Abstract
The pathogenesis of pulmonary hypoplasia associated with congenital diaphragmatic hernia (CDH) is unknown. The sonic hedgehog (Shh) cascade is crucial for the patterning of the early respiratory system in mice. To establish whether Shh plays a role in the pathogenesis of lung hypoplasia in CDH, we investigated the gestation-specific expression of Shh in normal rat and human lungs using in situ hybridization and immunohistochemistry. The expression pattern was compared with that of age-matched samples of hypoplastic lungs associated with CDH in humans and in the 2,4-dichlorophenyl-p-nitrophenylether (nitrofen) rat model. Our results showed that in normal controls the expression of Shh increased with advancing gestation, peaked in the late pseudoglandular stage, and declined thereafter. The expression of Shh is initially down-regulated in pulmonary hypoplasia associated with CDH and peaks instead during the late canalicular stage. These data indicate that maximal expression of Shh occurs when respiratory bronchioles develop and thinning of the interstitium takes place, suggesting that Shh may play a role in these processes. Furthermore, we observed that Shh inhibited fetal lung fibroblast proliferation in vitro. Therefore, it is tempting to speculate that alterations in Shh expression may affect these developmental processes, thereby contributing to the pulmonary abnormality in CDH.
Congenital diaphragmatic hernia (CDH) occurs with an approximate prevalence of 1 in 2500 to 3000 newborns. 1 Mortality for this anomaly has been variably reported from 20 to 40% recently 2,3 and this mortality is felt to be primarily because of associated pulmonary hypoplasia. 4 The current therapies that exist are purely supportive in nature. To develop new treatment modalities we require a better understanding of the pathogenesis of lung hypoplasia in the case of CDH. The lungs of babies who have succumbed from diaphragmatic hernia show a characteristic histological pattern of pulmonary hypoplasia. The lungs show a weight that is disproportionately small compared to birth weight. 5 In addition, they have a reduced number of airway branches and a reduction in vascularization, 6,7 which has been well correlated with animal models. 8 The lungs appear to be arrested at an earlier stage of development in that the interstitium has not thinned and there is a reduction in cellular maturity. There are fewer terminal bronchi associated with fewer terminal saccules. Furthermore, as the interstitium does not thin out normally, there is a greater distance from air sac to capillary. The cellular and molecular mechanisms responsible for the pulmonary immaturity are not understood.
Lung development is guided by tissue interactions. 9-11 Although the exact cell-cell signaling molecules remain to be identified, genetic analysis suggests that the sonic hedgehog (Shh) signaling cascade is important for murine lung development. Shh-null mutant (shh−/−) mice display pulmonary hypoplasia as well as tracheoesophageal fistulas. 12 Surprisingly, proximal-distal differentiation of lung epithelium was normal in shh−/− mice. 13 Overexpression of Shh in distal lung epithelium resulted in the absence of functional alveoli and an increase in interstitial tissue caused by an increased proliferation of both epithelial and mesenchymal cells. 14 Transgenic newborn mice die soon after birth, probably owing to respiratory failure. Cell differentiation was normal in Shh-overexpressing mice lungs. These data indicate that Shh is important for branching morphogenesis but not for proximal-distal differentiation. The significance of Shh in human lung development, however, has yet to be elucidated.
Shh is transcribed as a 48-kd molecule and subsequently cleaved into two subunits, a 19-kd NH2-terminal peptide and a 26- to 28-kd COOH-terminal peptide. 15 The NH2 moiety is modified by the addition of a cholesterol terminus that acts as a cell membrane anchor and, thus, Shh can have local effects. The COOH moiety is freely diffusible and can have downstream effects in a concentration-dependent manner. Shh binds to a multiple pass membrane-spanning receptor known as Patched. 16,17 Activation of the Ptc receptor releases an inhibition of a second membrane receptor known as Smoothened. 18 This then activates three zinc-finger transcription factors, Gli1, Gli2, and Gli3, 19 which are expressed in lung mesoderm rather than endoderm. Genetic manipulations have revealed important roles for Gli proteins in murine lung development. 20,21 The remainder of the signaling cascade remains somewhat elusive, however, it may involve the families of fibroblast growth factors and bone morphogenetic proteins. 22-25
These results indicate a role for Shh signaling in pulmonary branching morphogenesis in mice. We sought first to elucidate the spatial and temporal pattern of Shh expression in the normal rat and human developing lung. We then compared this to the expression pattern in lungs affected by pulmonary hypoplasia secondary to CDH. In the rat model, CDH was induced with nitrofen. 26 We show herein that Shh expression peaks once the tracheobronchial tree has been formed. Shh peak expression is down-regulated in hypoplastic lungs associated with CDH. In addition, the effect of Shh on fetal lung fibroblast proliferation was assessed and it appears that Shh inhibits fetal lung fibroblast proliferation. Thus, delayed pulmonary down-regulation of Shh in case of CDH may have a causative role in reduced branching and interstitial thickening observed in lung hypoplasias associated with CDH.
Materials and Methods
Materials
Culture media, antibiotics, fetal bovine serum, and ascorbic acid were purchased from Life Technologies, Inc. (Grand Islands, NY). Four-well culture dishes were from Nunc (Intermed, Denmark). Trypsin was from Becton Dickinson Labware (Bedford, MA) and [3H]thymidine from Amersham (Arlington Heights, IL). Recombinant Shh (rShh) protein (19-kd N-terminal) was expressed as a GST fusion protein in Escherichia coli DH5α (a gift from Dr. CC Hui, Hospital for Sick Children, Toronto, Canada). The GST-rShh protein was purified from E. coli by affinity chromatography on glutathione agarose. The beads were then treated with Factor Xa to cleave the rShh moiety and released rShh was collected. Recombinant Shh (full length) was also stably expressed in mammalian Cos-1 cells (a gift from Dr. CC Hui). Shh monoclonal antibodies (5E1) were obtained from Developmental Studies Hybridoma Bank (Iowa City, IA).
Animals
Female (220 to 250 g) and male (250 to 300 g) Wistar rats were obtained from Charles River (St. Constant, PQ, Canada). The animals were kept in a controlled light-dark cycle and food and water were supplied ad libitum. Rats were mated overnight and the finding of a sperm-positive vaginal smear was designated day 0 of gestation. Timed pregnant dams were euthanized with diethyl ether excess at day 12 to day 22 (full term) gestation. The fetuses were removed by cesarean section and washed in Hanks’ balanced salt solution. Pneumonectomy was performed with the aid of a dissecting microscope. The lungs were fixed in 4% (v/v) paraformaldehyde in phosphate-buffered saline (PBS), dehydrated in a graded series of ethanol, cleared in xylene, and embedded in paraplast. All protocols were evaluated and approved by the Animal Care Committee of the Hospital for Sick Children.
Nitrofen
CDH was created in fetal rats by administering orally 100 mg of the herbicide 2,4-dichlorophenyl-p-nitrophenylether (nitrofen) (Rohm & Haas Co., Philadelphia, PA) in 1 ml of olive oil to pregnant dams at 9 days of gestation. 8,26,27 The rats were euthanized with diethyl ether excess at 12 to 22 days of gestation and underwent cesarean section to remove the fetuses. The fetuses were transected and examined for the absence of a diaphragm (∼80% of fetuses were affected). The lungs of affected fetuses were dissected, fixed in 4% (v/v) paraformaldehyde in PBS, dehydrated in a graded series of ethanol, cleared in xylene, and embedded in paraplast. Disposal of all waste products containing nitrofen was done according to local regulations of the Hospital for Sick Children and all experimental procedures were approved by the Department of Occupational Health and Safety of the Hospital for Sick Children.
Human Lung Tissues
Human lung tissue was obtained from 6 weeks to 40 weeks (full term) of gestation. Fetuses between 6 weeks and 21 weeks of gestation were obtained with patient consent from elective terminations of pregnancies. Lungs were isolated, fixed, and embedded in paraplast. Lung tissue from 24 weeks to 40 weeks of gestation was obtained from archived material at the Hospital for Sick Children and Sophia Children’s Hospital. All of these children succumbed to stillbirth or asphyxial deaths. Their lungs appeared to be histologically normal by postmortem examination. Four human samples were tested for each of the embryonic (7 to 8 weeks), pseudoglandular (12 to 16 weeks), canalicular (18 to 21 weeks), and saccular (29 to 35 weeks) stages of lung development. Six samples were tested from the alveolar stage (38 to 40 weeks) of lung development. Human age-matched lung samples were obtained from fetuses with CDH (gestational age 8 to 21 weeks) that were the product of a termination of the pregnancy. The diagnosis of CDH was made by ultrasound scanning initially and confirmed microscopically on postmortem examination. Tissue was also obtained from the lungs of babies who died of pulmonary hypoplasia associated with CDH (gestational age of 24 to 40 weeks). The diagnosis of CDH was confirmed at postmortem. Furthermore, the diagnosis of pulmonary hypoplasia was confirmed by a reduced lung weight and a consistent histological appearance. We obtained five human CDH samples from the pseudoglandular stage (14 to 16 weeks), two CDH samples of the canalicular stage (18 to 21 weeks), three CDH samples of the saccular stage (32 to 35 weeks), and six CDH samples of the alveolar stage (38 to 40 weeks) of lung development. There were samples of lungs from babies with right, left, and bilateral diaphragmatic hernias. In all cases, the ipsilateral lung was studied. All human tissue samples were obtained in accordance with the ethical guidelines of the Research and Ethics Board of the Hospital for Sick Children and Sophia Children’s Hospital.
In Situ Hybridization
Sections of 5 μm were cut and mounted on Superfrost slides (Fisher Scientific, Unionville, Ontario, Canada). Human Shh cDNA (a gift from Dr. CC Hui) was subcloned in the PCRII vector (InVitrogen, Carlsbad, CA) to generate sense and anti-sense digoxigenin-labeled cRNA probes according to the RNA labeling and detection kits (nonradioactive) from Roche (Laval, Quebec, Canada). In situ hybridization to lung tissue from different normal gestational ages and from hypoplastic lungs associated with CDH was performed as previously described 27,28 with minor modifications. Briefly, the slides were dewaxed and rehydrated in a series of dilutions of ethanol. To enhance signal and facilitate probe penetration, sections were transferred to a pressure cooker containing 1 L of 0.1 mol/L Tris at pH 8.0 and heated at maximum power in a microwave for 18 minutes. The slides were cooled with the lid on for 15 minutes and then for an additional 30 minutes with the lid off. The tissue was then refixed with 4% (v/v) paraformaldehyde in PBS for 30 minutes. The slides, washed with PBS and 10× standard saline citrate, were blocked with a prehybridization solution for 1 hour at room temperature. The probe was added to each tissue section at a concentration of 1:500 and hybridized overnight at 37°C. After high stringency washing (2× standard saline citrate twice, 1× standard saline citrate twice, 0.5× standard saline citrate twice at 52°C), sections were incubated with an alkaline phosphatase-conjugated sheep anti-digoxigenin antibody, which catalyzed a color reaction when using a BCIP (5-bromo-4-chloro-3-indolyl phosphate) substrate system (Roche). All slides were stopped at the same time to make semiquantitative comparisons of Shh mRNA expression between different stages of development and normal versus CDH lungs. A purple/black precipitate indicated a positive signal. Sense probes served as control and did not give any signal. Slides were counterstained with nuclear fast red and mounted with Permount (Fisher Scientific). In situ hybridizations were repeated a minimum of three times on each tissue sample.
Immunohistochemistry
Sections of rat and human lungs were evaluated for the presence of Shh protein using a mouse monoclonal antibody to Shh (clone 5E1). Tissue sections were dewaxed in ethanol and rehydrated in a graded series of ethanol. Antigen retrieval was performed by boiling the sections in 10 mmol/L of sodium citrate solution, pH 6.0, for two periods of 5 minutes in a microwave at medium high. Between the boiling periods the sections were allowed to cool down for 20 minutes. After rinsing in PBS, endogenous peroxidase activity was quenched with 1% (v/v) hydrogen peroxide in methanol. Nonspecific binding sites were blocked using 5% (v/v) normal goat serum and 1% (w/v) bovine serum albumin. The slides were then incubated at 4°C overnight with a 1:50 dilution of Shh monoclonal antibody. After washing in PBS, the slides were incubated with a 1:300 dilution of biotinylated secondary sheep anti-mouse antibody at room temperature. After washing, sections were incubated with avidin-biotin complex (Vecastain) kit from Vector Laboratories (Burlinghame, CA) for 2 hours at room temperature. Subsequently, after washes in PBS and Tris-buffered saline (TBS), the sections were developed using a 3,3-diaminobenzidine substrate. Again, all slides were stopped at the same time to make semiquantitative comparisons of Shh protein expression between different stages of development. After washes with in TBS and PBS, sections were counterstained using Carazzi’s hematoxylin and mounted with Permount.
Isolation of Fetal Lung Fibroblasts
Pregnant rats were killed on day 18 of gestation by diethyl ether excess. The fetuses were aseptically removed from the mothers and the fetal lungs dissected out in cold Hanks’ balanced salt solution without calcium and magnesium [HBSS(−)] and cleared of major airways and vessels. The lungs were washed twice in HBSS(−), minced, and suspended in HBSS(−). Fibroblasts were isolated from the fetal lungs as described in detail previously. 29
[3H]Thymidine Incorporation into DNA
Fetal lung fibroblasts, cultured for 48 hours in Ham’s F12/Dulbecco’s modified Eagle’s medium (DMEM) plus 10% (v/v) fetal bovine serum, were incubated for 12 hours in serum-free MEM before a 24-hour incubation in the presence of 2 μCi/ml of [3H]thymidine with medium of Shh-expressing Cos-1 cells with and without pretreatment with 10 μg/ml of Shh monoclonal antibody (mAb). Serum-free MEM was used as control. Alternatively, fetal lung fibroblasts growing in Ham’s F12/DMEM plus 5% (v/v) fetal bovine serum were incubated for 24 hours with increasing dosages of rShh (19-kd fragment) in the presence of 2 μCi/ml of [3H]thymidine. At the end of the incubation period the media were aspirated and the cells were rinsed twice with ice-cold PBS. The amount of radioactive thymidine incorporated into DNA was then measured as previously described. 30 Thymidine incorporation into DNA was calculated as dpm/well.
Results
Developmental Pattern of Shh Expression in Rat Lung
In situ hybridization analysis revealed minimal Shh mRNA expression at 13 days of gestation (Figure 1A) ▶ . Overdeveloping the day-13 tissue sections, however, elucidated a positive signal. After day 13 of gestation, Shh mRNA expression increased and peaked at 18 days of gestation. Thereafter, Shh message decreased and disappeared by the first postnatal day. Shh mRNA expression localized to the airway epithelial cells. Initially Shh message was detected in the distal as well as in the proximal airways. As gestation advanced, the expression disappeared in the distal cells ahead of that of the proximal airway epithelial cells. Immunohistochemical analysis of the same tissue samples revealed a similar Shh protein expression pattern (Figure 1B) ▶ . Shh protein was detected in airway epithelial cells as early as at fetal day 13, increased to a maximal expression at day 18 with a rapid and progressive decline thereafter.
Figure 1.
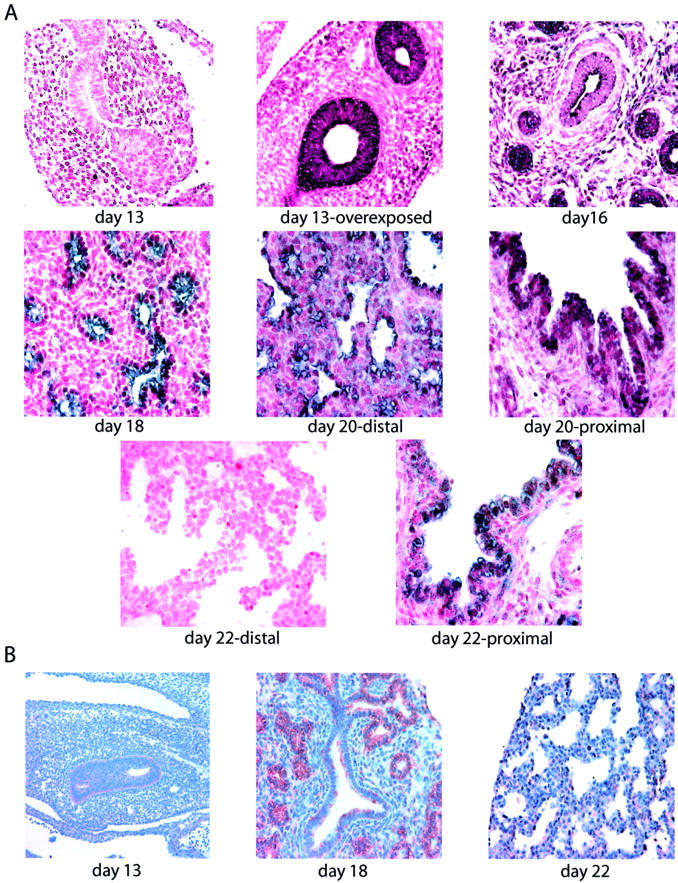
Expression pattern of Shh in developing rat lung. A:In situ hybridization showed that Shh expression (purple signal) localized to the airway epithelium. It could only be detected at day 13 by overexposing the section. The positive Shh signal increased in intensity during development until it peaked at day 18, and declined thereafter. As gestation advances, Shh expression disappeared in the distal airways ahead of the proximal airways. B: Immunohistochemical analysis confirmed the gestational pattern of Shh expression. All photomicrographs are shown at the same magnification.
Developmental Pattern of Shh Expression in Hypoplastic Rat Lung
Of the fetuses born to dams treated with nitrofen, ∼80% were affected with CDH. This was diagnosed by microscopic examination and only those with CDH were used for the purposes of this study. The lungs studied were on the ipsilateral side to the diaphragmatic hernia. Pulmonary hypoplasia was diagnosed based on consistent histological findings of delayed pulmonary maturation and in particular a delay in branching morphogenesis and a delay in thinning of the interstitium. In situ hybridization demonstrated the appearance of Shh mRNA at 13 days of gestation, however, again only by overdeveloping the tissue sections (Figure 2) ▶ . In comparison with control rat lung, progression of Shh expression was thereafter markedly delayed with maximal expression occurring at term. This held true for both proximal and distal airway expression patterns with relatively high levels of Shh message demonstrable throughout the hypoplastic rat lung at 22 days of gestation.
Figure 2.
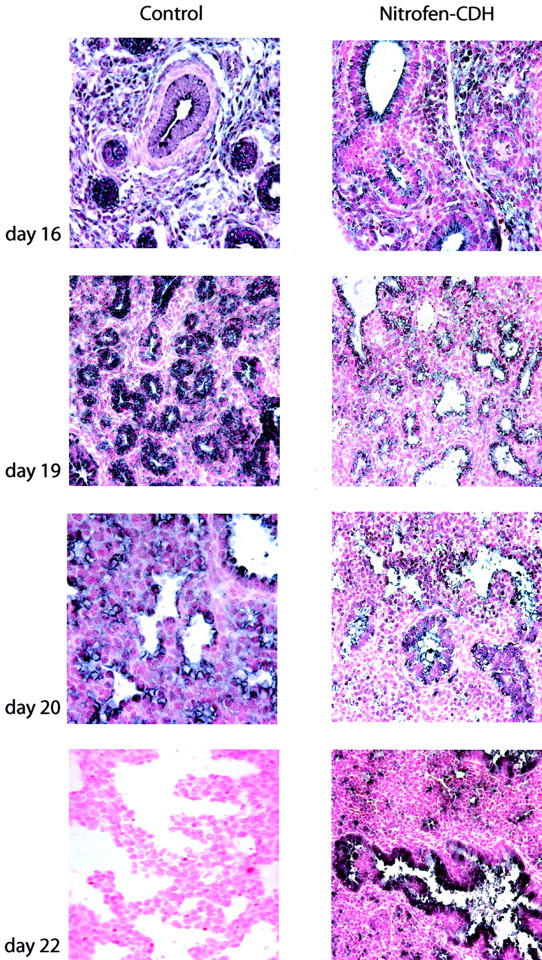
Expression pattern of Shh in hypoplastic rat lungs secondary to CDH. In situ hybridization revealed that Shh mRNA appeared at day 16 of gestation in CDH lungs, however, the signal was reduced in intensity when compared to age-matched control. In contrast to control, maximal expression was seen at term (day 22) instead of day 18. All photomicrographs are shown at the same magnification.
Developmental Pattern of Shh Expression in Human Lung
The earliest human sample that was analyzed was at 8 weeks of gestation. In situ hybridization demonstrated the presence of Shh mRNA in the primitive airway epithelial cells (Figure 3A) ▶ . The positive Shh signal increased in intensity to a maximum expression at 16 to18 weeks of gestational age. Thereafter Shh mRNA expression rapidly declined such that it was present only in the proximal large airways at 32 weeks of gestation and had completely disappeared by 35 weeks of gestation.
Figure 3.
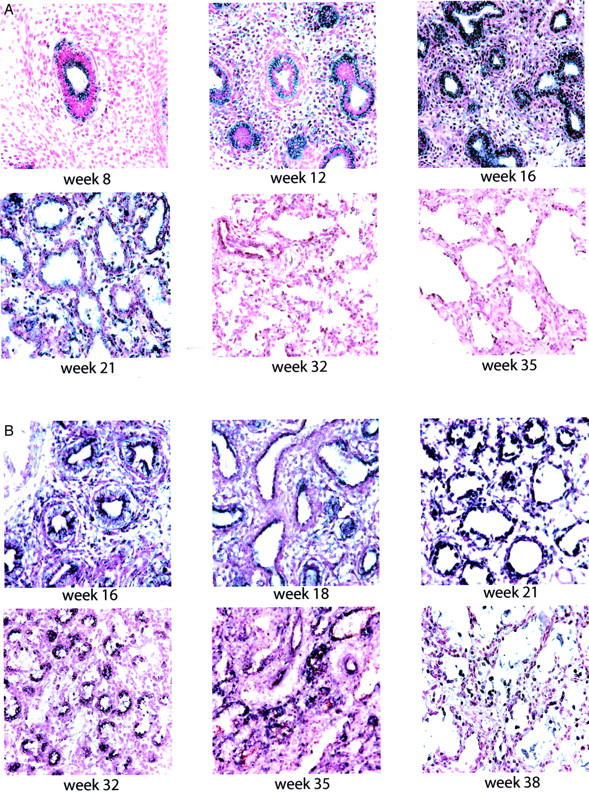
Developmental expression of Shh in normal human lung and hypoplastic lungs associated with CDH. A:In situ hybridization demonstrated Shh mRNA expression as early as 8 weeks of gestational age. The positive Shh signal increased in intensity with advancing gestation, reaching a peak at 16 weeks. At 32 weeks, a positive Shh signal was only detected in the large proximal airways. Shh expression disappeared at 35 weeks. B: In CDH lungs Shh message was detected as early as 16 weeks, however peak expression was markedly delayed until 21 to 32 weeks. At 35 weeks, positive Shh signal was present in many of the larger airways. All photomicrographs are shown at the same magnification.
Developmental Pattern of Shh Expression in Hypoplastic Human Lung
Shh mRNA expression was detected at very early stages of development, however, in a markedly reduced signal in comparison to that of control lung samples (Figure 3B) ▶ . The mRNA expression increased to a maximal demonstrable level in the late canalicular/saccular period (21 to 32 weeks of gestation). Shh message continued to be detectable in lower levels at 35 weeks and term, which is in contrast to control samples.
Shh Inhibits Fibroblast Proliferation
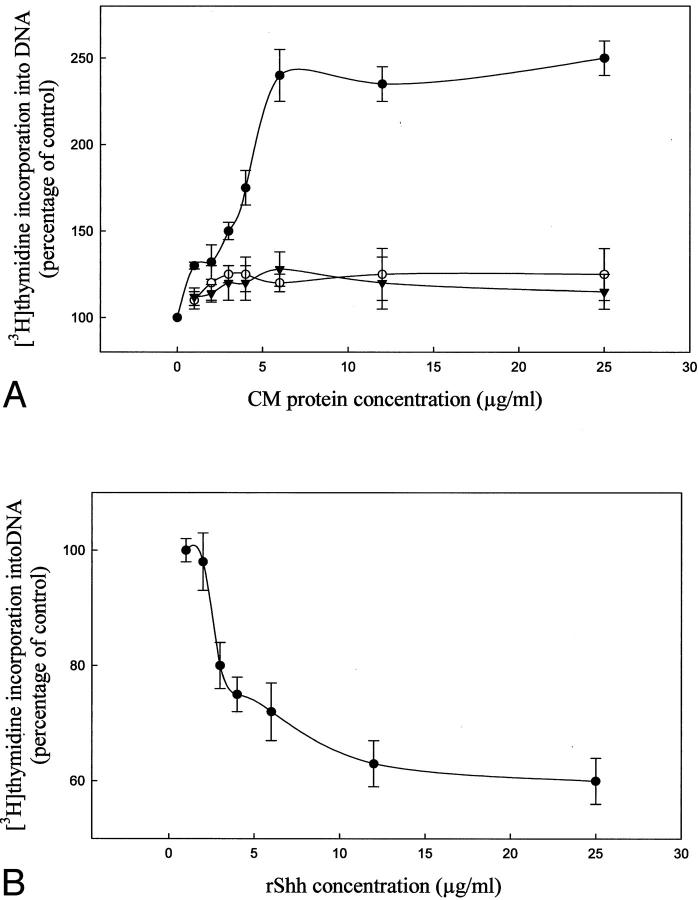
The mitogenic effect of Shh was investigated by incubating fetal lung fibroblasts with medium of Cos-1 cells stable-transfected with full-length Shh (Figure 4A) ▶ . Addition of increasing amounts of Cos-1 medium did not significantly alter [3H]thymidine incorporation into DNA. Preincubation of Cos-1 medium with Shh mAb resulted in a marked stimulation in DNA synthesis, whereas preincubation of MEM with Shh mAb had no effect on DNA synthesis. In a second series of experiments, fetal lung fibroblast were grown in MEM in the presence of 5% (v/v) serum and increasing amounts of recombinant Shh (rShh). As can be seen in Figure 4B ▶ , rShh inhibited DNA synthesis of fetal lung fibroblasts in a dose-dependent manner.
Figure 4.
Effect of Shh on fetal lung fibroblast proliferation. A: Addition of increasing amounts of conditioned MEM of Cos-1 cells stably transfected with full-length Shh did not significantly alter [3H]thymidine incorporation into DNA (○). Preincubation of this medium with Shh mAb resulted in a marked stimulation of DNA synthesis (•), whereas preincubation of unconditioned MEM with Shh mAb had no effect (▾), indicating that Shh inhibited fibroblast proliferation. B: Increasing amounts of recombinant rShh resulted in a dose-dependent inhibition of fetal lung fibroblast proliferation.
Discussion
Sonic hedgehog has been previously localized to airway epithelium of the developing rat lung by whole mount in situ hybridization. 13 The entire gestational expression profile in the lung, however, has not been described. Herein we show that Shh mRNA expression in the pulmonary epithelium peaks at days 18 to 19 of gestation in the fetal rat and declines steadily and rapidly thereafter. The developmental pattern for Shh immunostaining in fetal rat correlated very closely with that of the in situ hybridization. It can therefore be assumed that Shh mRNA expression reflects that of Shh protein. Immunostaining for Shh in the developing mouse lung revealed that Shh expression peaked at days 17 to 18 (term) of gestation. 31 Staining was intense in the epithelium of both conducting and distal airways. We have demonstrated a similar pattern in the rat with Shh expression disappearing in the distal cells ahead of that of the proximal airway epithelial cells. Days 18 to 19 of gestation in the rat correspond to the late pseudoglandular, early canalicular period of development, a time of terminal bronchial division, interstitial thinning, epithelial differentiation as well as of intense pulmonary vascularization. It is during this period that the pulmonary capillary system becomes intimately linked to the pulmonary airspaces. Although the exact function of Shh in later lung morphogenesis has yet to be elucidated, an attractive theory is that Shh is involved in regulating some of these developmental processes. As seen in mice, 31 we found a persistence of Shh expression in the proximal conducting airways after its disappearance in the distal airways. Its persistence in this region may be important in continued apposition of the airways with major vascular structures or in cytodifferentiation of the conducting airway epithelium. In mice, it has been shown that Shh staining in conducting airways co-localized with that of nonciliated Clara cell marker, CCSP, but not ciliated epithelial cell markers, hepatocyte nuclear factor/forkhead homologue-4 (HFH4), and β-tubulin. 31 In the present study, we also found that Shh inhibited fetal lung fibroblast growth and, thus, it is conceivable that Shh contributes to the interstitial thinning occurring at the late pseudoglandular and early canalicular stages of lung development.
The importance of Shh in human brain development has been a matter of intensive research. 32,33 Although Shh plays a crucial role in murine lung development, 12 its expression pattern in the developing human lung has not been previously reported. In the present study, we show a very tight association between the pulmonary expression patterns of Shh in the fetal rat and human. As mentioned earlier, the expression pattern of Shh in the developing rat lung is very similar to that of mice. Together these similarities in pulmonary Shh expression suggest that rodents are suitable models for elucidating the role of Shh in human lung development.
The pathological findings in pulmonary hypoplasia associated with CDH are that of a reduced number of terminal bronchial divisions, delayed interstitial thinning, as well as a reduction in pulmonary vascularization. 5-7,25 Many authors have speculated that the pulmonary hypoplasia of CDH is not only related to a pressure phenomenon secondary to herniation of the bowel contents into the thoracic cavity. 8,28,34 Nitrofen has been used in pregnant rat dams to induce a diaphragmatic hernia in attempts to further delineate the pathophysiology of the associated pulmonary hypoplasia. It has been shown in separate studies, 27 that nitrofen alone negatively influences lung-branching morphogenesis regardless of the presence or absence of an intact diaphragm. 8 It is thus speculated that the biochemical signaling process involved in abnormal diaphragm development may also be involved in abnormal pulmonary morphogenesis or that they may be two independent developmental processes affected by nitrofen. Sonic hedgehog is an attractive morphogen to implicate in these processes because it appears to be most highly expressed during a period of gestation coincident with the abnormalities seen histologically on examination of hypoplastic lungs. In the present study, we compared rat and human fetuses with CDH and again observed a dramatically similar pattern between the two species although quite different from the control samples. The early-onset rat model of nitrofen-induced pulmonary hypoplasia showed a maturational delay in the expression of Shh. Instead of a peak mRNA expression at the late pseudoglandular-early canalicular stage, we observed a maximal Shh expression at term. As in the rat, Shh expression in human pulmonary hypoplasia associated with CDH was delayed. Maximal expression of Shh was at ∼21 to 32 weeks of gestation corresponding to the late canalicular/early saccular period of development. The expression of Shh subsequently diminished quite rapidly in both rat and human pulmonary hypoplasias with advancing gestation.
We speculate that the maturational delay in Shh expression may be critical to the development of pulmonary hypoplasia associated with CDH. It is well known that surgical decompression of the abdominal contents postnatally does not induce rapid lung growth. 35 Although prenatal tracheal ligation in experimental models in vivo 36-38 and in vitro 39 of CDH has resulted in accelerated lung growth, significant variability in lung growth has been observed in early clinical trials of tracheal ligation including a negative effect on type II cell differentiation. One must therefore hypothesize that aberrant biochemical signaling inherent to CDH that cannot be altered by physical and mechanical forces alone or their reversal. It is evident that a better understanding of Shh signaling in normal lung growth and development is required to understand the impact of its aberrant expression in CDH.
Acknowledgments
We thank Ronald de Krijger and Richard Kijzer for the early human CDH samples.
Footnotes
Address reprint requests to Martin Post, Program in Lung Biology Research, Hospital for Sick Children Research Institute, 555 University Ave., Toronto, Ontario M5G 1X8, Canada. E-mail: martin.post@sickkids.ca.
Supported by Canadian Institutes of Health Research (CIHR) (grant no. 15272).
References
- 1.Avery GB, Fletcher MA, MacDonald MG: Neonatology: Pathophysiology and Management of the Newborn. Avery GB eds. :pp 921-922 Lippincott Company, Philadelphia
- 2.Kays DW, Langham MR, Jr, Ledbetter DJ, Talbert JL: Detrimental effects of standard medical therapy in congenital diaphragmatic hernia. Ann Surg 1999, 230:340-348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boloker J, Bateman DA, Wung JT, Stolar CJ: Congenital diaphragmatic hernia in 120 infants treated consecutively with permissive hypercapnea/spontaneous respiration/elective repair. J Pediatr Surg 2002, 37:357-366 [DOI] [PubMed] [Google Scholar]
- 4.Azarow K, Messineo A, Pearl R, Filler R, Barker G, Bohn D: Congenital diaphragmatic hernia—a tale of two cities: the Toronto experience. J Pediatr Surg 1997, 32:395-400 [DOI] [PubMed] [Google Scholar]
- 5.Reale FR, Esterly JR: Pulmonary hypoplasia: a morphometric study of the lungs of infants with diaphragmatic hernia, anencephaly, and renal malformations. Pediatrics 1973, 51:91-96 [PubMed] [Google Scholar]
- 6.Lipsett J, Cool JC, Runciman SI, Ford WD, Kennedy JD, Martin AJ, Parsons DW: Morphometric analysis of preterm fetal pulmonary development in the sheep model of congenital diaphragmatic hernia. Pediatr Dev Pathol 2000, 3:17-28 [DOI] [PubMed] [Google Scholar]
- 7.Shehata SM, Tibboel D, Sharma HS, Mooi WJ: Impaired structural remodelling of pulmonary arteries in newborns with congenital diaphragmatic hernia: a histological study of 29 cases. J Pathol 1999, 189:112-118 [DOI] [PubMed] [Google Scholar]
- 8.Cilley RE, Zgleszewski SE, Krummel TM, Chinoy MR: Nitrofen dose-dependent gestational day-specific murine lung hypoplasia and left-sided diaphragmatic hernia. Am J Physiol 1997, 272:L362-L371 [DOI] [PubMed] [Google Scholar]
- 9.Post M, Tanswell K: Embryonic lung development. Hamid Q Wardlaw AJ eds. Textbook of Respiratory and Molecular Biology. 2002:pp 3-14 Martin Dunitz Ltd. London
- 10.Chinoy M, Chi X, Cilley RE: Down-regulation of regulatory proteins for differentiation and proliferation in murine fetal hypoplastic lungs: altered mesenchymal-epithelial interactions. Pediatr Pulmonol 2001, 32:129-141 [DOI] [PubMed] [Google Scholar]
- 11.Shannon JM, Deterding RR: Epithelial-mesenchymal interactions in lung development. McDonald JA eds. Lung Growth and Development. 1997:pp 81-117 Dekker New York
- 12.Litingtung Y, Lei L, Westphal H, Chiang C: Sonic hedgehog is essential to foregut development. Nat Genet 1998, 20:58-61 [DOI] [PubMed] [Google Scholar]
- 13.Pepicelli CV, Lewis PM, McMahon AO: Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr Biol 1998, 8:1083-1086 [DOI] [PubMed] [Google Scholar]
- 14.Bellusci S, Furuta Y, Rush MG, Henderson R, Winnier G, Hogan BL: Involvement of sonic hedgehog (Shh) in mouse embryonic lung growth and morphogenesis. Development 1997, 124:53-63 [DOI] [PubMed] [Google Scholar]
- 15.Hammerschmidt M, Brook A, McMahon AP: The world according to hedgehog. Trends Genet 1997, 13:14-21 [DOI] [PubMed] [Google Scholar]
- 16.Stone DM, Hynes M, Armanini M, Swanson TA, Gu Q, Johnson RL, Scott MP, Pennica D, Goddard A, Phillips H, Noll M, Hooper JE, deSauvage F, Rosenthal A: The tumor-suppressor gene patched encodes a candidate receptor for sonic hedgehog. Nature 1996, 384:129-132 [DOI] [PubMed] [Google Scholar]
- 17.Marigo V, Davey RA, Zuo Y, Cunningham JM, Tabin CJ: Biochemical evidence that Patched is the hedgehog receptor. Nature 1996, 384:176-179 [DOI] [PubMed] [Google Scholar]
- 18.Van den Heuvel M, Ingham PW: Smoothened encodes a receptor-like serpentine protein required for hedgehog signalling. Nature 1996, 382:547-551 [DOI] [PubMed] [Google Scholar]
- 19.Grindley JC, Bellusci S, Perkins D, Hogan BL: Evidence for the involvement of the Gli gene family in embryonic mouse lung development. Dev Biol 1997, 188:337-348 [DOI] [PubMed] [Google Scholar]
- 20.Motoyama J, Liu J, Mo R, Ding Q, Post M, Hui CC: Essential function of Gli2 and Gli3 in the formation of lung, trachea and oesophagus. Nat Genet 1998, 20:54-57 [DOI] [PubMed] [Google Scholar]
- 21.Park HL, Bai C, Platt KA, Matise MP, Beeghly A, Hui CC, Nakashima M, Joyner AL: Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2 mutation. Development 2000, 127:1593-1605 [DOI] [PubMed] [Google Scholar]
- 22.Bellusci S, Grindley J, Emoto H, Itoh N, Hogan B: Fibroblast growth factor 10 (FGF 10) and branching morphogenesis in the embryonic mouse lung. Development 1997, 124:4867-4878 [DOI] [PubMed] [Google Scholar]
- 23.Sekine K, Ohuchi H, Fujiwara M, Yamasaki M, Yoshizawa T, Sato T, Yagishita N, Matsui D, Koga Y, Itoh N, Kato S: Fgf10 is essential for limb and lung formation. Nat Genet 1999, 21:138-141 [DOI] [PubMed] [Google Scholar]
- 24.Bellusci S, Henderson R, Winnier G, Oikawa T, Hogan B: Evidence from normal expression and targeted misexpression that bone morphogenetic protein-4 (Bmp-4) plays a role in mouse embryonic lung morphogenesis. Development 1996, 122:1693-1702 [DOI] [PubMed] [Google Scholar]
- 25.Chinoy MR: Pulmonary hypoplasia and congenital diaphragmatic hernia: advances in the pathogenetics and regulation of lung development. J Surg Res 2002, 106:209-223 [DOI] [PubMed] [Google Scholar]
- 26.Tenbrinck R, Tibboel D, Gaillard JL, Kluth D, Bos AP, Lachmann B, Molenaar JC: Experimentally induced congenital diaphragmatic hernia in rats. J Pediatr Surg 1990, 25:426-429 [DOI] [PubMed] [Google Scholar]
- 27.Keijzer R, Liu J, Deimling J, Tibboel D, Post M: Dual-hit hypothesis explains pulmonary hypoplasia in the nitrofen model of congenital diaphragmatic hernia. Am J Pathol 2000, 156:1299-1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith DE, Otulakowski G, Yeger H, Post M, Cutz E, O’Brodovich HM: Epithelial Na+ channel (EnaC) expression in the normal and abnormal human perinatal lung. Am J Respir Crit Care Med 2000, 161:1322-1331 [DOI] [PubMed] [Google Scholar]
- 29.Caniggia I, Tseu I, Han RNN, Smith BT, Tanswell AK, Post M: Spatial and temporal differences in fibroblast behaviour in fetal rat lung. Am J Physiol 1991, 261:L424-L433 [DOI] [PubMed] [Google Scholar]
- 30.Greenstein LA, Nissley SP, Moses AC, Short PA, Yang WHY, Lee L, Rechler MM: Purification of multiplication-stimulating activity. Barnes DW Sirbasku SA Sato GH eds. Methods for Preparation of Media, Supplements, and Substrata for Serum-Free Animal Cell Culture. 1984:pp 111-138 Alan R. Liss New York
- 31.Miller LAD, Wert SE, Whitsett JA: Immunolocalization of sonic hedgehog (Shh) in developing mouse lung. J Histochem Cytochem 2001, 49:1593-1603 [DOI] [PubMed] [Google Scholar]
- 32.Roessler E, Belloni E, Gaudenz K, Jay P, Berta P, Scherer SW, Tsui LC, Muenke M: Mutations in the human sonic hedgehog gene cause holoprosencephaly. Nat Genet 1996, 14:357-360 [DOI] [PubMed] [Google Scholar]
- 33.Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP: Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 1993, 75:1417-1430 [DOI] [PubMed] [Google Scholar]
- 34.Iritani I: Experimental study on embryogenesis of congenital diaphragmatic hernia. Anat Embryol 1984, 169:133-139 [DOI] [PubMed] [Google Scholar]
- 35.Nagaya M, Akatsuka H, Kato J, Niimi N, Ishiguro Y: Development in lung function of the affected side after repair of congenital diaphragmatic hernia. J Pediatr Surg 1996, 31:349-356 [DOI] [PubMed] [Google Scholar]
- 36.O’Toole SJ, Sharma A, Karamanoukian HL, Holm B, Azizkhan RG, Glick PL: Tracheal ligation does not correct the surfactant deficiency associated with congenital diaphragmatic hernia. J Pediatr Surg 1996, 31:546-550 [DOI] [PubMed] [Google Scholar]
- 37.Kitano Y, Davies P, von Allmen D, Adzick NS, Flake AW: Fetal tracheal occlusion in the rat model of nitrofen-induced congenital diaphragmatic hernia. J Appl Physiol 1999, 87:769-775 [DOI] [PubMed] [Google Scholar]
- 38.Wu J, Ge X, Verbeken EK, Gratacos E, Yesildaglar N, Deprest JA: Pulmonary effects of in utero tracheal occlusion are dependent on gestational age in a rabbit model of diaphragmatic hernia. J Pediatr Surg 2002, 37:11-17 [DOI] [PubMed] [Google Scholar]
- 39.Zgleszewski SE, Cilley RE, Krummel TM, Chinoy MR: Effects of dexamethasone, growth factors, and tracheal ligation on the development of nitrofen-exposed hypoplastic murine fetal lungs in organ culture. J Pediatr Surg 1999, 34:1187-1195 [DOI] [PubMed] [Google Scholar]