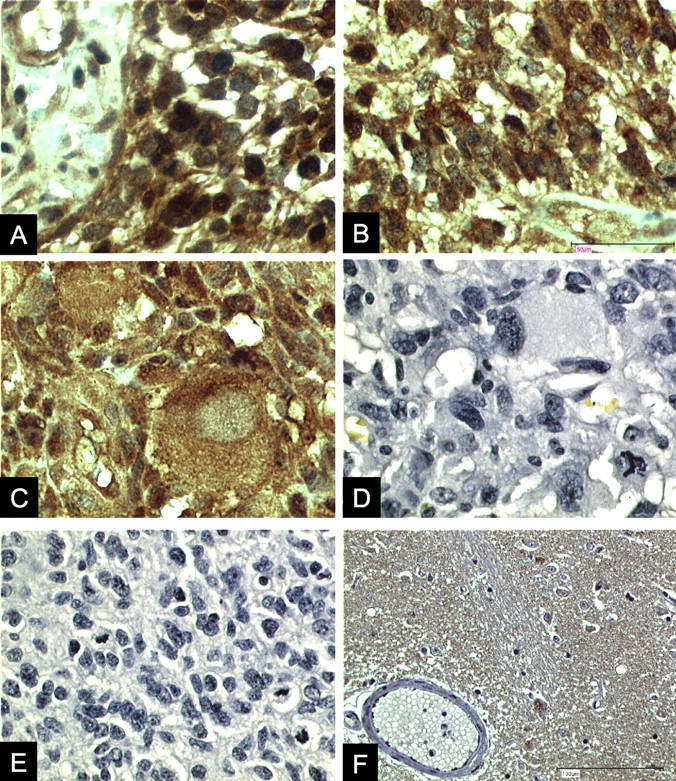
MIF immunolabel in human GBM sections A to C. A and B: Prominent MIF immunostain (brown label) was frequently detected in the vicinity of tumor-associated blood vessels. MIF immunostain was also detected in tumor vasculature endothelial cells. C: MIF immunostain was frequently detected within the cytoplasm of large tumor cells. D and E: Control sections of GBM samples in which the primary anti-MIF antibodies were preabsorbed with rMIF. F: MIF immunoreactivity within a section of normal brain. MIF protein was only localized in a few scattered cells. We observed a complete lack of MIF in cerebral vasculature endothelial cells. Immunolabel was performed using the labeled streptavidin biotin (LSAB) kit from DAKO. Original magnifications: ×200 (A–E); ×100 (F).
Table 1.
Analysis of MIF and p53 Immunoreactivity in 49 GBM Samples
| MIF+/p53+ | MIF+/p53− | MIF−/p53+ | MIF−/p53− |
|---|---|---|---|
| 11 | 6 | 5 | 27 |
Tissues were scored under very stringent condition. Samples were scored positive only when the majority of tumor cells were clearly labeled. In a few cases in which only discrete staining was detected, these samples were scored negative.
Figure 2.
Immunolocalization of p53 in human GBM samples A to B. A: Profound p53 protein accumulation within the nuclei of tumor cells. B: Strong p53 immunostain was frequently observed in large tumor cells. A monoclonal antibody against human p53 (DAKO) was used as primary antibody. Original magnifications, ×200.
MIF Expression in Normal Human Brain
Analysis of normal human brain sections revealed MIF immunolabel only within a few scattered cells and complete lack of MIF immunostain within cerebral vasculature endothelial cells (Figure 1D) ▶ .
Up-Regulation of MIF Gene and Protein Expression in a Human Glioma Cell Line during Hypoxia and Hypoglycemia
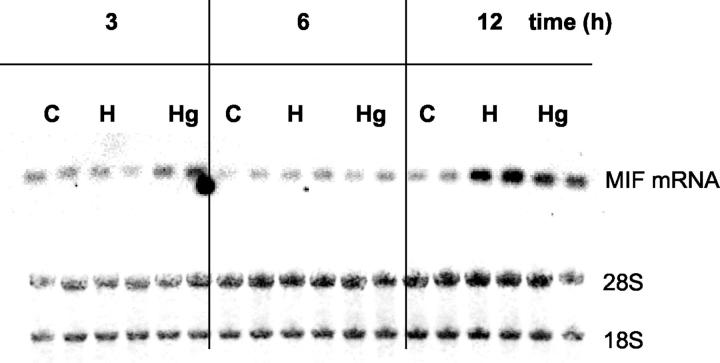
We next examined MIF mRNA expression in normal, hypoxic, or hypoglycemic LN-18 glioma cells at 3, 6, and 12 hours by Northern blot. There was a clear increase of MIF mRNA within 12 hours of hypoxic conditions, whereas under hypoglycemic conditions MIF mRNA was only slightly enhanced (Figure 3) ▶ .
Figure 3.
Kinetics of MIF steady-state mRNA levels in a human glioblastoma cell line (LN18) after normal (C), hypoxic (H), and hypoglycemic (Hg) conditions. Total RNA was isolated from duplicate cultures at the indicated time points. Five μg of RNA per sample was blotted onto Nylon membranes and hybridized with a DIG-labeled human MIF cRNA probe (top). To assess integrity and equal loading of all RNA samples, the same amount of RNA was also transferred onto nitrocellulose membranes and stained with methylene blue (bottom).
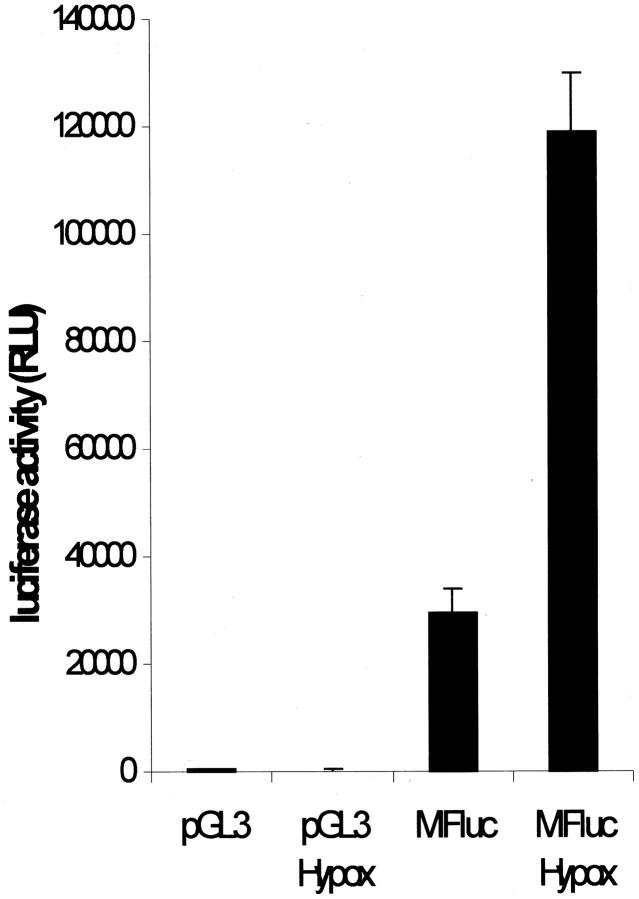
To investigate whether the increase of MIF steady-state mRNA during hypoxia was paralleled by an induced transcriptional activity, we performed a promoter analysis. To this end, the murine MIF promoter fused to a luciferase reporter gene (MIFluc) was transiently transfected into rat RGL3 glioma cells. This construct has been previously shown to be sufficient to drive reporter gene expression in pituitary cells. 22 A prominent basal transcriptional activity was detected in unstimulated rat glioma cells (29.3 ± 4.5 × 103 relative light units (RLU), ∼80-fold more than the promoterless parent vector). Under hypoxic conditions, the transcriptional activity was found to be 3.9-fold induced in the MIFluc-transfected cells (118.9 ± 11.1 × 103 RLU) (Figure 4) ▶ .
Figure 4.
Transcriptional activity of the MIF promoter in rat glioma RGL3 cells. The MIF promoter fused to the luciferase gene was transiently transfected into rat glioma cells. The construct drove very high transcriptional activity (29.3 ± 4.5 × 103 RLU, ∼80-fold more than the promoterless plasmid), which was further enhanced (3.9-fold) when the cell cultures were incubated for 8 hours under hypoxic conditions (118.9 ± 11.1 × 103 RLU). The bars represent the mean ± SD of values obtained from duplicate culture in eight separate experiments.
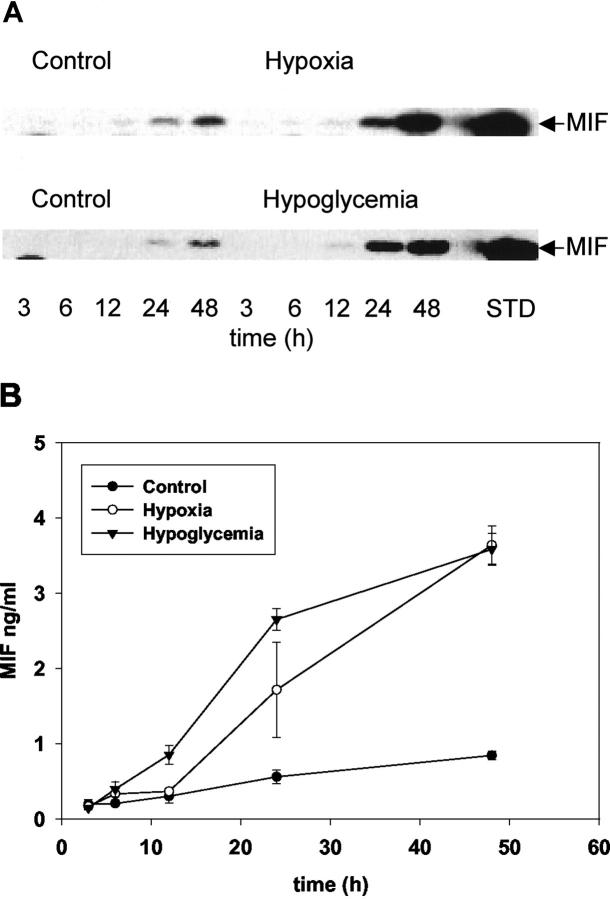
We then analyzed the corresponding supernatants for MIF protein content by Western blot or ELISA. In normal cells, MIF started to accumulate after 24 hours in culture, and was further increased after 48 hours (Figure 5) ▶ . However, the extracellular MIF content was markedly induced at these time points under hypoxic or hypoglycemic conditions (Figure 5) ▶ .
Figure 5.
Analysis of extracellular MIF production in normal LN18 cells, and after hypoxic or hypoglycemic conditions by Western blot (A) and ELISA (B). A: Supernatants were prepared as described in Materials and Methods. Ten-μl aliquots of the supernatants at the indicated times were electrophoresed and transferred onto nitrocellulose membranes and the MIF content was analyzed by reaction with an anti-MIF antibody as described in Materials and Methods. Recombinant MIF (50 ng) was electrophoresed as standard (STD). B: MIF release was analyzed by sandwich ELISA of supernatants collected at the indicated time points from control cells (filled circles), after hypoxia (open circles), and after hypoglycemia (inverted filled triangle). Measurements were performed in triplicate cultures; the data shown are the mean ± SD of one representative experiment of a group of five separate experiments with similar results.
Discussion
Necroses and vascular proliferates are the most prominent histopathological features in human malignant gliomas. It has been shown that withdrawal from blood supply and hypoxia induce glioma angiogenesis and that vascular endothelial growth factor and its receptor play an important role in this process. 1 The regulation, however, seems to be more complex and other factors are also involved. The identification of these factors is of clinical importance because interference with glial tumor angiogenesis might open novel approaches to targeted tumor therapies.
Here we show that MIF is expressed in one third of GBM samples investigated. Moreover, MIF was mainly expressed in the surrounding structures of necrosis and/or vascular proliferates. These are the regions within the tumor tissue where hypoxia plays an important role. Consequently, hypoxia-induced factors are mainly expressed in these structures indicating that MIF expression in gliomas in vivo could also be regulated by hypoxia. This hypothesis is corroborated by the experimental data of the present study that demonstrate an up-regulation of MIF mRNA and protein expression in LN18 glioma cells after hypoxia. Thus, MIF seems to be one of the factors involved in the complex response of glioma cells to hypoxia. The induction of MIF mRNA during hypoxia has been recently reported in cell lines derived from squamous cell carcinomas. 17 In contrast, when LN18 glioma cells were stressed by hypoglycemia, the increase of extracellular MIF protein levels was not because of a transcriptional activation of MIF expression. These data imply that MIF is functionally regulated by alternative pathways in hypoxia and hypoglycemia, respectively. Hypoxia clearly leads to an up-regulation of MIF expression by transcriptional activation.
Expression of MIF has been shown in several tumors including lymphomas and solid tumors with a prominent inflammatory reaction. 15,16,23 The demonstration of MIF expression in malignant gliomas by the present study adds another tumor type to the list of MIF-associated tumors. Inflammatory response to tumor growth, however, is not a characteristic feature of glial tumors. The distribution of MIF expression around necroses and vascular proliferates in the GBM and the induction of MIF in LN18 GBM cells in vitro clearly demonstrates that hypoxia stimulates MIF expression in glioma cells. Thus, MIF release because of inflammation in lymphoma and solid tumors with strong inflammatory reaction or because of hypoxia and necrosis in glial tumors could be functional in tumor tissues.
This might have at least two consequences. First, MIF might be directly involved in the complex regulation of glial tumor angiogenesis. It has been shown in experimental colon cancer that treatment with antibodies directed against MIF suppresses angiogenesis in these tumors. The demonstration of the angiogenic potential of MIF not only indicates a comparable role in glial tumors but also could open novel therapeutic options by interference with glial tumor neovascularization. Second, MIF expression could be effective in inactivating p53 in gliomas. The latter concept has been recently established by two investigations demonstrating the ability of MIF to inhibit p53-dependent apoptosis in rat fibroblasts and murine macrophages. 19,20
The p53 tumor suppressor gene product plays an important role in glial tumorigenesis. It has been shown, that a clinical and genetic subtype (secondary GBM) of glial tumors is characterized by p53 mutations. 24 This type however constitutes only a minor part of GBMs and none of the MIF-positive tumors of the present study belongs to this subtype. The other GBM subtype, the de novo occurring primary GBM, does not harbor p53 mutations. However, we and others have shown previously that p53 is functionally altered in these tumors with wild-type p53. 25 The inactivation of p53 by mutation or functional alterations demonstrates the important role of p53 in glial tumorigenesis. 26 Therefore, MIF-induced inactivation of p53 in tumors with wild-type p53 could be functional in malignant gliomas and could protect cells against hypoxia-induced apoptosis. Although, we did not obtain a strict correlation between MIF and p53 expression pattern, the high incidence (65%) of MIF and p53 co-localization could provide a first hint that MIF and p53 could be functionally associated in GBM.
We would like to emphasize that this is a preliminary study about a possible role for MIF in GBM tumorigenesis and a correlation between MIF staining and other clinical features has not yet been analyzed. Further studies are required to substantiate and extend the link between MIF and human glial tumors.
Footnotes
Address reprint requests to Dr. Michael Bacher, Institute of Immunology, Robert Koch-Str.17 35037, Marburg, Germany. E-mail: bacher@mailer.uni-marburg.de.
M. B. and J. S. contributed equally to this work.
References
- 1.Holland EC: Glioblastoma multiforme: the terminator. Proc Natl Acad Sci USA 2000, 97:6242-6244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plate KH, Breier G, Weich HA, Risau W: Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature , 359:845-848 [DOI] [PubMed] [Google Scholar]
- 3.David JR, Bozza M, Carini C: Macrophage migration inhibitory factor. Aggraval BB Gutterman JU eds. Human Cytokines. Handbook for Basic and Clinical Research, 1996, vol 2.:pp 222-256 Blackwell Science, Cambridge [Google Scholar]
- 4.Bucala R: MIF rediscovered: cytokine, pituitary hormone, and glucocorticoid regulator of the immune response. EMBO J 1996, 10:1607-1613 [DOI] [PubMed] [Google Scholar]
- 5.Lan HY, Mu W, Yang N, Meinhardt A, Nikolic-Paterson DJ, Ng YY, Bacher M, Atkins RC, Bucala R: De novo renal expression of macrophage migration inhibitory factor during the development of rat crescentic glomerulonephritis. Am J Pathol 1996, 149:1119-1127 [PMC free article] [PubMed] [Google Scholar]
- 6.Meinhardt A, Bacher M, McFarlane JR, Metz CN, Seitz J, Hedger MP, de Kretser DM, Bucala R: The macrophage migration inhibitory factor (MIF) production by Leydig cells: evidence for a role in regulation of testicular function. Endocrinology 1996, 137:5090-5095 [DOI] [PubMed] [Google Scholar]
- 7.Bacher M, Meinhardt A, Lan HY, Mu W, Metz CN, Chesney JA, Calandra T, Gemsa D, Donnelly T, Atkins RC, Bucala R: MIF expression in experimentally-induced endotoxemia. Am J Pathol 1997, 150:235-246 [PMC free article] [PubMed] [Google Scholar]
- 8.Waeber G, Calandra T, Roduit R, Haefliger JA, Thompson N, Thorens B, Temler E, Meinhardt A, Bacher M, Metz CN, Nicod P, Bucala R: Insulin secretion is regulated by the glucose-dependent production of islet beta cell macrophage migration inhibitory factor. Proc Natl Acad Sci USA 1997, 94:4782-4787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernhagen J, Calandra T, Mitchell RA, Martin SB, Tracey KJ, Voelter W, Manogue KR, Cerami A, Bucala R: MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature 1993, 365:756-759 [DOI] [PubMed] [Google Scholar]
- 10.Calandra T, Bernhagen J, Metz CN, Spiegel LA, Bacher M, Donnelly T, Cerami A, Bucala R: MIF as a glucocorticoid-induced modulator of cytokine production. Nature 1995, 377:68-71 [DOI] [PubMed] [Google Scholar]
- 11.Mikulowska A, Metz CN, Bucala R, Holmdahl R: Macrophage migration inhibitory factor is involved in the pathogenesis of collagen type II-induced arthritis in mice. J Immunol 1997, 158:5514-5517 [PubMed] [Google Scholar]
- 12.Lan HY, Bacher M, Yang N, Mu W, Nikolic-Paterson DJ, Metz C, Meinhardt A, Bucala R, Atkins RC: The pathogenic role of macrophage migration inhibitory factor in immunological induced kidney disease in the rat. J Exp Med 1997, 185:1455-1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimizu T, Ohkawara A, Nishihira J, Sakamoto W: Identification of macrophage migration inhibitory factor (MIF) in human skin and its immunohistochemical localization. FEBS Lett 1996, 381:199-202 [DOI] [PubMed] [Google Scholar]
- 14.Mitchell RA, Metz CN, Peng T, Bucala R: Sustained mitogen-activated protein kinase (MAPK) and cytoplasmic phospholipase A2 activation by macrophage migration inhibitory factor (MIF). J Biol Chem 1999, 274:18100-18106 [DOI] [PubMed] [Google Scholar]
- 15.Chesney J, Metz C, Bacher M, Peng T, Meinhardt A, Bucala R: An essential role for macrophage migration inhibitory factor (MIF) in angiogenesis and the growth of a murine lymphoma. Mol Med 1999, 5:181-191 [PMC free article] [PubMed] [Google Scholar]
- 16.Shimizu T, Abe R, Nakamura H, Ohkawara A, Suzuki M, Nishihira J: High expression of macrophage migration inhibitory factor in human melanoma cells and its role in tumor cell growth and angiogenesis. Biochem Biophys Res Commun 1999, 264:751-758 [DOI] [PubMed] [Google Scholar]
- 17.Koon AC, Denko NC, Hudson KM, Schindler C, Swiersz L, Koch C, Evans S, Ibrahim H, Le QT, Terris DJ, Giaccia AJ: Candidate genes for the hypoxic phenotype. Cancer Res 2000, 60:883-887 [PubMed] [Google Scholar]
- 18.Markert JM, Fuller CM, Gillespie GY, Bubien JK, McLean LA, Hong RL, Lee K, Gullans SR, Mapstone TB, Benos DJ: Differential gene expression profiling in human brain tumors. Physiol Genomics 2001, 5:21-33 [DOI] [PubMed] [Google Scholar]
- 19.Hudson JD, Shoaibi MA, Maestro R, Carnero A, Hannon GJ, Beach DH: A proinflammatory cytokine inhibits p53 tumor suppressor activity. J Exp Med 1999, 190:375-382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell RA, Liao H, Chesney J, Fingerle-Rowson G, Baugh J, David J, Bucala R: Macrophage migration inhibitory factor (MIF) sustains macrophage proinflammatory function by inhibiting p53: regulatory role in the innate immune response. Proc Natl Acad Sci USA 2002, 99:345-350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sambrook J: Fritsch EF Maniatis T eds. Molecular Cloning: A Laboratory Manual. 1989:p 7.51 Cold Spring Harbor Laboratory Press, Cold Spring Harbor
- 22.Waeber G, Thompson N, Chautard T, Steinmann M, Nicod P, Pralong FP, Calandra T, Gaillard RC: Transcriptional activation of the macrophage migration-inhibitory factor gene by the corticotropin-releasing factor is mediated by the cyclic adenosine 3′,5′-monophosphate responsive element-binding protein CREB in pituitary cells. Mol Endocrinol 1998, 12:698-705 [DOI] [PubMed] [Google Scholar]
- 23.Takahashi N, Nishihira J, Sato Y, Kondo M, Ogawa H, Ohshima T, Une Y, Todo S: Involvement of macrophage migration inhibitory factor (MIF) in the mechanism of tumor growth. Mol Med 1998, 4:707-714 [PMC free article] [PubMed] [Google Scholar]
- 24.Kleihues P, Ohgaki H: Primary and secondary glioblastoma: from concept to clinical diagnosis. Neuro-Oncology 1999, 1:44-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kraus A, Gross MW, Knuechel R, Munkel K, Neff F, Schlegel J: Aberrant p21 regulation in radioresistant primary glioblastoma multiforme cells bearing wild-type p53. J Neurosurg 2000, 93:863-872 [DOI] [PubMed] [Google Scholar]
- 26.Bogler O, Huang HJ, Kleihues P, Cavenee WK: The p53 gene and its role in human brain tumors. Glia 1995, 15:308-327 [DOI] [PubMed] [Google Scholar]