Abstract
CC chemokine receptor 8 (CCR8) has been detected in vitro on type 2 helper and regulatory lymphocytes, which might exert beneficial functions in multiple sclerosis (MS) and on macrophages and microglia, possibly promoting tissue injury in MS lesions. To discriminate the relevant expression pattern in vivo, we defined the cell types that expressed CCR8 in MS lesions and determined the relationship of CCR8 expression and demyelinating activity. CCR8 was not expressed on T cells but was associated with phagocytic macrophages and activated microglia in MS lesions and directly correlated with demyelinating activity. To identify factors associated with CCR8 expression, the study was extended to other central nervous system (CNS) pathologies. CCR8 was consistently expressed on phagocytic macrophages and activated microglia in stroke and progressive multifocal leukoencephalopathy, but not expressed on microglia in pathologies that lacked phagocytic macrophages such as senile change of the Alzheimer’s type. CCR8 was up-regulated by macrophage differentiation and activating stimuli in vitro. In summary CNS CCR8 expression was associated with phagocytic macrophages and activated microglial cells in human CNS diseases, suggesting that CCR8 may be a feasible target for therapeutic intervention in MS. CCR8 expression may also indicate a selective program of mononuclear phagocyte gene expression.
Chemokines constitute a superfamily of structurally and functionally related small proteins associated with inflammatory cell recruitment. Besides their well-characterized roles in inflammation and the immune system, chemokines modulate a number of biological responses, including enzyme secretion, cellular adhesion, cytotoxicity, tumor cell growth, degranulation, and T cell activation. Chemokines act through plasma membrane G-protein-coupled chemokine receptors on cellular targets. 1,2 Chemokines and their receptors have been implicated in various pathologies of the human central nervous system (CNS) and have emerged as salient targets for investigation. 3-6 In vitro studies have shown that CC chemokine receptor (CCR) 8 is expressed by polarized T helper type 2 (Th2) lymphocytes, natural killer cells, and monocytes. 7-11 Recent studies suggest that CCR8 is present on CD4+/CD25+ T-regulatory lymphocytes. 12 CCL1 is the unique human and mouse ligand for CCR8. 10,13,14 Human CCL1 was previously designated I-309; mouse CCL1 was termed TCA-3.
This diverse pattern of expression in vitro left unclear what parts the CCR8/CCL1 system might play in any specific immune or inflammatory process. The role of CCR8 and CCL1 in the pathogenesis of CNS diseases has recently been clarified significantly through studies in experimental autoimmune encephalomyelitis (EAE), a model of CNS inflammatory demyelination. Initial experiments a decade ago showed that production of the murine CCL1 homologue was tightly associated with the capacity of proteolipid protein-specific T-cell clones to mediate EAE. 15,16 Analysis of eae7, a polymorphic locus that controls susceptibility to EAE in mice revealed amino acid substitutions in CCL1, and two other β-chemokines. 16 Both CCL1 and CCR8 mRNA were highly expressed in the CNS of mice with EAE and this up-regulation was entirely contingent on secretion of tumor necrosis factor-α by infiltrating hematogenous leukocytes 17,18 (personal communication, J. Sedgwick, DNAX Research, Palo Alto, CA). Microglia were the predominant cellular source of CCL1 in the brains of mice with EAE, suggesting the possibility of an autocrine signaling to these cells through CCR8 (personal communication, J. Sedgwick). CCR8−/− mice exhibited a significant delay in the onset of EAE as compared with controls. The severity of EAE was significantly attenuated in CCR8−/− mice, with knockouts never achieving the mean neurological disease scores observed in wild-type animals. Leukocyte infiltration into the parenchyma of CCR8−/− mice with EAE was not impaired, suggesting that reduced EAE severity in CCR8 knockouts was because of defective activation of microglia and macrophages (personal communication, J. Sedgwick). A previous analysis of CCR8−/− mice described a specific defect in the generation of type 2 inflammatory responses to protozoal pathogens or airway-allergen challenge, while type 1 reactions were unaffected. 19 Therefore, it was plausible that the afferent limb of the type 1 immune response to myelin antigens was not impaired in CCR8−/− mice with EAE. Furthermore, these studies demonstrated that the effects of deleting CCR8 on macrophage activation in the CNS were dominant over the type 2 cytokine defect, as revealed in the EAE model.
The current study addressed the expression of CCR8 in multiple sclerosis (MS). We found that CCR8 was not expressed on T cells but was associated with phagocytic macrophages and activated microglia in both pattern II and pattern III lesions. To establish possible factors that may govern CCR8 expression in vivo, the study was extended to analysis of tissue sections from other CNS pathologies. CCR8 was consistently expressed on phagocytic macrophages and activated microglia in stroke and progressive multifocal leukoencephalopathy (PML). In other disorders that lacked phagocytic macrophages, such as Rasmussen’s encephalitis, neurosarcoidosis, or herpes simplex viral encephalitis, microglia failed to express CCR8. In two cases (one each of cerebral lymphoma and cerebral toxoplasmosis) in which concentrations of intense CCR8 immunoreactivity were detected, focal cerebral infarcts were also identified. Further, CCR8 was not expressed on activated microglia in cases with senile changes of the Alzheimer’s type, raising the possibility that such microglial activation differs from that observed in MS, PML, and stroke. CCR8 was not expressed by T cells or monocytes in peripheral blood, but its expression at protein and message levels was positively modulated by macrophage differentiation and activating stimuli in vitro. Our data demonstrated that CNS CCR8 expression was associated with phagocytic macrophages and activated microglial cells, but not with T cells, in these human CNS diseases. These findings suggest that CCR8 expression may be a marker for a selective program of mononuclear phagocyte gene expression, present in some but not all CNS pathologies that induce microglial activation and monocyte infiltration.
Materials and Methods
Autopsy Material from Patients with MS
CCR8 expression was analyzed in paraffin-embedded archival autopsy material of eight patients with MS. Five patients had lesions consistent with pattern II and three patients with pattern III. 20 A total of 16 tissue sections with 38 active lesions were available for this study (Table 1) ▶ . The material was collected and characterized at the Brain Research Institute, University of Vienna, Vienna, Austria.
Table 1.
Characteristics of Autopsy Material of Patients with MS
| Case number | Lesion pattern | Gender | Age, years | Disease course | Disease duration, months | Number of tissue sections | Number of lesions | Early-active regions | Late-active regions | Inactive regions | PW active | PW inactive | NAWM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | II | F | 47 | Acute | 3.5 | 2 | 3 | 3 | 2 | 2 | 3 | 0 | 1 |
| 2 | II | F | 46 | Acute | 0.4 | 3 | 4 | 4 | 4 | 4 | 5 | 0 | 3 |
| 3 | II | F | 28 | SP | 12 | 2 | 6 | 6 | 4 | 3 | 3 | 1 | 1 |
| 4 | II | M | 52 | Acute | 1.5 | 2 | 6 | 5 | 3 | 2 | 4 | 2 | 2 |
| 5 | II | F | 34 | SP | 144 | 1 | 2 | 2 | 2 | 2 | 1 | 0 | 1 |
| 6 | III | M | 35 | Acute | 1.5 | 3 | 8 | 7 | 5 | 7 | – | – | 3 |
| 7 | III | M | 45 | Acute | 0.8 | 2 | 6 | 5 | 3 | 3 | – | – | 2 |
| 8 | III | F | 46 | Acute | 0.3 | 1 | 3 | 4 | 2 | 2 | – | – | 1 |
| 8 patients | 5F/3M | 41.6 | 6 Acute/ | 20.5 | 16 | 38 | 36 | 25 | 25 | 16 | 3 | 8 | |
| (28–52) | 2 SP | (0.3–144) |
F, female; M, male; SP, secondary progressive; PW active, periplaque white matter at the border of active lesion; PW inactive, periplaque white matter at the border of inactive lesion; NAWM, normal appearing white matter.
After analysis of these materials, one additional case was analyzed, to determine whether CCR8-positive T cells could be identified in pattern II lesions whose unusual characteristics suggested a putative Th2 environment. This case presented as mild relapsing-remitting disease, followed by several months of rapidly progressive deterioration before death at age 20, with total disease duration being 4 years. In autopsy sections from this case, active lesions exhibited pattern II pathology with IgG and C9 neoantigen deposition on degenerating myelin sheaths. The cellular infiltrate contained unusually large numbers of eosinophil and neutrophil granulocytes along with anticipated numbers of macrophages and T cells. In one representative early active (EA) zone (shown in Figure 3 ▶ ), there were 172 eosinophils/mm2, 2838 granulocytes/mm2, 1161 macrophages/mm2, and 301 T cells/mm2.
Figure 3.
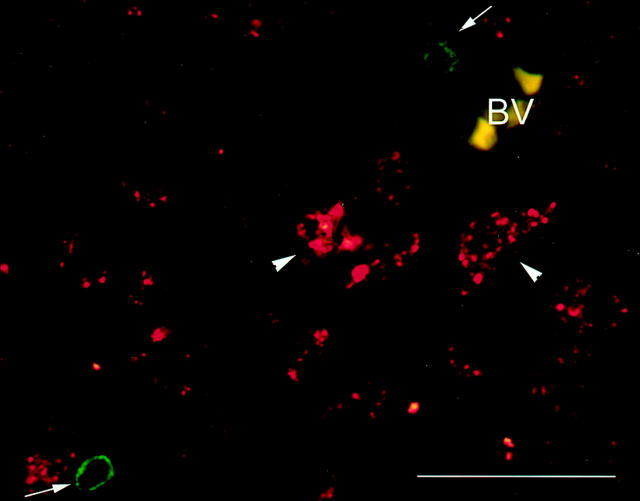
CCR8 is not expressed by lymphocytes in MS lesions. Co-localization of CD3 (green) and CCR8 (red) was performed in an EA area of a pattern II MS lesion. CD3 immunoreactivity did not co-localize with CCR8 (arrows). CCR8 was found associated with macrophages (arrowheads). BV, blood vessel. Scale bar, 50 μm.
Within active MS lesions, individual regions of different demyelinating activity were classified according to previously published criteria. 20,21 EA demyelinating regions were diffusively infiltrated by macrophages immunoreactive for all myelin proteins including myelin oligodendrocyte glycoprotein. Late active (LA) demyelinating regions were more advanced with respect to myelin degradation, and were immunoreactive for the major myelin proteins major basic protein and proteolipid protein, but not for myelin oligodendrocyte glycoprotein. Inactive (IA) demyelinated regions were completely demyelinated with no signs of active demyelination. Pattern II lesions showed typical perivenular distribution with a distinct lesion edge, simultaneous loss of all types of myelin, and without apoptotic oligodendrocytes. Pattern III lesions were distinguished from pattern II because of the presence of preferential loss of myelin-associated glycoprotein associated with oligodendrocyte apoptosis. 20
Normal appearing white matter was defined as an area that showed no evidence of demyelination by macroscopic inspection and histology within the area and the surrounding tissue. Periplaque white matter (PW) represented a strip of tissue of 5 mm adjacent to the border of active (PW active) or inactive (PW inactive) plaques.
Control Autopsy Material
Thirteen paraffin-embedded archival brain tissue sections from three individuals without known neurological, inflammatory, or metastatic disorder were collected at the Cleveland Clinic Foundation and served as noninflammatory controls. All three individuals (mean age, 69 years; two females and one male) died from sudden cardiac attack.
Autopsy and Biopsy Material from Other CNS Pathologies
Archival autopsy and biopsy material from a variety of other cases with neurological disorders were collected at the Cleveland Clinic Foundation, the University of California at San Francisco, and the Johns Hopkins University. All cases underwent routine gross and histopathological evaluation at the sites of collection. Diagnoses were based on established criteria. A total of 43 tissue sections of 31 individual cases were included in our analysis of CCR8 expression (Table 2) ▶ . Diagnoses were as follows: Rasmussen’s encephalitis (one case), lymphoma (two cases), chronic encephalitis (two cases), cerebral toxoplasmosis (two cases), Herpes simplex virus encephalitis (three cases), neurosarcoidosis (two cases), PML (one case), rabies encephalitis (two cases), CNS malaria (one case), senile changes of the Alzheimer’s type (eight cases), and ischemic stroke (seven cases).
Table 2.
Quantitation and Morphology of CCR8+ Cells in Cases with a Variety of Neurological Disorders and in Control Brain Sections
| Diagnosis | Number of cases | Gender, M:F | Median age | Number of lesions | Mean number of CCR8+ cells/mm2 | Mean number of CD68+ cells/mm2 | Morphology of CCR8+ cells |
|---|---|---|---|---|---|---|---|
| Rasmussen’s encephalitis | 1 | 0:1 | 13 | 1 | 0 | 809 | – |
| Neurosarcoidosis | 2 | 0:2 | 28 | 3 | 0 | 657 | – |
| PML | 1 | 0:1 | 79 | 2 | 468 | 805 | Microglia and macrophages |
| HSV encephalitis | 3 | 2:1 | 39 | 6 | 111 | 746 | Microglia |
| Rabies encephalitis | 2 | 2:0 | 10 | 2 | 25 | 1041 | Microglia |
| Cerebral toxoplasmosis | 1 | 0:1 | 30 | 2 | 280 | 683 | Microglia nodules |
| Cerebral malaria | 1 | 1:0 | na | 1 | 0 | 246 | – |
| Ischemic stroke | 7 | 4:3 | 66 | 10 | 856 | 1342 | Macrophages |
| Status epilepticus, posttreatment for malignant lymphoma complicated by cerebral toxoplasmosis and infarction | 1 | 1:0 | 62 | 1 | 963 | 1625 | Microglia and macrophages |
| Chronic encephalitis of undetermined etiology | 2 | 1:1 | 58 | 3 | 0 | 675 | – |
| Cerebral lymphoma | 1 | F | 87 | 2 | 0 | 469 | |
| Cerebral lymphoma complicated by acute and subacute infarction | 1 | F | 36 | 1 | 455 | 1059 | Microglia |
| Senile changes of the Alzheimer’s type | 8 | 3:5 | 78 | 9 | 0 | 79 | – |
| Control | 3 | 1:2 | 63 | 13 | 0 | 132 | – |
PML, progressive multifocal leukoencephalopathy; HSV, herpes simplex virus; na, not available.
Under guidelines established by the National Institutes of Health, this study was exempt from review, as determined by the Institutional Review Board of the Cleveland Clinic Foundation.
Immunohistochemistry
Immunohistochemistry was performed as previously described. 22,23 In brief, 5-μm sections were placed on Superfrost (Fisher Scientific, Pittsburgh, PA) slides. Paraffin-embedded tissue sections were deparaffinized with xylenes and rehydrated in ethanol. After antigen retrieval by steaming in citrate buffer, slides were incubated overnight with primary antibody at 4°C, washed in phosphate-buffered saline (PBS), incubated with biotinylated secondary antibody at room temperature for 40 minutes, washed, and incubated with avidin-biotin-horseradish peroxidase complex (Vectastain Elite; Vector Laboratories, Burlingame, CA). After development with 3,3-diaminobenzidine substrate (Sigma Chemical Co., St. Louis, MO), slides were dehydrated and mounted in Permount (Fisher Scientific, Pittsburgh, PA). For analysis of co-localization of CCR8 with CD68 and CCR8 with CD3, sections were simultaneously labeled with primary antibodies, followed by incubation with species-specific Texas Red- and fluorescein isothiocyanate-conjugated secondary antibodies (Southern Biotechnology Associates, Inc., Birmingham, AL). In controls, primary antibodies were omitted, and tests for cross-reactivity by secondary antibodies were performed. A rabbit polyclonal anti-CCR8 antibody was prepared at Berlex Biosciences (Richmond, CA). 24 Confirmatory stainings were done with a goat polyclonal antibody preparation from Alexis Biochemicals (catalog no. 210-764-R100). This reagent has been shown to stain T-regulatory cells generated in vitro. 12 Murine monoclonal anti-CD68 (clone KP1, mouse IgG1) was obtained from DAKO Corp., Carpinteria, CA; a goat polyclonal antibody anti-CCR3 from Santa Cruz Biotechnology, Santa Cruz, CA; and a rat monoclonal anti-CD3 (clone CD3–12) from Serotec Inc., Raleigh, NC.
Identification and Quantitation of CCR8-Positive Cells
CD68+ cells were classified as either monocytes, phagocytic macrophages, or activated microglial cells based on morphological criteria. Within CD68+ cells, the presence of vesicular structures and foamy cytoplasmic inclusions were considered as histological criteria for phagocytic macrophages. Small round CD68+ cells in the perivascular space and in the parenchyma, with no indicators of phagocytosis, were classified as monocytes. Activated microglial cells were defined as CD68+ cells with either elongated or round cell bodies and thickened asymmetric processes.
The number of immunostained cells was determined in at least four standardized fields (146,200 μm2, defined by a morphometric grid) from each lesional area. Immunostained sections were photographed using a Leica DMR microscope (Leica Wetzlar, Heidelberg, Germany) microscope and an Optronix Magnafire digital camera system and analyzed using Image Pro Plus (Media Cybernetics, Silver Spring, MD).
Isolation and Culture of Human Monocytes
Monocytes were obtained from freshly donated human peripheral blood from three healthy donors (two females and one male; mean age, 28 years (range, 22 to 33 years). The blood was immediately diluted 1:1 with PBS and underlayered with Ficoll-Paque (Pharmacia, Piscataway, NJ) for separation of mononuclear cells by density centrifugation. Monocytes were separated by adherence to serum-coated flasks according to the method of Kumagai and colleagues 26 and subsequently detached using 0.5 mmol/L of ethylenediaminetetraacetic acid. Isolated monocytes were cultured in Dulbecco’s modified Eagle medium (Mediatech Cellgro Inc., Herndon, VA) supplemented with l-glutamine, 4.5 mg/L glucose, and 10% bovine calf serum (Hyclone, Logan, UT) at 37°C and 10% CO2. Under these conditions monocytes differentiate into macrophages after 7 days. 26,27 Granulocyte-macrophage colony-stimulating factor was omitted from the cell culture medium because this might alter chemokine surface expression levels. 27 Where indicated, monocytes were stimulated with opsonized zymosan (ZOP) for 24 hours. Zymosan was obtained from ICN Biochemicals (Cleveland, OH) and opsonized and used at a concentration of 2 mg/ml to activate human monocytes. 28
CCR8 Expression on Monocytes in Vitro
CCR8 expression on cultured monocytes was examined by quantitative immunohistochemistry as previously described. 29 Live cells were rinsed with PBS and then fixed in situ with 4% paraformaldehyde (pH 7.3). After 1 hour the fixative was removed and the cells carefully washed with PBS and stored in PBS at 4°C until the time of staining. Before staining, cells were blocked overnight at 4°C with 0.2 mg/ml of normal goat IgG (Caltag Laboratories, Burlingame, CA). The blocking solution was removed, and the primary antibody solution was added without a rinse step. After incubation for 2 hours at 37°C, cells were rinsed and incubated with a biotinylated goat anti-rabbit secondary antibody for 1 hour. Finally, a fluorescein isothiocyanate-conjugated avidin (Southern Biotechnology Associates, Inc.) was added for 1 hour. After a final rinse step, cells were mounted with Vectashield mounting media containing 4′-6-diamidine-2′-phenylindole (DAPI) for nuclear counterstain. Analysis was performed on a Leica DMR microscope. CCR8-immunostained cells were counted in at least five entire microscopic fields at high (×40) magnification (diameter, 620 μm). Immunostained cells were expressed as percentages of absolute number of cells per field as assessed by DAPI nuclei counterstain.
RNA Extraction
RNA was extracted from monocyte cultures and nonadherent cells using TRIzol (Life Technologies, Inc., Carlsbad, CA) according to the manufacturer’s instructions. Briefly, cells were washed once with PBS and mixed with 250 μl of TRIzol. RNA was precipitated with isopropanol and washed with 75% ice-cold ethanol. The supernatant was removed with a Pasteur pipette after centrifugation at 7500 × g for 5 minutes at 4°C. RNA was resuspended in sterile MilliQ-water and the concentration determined by spectrophotometry. One μg of RNA was DNase treated (Life Technologies, Inc.) according to the manufacturer’s instructions.
Reverse Transcription-Coupled Polymerase Chain Reaction (PCR)
First-strand cDNA was synthesized using 1 μg of DNase treated RNA, oligo dT primers, and Super-Script II (Life Technologies, Inc.) according to the manufacturer’s instructions. The product of this reaction was amplified by PCR using primer pairs and TaqDNA polymerase (Boehringer Mannheim Corp., Indianapolis, IN). Primer pair sequences were as follows: 5′-TTT ACC AAG TGG CCT CTG AA; 3′-CCA AGA TGT GCA TAC TGT GCA A. 30 PCR conditions were set as follows: first denaturation, 94°C, 3 minutes; annealing, 60°C, 1 minute; extension, 72°C, 1 minute; denaturation, 94°C, 1 minute. Optimal concentration of Mg2+ was empirically determined to be 1.5 mmol/L. The product of 40 cycles of PCR was a unique band of expected length (297 bp) as revealed by electrophoretic analysis on ethidium bromide-stained agarose gels. Cloning and subsequent sequencing of the product verified amplification of CCR8 mRNA.
Flow Cytometry
Freshly isolated peripheral blood mononuclear cells, 24-hour monocytes after adherence, and 7-day macrophages after culture were isolated and diluted to 106 cells/ml in cold fluorescence-activated cell sorting (FACS) buffer (PBS containing 1% fetal calf serum and 0.1% sodium azide). One hundred μl of the cell suspension was blocked with 0.2 mg/ml normal goat IgG (Caltag Laboratories) for 15 minutes at room temperature. Cells were incubated with anti-CCR8 (Berlex Biosciences) for 15 minutes at room temperature and washed twice with FACS buffer. In a second staining step, cells were incubated with anti-CD3 PE (clone SK7; BD Biosciences, San Jose, CA) or anti-CD14 PE (SK3, BD Biosciences) and goat anti-rabbit fluorescein isothiocyanate (Southern Biotechnology Associates) for 15 minutes at room temperature, followed by two washes with FACS buffer and subsequent fixation with 1% paraformaldehyde. Cells were acquired on a FACScan flow cytometer (BD Biosciences) and analysis performed using WinList software (Verity Software House Inc., Topsham, ME). Cells were gated according to forward- and side light-scattering properties, and were positively selected for CD3 or CD14.
Statistics
Nonparametric tests (Mann-Whitney test and Wilcoxon signed rank test) were applied because the data were not normally distributed (Kolmogorov-Smirnov test). Reported P values are two-tailed and considered statistically significant at a P value <0.05.
Results
CCR8 Is Highly Expressed by Activated Microglia and Phagocytic Macrophages, but Not CD3-Positive Cells in Tissue Sections from MS Autopsy Brains
CCR8 expression was analyzed by immunohistochemistry in 10 tissue sections of five patients with pattern II MS lesions and in six sections of three patients with pattern III lesions. Twenty-one active lesions exhibiting pattern II and 17 exhibiting pattern III were identified within the available sections. In both pattern types, CCR8 immunoreactivity was predominantly found within MS lesions, as compared to the periplaque and normal appearing white matter (Figure 1) ▶ . CCR8 immunoreactivity was detected on cells with the morphology of phagocytic macrophages (Figure 1, c and g) ▶ at the expanding lesion edge and in areas of active ongoing demyelination. The distributions of CD68 and CCR8 immunoreactivity were similar (Figure 1; a, b, e, and f) ▶ . Within the periplaque and normal appearing white matter, CCR8 expression was observed on cells morphologically consistent with activated microglia (Figure 1, d and h) ▶ .
Figure 1.
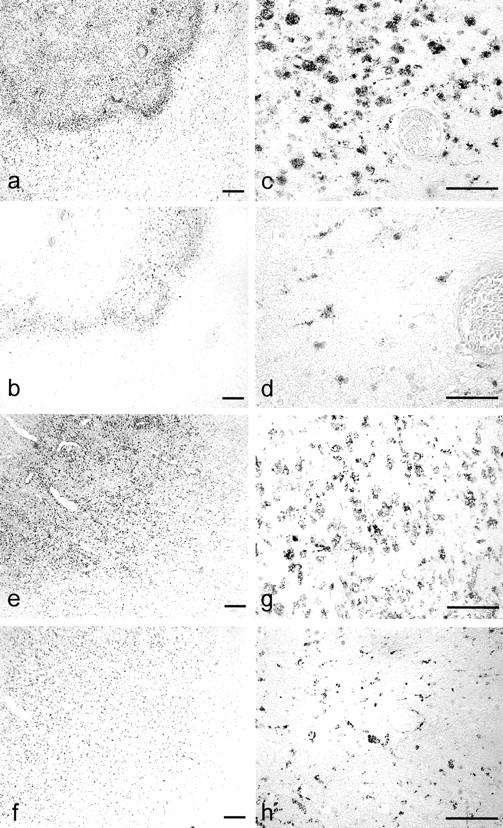
Distribution and morphology of CCR8 expression in patterns II (a–d) and III (e–h) MS lesions. a and b: Serial sections of an actively demyelinating lesion edge of an EA pattern II MS lesion and adjacent PW immunostained for CD68 (a) and CCR8 (b, c, d). b: Immunohistochemistry for CCR8 located CCR8+ cells to the lesion edge in EA regions. c: Within areas of active demyelination CCR8 was predominately found on phagocytic macrophages. d: In the PW CCR8 immunoreactivity was associated with activated microglial cells. e and f: Serial sections of an EA region of a pattern III MS lesion. Immunostaining for CD68 (e) and CCR8 (f) shows irregular lesion boarder of pattern III lesion. CCR8 was found on phagocytic macrophages and microglia within EA region (g) and predominantly on microglia in PW (h). Scale bars: 100 μm (a, b, e and f); 50 μm (c, d, g and h).
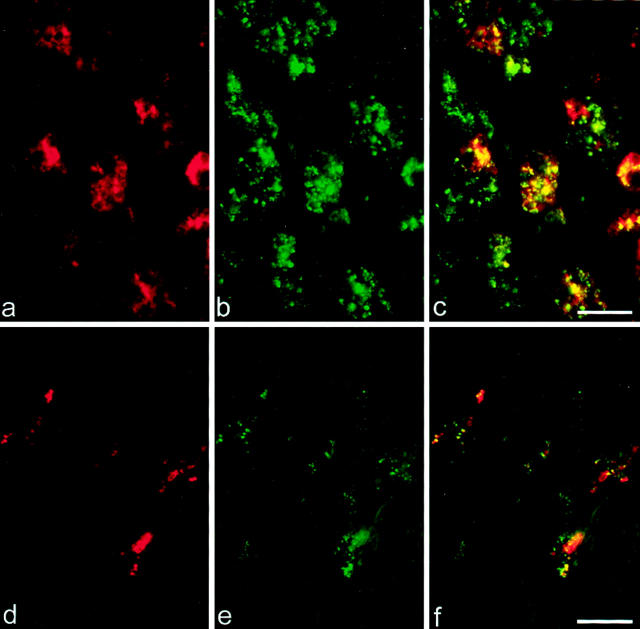
Analyses on serial sections and co-localization studies demonstrated CCR8 immunoreactivity associated with CD68-positive cells of the monocyte/macrophage lineage (Figure 2, c and f) ▶ .
Figure 2.
Co-localization of CD68 and CCR8 in active MS lesions. Dual-label immunofluorescence histochemistry for CD68 and CCR8 was performed on an area of EA demyelination (a–c) and on PW at the border to an active pattern II lesion (d–f). CD68 immunoreactivity is shown in red and green indicates CCR8 immunoreactivity. c: CD68+CCR8+ phagocytic macrophages were found in EA demyelinating areas. f: CD68+CCR8+-activated microglial cells were found within PW at the border of active pattern II lesions. Scale bars, 20 μm.
CCR8 immunoreactivity did not co-localize with CD3+ cells in any of 38 lesions in the above eight MS cases (data not shown). This latter observation was noteworthy, as CCR8 has been associated with type 2 lymphocytes or with CD4+/CD25+ T-regulatory cells in vitro. 11 It remained plausible that there were no type 2 or regulatory lymphocytes in the active MS lesions under investigation. Therefore, we extended our analysis to one additional MS case, whose outstanding feature was the presence of eosinophils within areas of active demyelination, in the context of pattern II tissue injury (not shown). To evaluate the presence of type 2 cells in this case, we analyzed CCR3, a chemokine receptor present on lymphocytes polarized to a type 2 cytokine profile 31 as well as on both T cells and eosinophils associated with allergic skin lesions. 32 We detected CCR3 on lymphocytic cells in zones of ongoing demyelination in this atypical MS case (not shown). In contrast, CCR8 was not detected on lymphocytes in this case (Figure 3) ▶ . CCR3 immunoreactivity was also expressed on T cells in cases of Devic’s neuromyelitis optica, associated with other indicators of humoral immunity. 33
CCR8 Expression on Phagocytic Macrophages Is Associated with Active Demyelination in MS Lesions
Quantitative immunohistochemistry was applied to establish the relationship between CCR8 expression and demyelinating activity. Within the 38 actively demyelinating lesions, 86 distinct areas of different demyelinating activity were identified: 36 EA areas, 25 LA areas, and 25 inactive (IA) areas (Table 1) ▶ . There were significantly more cells expressing CCR8 in areas of ongoing demyelination (EA and LA areas) than in normal appearing white matter in both pattern II and pattern III lesions (data not shown).
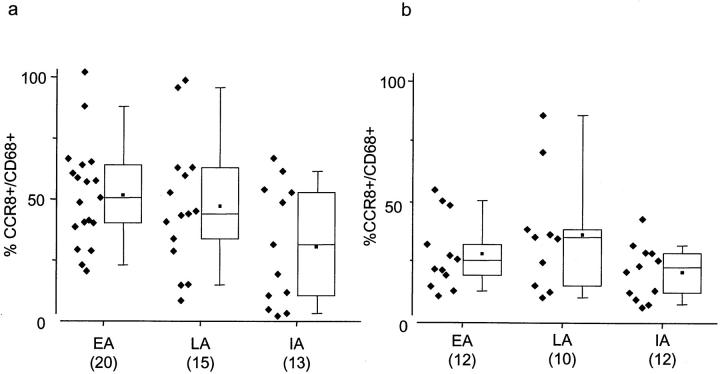
Because CCR8 was co-localized with CD68+ on cells in these tissues (Figure 2) ▶ , numbers of CCR8+ cells were normalized to the total CD68+ macrophage-lineage cells. In pattern II lesions, approximately half of all CD68+ cells in EA and LA areas expressed CCR8; EA, 52% (SEM = 6.7); LA, 47% (SEM = 9.4) with no significant differences between areas (Figure 4a) ▶ . Only 23% (SEM = 8.2) of CD68+ cells in IA areas of pattern II lesions expressed CCR8, which was significantly less (P = 0.01) than in EA areas (Figure 4a) ▶ . In pattern III lesions, CCR8 expression normalized for CD68+ cells, was less than in pattern II; EA, 31% (SEM = 4.7); LA, 37% (SEM = 7.8); IA, 20% (SEM = 3.0) [EA(II) versus EA(III); P = 0.001, Figure 4b ▶ ]. The total numbers of CD68+ cells in EA, IA, and LA zones of individual pattern II and III lesions were not significantly different (data not shown). 23
Figure 4.
a and b: Percentage of CD68+ cells that express CCR8 in EA, LA, and IA zones of demyelination in pattern II and III lesions. CD68 and CCR8 expressions were quantified in serial sections of 20 EA, 15 LA, and 13 IA regions in pattern II lesions (a) and 12 EA, 10 LA, and 12 IA regions in pattern III lesions (b). Shown are individual data points of percentages of CCR8/CD68+ cells/mm2 besides box-plots depicting median, 10th, 25th, 50th, and 90th percentiles. Means are shown in dots within box plots. CCR8 expression normalized for CD68 in EA region of pattern II is significantly greater than IA in pattern II lesions and all regions in pattern III lesions [EA(II) versus IA(II); P = 0.014, EA(II) versus EA(III); P = 0.001]. Normalized values for normal appearing white matter are not shown, as most regions did not contain significant numbers of CD68- or CCR8-positive cells.
CCR8 Is Expressed by Phagocytic Macrophages Associated with Cerebral Ischemia and Virus-Induced Demyelination
To characterize CCR8 expression in other neurological disorders, tissue sections from CNS inflammatory conditions such as Rasmussen’s encephalitis and neurosarcoidosis; from viral infections such as PML, herpes simplex virus encephalitis, and rabies encephalitis; from nonviral infectious conditions such as cerebral toxoplasmosis and CNS malaria; and from patients with ischemic stroke, senile changes of the Alzheimer’s type, chronic encephalitis of undetermined etiology, and cerebral lymphoma were analyzed (Table 2) ▶ .
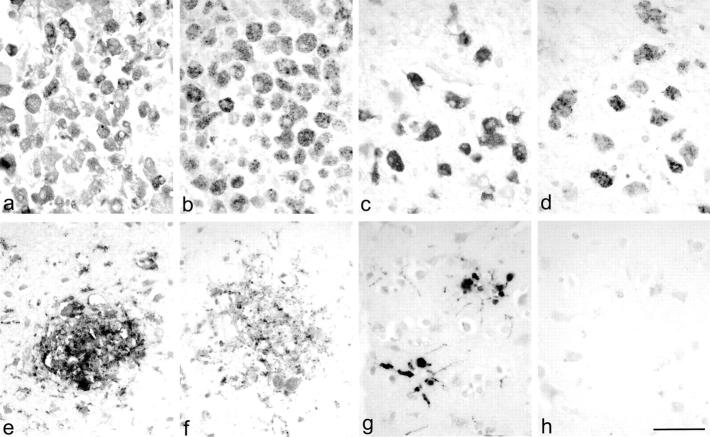
The most prominent CCR8 expression was observed in cases of ischemic stroke (Figure 5, a and b) ▶ and in one case of PML (Figure 5, c and d) ▶ . In both instances, CCR8 was expressed on the majority of macrophages within lesions (PML, 62% of CD68+ cells expressed CCR8; an average of 67% in ischemic stroke cases). CCR8 expression on activated microglia was also detected in nondemyelinated areas in PML.
Figure 5.
CCR8 expression in inflammatory CNS pathologies. CD68 (a, c, e, g) and CCR8 (b, d, f, h) immunohistochemistry was performed on serial sections of a case with ischemic stroke (a, b), PML (c, d), cerebral toxoplasmosis (e, f), and with senile changes of the Alzheimer’s type (g, h). CCR8 expression was observed on the majority of phagocytic macrophages in all cases with stroke (a, b) and in demyelinated lesions in PML (c, d). CCR8 expression on activated microglial cells was not observed in all cases with microglial activation. The majority of microglial cells within microglial nodules in cerebral toxoplasmosis expressed CCR8 (e, f). It was striking that even though microglial nodules were also observed in brain sections with senile changes of the Alzheimer’s type (g), no CCR8 expression could be detected (h). Scale bar, 50 μm.
CCR8 expression was observed on the majority of macrophages (55%) in one case with cerebral toxoplasmosis and infarction. In this case, CCR8 expression occurred most prominently in areas with signs of ischemic infarction because of a lymphomatous embolus and not in areas of acute necrosis associated with toxoplasma tachyzoites. CCR8 expression on microglial cells was observed in one case of cerebral lymphoma complicated by acute and subacute infarction. Interestingly, CCR8 expression in this case was limited to the area of ischemic infarction.
CCR8 Is Expressed by Activated Microglia in Some but Not All CNS Pathological Processes
In the remaining cases, CCR8 expression was inconsistently present, despite the presence of activated microglia. CCR8 expression was readily identified in the microglial nodules of cerebral toxoplasmosis, where 47% of microglia within nodules expressed CCR8 (Figure 5, e and f) ▶ . The dissociation between microglial activation and CCR8 expression was most striking in change of the Alzheimer’s type, in which diffuse, prominent microglial activation was observed, with clusters of such cells around senile plaques, but CCR8 expression was not detected (Figure 5, g and h) ▶ . Further, CCR8 expression was not detected in either Rasmussen’s encephalitis and neurosarcoidosis, despite a report of abundant activated CD68+ microglial cells in the former pathology. 34 In viral encephalitides, CCR8 expression by CD68-positive microglial cells was variable (Table 2) ▶ . CCR8 expression was not detected in three control brains.
In Vitro, CCR8 Expression Is Up-Regulated by Macrophage Differentiation and Activating Stimuli
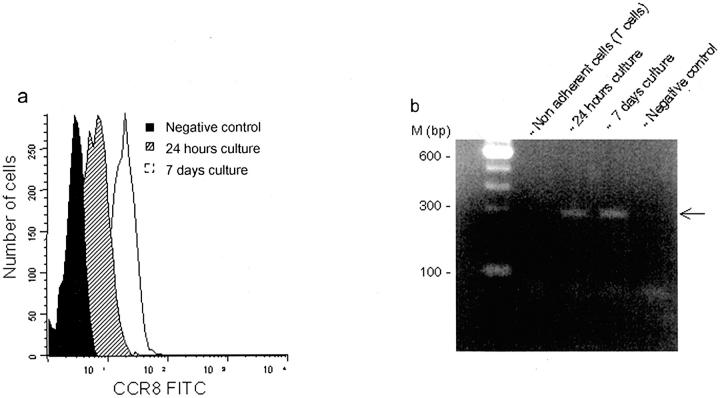
Using flow cytometry, the expression of CCR8 was analyzed on monocytes cultured for 24 hours and 7 days. The expression of CCR8 was detected on macrophages derived from monocytes after 24 hours of culture and the expression of CCR8 up-regulated after 7 days of culture (Figure 6a) ▶ . CD3+ T cells and CD14+ monocytes in preparations of freshly isolated peripheral blood mononuclear cells showed negligible levels of CCR8 expression (data not shown). The absence of CCR8 immunoreactivity in freshly isolated T cells was confirmed by the lack of CCR8 mRNA using reverse transcriptase (RT)-PCR, whereas CCR8 transcripts were detected in monocytes obtained after 24 hours and 7 days of culture (Figure 6b) ▶ .
Figure 6.
a and b: CCR8 expression during monocyte maturation in vitro and in response to monocyte-activating stimuli by flow cytometry and RT-PCR. Flow cytometry was performed on 24-hour monocytes after adherence and 7-day monocytes after culture from three controls. Histograms of CCR8 expression on cells at the two time points and the sample stained without the primary antibody as a negative control are shown (a). RT-PCR analysis confirmed the presence of a 297-bp band at 24 hour and 7-day time points (b), consistent with the findings from flow cytometry and immunohistochemistry. PCR buffer without cDNA was used as the negative control for PCR and secondary antibody without the primary for flow cytometry.
The expression of CCR8 during monocyte maturation in vitro and in response to monocyte-activating stimuli was further analyzed in monocytes obtained after 2 hours of adherence and after 24 hours stimulation with ZOP by quantitative fluorescence immunohistochemistry (Figure 7) ▶ . Monocytes cultured for 24 hours in the absence of any stimuli served as controls for the stimulation experiments. In other cultures, monocytes were allowed to differentiate into macrophages by maintenance for 7 days.
Figure 7.
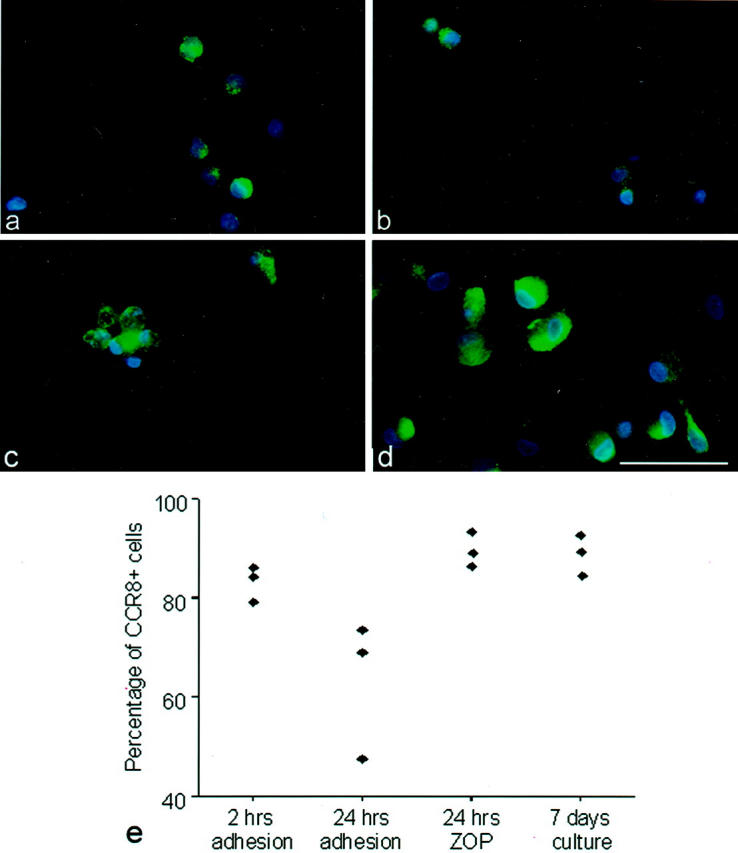
CCR8 expression during monocyte maturation in vitro and in response to monocyte-activating stimuli by immunofluorescence. Monocytes were obtained from three healthy donors by density centrifugation and adherence to serum-coated flasks. CCR8 expression was examined by quantitative immunofluorescence microscopy. CCR8 immunoreactivity is shown in green. Cells were counterstained with DAPI (blue). Depicted are monocytes after 2 hours of initial adherence (a), after 24 hours without any stimulation (b), and after 24 hours of stimulation with ZOP (c). Monocytes were allowed to further differentiate in culture to mature macrophages (d). Results of three individual experiments quantitating CCR8 expression are shown in the graph in e. Scale bar, 50 μm.
On average, 83% of monocytes obtained after 2 hours of adherence expressed CCR8 (Figure 7, a and e) ▶ . Without stimulation, CCR8 expression decreased to an average of 63% after 24 hours in culture (Figure 7, b and e) ▶ . In contrast, monocytes stimulated with ZOP for 24 hours (Figure 7c) ▶ maintained the expression of CCR8 (mean 90% positive cells; Figure 7e ▶ ). ZOP-stimulated monocytes displayed evidence of cell maturation with changed morphology, characterized by increased number of vesicles and formation of cell clusters (Figure 7c) ▶ . Mature macrophages derived from monocytes by 7 days of culture (without further stimulation) had become enlarged or spindle shaped with extended processes (Figure 7d) ▶ and 90% expressed CCR8 (Figure 7e) ▶ .
Discussion
We found CCR8 expression on microglial cells and phagocytic macrophages but not T cells in brain sections of patients with MS and other neurological disorders. The current study was undertaken to discriminate whether this receptor was expressed on Th2-polarized and T-regulatory lymphocytes or mononuclear phagocytes in human CNS inflammation. This investigation included all stages of active demyelination in MS, encompassing the two patterns (II and III) that together comprise about 90% of available material, as well as one atypical case, with pattern II demyelination but abundant eosinophilic infiltrates. Despite the presence in this atypical case of T cells immunoreactive for CCR3, another chemokine receptor associated with Th2 inflammatory reactions, we did not observe CCR8-positive T cells. Further, CCR8-positive T cells were not detected in CNS toxoplasmosis, a pathology in which type 2 helper cells might be implicated. Our current data also indicate that CCR8-positive T cells are essentially undetectable in the peripheral blood of human patients. Because negative results can be extrapolated only with caution, we tentatively interpret our inability to detect CCR8-positive T cells in CNS inflammatory pathologies to indicate that type 2 and regulatory lymphocytes, if present in CNS tissues, do not express CCR8.
In MS tissues, CCR8 expression by CD68+ macrophages was primarily associated with ongoing active demyelination. CCR8 expression decreased significantly during lesion evolution in pattern II and III. In IA areas of both pattern II and pattern III lesions, where myelin phagocytosis was completed, only a minority of CD68+ macrophages expressed CCR8 (Figure 4) ▶ . We concluded that macrophages expressed CCR8 only transiently during active myelin phagocytosis in MS lesions. In this regard, it is important to note that all lesions analyzed here were highly active as required for classification according to patterns of demyelination. Furthermore, in the majority of these cases, MS was fatal within months. Therefore the expression of CCR8 in the chronic lesions of MS remains to be defined.
CCR8 was also observed on microglial cells within MS lesions and in the periplaque and normal appearing white matter. Furthermore, significantly more cells expressed CCR8 in the periplaque white matter (PW) at the borders of active demyelinating lesions (PW active), when compared to PW IA and normal appearing white matter (data not shown). CCR8 expression was not observed on microglial cells in normal control brain sections. These data suggested that CCR8 expression on microglial cells in MS lesions is a reflection of specific microglial activation rather than an intrinsic property of microglial cells.
There are several lines of evidence that microglial cells in normal appearing and PW in MS brains are activated. 35-37 Immune-mediated inflammation is thought to play a critical role in the pathogenesis of pattern II lesions, whereas primary injury to the oligodendrocyte is proposed to initiate the pattern III lesion. 20 We found similar profiles of CCR8 expression in pattern II and pattern III lesions, and also found extensive CCR8 expression in cerebral ischemia and PML. These results indicate that varied pathways to destruction of neural tissue, and myelin in particular, can induce CCR8 expression on the resident and infiltrating mononuclear phagocytes.
In the current study, CCR8 expression was strikingly associated with phagocytic macrophages. CCR8+ macrophages were observed in all MS cases, in PML, and in all cases of stroke. Furthermore, we unexpectedly found CCR8-positive macrophages co-localized with foci of incidental ischemic pathology in two additional cases (one case each with primary CNS lymphoma and toxoplasmosis). Despite the common endpoint of CCR8 expression, molecular mechanisms that lead to generation of phagocytic macrophages in these cases are likely to differ. In MS, macrophages actively strip myelin from axons, although the factors that target the process to myelin internodes remain uncertain. PML is a primary viral infection of oligodendrocytes, which leads to oligodendrocyte cell death and demyelination. In PML, accumulation of phagocytes to remove myelin debris is presumed to be a secondary process. 38,39 Most leukocytes found in lesions of focal cerebral ischemia are macrophages, derived either from monocytes or microglia. 40 Despite different activation mechanisms and origins (ie, resident microglia versus hematogenous monocytes), most phagocytic macrophages in MS, PML, and stroke expressed CCR8.
Expression of chemokine receptors on the cell surface is a result of a complex interplay of regulatory mechanisms including cytokine-regulated transcription and ligand-induced internalization, occurring in the context of differentiation of receptor-bearing cells. We previously reported that other chemokine receptors, CCR1 and CCR5, are differentially regulated during monocyte maturation in vitro. 23 Here, we report that the expression of CCR8 on monocytes in vitro is dependent on the activation state of the cells. After 2 hours of adherence to serum-coated flasks, 83% of cultured monocytes expressed CCR8. Without further stimulation CCR8 expression decreased during initial culture (63% after 24 hours), whereas stimulation with ZOP or during 24 hours of culture maintained CCR8 expression. The presence of increased CCR8 immunoreactivity was confirmed by the detection of increased mRNA.
Various types of injury to the CNS, such as infection, trauma, autoimmune inflammation, and neurodegeneration are known to elicit microglial activation. 41 Regardless of the nature of damage inflicted on the CNS, microglial activation is generally associated with a change in morphology into an amoeboid appearance with shortened cytoplasmic processes and a rounded cell body accompanied by increased expression of genes involved in immune reactions. It has become clear that microglia may display different activity states and have different functional properties under different pathological conditions. 42 For example, microglial cells play an important role in the defense against toxoplasma infection. Because cytokines secreted by human microglial prevent the intracellular entry of Toxoplasma gondii. 43 Our detection of CCR8 expression in cerebral toxoplasmosis is consistent with this function and supports the importance of CCR8 in defense against protozoal pathogens, recently demonstrated in CCR8−/−. 19
A different, detrimental role of microglia has been suggested in degenerative CNS diseases such as Alzheimer’s dementia. It is hypothesized that β-amyloid precursor protein can directly activate microglia cells and enhance their neurotoxic capabilities. 41 Unexpectedly, we did not detect CCR8 in brain sections with changes of the Alzheimer’s type. This observation is provocative, in view of the recent proposal that microglial activation may be deficient, rather than excessive, in Alzheimer’s dementia. 44
In summary, we report that CCR8 expression in the human CNS is limited to cells of the macrophage lineage and is found in actively demyelinating MS lesions, in PML, and in cerebral ischemia. CCR8 expression was furthermore observed on activated microglial cells in some, but not all cases with diffuse microglial activation. We conclude that CCR8 expression is strongly associated with phagocytic properties of monocytes and activated microglia cells and that CCR8 identifies a subset of specifically activated microglia cells in different CNS pathologies. These interpretations are limited, however, by insufficient knowledge concerning distribution of CCL1 in human CNS disorders, because of lack of suitable reagents for its detection. CCR8 was not found on T cells, making it unlikely that CNS entry by polarized type 2 lymphocytes or CD4+/CD25+ T cells is mediated by CCR8. Taken in the context of reduced EAE severity in CCR8−/− mice, these findings suggest that CCR8 may be considered a target for therapeutic intervention in CNS diseases in which phagocytic macrophages are deemed pathogenic.
Acknowledgments
We thank the Nancy Davis Center Without Walls for providing support and the Cleveland MS Women’s Committee for a morphometric image analysis station.
Note Added in Proof: Sedgewick J: Interactions between hemotopoietically-derived TNF and central nervous system resident glial chemokines underlies initiation of autoimmune inflammation in the brain. J Immunol, in press.
Footnotes
Address reprint requests to Richard M. Ransohoff, M.D., Department of Neurosciences, Mail Code NC30, The Lerner Research Institute, The Cleveland Clinic Foundation, 9500 Euclid Ave., Cleveland, OH 44195. E-mail: ransohr@ccf.org.
Supported by National Institutes of Health (PO1NS38667 to R. M. R.) and Bundesministerium für Bildung, Wissenschaft, und Kultur, Austria (GZ 70.056/2-Pr/4/99 to H. L.).
Current address of K. D. A.: MD Anderson Cancer Center, University of Texas, Houston, TX.
References
- 1.Luster AD: Chemokines—chemotactic cytokines that mediate inflammation. N Engl J Med 1998, 338:436-445 [DOI] [PubMed] [Google Scholar]
- 2.Rollins BJ: Chemokines. Blood 1997, 90:909-928 [PubMed] [Google Scholar]
- 3.Asensio VC, Campbell IL: Chemokines in the CNS: plurifunctional mediators in diverse states. Trends Neurosci 1999, 22:504-512 [DOI] [PubMed] [Google Scholar]
- 4.Glabinski AR, Ransohoff RM: Chemokines and chemokine receptors in CNS pathology. J Neurovirol 1999, 5:3-12 [DOI] [PubMed] [Google Scholar]
- 5.Mennicken F, Maki R, de Souza EB, Quirion R: Chemokines and chemokine receptors in the CNS: a possible role in neuroinflammation and patterning. Trends Pharmacol Sci 1999, 20:73-78 [DOI] [PubMed] [Google Scholar]
- 6.Trebst C, Ransohoff RM: Investigating chemokines and chemokine receptors in patients with multiple sclerosis: opportunities and challenges. Arch Neurol 2001, 58:1975-1980 [DOI] [PubMed] [Google Scholar]
- 7.Inngjerdingen M, Damaj B, Maghazachi AA: Expression and regulation of chemokine receptors in human natural killer cells. Blood 2001, 97:367-375 [DOI] [PubMed] [Google Scholar]
- 8.Murphy PM, Baggiolini M, Charo IF, Hebert CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ, Power CA: International union of pharmacology. XXII Nomenclature for chemokine receptors. Pharmacol Rev 2000, 52:145-176 [PubMed] [Google Scholar]
- 9.Napolitano M, Zingoni A, Bernardini G, Spinetti G, Nista A, Storlazzi CT, Rocchi M, Santoni A: Molecular cloning of TER1, a chemokine receptor-like gene expressed by lymphoid tissues. J Immunol 1996, 157:2759-2763 [PubMed] [Google Scholar]
- 10.Tiffany HL, Lautens LL, Gao JL, Pease J, Locati M, Combadiere C, Modi W, Bonner TI, Murphy PM: Identification of CCR8: a human monocyte and thymus receptor for the CC chemokine I-309. J Exp Med 1997, 186:165-170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zingoni A, Soto H, Hedrick JA, Stoppacciaro A, Storlazzi CT, Sinigaglia F, D’Ambrosio D, O’Garra A, Robinson D, Rocchi M, Santoni A, Zlotnik A, Napolitano M: The chemokine receptor CCR8 is preferentially expressed in Th2 but not Th1 cells. J Immunol 1998, 161:547-551 [PubMed] [Google Scholar]
- 12.Iellem A, Mariani M, Lang R, Recalde H, Panina-Bordignon P, Sinigaglia F, D’Ambrosio D: Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J Exp Med 2001, 194:847-853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goya I, Gutierrez J, Varona R, Kremer L, Zaballos A, Marquez G: Identification of CCR8 as the specific receptor for the human beta-chemokine I-309: cloning and molecular characterization of murine CCR8 as the receptor for TCA-3. J Immunol 1998, 160:1975-1981 [PubMed] [Google Scholar]
- 14.Roos RS, Loetscher M, Legler DF, Clark-Lewis I, Baggiolini M, Moser B: Identification of CCR8, the receptor for the human CC chemokine I-309. J Biol Chem 1997, 272:17251-17254 [DOI] [PubMed] [Google Scholar]
- 15.Kuchroo VK, Martin CA, Greer JM, Ju ST, Sobel RA, Dorf ME: Cytokines and adhesion molecules contribute to the ability of myelin proteolipid protein-specific T cell clones to mediate experimental allergic encephalomyelitis. J Immunol 1993, 151:4371-4382 [PubMed] [Google Scholar]
- 16.Teuscher C, Butterfield RJ, Ma RZ, Zachary JF, Doerge RW, Blankenhorn EP: Sequence polymorphisms in the chemokines Scya1 (TCA-3), Scya2 (monocyte chemoattractant protein (MCP)-1), and Scya12 (MCP-5) are candidates for eae7, a locus controlling susceptibility to monophasic remitting/nonrelapsing experimental allergic encephalomyelitis. J Immunol 1999, 163:2262-2266 [PubMed] [Google Scholar]
- 17.Fischer FR, Santambrogio L, Luo Y, Berman MA, Hancock WW, Dorf ME: Modulation of experimental autoimmune encephalomyelitis: effect of altered peptide ligand on chemokine and chemokine receptor expression. J Neuroimmunol 2000, 110:195-208 [DOI] [PubMed] [Google Scholar]
- 18.Godiska R, Chantry D, Dietsch GN, Gray PW: Chemokine expression in murine experimental allergic encephalomyelitis. J Neuroimmunol 1995, 58:167-176 [DOI] [PubMed] [Google Scholar]
- 19.Chensue SW, Lukacs NW, Yang TY, Shang X, Frait KA, Kunkel SL, Kung T, Wiekowski MT, Hedrick JA, Cook DN, Zingoni A, Narula SK, Zlotnik A, Barrat FJ, O’Garra A, Napolitano M, Lira SA: Aberrant in vivo T helper type 2 cell response and impaired eosinophil recruitment in CC chemokine receptor 8 knockout mice. J Exp Med 2001, 193:573-584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H: Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol 2000, 47:707-717 [DOI] [PubMed] [Google Scholar]
- 21.Bruck W, Porada P, Poser S, Rieckmann P, Hanefeld F, Kretzschmar HA, Lassmann H: Monocyte/macrophage differentiation in early multiple sclerosis lesions. Ann Neurol 1995, 38:788-796 [DOI] [PubMed] [Google Scholar]
- 22.Sorensen TL, Tani M, Jensen J, Pierce V, Lucchinetti C, Folcik VA, Qin S, Rottman J, Sellebjerg F, Strieter RM, Frederiksen JL, Ransohoff RM: Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J Clin Invest 1999, 103:807-815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trebst C, Sorensen TL, Kivisakk P, Cathcart MK, Hesselgesser J, Horuk R, Sellebjerg F, Lassmann H, Ransohoff RM: CCR1+/CCR5+ mononuclear phagocytes accumulate in the central nervous system of patients with multiple sclerosis. Am J Pathol 2001, 159:1701-1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horuk R, Hesselgesser J, Zhou Y, Faulds D, Halks-Miller M, Harvey S, Taub D, Samson M, Parmentier M, Rucker J, Doranz BJ, Doms RW: The CC chemokine I-309 inhibits CCR8-dependent infection by diverse HIV-1 strains. J Biol Chem 1998, 273:386-391 [DOI] [PubMed] [Google Scholar]
- 25.Kumagai K, Itoh K, Hinuma S, Tada M: Pretreatment of plastic Petri dishes with fetal calf serum. A simple method for macrophage isolation. J Immunol Methods 1979, 29:17-25 [DOI] [PubMed] [Google Scholar]
- 26.Hariharan D, Douglas SD, Lee B, Lai JP, Campbell DE, Ho WZ: Interferon-gamma upregulates CCR5 expression in cord and adult blood mononuclear phagocytes. Blood 1999, 93:1137-1144 [PubMed] [Google Scholar]
- 27.Tuttle DL, Harrison JK, Anders C, Sleasman JW, Goodenow MM: Expression of CCR5 increases during monocyte differentiation and directly mediates macrophage susceptibility to infection by human immunodeficiency virus type 1. J Virol 1998, 72:4962-4969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson R: Secretion of superoxide anion. Adams D eds. Methods of Studying Mononuclear Phagocytes. 1981:pp 489-497 Academic Press, Boston
- 29.Hesselgesser J, Halks-Miller M, DelVecchio V, Peiper SC, Hoxie J, Kolson DL, Taub D, Horuk R: CD4-independent association between HIV-1 gp120 and CXCR4: functional chemokine receptors are expressed in human neurons. Curr Biol 1997, 7:112-121 [DOI] [PubMed] [Google Scholar]
- 30.Baranzini SE, Elfstrom C, Chang SY, Butunoi C, Murray R, Higuchi R, Oksenberg JR: Transcriptional analysis of multiple sclerosis brain lesions reveals a complex pattern of cytokine expression. J Immunol 2000, 165:6576-6582 [DOI] [PubMed] [Google Scholar]
- 31.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A: Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med 1998, 187:875-883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerber BO, Zanni MP, Uguccioni M, Loetscher M, Mackay CR, Pichler WJ, Yawalkar N, Baggiolini M, Moser B: Functional expression of the eotaxin receptor CCR3 in T lymphocytes co-localizing with eosinophils. Curr Biol 1997, 7:836-843 [DOI] [PubMed] [Google Scholar]
- 33.Lucchinetti CF, Mandler RN, McGavern D, Bruck W, Gleich G, Ransohoff RM, Trebst C, Weinshenker B, Wingerchuk D, Parisi JE, Lassmann H: A role for humoral mechanisms in the pathogenesis of Devic’s neuromyelitis optica. Brain 2002, 125:1450-1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bien CG, Bauer J, Deckwerth TL, Wiendl H, Deckert M, Wiestler OD, Schramm J, Elger CE, Lassmann H: Destruction of neurons by cytotoxic T cells: a new pathogenic mechanism in Rasmussen’s encephalitis. Ann Neurol 2002, 51:311-318 [DOI] [PubMed] [Google Scholar]
- 35.Ciccarelli O, Werring DJ, Wheeler-Kingshott CA, Barker GJ, Parker GJ, Thompson AJ, Miller DH: Investigation of MS normal-appearing brain using diffusion tensor MRI with clinical correlations. Neurology 2001, 56:926-933 [DOI] [PubMed] [Google Scholar]
- 36.De Groot CJ, Bergers E, Kamphorst W, Ravid R, Polman CH, Barkhof F, van der Valk P: Post-mortem MRI-guided sampling of multiple sclerosis brain lesions: increased yield of active demyelinating and (p)reactive lesions. Brain 2001, 124:1635-1645 [DOI] [PubMed] [Google Scholar]
- 37.Kapeller P, McLean MA, Griffin CM, Chard D, Parker GJ, Barker GJ, Thompson AJ, Miller DH: Preliminary evidence for neuronal damage in cortical grey matter and normal appearing white matter in short duration relapsing-remitting multiple sclerosis: a quantitative MR spectroscopic imaging study. J Neurol 2001, 248:131-138 [DOI] [PubMed] [Google Scholar]
- 38.Hou J, Major EO: Progressive multifocal leukoencephalopathy: JC virus induced demyelination in the immune compromised host. J Neurovirol 2000, 6(Suppl 2):S98-S100 [PubMed] [Google Scholar]
- 39.Wasmuth JC, Wasmuth-Pietzuch A, Spengler U, Rockstroh JK: Progressive multifocal leukoencephalopathy. Med Klin 1999, 94:264-273 [DOI] [PubMed] [Google Scholar]
- 40.Stoll G, Jander S, Schroeter M: Inflammation and glial responses in ischemic brain lesions. Prog Neurobiol 1998, 56:149-171 [DOI] [PubMed] [Google Scholar]
- 41.Stoll G, Jander S: The role of microglia and macrophages in the pathophysiology of the CNS. Prog Neurobiol 1999, 58:233-247 [DOI] [PubMed] [Google Scholar]
- 42.Slobodov U, Reichert F, Mirski R, Rotshenker S: Distinct inflammatory stimuli induce different patterns of myelin phagocytosis and degradation in recruited macrophages. Exp Neurol 2001, 167:401-409 [DOI] [PubMed] [Google Scholar]
- 43.Chao CC, Gekker G, Hu S, Peterson PK: Human microglial cell defense against Toxoplasma gondii. The role of cytokines. J Immunol 1994, 152:1246-1252 [PubMed] [Google Scholar]
- 44.Streit WJ, Conde JR, Harrison JK: Chemokines and Alzheimer’s disease. Neurobiol Aging 2001, 22:909-913 [DOI] [PubMed] [Google Scholar]