Abstract
The high rate of prostate cancer mortality invariably reflects the inability to control the spread of the disease. The urokinase-type plasminogen activator and its receptor (u-PAR) contribute to prostate cancer metastases by promoting extracellular matrix degradation and growth factor activation. The current study was undertaken to determine the efficacy of a urokinase-derived peptide (Å6) in reducing the lymph node metastases of prostate cancer using a model in which prostatic tumors established in nude mice from orthotopically implanted PC-3 LN4 prostate cancer cells disseminate to the lymph nodes. As a first step in evaluating the in vivo effectiveness of Å6, we determined its effect on in vitro invasiveness. In vitro, Å6 reduced the invasiveness of PC-3 LN4 cells through a Matrigel-coated filter without affecting growth rate. A first in vivo survival experiment showed that all Å6-treated mice were alive after 57 days, and half of them tumor-free, whereas all control mice receiving vehicle had died. In a second experiment with a larger tumor inoculum and a longer delay until treatment, whereas 71% of control mice and 83% of mice treated with a scrambled peptide developed lymph node metastases, only 22 to 25% of Å6-treated mice had positive lymph nodes. Further, lymph node volume, reflective of tumor burden at the secondary site, was diminished 70% in Å6-treated mice. In conclusion, we provide definitive evidence that a peptide spanning the connecting region of urokinase suppresses metastases and, as a single modality, prolongs the life span of prostate tumor-bearing mice.
Prostate cancer afflicts 209,000 American men every year and is second only to lung cancer as the leading cause of cancer deaths in the male population. The high rate of mortality invariably reflects spread of this disease to the secondary sites and consequently, effective treatments in the future will require a means of combating prostate cancer dissemination.
It is well established that the spread of virtually all malignancies require the expression of one or more proteases which serve to cleave extracellular matrix and activate growth factors. 1 The urokinase-type plasminogen activator 2 contributes to tumor progression by converting plasminogen into plasmin a widely acting serine protease that cleaves several basement membrane components including laminin and fibronectin 3 as well as type IV collagen indirectly via activation of metalloproteinases. 4 Urokinase, achieves this by binding to a cell surface receptor (u-PAR) 5,6 which increases the efficiency by which plasminogen is converted into plasmin. 7 Further, urokinase cleaves u-PAR thereby promoting chemotaxis. 8,9
There is currently strong evidence implicating the urokinase-u-PAR axis in prostate cancer progression. For example, the urokinase gene is amplified in some hormone-refractory prostate cancers 10 and overexpression of this protease increases skeletal metastases of this malignancy. 11 Additionally, in two separate studies, 12,13 the expression of an exogenous plasmid encoding urokinase lacking an enzyme active site, prevented metastases of human (PC-3) and murine (MAT-LyLu) prostate cancers. Further, independent studies by Festuccia et al 14 and Hollas et al 15 reported that urokinase-u-PAR complexes characterized the invasive phenotype of cultured prostate cancer cells and that antibodies that prevented this interaction blocked in vitro invasion. Moreover, high u-PAR levels in the serum is predictive of metastatic prostate cancer and shortened survival time. 16 Taken together, these reports would suggest that the urokinase-u-PAR axis represents a therapeutic target for controlling prostate cancer metastases. We therefore determined the potential of a urokinase-derived peptide (Ac-KPSSPPEE-amide, hereafter referred to as Å6) spanning amino acids 136–143 to counter the metastases of orthotopically grown prostate cancer. This peptide, which non-competitively blocks the interaction of urokinase with soluble u-PAR in vitro, 17 has proven efficacious in reducing glioblastoma growth and angiogenesis. 18
Materials and Methods
Cell Culture
Establishment of the highly metastatic PC-3 LN4 cell line has been described elsewhere. 19 Cells were grown in DMEM/F12 culture medium containing 10% FBS.
Western Blotting for u-PAR
Western blotting for u-PAR was performed as described previously. 20 Briefly, cells were extracted into a Triton X-100-containing buffer supplemented with protease inhibitors. Insoluble material was removed by centrifugation and the cell extract immunoprecipitated with a polyclonal anti-u-PAR antibody. The immunoprecipitated material was then subjected to standard Western blotting and the blot probed with 5 μg/ml of an anti-u-PAR monoclonal antibody (no. 3931, American Diagnostica, Greenwich, CT) and an HRP-conjugated goat anti-mouse IgG. Bands were visualized by enhanced chemiluminescence (Amersham, Arlington Heights, IL).
Northern Blotting
The level of steady state mRNAs was determined by Northern analysis. 20 Total cellular RNA was extracted from 90% confluent cultures using 5.0 mol/L guanidinium isothiocyanate and purified on a cesium chloride cushion (5.7 mol/L) by centrifugation at 150,000 × g for 20 hours. Purified RNA was electrophoresed in a 1.5% agarose-formaldehyde gel and transferred to Nytran-modified nylon by capillary action using 10X SSC. The Northern blot was probed at 42°C with a random primed radiolabeled u-PAR cDNA which starts at the transcription start site and extends 0.65 kb downstream. The blots were then washed at 65°C using 0.25X SSC in the presence of 0.75% SDS.
In Vitro Invasion Assays
These were performed as described by this laboratory previously, 21 but with modifications. Briefly, cells are dispersed with 4 mmol/L EDTA and 250,000 cells dispensed into a BD BioCoat Falcon cell culture chamber (BD Biosciences Bedford, MA). The chamber was subsequently inserted into the outer well, the latter also containing culture medium. The cells were incubated at 37°C for 2 days after which the cells on the upper aspect of the filter were removed with a cotton swab and cells on the lower aspect stained using the DifQuik kit. Invaded cells were enumerated.
Orthotopic Model to Assess Prolongation of Life Span
Nu/Nu mice (8–12 weeks of age) were injected with 2 × 105 PC-3 LN4 cells/50 μl in Ca2+, Mg2+-free HBSS into the prostate as described previously. 19 After 3 days to allow for tumor establishment, mice were injected every 12 hours with either vehicle (PBS) or Å6. Each Å6 injection contained 37.5 mg/kg such that the daily dose was 75 mg/kg.
Orthotopic Model for Measuring in Vivo Metastases
These were carried out as above, except that the tumor inoculum was 5 × 105 PC-3 LN4 cells. After 7 days, when primary tumors were palpable, Å6 or Å16 was administered i.p. twice daily at 25 or 75 mg/kg/day body weight. In the male mouse, the prostate is located in the bladder neck (outlet), allowing for easy palpation of an enlarged prostatic mass from the outside. 22 The prostate mass is round, fixed and hard. At the end of the experiment, mice were sacrificed and prostate and lymph nodes examined both macroscopically and microscopically for the presence of tumor cells. For histological examination, tissues were stained with hematoxylin and eosin.
Statistics
Statistical analysis was performed using the Instat (version 3.05) and Prism statistical software (GraphPad, San Diego, CA). The Mann-Whitney non-parametric test (two-tailed) was used to test for statistical significant differences between Å6-treated and untreated cells in the in vitro invasion assays as well as reductions in lymph node volumes in the in vivo model. Survival curves were tested for statistically significant differences using the log rank test.
Results
Elevated u-PAR Expression in Locally Advanced Prostatic Cancer
Previous studies with prostate cancer have strongly suggested a role for the urokinase-u-PAR axis in the progression of this disease. Therefore, u-PAR protein levels were compared in resected prostate cancer derived from patients with organ confined or locally advanced (demonstrating seminal vesicle involvement) disease. All tumors were resected at the M.D. Anderson Cancer Center. Three tumors had bilateral extracapsular tumor extension without regional lymph node metastases (pT3bN0) while two other tumors were palpable but confined to the prostate (pT2N0). While u-PAR protein was abundant in tumor extracts from three patients with locally advanced disease (Figure 1A) ▶ , the amount of this protein derived from patients with organ-confined disease was at, or below, the detection limit of Western blotting. These results are consistent with previous studies demonstrating that the urokinase/u-PAR axis contributes to prostate tumor progression, and that inhibition of this proteolytic axis might limit this process.
Figure 1.
u-PAR expression is increased in locally advanced prostate cancer. A: Equal protein (500 μg) from resected prostate tumor extracts was immunoprecipitated with an anti-u-PAR antibody and analyzed for u-PAR protein by Western blotting. The residual supernatant from the immunoprecipitated was immunoblotted for actin. B: PC-3 LN4 cells were analyzed for u-PAR protein (left panel) or for mRNA (right panel). Western blotting was as described for panel A with the exception that the amount of protein immunoprecipitated was varied. For the Northern blotting, total RNA (20 μg) from PC-3 LN4 cells was subjected to Northern blotting for u-PAR mRNA.
Reduction of in Vitro PC-3 LN4 Invasion by Å6
We first determined the ability of Å6 to diminish in vitro invasiveness of PC-3 LN4 cells. These cells express both u-PAR as evidenced by both Northern and Western blotting (Figure 1B) ▶ and urokinase as shown previously. 14,15 Cells were plated on Matrigel-coated porous filters and after 6 hours to allow for cell attachment, Å6 added at varying concentrations shown previously to reduce in vitro breast cancer invasiveness. 17 In the absence of the peptide, PC-3 LN4 showed a pronounced invasiveness through the extracellular matrix-coated barrier (Figure 2A) ▶ . However, addition of the urokinase-derived peptide resulted in a dose-dependent decrease in the number of cells traversing the Matrigel-coated filter (Figure 2A) ▶ . At 50 μmol/L, Å6 caused about a 60% diminution in in vitro invasion by PC-3 LN4 cells. This difference was statistically significant (P < 0.05). The reduced invasiveness was not due to a diminished cell proliferation (Figure 2B) ▶ . The diminished invasion of PC-3 LN4 cells was less impressive than previously published for the urokinase inhibitor amiloride. 10 It may be that the greater effect with amiloride reflects its multiple mechanisms of action. Indeed, in addition to inhibiting urokinase activity, 23 amiloride also reduces mRNA levels for this plasminogen activator and its receptor. 24,25
Figure 2.
Å6 reduces in vitro invasiveness of PC-3 LN4 cells. A: PC-3 LN4 (2.5 × 105 cells) were plated on Matrigel-coated porous filters. After 6 hours to allow for cell attachment, Å6, was added at the indicated concentration. Two days later, the cells on the upper aspect of the filter were removed and invaded cells stained, photographed and then enumerated. Data are presented as mean ± SD of three independent assays. B: Growth curve of PC-3 LN4 grown in the presence, or absence, of 50 μmol/L Å6.
Å6 Prolongs the Survival Time of PC-3 LN4-Bearing Mice
To assess the efficacy of Å6 in prolonging life span, we used an in vivo model in which 2 × 105 highly metastatic PC-3 LN4 cells are injected orthotopically into the prostate. After 3 days to allow for tumor establishment, mice were injected every 12 hours with either vehicle (PBS) or Å6 (75 mg/kg/day). After 38 days, all of the mice in the control group had palpable prostatic tumors whereas none of the mice in the Å6-treated group had palpable tumors. The severe cachexia demonstrated by the control group, but not by the Å6-treated mice, is apparent at day 50 (Figure 3A) ▶ . The control mice were sacrificed when moribund (Figure 3B) ▶ to keep the study within IACUC guidelines. While there were 2 deaths in the Å6-treated mice after 59 days (Figure 3B) ▶ , these deaths were not due to tumor, since at autopsy, both the prostate and lymph nodes were observed to be tumor-free. Deaths in these Å6-treated mice were due to systemic infection originating at the injection site as a consequence of inadequate sterile technique compounded by the frequent injection schedule. The surviving Å6-treated mice were sacrificed at day 80 and autopsied. Of these, 4 of 10 mice were deemed to be tumor-free. Of the 6 mice that developed primary tumors, only 2 showed lymph nodes positive for disease. It is noteworthy that the reduced incidence (50%) of primary tumor development in the Å6-treated mice compares with 100% tumor development in mice receiving the vehicle alone. Log rank analysis indicated that the prolonged survival of the Å6-treated mice compared with the controls was statistically significant (P < 0.0001).
Figure 3.
Untreated, but not Å6-treated, PC-3 LN4-bearing mice show severe cachexia and decreased survival. A: Pictures of mice bearing orthotopically grown PC-3 LN4 tumors after daily treatment for 50 days with carrier (PBS-control) or 75 mg/kg Å6. B: Mice were orthotopically injected with 2 × 105 cells. When primary tumors were palpable, mice were divided into two groups with one group receiving PBS alone and the other administered with 75 mg/kg Å6 on a daily basis. Mice were sacrificed when moribund as determined by standard IACUC criteria. Differences in the survival rates were tested for statistical significance using the log rank test.
Å6 Attenuates the Metastasis of Prostate Cancer in an Orthotopic Model
Since Å6 effectively prevented the establishment of primary prostatic tumors in the model described above, it was not possible to assess the drug’s activity against their metastasis. Therefore, a second study was conducted using the above model, but, with a larger tumor inoculum and withholding treatment for 7 days until the primary tumor mass was definitely palpable. Inoculation of 5 × 105 PC-3 LN4 cells, with no treatment, resulted in the formation of an enlarged prostatic tumor mass at the primary site (Figure 4, A and B) ▶ at a rate of between 85 and 100% after 6 weeks. The majority of these mice (71%) demonstrated spread of the disease to the regional lymph nodes as evidenced both macroscopically (Figure 4A) ▶ and microscopically (Figure 4C) ▶ . In the treated group, mice were injected twice daily with 25 or 75 mg/kg/day of Å6 or as a control, Å16, a peptide comprised of the scrambled Å6 amino acid sequence (Ac-PSESPEKP-NH2). After 6 weeks, mice were sacrificed and tumor weights and lymph node involvement determined. Å6 treatment had little effect on primary tumor mass (Figure 5, A and C) ▶ . However, Å6, but not the control peptide Å16, reduced the percentage of mice with tumor cell-positive lymph nodes from 71 to as low as 22% (Figure 5, B and C) ▶ . Further, while Å6 diminished lymph node volume (Figure 5, A–C) ▶ by up to 70% (P = 0.004), the control peptide, Å16, had no effect on this parameter.
Figure 4.

Extra-prostatic spread of orthotopically grown PC-3 LN4 to the lymph nodes. PC-3 LN4 cells (5 × 105) were injected into the prostate of male nu/nu mice. After 6 weeks, mice were sacrificed and prostate and lymph node tissues fixed and stained for histological examination. A depicts the prostate tumor mass and enlarged lymph nodes while histological evidence of tumor cells in the prostatic mass are illustrated in B. The presence of tumor cells in the enlarged lymph nodes is apparent by histology (C). These data confirm the spread of the orthotopically implanted prostate cancer cells to the lymph nodes.
Figure 5.
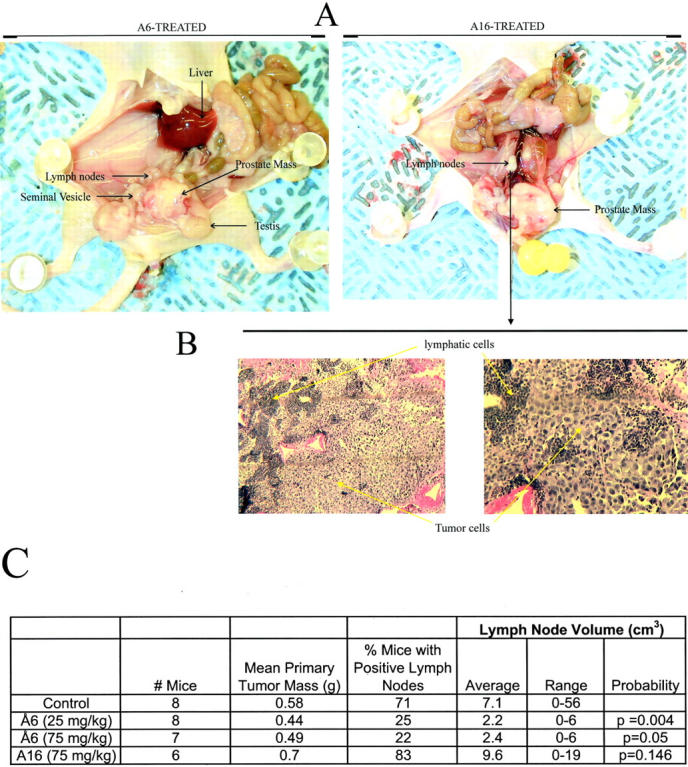
Å6 reduces the lymph node-spread of orthotopically grown PC-3 LN4 cells. A, B: PC-3 LN4 cells (5 × 105 cells) were injected into the prostates of nu/nu mice. When primary tumors were established, as determined by palpation, mice were injected twice daily with the indicated doses of Å6 or Å16. After 6 weeks, mice were sacrificed, and examined macroscopically and microscopically for spread of the prostate cancer to the lymph nodes. C: Statistical analysis of the effect of Å6 and Å16 on the spread of PC-3 LN4 to the lymph nodes. Probability levels are given with respect to the control.
Discussion
Effective therapy of prostate cancer is hampered by the lack of suitable agents for controlling the spread of the disease. Indeed the poor prognosis of those patients who present with metastatic disease compared with those individuals with organ-confined prostate cancer (81% and 25% disease-free at 10 years, respectively) indicate the urgent need for strategies to combat the metastatic disease. We demonstrate, herein, the efficacy of Å6, a urokinase-derived peptide previously shown to interfere with the urokinase-u-PAR system, 17 in reducing the metastatic spread of orthotopically grown prostate cancer and prolonging the life span of prostate cancer-bearing mice.
Å6 has previously been shown to have anti-tumor effects on other experimental cancers including mammary adenocarcinoma 17,26 and glioblastoma. 18 However, while the previous study of Guo and co-workers 17 indicated an anti-metastatic effect of Å6, since the primary tumor mass was reduced in both cases, it was difficult to determine whether reduced metastases was a direct effect of the agent on the metastatic process or secondary to a smaller primary tumor size. Our study was intended to resolve this issue and determine whether Å6 has an anti-metastatic effect. In our first experiment with the PC-3 LN4 highly metastatic orthotopic prostatic carcinoma model that used treatment 3 days after a tumor inoculum of 2 × 105 cells, we fully expected the primary tumor to establish itself and metastasize despite treatment with Å6. It was therefore a surprise to note that the treated animals fared very well compared to the untreated controls, and we therefore extended the duration of treatment of 10 mice to 80 days. It is noteworthy that 4 of these 10 mice were tumor-free on autopsy. Of the 6 mice that did have a primary tumor, only 2 had lymph node metastases. This result was in pronounced contrast to the result from the untreated control animals, all of which succumbed to metastatic disease and exhibited severe cachexia before their sacrifice. These findings are consistent with other studies demonstrating an attenuating effect of Å6 on primary tumor volume. 18,26 In a second experiment, we therefore made a determined effort to establish the primary tumor before start of treatment. This was achieved with a larger tumor inoculum (5 × 105 cells) and waiting until the primary tumor was clearly palpable; this took 7 days. Å6 treatment started on day 8, continued for 6 weeks, and resulted in little effect on the primary tumor mass relative to controls but a marked effect on lymph node metastasis.
Thus, our findings are the first to show that Å6 administered alone effectively prolongs the survival of tumor-bearing mice, in this case orthotopically grown prostate cancer. We can only speculate as to how Å6 prolongs survival of the tumor-bearing mice. Certainly, in the clinical situation in humans, tumor progression is generally associated with protein wasting (negative nitrogen balance) and reduced food intake, all events that are self-reinforcing, thereby hastening the demise of the individual. Additionally, renal failure and bilateral ureter obstruction from lymph node enlargement and hepatic failure from spread of the tumor cells to the liver may also contribute to the increased morbidity of the control animals.
Our results contrast with a study 18 where it was reported that Å6 combined with cisplatin, but not as a single modality, increased survival of glioblastoma-bearing mice. Second, our findings demonstrate that in the second, more difficult to treat, model the effect of inhibiting the urokinase-u-PAR axis is entirely on the development of lymph node metastases and not growth of tumor at the primary site. A third, broad, conclusion that can be drawn by comparison of the two experiments is that earlier treatment with Å6 on smaller PC-3 LN4 tumors was much more effective than later treatment on larger tumors. This may have implications for how Å6 may best be used in cancer patients.
At the present time, there are few studies to identify candidate metastases-suppressing drugs. One exception is a previous report by Rabbani and colleagues, 27 who demonstrated the ability of the nucleoside analogue β-l-(−)dioxolane-cytidine to reduce the incidence and spread of the highly metastatic Dunning R3227 Mat Ly Lu cells to the adrenals. However, as in previous studies with Å6, it was not clear whether the benefit of this agent was due to its effect on the metastatic process or secondary to the marked decrease in tumor volume.
Considering the role of the urokinase-u-PAR axis in promoting extracellular matrix degradation, cell migration and chemotaxis 8,21,28 as well as preventing tumor dormancy, 29,30 antagonism of the urokinase-u-PAR axis represents one potential means of controlling spread of cancer. Some evidence for this possibility has been derived from the study of various malignancies including prostate cancer. In one study, the epidermal growth factor domain of murine urokinase fused to the Fc portion of human IgG proved to be a high affinity antagonist of the murine u-PAR and inhibited neovascularization and growth of B16 melanoma in syngeneic mice. 31 In another study, Goodson and others isolated a family of u-PAR-binding ligands by affinity selection of a 15-mer random peptide library displayed on bacteriophage M13. 32 Further, small molecular weight inhibitors of urokinase such as amiloride and p-aminobenzamidine 33 have proven effective in reducing LNCaP and DU145 prostate tumor growth in mice. While it is not possible at the present time to determine which of these agents would prove most efficacious in blocking the urokinase-u-PAR axis, one practical benefit of Å6 is its high aqueous solubility compared with the bacteriophage peptides isolated previously. 32
The mechanism by which Å6 exerts its anti-tumor and anti-metastatic effects is presently unclear. In a previous study, 17 it was shown that Å6, which is derived from the connecting peptide of urokinase (amino acids 136–143) acts as a non-competitive antagonist of the u-PAR using a non-cell based biochemical assay in which soluble u-PAR and immobilized urokinase were used. However, it is also possible that Å6 mediates its anti-metastatic effects via other mechanisms. For example, phosphorylation of urokinase at serine 138 has previously been shown to modulate cell adhesion and motility 34 and Å6 might act as a phosphorylation substrate in competition with endogenous urokinase.
In conclusion, we have demonstrated the ability of Å6, a urokinase-derived peptide, to increase the survival of mice bearing orthotopically grown prostate cancer and to reduce the metastatic spread of this cancer to lymph nodes. Å6 can be added to a short list of agents that perturb the urokinase-u-PAR axis to counter tumor invasiveness. Our study sets the stage for future investigations to determine the clinical utility of Å6, or congeners, in reducing prostate cancer dissemination.
Acknowledgments
We are grateful to Dr. Janet Price for assistance with the statistical analysis. We also acknowledge the excellent technical assistance of Nila Parikh, Kristen Kreuger, Karen Shannahan, Hector Avila, and Marjorie Johnson.
Footnotes
Address reprint requests to Douglas Boyd, Department of Cancer Biology, Box 179, M.D. Anderson Cancer Center, 1515 Holcombe Blvd., Houston, TX 77030. E-mail: dboyd@mdanderson.org.
Supported by NIH grants RO1 CA58311 and R01 DE10845 (to D.B.) and DAMD17–00-1–9524 (to G.E.G.).
References
- 1.Naldini L, Tamagnone L, Vigna E, Sachs M, Hartmann G, Birchmeier W, Daikuhara Y, Tsubouchi H, Blasi F, Comoglio PM: Extracellular proteolytic cleavage by urokinase is required for activation of hepatocyte growth/scatter factor. Eur Mol Biol Org 1992, 11:4825-4833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wun T, Ossowski L, Reich E: A proenzyme form of human urokinase. J Biol Chem 1982, 257:7262-7268 [PubMed] [Google Scholar]
- 3.Liotta L, Goldfarb R, Brundage R, Siegel G, Terranova V, Garbisa S: Effect of plasminogen activator (urokinase), plasmin, and thrombin on glycoprotein and collagenous components of basement membrane. Cancer Res 1981, 41:4629-4636 [PubMed] [Google Scholar]
- 4.Ginestra A, Monea S, Seghezzi G, Dolo V, Nagase H, Mignatti P, Vittorelli M: Urokinase plasminogen activator and gelatinases are associated with membrane vesicles shed by human HT1080 cells. J Biol Chem 1997, 272:17216-17222 [DOI] [PubMed] [Google Scholar]
- 5.Mackay AR, Corbitt RH, Hartzler JL, Thorgeirsson UP: Basement membrane type IV collagen degradation: evidence for the involvement of a proteolytic cascade independent of metalloproteinases. Cancer Res 1990, 50:5997-6001 [PubMed] [Google Scholar]
- 6.Nielsen L, Kellerman GM, Behrendt N, Picone R, Dano K, Blasi F: A 55,000–60,000 Mr receptor protein for urokinase-type plasminogen activator. J Biol Chem 1988, 263:2358-2363 [PubMed] [Google Scholar]
- 7.Ellis V, Behrendt N, Dano K: Plasminogen activation by receptor-bound urokinase. J Biol Chem 1991, 266:12752-12758 [PubMed] [Google Scholar]
- 8.Resnati M, Pallavicini I, Wang JM, Oppenheim J, Serhan CN, Romano M, Blasi F: The fibrinolytic receptor for urokinase activates the G protein-coupled chemotactin receptor FPRL1/LXA4R. Proc Natl Acad Sci USA 2002, 99:1359-1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yebra M, Parry GCN, Stromblad S, Mackman N, Rosenberg S, Mueller BM, Cheresh DA: Requirement of receptor-bound urokinase-type plasminogen activator for integrin α vβ5-directed cell migration. J Biol Chem 1996, 271:29393-29399 [DOI] [PubMed] [Google Scholar]
- 10.Helenius MA, Saramaki OR, Linja MJ, Tammela TLJ, Visakorpi T: Amplification of urokinase gene in prostate cancer. Cancer Res 2001, 61:5340-5344 [PubMed] [Google Scholar]
- 11.Achbarou A, Kaiser S, Tremblay G, Ste-Marie L, Brodt P, Goltzman D, Rabbani SA: Urokinase overproduction results in increased skeletal metastasis by prostate cancer cells in vivo. Cancer Res 1994, 54:2372-2377 [PubMed] [Google Scholar]
- 12.Crowley CW, Cohen R, Lucas BK, Liu G, Shuman MA, Levinson AD: Prevention of metastasis by inhibition of the urokinase receptor. Proc Natl Acad Sci USA 1993, 90:5021-5025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans CP, Elfman F, Parangi S, Conn M, Cunha G, Shuman M: Inhibition of prostate cancer neovascularization and growth by urokinase-plasminogen activator receptor blockade. Cancer Res 1997, 57:3594-3599 [PubMed] [Google Scholar]
- 14.Festuccia C, Dolo V, Guerra F, Violini S, Muzi P, Pavan A, Bologna M: Plasminogen activator system modulates invasive capacity and proliferation in prostatic tumor cells. Clin Exp Metast 1998, 16:513-528 [DOI] [PubMed] [Google Scholar]
- 15.Hollas W, Hoosein N, Chung LWK, Mazar A, Henkin J, Kariko K, Barnathan E, Boyd D: Expression of urokinase and its receptor in invasive and non-invasive prostate cancer cell lines. Thromb Haemost 1992, 68:662-666 [PubMed] [Google Scholar]
- 16.Miyake H, Hara I, Yamanaka K, Gohji K, Arakawa S, Kamidono S: Elevation of serum levels of urokinase-type plasminogen and its receptor is associated with disease progression and prognosis in patients with prostate cancer. Prostate 1999, 39:123-129 [DOI] [PubMed] [Google Scholar]
- 17.Guo Y, Higazi A, Arakelian A, Sachais BS, Cines D, Goldfarb R, Jones T, Kwaan H, Mazar A, Rabbani SA: A peptide derived from the nonreceptor binding region of urokinase plasminogen activator (uPA) inhibits tumor progression and angiogenesis and induces tumor cell death in vivo. EMBO J 2000, 14:1400-1410 [DOI] [PubMed] [Google Scholar]
- 18.Mishima K, Mazar A, Gown A, Skelly M, Ji X-D, Wang X-D, Jones TR, Cavenee WK, Huang H-J: A peptide derived from the non-receptor-binding region of urokinase plasminogen activator inhibits glioblastoma growth and angiogenesis in vivo in combination with cisplatin. Proc Natl Acad Sci USA 2000, 97:8484-8489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pettaway CA, Pathak S, Greene G, Ramirez E, Wilson MR, Killion JJ, Fidler IJ: Selection of highly metastatic variants of different human prostatic carcinomas using orthotopic implantation in nude mice. Clin Cancer Res 1996, 2:1627-1636 [PubMed] [Google Scholar]
- 20.Allgayer H, Wang H, Gallick GE, Crabtree A, Mazar A, Jones T, Kraker AJ, Boyd DD: Transcriptional induction of the urokinase receptor gene by a constitutively active Src. Requirement of an upstream motif (−152/−135) bound with Sp1 J Biol Chem 1999, 274:18428-18445 [DOI] [PubMed] [Google Scholar]
- 21.Hollas W, Blasi F, Boyd D: Role of the urokinase receptor in facilitating extracellular matrix invasion by cultured colon cancer. Cancer Res 1991, 51:3690-3695 [PubMed] [Google Scholar]
- 22.Popesko P, Rajtova V, Horak J: A Color Atlas of Anatomy of Small Laboratory Animals. Rat, Mouse, Hamster. 1992, Volume II:pp 157-159 Wolfe Publishing Ltd., London [Google Scholar]
- 23.Vassalli J-D, Belin D: Amiloride selectively inhibits the urokinase-type plasminogen activator. FEBS Lett 1987, 214:187-191 [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Dang J, Liang X, Doe WF: Amiloride modulates urokinase gene expression at both transcription and post-transcription levels in human colon cancer cells. Clin Exp Metast 1995, 13:196-202 [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Jones CJ, Dang J, Liang X, Olsen JE, Doe WF: Human urokinase receptor expression is inhibited by amiloride an induced by tumor necrosis factor and phorbol ester in colon cancer cells. FEBS Lett 1994, 353:138-142 [DOI] [PubMed] [Google Scholar]
- 26.Guo Y, Mazar A, Lebrun J, Rabbani SA: An antiangiogenic urokinase-derived peptide combined with tamoxifen decreases tumor growth and metastasis in a syngeneic model of breast cancer. Cancer Res 2002, 62:4678-4684 [PubMed] [Google Scholar]
- 27.Rabbani SA, Harakidas P, Bowlin T, Attardo G: Effect of nucleoside analogue BCH-4556 on prostate cancer growth and metastases in vitro and in vivo. Cancer Res 1998, 58:3461-3465 [PubMed] [Google Scholar]
- 28.Wei Y, Yang X, Liu Q, Wilkins JA, Chapman HA: A role for caveolin and the urokinase receptor in integrin-mediated adhesion and signaling. J Cell Biol 1999, 144:1285-1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghiso JA, Kovalski K, Ossowski L: Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling. J Cell Biol 1999, 147:89-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aguirre-Ghiso JA, Liu D, Mignatti A, Kovalski K, Ossowski L: Urokinase receptor and fibronectin regulate the ERK to p38 activity ratios that determine carcinoma cell proliferation or dormancy in vivo. Mol Biol Cell 2001, 12:863-879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Min HY, Doyle LV, Vitt CR, Zandonella CL, Stratton-Thomas JR, Shuman MA, Rosenberg S: Urokinase receptor antagonists inhibit angiogenesis and primary tumor growth in syngeneic mice. Cancer Res 1996, 56:2428-2433 [PubMed] [Google Scholar]
- 32.Goodson RJ, Doyle MV, Kaufman SE, Rosenberg S: High-affinity urokinase receptor antagonists identified with bacteriophage peptide display. Proc Natl Acad Sci USA 1994, 91:7129-7133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jankun J, Keck RW, Skzypczak-Jankun E, Swiercz R: Inhibitors of urokinase reduce size of prostate cancer xenografts in severe combined immunodeficient mice. Cancer Res 1997, 57:559-563 [PubMed] [Google Scholar]
- 34.Franco P, Iaccarino C, Chiaradonna F, Frandazza A, Iavarone C, Mastronicola MR, Nolli ML, Stoppelli MP: Phosphorylation of human pro-urokinase on Ser 138/303 impairs its receptor-dependent ability to promote myelomonocytic adherence and motility. J Cell Biol 1997, 137:779-791 [DOI] [PMC free article] [PubMed] [Google Scholar]





