Abstract
The expression profiles of nine bladder cancer cell lines were compared against a pool containing equal total RNA quantities of each of them. Lower expression of KiSS-1 was revealed in cells derived from the most advanced bladder tumors. When comparing 15 primary bladder tumors versus a pool of four bladder cancer cell lines, lower transcript levels of KiSS-1 were observed in the invasive bladder carcinomas as compared to superficial tumors. KiSS-1 expression ratios provided prognostic information. The expression pattern of KiSS-1 transcripts was analyzed using in situ hybridization in nine bladder cancer cells, paired normal urothelium and bladder tumor samples (n = 25), and tissue microarrays of bladder tumors (n = 173). We observed complete loss of KiSS-1 in all invasive tumors under study as compared to their respective normal urothelium. The expression of KiSS-1 was found to be significantly associated with histopathological stage. Patients with lower KiSS-1 expression showed a direct correlation with overall survival in a subset of bladder tumors whose follow-up was available (n = 69). We did not observe any significant differential KiSS-1 expression along cell cycle by sorting analysis. A potential tumor suppressor role in bladder cancer was revealed for KiSS-1. Moreover, it showed predictive value by identifying patients with poor outcome.
Transitional cell carcinomas (TCCs) have been classified into two groups with distinct clinical behavior and different molecular profiles. Low-grade tumors are always papillary and usually superficial, whereas high-grade tumors can be either papillary or nonpapillary and are often invasive. Patients diagnosed with localized TCC have a 5-year relative survival rate of 93%. However, patients presenting with regional and distant disease spread have 5-year relative survival rates of 49% and 6%, respectively. 1 Bladder cancer progression and development of secondary metastases follow complex sequential steps characterized by alterations of many genes involved in critical cell functions, definitively not being completely understood. 2 It has been reported that genes on chromosome 6 could regulate expression of metastasis suppressor genes encoded elsewhere within the genome. 3-8 KiSS-1, mapping to chromosome 1q32, 8 is one of these genes regulated at chromosome 6. KiSS-1 expression was discovered to be reduced in metastatic melanoma. 3-6 Experimental and clinical studies have revealed its role as a functionally active metastasis suppressor gene in certain tumors. 5-9 Regulation of events downstream of cell-matrix adhesion involving cytoskeleton reorganization has been attributed to KiSS-1. 7,10-14 However, the mechanism by which KiSS-1 is involved in the invasive/metastatic phenotype has not been elucidated. A differential expression of KiSS-1 and a clone representing the regulatory counterpart of KiSS-1 on chromosome 6 3,4 were identified through molecular profiling analysis of bladder cancer cell lines and primary bladder tumors. We further evaluated the role of KiSS-1 in bladder cancer progression by characterizing its mRNA expression levels in a comprehensive collection of bladder cancer cell lines, as well as in normal urothelium, and in a large cohort of bladder tumors.
Materials and Methods
Cell Culture and RNA Extraction
Nine bladder cancer cell lines including T24, J82, 5637, HT-1376, RT4, SCaBER, TCCSUP, UMUC-3, and HT1197, were obtained from the American Type Culture Collection (Rockville, MD) and maintained following standard procedures. All cells were grown and harvested at 75 to 90% confluence no longer than four to six passages for the extraction of total RNA using RNeasy protocol (Qiagen, Valencia, CA). Cytospins were also prepared and later used for analysis of the expression of the targets under study. Total RNA from bladder tumors was isolated in two steps using TRIzol (Life Technologies, Inc., Grand Island, NY), followed by RNeasy (Qiagen) purification.
Preparation of cDNA Microarrays
Two separate studies of expression profiling in bladder cancer on bladder cancer cell lines and clinical material using different sets of cDNA microarrays were performed. First, a set of 8976 sequence-verified human IMAGE cDNA clones, representing both known genes and expressed sequence tags, was used to compare the expression profiles of nine bladder cancer cell lines (T24, J82, 5637, HT-1376, RT4, SCaBER, TCCSUP, UMUC-3, and HT1197) against a pool containing equal RNA quantities of each of them. The second set contained 17,842 known genes and expressed sequence tags and was used to study the transcriptome of 15 bladder cancer tumors against a pool containing equal RNA quantities of four selected bladder cancer cell lines: T24, J82, RT4, and HT1197. Clones were polymerase chain reaction amplified and spotted onto poly-l-lysine-coated microscope slides using a custom robot designed and built at Albert Einstein College of Medicine, New York, NY (sequence.aecom.yu.edu/bioinf/funcgenomic.html). 15
Labeling of cDNA, Hybridization to Arrays, Image Acquisition, and Normalization of the cDNA Microarrays
In the first gene expression analysis of the bladder cancer cell lines, 10 μg of total RNA of each cell line was labeled with Cy5 and hybridized against 10 μg of total RNA of a pool containing equal RNA quantities of each of these cell lines labeled with Cy3. 16 In the expression-profiling study of the bladder tissues, 5 μg of total RNA of each bladder tissue and pool of cell lines was linearly amplified using a single amplification round. 17 Amplified cRNA obtained from bladder tissues (normal or tumor) were labeled with Cy5 and hybridized against amplified cRNA obtained from a pool containing equal RNA quantities of the four cell lines labeled with Cy3. After hybridization, slides were washed, dried, and scanned by an Axon automated laser scanner. GenePix software was used for gridding and calculation of red and green signal intensities. 18 Before any analysis, plots of the fold change versus the average intensity were examined to look for abnormalities in single-array data. Samples were normalized using this intensity-dependent normalization using the Splus function lowess. 19 We focused on the expression results in these two independent studies of two clones representing KiSS-1 and 6q16.2-21, whose accession numbers were AA464595 and N76881, respectively. We collected the red to green ratio (R/G) expression data of these two clones for the analysis of this study.
Clinical Evaluation of the Expression of KiSS-1
Tissue Samples and Microarrays
Twenty-five paired normal urothelium and respective bladder tumors were first evaluated. All these tumors presented high tumor grade. Three different bladder cancer microarrays also were constructed for this study. All these specimens were obtained under Institutional Review Board-approved protocols. Normal and tumor tissues were embedded in paraffin and 5-μm sections were stained with hematoxylin and eosin to identify viable, morphologically representative areas of the specimen from which needle core samples were taken, using a precision instrument (Beecher Instruments, Silver Spring, MD). From each specimen triplicate cores with diameters of 0.6 mm were punched and arrayed on the recipient paraffin block. 20 Five-μm sections of these tissue array blocks were cut and placed on charged poly-l-lysine-coated slides and used for in situ hybridization analysis.
These tissue microarrays included a total of 173 bladder primary TCC tumors. Tumor stage and grade were defined according to consensus criteria. 21,22 A total of 40 superficial (TIS, pTa, T1) and 64 invasive (pT2, pT3, pT4) TCC tumors were analyzed in two microarrays. These tumors corresponded to 14 grade 1, 8 grade 2, and 82 grade 3 lesions. The third tissue microarray comprised a cohort of 69 well-characterized bladder primary TCC cases with known p53 and pRB status, and consisted of two superficial (TIS, pTa, T1) and 67 invasive lesions (pT2, pT3, pT4).
In Situ Hybridization
Human KiSS-1 cDNA sequence (GenBank accession number, AA464595) was obtained from the set of clones used in the cDNA microarrays. Recombinant plasmid (1 μg) was linearized by EcoRI and NotI. Riboprobes were generated with T7 and T3 polymerases for 2 hours at 37°C in 1× transcription buffer (Boehringer Mannheim, Indianapolis, IN) containing 20 U of RNase inhibitor; 1 mmol/L each of ATP, GTP, and CTP; 6.5 mmol/L of UTP; and 3.5 mmol/L of digoxigenin-UTP. Deparaffinized tissue sections were rinsed in water and phosphate-buffered saline for 10 minutes. Slides were digested in prewarmed in citrate buffer (pH = 6) for 5 minutes in microwave at full power. Prehybridization was performed for 30 minutes at 45°C in 50% deionized formamide and 2× standard saline citrate. For hybridization, 10 pmol/L of digoxigenin-labeled riboprobe was added to 50 μl of hybridization buffer [50% deionized formamide (v/v), 10% dextran sulfate, 2× standard saline citrate, 1% sodium dodecyl sulfate, and 0.25 mg/ml of herring sperm DNA]. After overnight incubation at 45°C, slides were washed twice for 20 minutes in prewarmed 2× standard saline citrate at 42°C, followed by two washes in prewarmed 1× standard saline citrate at 42°C for 20 minutes. Slides were then incubated in normal sheep serum diluted in buffer 1 (2 mol/L Tris-HCl, 5 mol/L NaCl, pH 7.5) for 30 minutes followed by incubation in the same buffer with antibody digoxigenin-AP (Boehringer Mannheim) at a dilution of 1:500 for 2 hour at room temperature. Visualization was accomplished by nitro blue tetrazolium 5-bromo-4-chloro-3-indolylphosphate. The slides were counterstained with methyl green and mounted. 23
Immunohistochemistry
Protein patterns of expression of p53, pRB, and certain adhesion molecules were assessed at the microanatomical level, using both cytospin from all cell lines and tissue samples outlined above. Standard immunoperoxidase procedures were used for immunohistochemistry. The following antibodies were used: E-cadherin, mouse monoclonal clone 36 at 1:1000 (2.5 μg/ml) (BD Transductions Labs, Lexington, KY); moesin, mouse monoclonal clone 38/87 at 1:50 (4 μg/ml) with microwave pretreatment of the slides (Neomarkers, Fremont, CA); zyxin, mouse monoclonal clone 21 at 1:25 (10 μg/ml) with microwave pretreatment of the slides (Transduction Labs); underphosphorylated pRB, mouse monoclonal clone G99-549 at a final concentration of 10 μg/ml (PharMingen, San Diego, CA); total RB, mouse monoclonal clone 3C8 at a final concentration of 1.2 μg/ml (QED Bioscience, San Diego, CA); p53 mouse monoclonal clone 1801 at 0.2 μg/ml (Calbiochem, Cambridge, MA); p21/WAF1, mouse monoclonal Ab-1 at 5 μg/ml (Calbiochem); cyclin D1, mouse monoclonal 1 μg/ml (Calbiochem); cyclin E, purified rabbit antiserum (1:500, supplied as a tissue culture supernatant by Dr. A. Koff, Memorial Sloan-Kettering Cancer Center). Staining conditions were optimized on sections from formalin-fixed, paraffin-embedded tissue controls for each antibody as specified by manufacturers. Antibody reactivity was detected by using diaminobenzidine as chromogen, and sections were counterstained with hematoxylin. The primary antibody was omitted for negative controls.
Data Analysis
All TCCs (n = 173) were used for the analysis of association between KiSS-1 p53, pRB, and other cell-cycle and adhesion molecules evaluated by immunohistochemistry. These cases were also used for evaluating KiSS-1 mRNA expression versus histopathological stage and tumor grade, using the nonparametric Wilcoxon-Mann-Whitney and Kruskall-Wallis tests. 24 The consensus value of the three representative cores from each tumor sample arrayed was used for statistical analyses. KiSS-1 expression values were displayed as negative to low (0 to 20%), intermediate (20 to 40%), and high (>40%). These cutoffs were selected to represent low different degrees of expression of KiSS-1.
The prognostic value of the ratios of KiSS-1 and 6q16.2-21 was evaluated in the subsets of 15 patients to whom cDNA microarray analysis were performed. The relationship of the in situ hybridizations of KiSS-1 to outcome was also evaluated using a subset of 69 TCC cases for which follow-up was available. Specific of disease overall survival time was defined as the months elapsed between transurethral resection (two superficial lesions) or cystectomy (rest of cases) and death from disease (or the last follow-up date). Patients who were alive at the last follow-up or lost to follow-up were censored. For survival analysis of the patients to whose tumors expression profiles were analyzed using cDNA microarrays, ratios of KiSS-1 and 6q16.2-21 and mRNA levels of KiSS-1 were analyzed as categorical variables taking the cutoffs of 1.5 and 4.0, respectively. When we evaluated the prognostic value of the mRNA levels of KiSS-1 obtained by in situ hybridization on the cohort of 69 patients, we used the expression cutoff of 40% to define the expression of KiSS-1 as high or positive. This cutoff was selected to represent low and high degrees of expression of KiSS-1. The association of the KiSS-1 expression levels with overall survival was analyzed using the log-rank test. Survival curves were plotted using standard Kaplan-Meier methodology. 24 Associations between biomarkers were analyzed using Kendall’s tau test using the SPSS statistical package (version 8.0).
Cell Sorting
Cells were grown and collected with trypsin at 80% confluency. They were washed with phosphate-buffered saline and stained with Hoechst 33342 for 2 hours at 37°C. Cell cycle subpopulations G1, S, and G2M were sorted using a MoFlow sorter. Cytospins of each cell cycle phase were fixed with methanol:acetone (1:1 v/v) during 10 minutes at −20°C and kept at −80°C until the analysis of KiSS-1 by in situ hybridization.
Results
Experimental Design
The present analysis was conducted under four major sets of experiments. Initially, bladder cancer cell lines and primary bladder tumor samples were analyzed using cDNA microarrays and KiSS-1 was identified as a differentially expressed gene between distinct histopathological tumor types and stages of the disease. Paired normal urothelium and tumor were evaluated to assess the potential involvement of KiSS-1 in the progression of the disease. Third, tissue microarrays were used to validate the potential clinical significance of KiSS-1 at the microanatomical detail using in situ hybridization on tissue samples of well-characterized cases. A cohort of superficial and invasive bladder neoplasms was used to evaluate the association between KiSS-1 with histopathological stage and grade. An additional tissue microarray, containing bladder tumors from cases with annotated follow-up, was used to delineate associations of KiSS-1 with clinical outcome. Finally, KiSS-1 expression along cell cycle was explored using cytospins containing enriched cell-cycle populations of the bladder cancer cell lines under study obtained by sorting analysis.
Identification of a Differential Expression of KiSS-1 in Bladder Cancer Using cDNA Microarrays
We identified differential expression of KiSS-1 in bladder cancer in two independent profiling approaches. First, the expression profiling of nine bladder cancer cell lines: T24, J82, 5637, HT-1376, RT4, SCaBER, TCCSUP, UMUC-3, and HT1197, was compared against a pool containing equal RNA quantities of each of them using cDNA arrays containing 8976 clones. This analysis revealed low expression of KiSS-1 in most of the cell lines derived from the most advanced bladder carcinomas as compared to the ones obtained from a papillary tumor (RT4) (Table 1) ▶ .
Table 1.
Differential Red-to-Green Ratios of KiSS-1 and 6q16.2-21 among Bladder Cancer Cell Lines Out of the cDNA Microarrays Comparing Each of the Cell Lines (Cy5) Versus a Pool Containing Equal RNA Quantities of Each of Them (Cy3)
| Bladder cancer cell line | R/G ratio of KiSS-1 (AA464595) | R/G ratio of 6q16.2-21 (N76881) |
|---|---|---|
| UMUC-3 | 0.9 | 0.98 |
| 5637 | 1.22 | 1.15 |
| HT1197 | 1.03 | 0.88 |
| HT1376 | 0.74 | 0.97 |
| J82 | 2.42 | 1.1 |
| T24 | 1.04 | 1.05 |
| SCABER | 0.76 | 1.32 |
| TCCSUP | 0.8 | 1.04 |
| RT4 | 2.82 | 1.88 |
The expression profiles of 15 bladder cancer tumors was analyzed versus a pool containing equal RNA quantities of four selected bladder cancer cell lines: T24, J82, RT4, and HT1197 using cDNA microarrays containing 17,842 known genes and expressed sequence tags (Table 1) ▶ . We observed that transcripts of KiSS-1 were highly expressed in superficial noninvasive tumors when compared to organ-confined invasive and primary invasive TCCs of patients that developed metastatic lesions (Table 2) ▶ . We also observed that messenger expression levels of the 6q16.2-21 clone displayed similar variations as the KiSS-1 gene in both tumor cell lines and clinical samples analyzed (Tables 1 and 2) ▶ ▶ .
Table 2.
Differential Red-to-Green Ratios (R/G) of KiSS-1 and 6q16.2-21 Obtained from the cDNA Microarray Analysis Comparing Bladder Tumors (R) and a Pool of Bladder Cancer Cell Lines (G)
| Patient ID | KiSS-1 R/G (AA464595) | 6q16.2-21 R/G (N76881) | Age | Sex | TNM | Carcinoma in situ | Smoking habit | Familial cancer history | Follow-up (months) | Clinical outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 174 | 4.58 | 12.77 | 67 | M | TISG3N0 | + | − | − | 12 | NED |
| 160 | 2.60 | 7.10 | 83 | M | TAG1N0 | + | − | − | 20 | NED |
| 157 | 3.01 | 15.36 | 61 | M | TISG3N0 | + | − | − | 44 | NED |
| 134 | 2.60 | 15.64 | 80 | M | T3BG3NO | + | + | + | 13 | NED |
| 170 | 0.92 | 1.50 | 55 | M | T4G3N0M1 | + | + | NK | 4 | DOD |
| 168 | 1.51 | 2.71 | 61 | M | T4BG3N1M2 | + | + | NK | 1 | DOD |
| 169 | 1.98 | 4.11 | 75 | F | TAG3NO | − | − | NK | 41 | NED |
| 165 | 1.41 | 4.26 | 75 | M | TAG1NO | − | − | − | 11 | NED |
| 163 | 1.69 | 3.54 | 65 | M | TAG1NO | − | + | + | 17 | NED |
| 162 | 3.34 | 19.26 | 60 | M | TISG3NO | + | + | + | 15 | NED |
| 141 | 1.25 | 5.18 | 49 | F | TAG3M1 | − | + | + | 3 | DOD |
| 135 | 1.48 | 1.90 | 64 | F | T4BG3N1 | + | + | + | 11 | DOD |
| 133 | 1.27 | 5.43 | 83 | M | T3BG3NO | + | + | + | 1 | DOD |
| 130 | 1.11 | 2.30 | 72 | M | T3BG3N0 | − | − | − | 13 | NED |
| 124 | 1.82 | 2.00 | 59 | F | T3AG3N1M0 | − | + | − | 9 | NED |
Accession numbers of KiSS-1 and 6q16.2-21 are included in brackets. NK, not known; NED, no evidence of disease; DOD, death of disease.
We evaluated whether the expression ratios of KiSS-1 and 6q16.2-21 obtained from the cDNA microarray analysis of the patients with bladder tumors could provide additional predictive information. The overall survival analysis revealed that KiSS-1 ratios were significantly associated with overall survival in this subset of 15 bladder lesions (Figure 1) ▶ . Patients whose tumor samples were subjected to gene profiling had a median follow-up of 12 months (mean, 14.3 months; range, 1 to 44 months) (Table 2) ▶ . We further investigated the prognostic utility of KiSS-1 for overall survival in a separate set of patients with bladder cancer (see below).
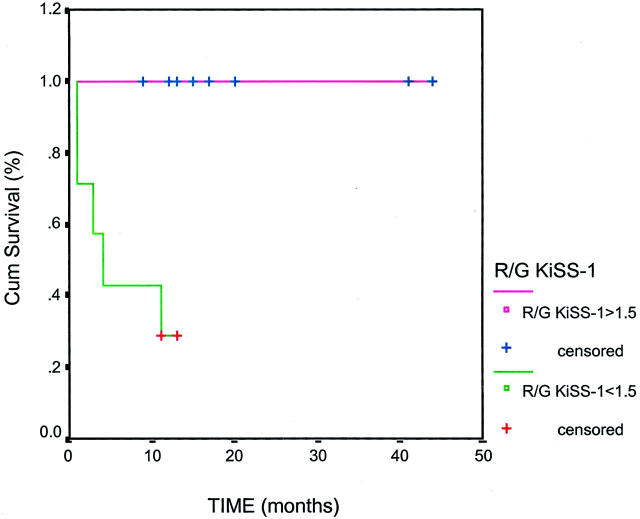
Figure 1.
Kaplan-Meier curves obtained from the survival analysis of R/G ratios of KiSS-1 in the patients with bladder tumors to whom cDNA microarrays were performed. Low expression of KiSS-1 is represented by bladder tumors displaying a R/G ratio <1.5 versus those patients with high expression of KiSS-1 and R/G ratio >1.5. KiSS-1 was found to be significantly associated with overall survival in this subset of 15 bladder lesions whose expression profiles were analyzed (P = 0.008) (median follow-up time, 2 months). No association with overall survival was found for the R/G ratios of 6q16.2-21.
KiSS-1 Expression Studies in Normal Urothelium and Bladder Tumors
The differential expression of KiSS-1 in the progression of bladder cancer was evaluated in 25 paired normal urothelium and respective bladder tumors. High positive expression of KiSS-1 was observed in all normal urothelium samples (Figure 2) ▶ . All superficial tumors showed intermediate or high expression of KiSS-1. Three invasive patients displayed intermediate expression of KiSS-1, whereas undetectable to low expression was observed in 80% (14 of 17) of the invasive tumors. All primary bladder tumors that developed distant metastases showed complete loss of KiSS-1. KiSS-1 expression levels between superficial and invasive tumors were found to reach a significant association (Mann-Whitney, P = 0.001). All these tumors presented high tumor grade. We evaluated the potential correlation between KiSS-1 expression and the suspicion of vascular invasion, positive nodes, multifocality, squamous differentiation, and carcinoma in situ (Table 3) ▶ . Interestingly, we only observed a significant association of KiSS-1 expression with those patients in which vascular invasion was reported (Mann-Whitney, P = 0.012).
Figure 2.
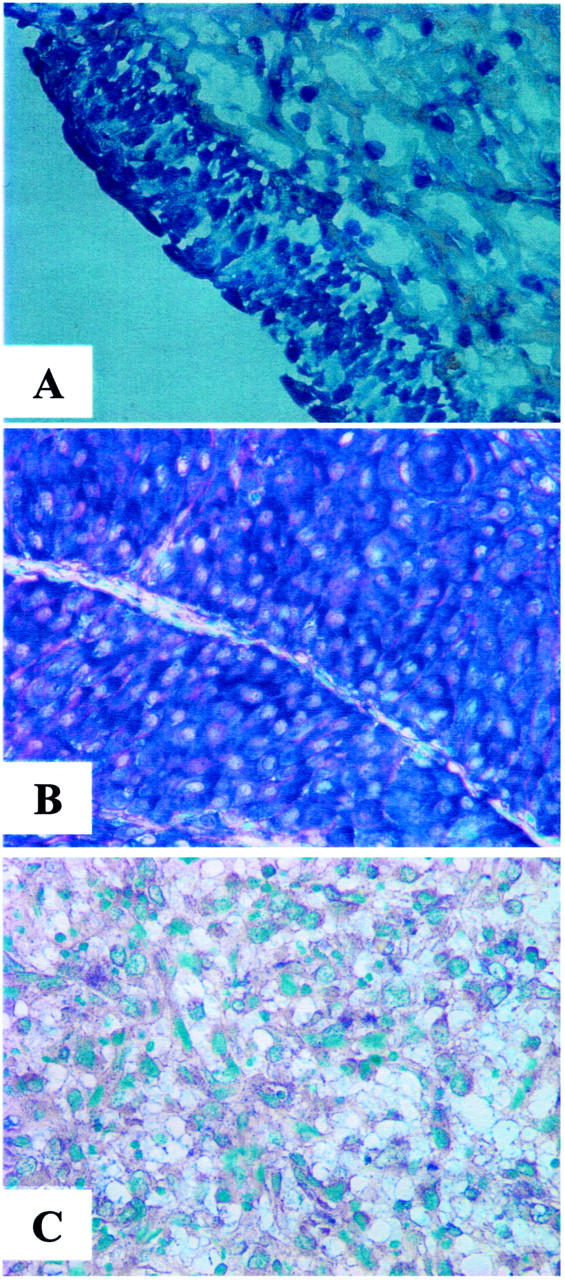
Representative expression patterns of KiSS-1 in normal urothelium (A), and transitional superficial (B) and invasive (C) bladder tumors. High expression levels of KiSS-1 were observed in normal urothelium and superficial bladder tumors as compared to the invasive bladder tumor. There was a significant difference regarding the expression of KiSS-1 regarding tumor stage and grade. Original magnifications, ×200.
Table 3.
Messenger Expression of KiSS-1 by in Situ Hybridization in the Paired Normal Urothelium (NU) and Bladder Tumors (TM) Regarding Their Histopathological Stage, Grade, the Presence of Positive Lymph Nodes (LN) and Vascular Invasion (VI), Multifocality (MF), Carcinoma in Situ (IS), and Squamous Differentiation (SQ)
| ID | KiSS-1 NU | KiSS-1 TM | Age | Sex | Stage | LN | VI | MF | IS | SQ |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | >40% | <20% | 64 | M | PT2 | − | − | + | + | − |
| 2 | >40% | 20–40% | 42 | M | PTA | − | − | − | − | − |
| 3 | >40% | 20–40% | 58 | F | PT3B | + | + | − | + | + |
| 4 | >40% | 20–40% | 64 | M | PT3B | + | − | − | + | − |
| 5 | >40% | <20% | 79 | M | PT3A | − | − | − | − | + |
| 6 | >40% | >40% | 43 | M | IS | − | − | + | + | − |
| 7 | >40% | <20% | 69 | M | IS | − | − | − | + | − |
| 8 | >40% | 20–40% | 78 | F | PT3B | − | − | − | + | + |
| 9 | >40% | >40% | 75 | M | PT1 | − | − | − | − | − |
| 10 | >40% | >40% | 73 | M | IS | − | − | − | + | − |
| 11 | >40% | <20% | 69 | F | PT2 | − | − | + | + | − |
| 12 | >40% | <20% | 66 | F | PT4A | + | + | − | − | + |
| 13 | >40% | >40%(NT) | 61 | M | PT1 | − | − | − | − | − |
| 14 | >40% | >20% | 66 | M | PT1 | − | − | − | − | − |
| 15 | >40% | <20% | 69 | M | PT3B | + | + | − | − | − |
| 16 | >40% | <20% | 79 | M | PT3A | − | − | − | + | − |
| 17 | >40% | <20% | 57 | M | PT3B | − | + | + | + | + |
| 18 | >40% | <20% | 71 | M | PT2 | − | − | − | + | − |
| 19 | >40% | <20% | 64 | F | PT4A | + | + | + | − | − |
| 20 | >40% | <20% | 86 | F | PT3B | − | + | + | + | − |
| 21 | >40% | <20% | 61 | M | PT3B | + | + | + | + | − |
| 22 | >40% | <20% | 68 | M | PT3B | + | + | − | + | − |
| 23 | >40% | <20% | 74 | M | PT3B | − | + | − | + | − |
| 24 | >40% | <20% | 66 | M | PT3B | − | + | + | + | − |
| 25 | >40% | 20–40% | 67 | M | PT1 | − | − | + | − | − |
NT, No tumor was detected in one of the patient specimens. However, the specimen analyzed lacking tumor cells showed a positive expression of KiSS-1.
We studied the potential role of KiSS-1 expression to identify patients with superficial and invasive disease. KiSS-1 expression was analyzed by in situ hybridization in primary tumors spotted onto the superficial and invasive tissue microarrays. The majority of superficial tumors displayed high expression of KiSS-1 in 20 of 32 (62.5%) of the cases. In contrast, intermediate or undetectable levels of KiSS-1 were detected in 56.1% of the invasive tumors. KiSS-1 expression was significantly associated with stage (Mann Whitney, P = 0.031) and not with tumor grade in our series (Table 4) ▶ .
Table 4.
Descriptive Analysis of the Percentage of Patients Displaying Differential KiSS-1 mRNA Expression by in Situ Hybridization Regarding with Stage and Grade in the Bladder Tumors that Provided Evaluable KiSS-1 Contained in the Three Tissue Microarrays
| KiSS-1, <20% (low) | KiSS-1, 20–40% (intermediate) | KiSS-1, >40% (high) | |
|---|---|---|---|
| Stage | |||
| Superficial (n = 32) | 5 (15.6%) | 7 (21.9%) | 20 (62.5%) |
| Invasive (n = 114) | 40 (35.1%) | 24 (21.1%) | 50 (43.8%) |
| Grade | |||
| I (n = 20) | 4 (20.0%) | 5 (25.0%) | 11 (55.0%) |
| II (n = 8) | 1 (12.5%) | 2 (25.0%) | 5 (62.5%) |
| III (n = 118) | 40 (33.9%) | 24 (20.3%) | 54 (45.8%) |
The association of the expression of KiSS-1 with overall survival was evaluated in a subset of 69 bladder tumors spotted onto a tissue microarray whose follow-up was available. There were 16 patients that displayed a KiSS-1 expression lower than 20% in this series. Their mean survival time was 14.7 months (95% CI, 8.9 to 20.4) with a median survival time of 9.0 months. Only one patient (6.25%) was alive among those tumors showing less than 20% in KiSS-1. Patients showing expression of KiSS-1 higher than 20% showed a mean survival time was 47.3 months (95% CI, 33.5 to 61.2) with a median survival time of 37.0 months. In this case, 23 of 48 patients showing KiSS-1 higher than 20% were censored alive (47.92%). KiSS-1 expression reached a significant association with overall survival (Figure 3) ▶ . We also observed that all bladder tumors developing distant metastases showed a complete loss of KiSS-1 within this cohort of follow-up patients.
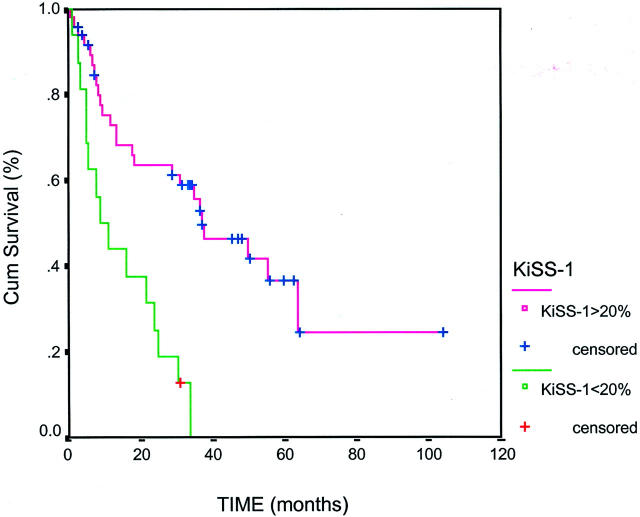
Figure 3.
Kaplan-Meier curve obtained from the survival analysis of the expression of KiSS-1 in a subset of 69 bladder lesions contained in a tissue microarray. KiSS-1 expression was analyzed by in situ hybridization in this separate subset of patients whose median follow-up time of 36 months. KiSS-1 was found to be significantly associated with overall survival (P = 0.03).
We evaluated the association of KiSS-1 expression with a selected group of genes previously reported to be altered during bladder cancer progression, including p53, total and underphosphorylated retinoblastoma, cyclin E, p21, moesin, zyxin, and E-cadherin. Immunohistochemical expression levels of these proteins were characterized in the bladder tumors contained in our tissue microarrays. The loss of KiSS-1 expression was significantly associated with total retinoblastoma (P = 0.034, n = 142) and E-cadherin expression (P = 0.002, n = 144). Thus, the expression of KiSS-1 was associated with the phosphorylation status of pRB and altered expression of adhesion E-cadherin.
Expression of KiSS-1 along Cell Cycle
We were interested in evaluating the potential association of KiSS-1 expression along cell cycle. The expression of KiSS-1 was evaluated on cytospins by in situ hybridization containing each cell cycle phase-enriched population obtained by sorting analysis of each of the cell lines under study. We noted a differential expression of KiSS-1 among samples in accordance with the expression levels obtained by the DNA analysis. We confirmed that the differential expression of KiSS-1 among the various bladder tumor cells lines analyzed by Northern blotting and quantitative reverse transcriptase-polymerase chain reaction (data not shown). However, we did not observe any significant differential messenger expression of KiSS-1 among the cell-cycle cytospins (Figure 4) ▶ .
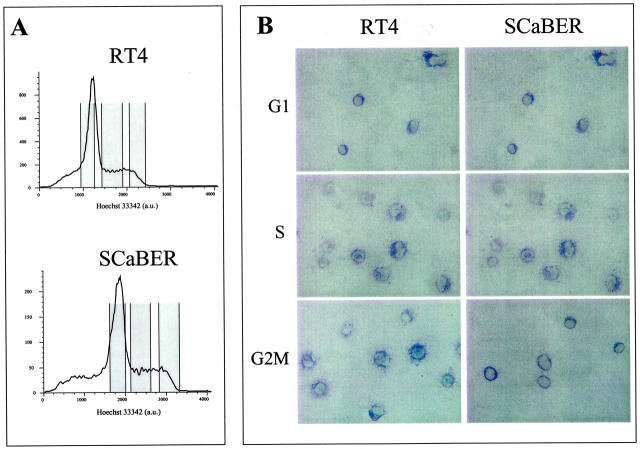
Figure 4.
A: Distribution of cell-cycle phases obtained from the cell-sorting analysis of RT4 and SCaBER. Cell-cycle-specific cytospins including cell-cycle subpopulations were obtained to evaluate the expression of KiSS-1 along cell cycle by in situ hybridization. B: Representative patterns of KiSS-1 in bladder cancer cell lines under study by in situ hybridization derived from a transitional superficial tumor (RT4) and a squamous bladder tumor (SCaBER). No differential expression of KiSS-1 by in situ hybridization was observed in these sorted cells spotted on cytospins containing enriched populations of each cell cycle phase. Original magnifications, ×200.
Discussion
Expression profiles analyses using cDNA microarrays identified in this study a differential expression of KiSS-1 in bladder cancer, providing clinical information of added predictive nature. KiSS-1 expression was high in normal urothelium, while transcript levels of KiSS-1 were shown to be associated with stage and grade in primary bladder cancer. Moreover, undetectable to low KiSS-1 expression was related to survival when validated in tissue microarrays containing primary bladder tumors.
The advent of high-throughput methods of molecular analysis is allowing the further characterization of distinct gene expression profiles of specific tumor subtypes. 25,26 The use of cDNA microarrays aims at identifying prevalent and potentially clinically relevant alterations within human tumors. In this study, the combination of cDNA and tissue microarray validation addressed the relevance of the KiSS-1 in bladder cancer. This combination of technologies has been shown to accelerate molecular studies that seek associations between genetic and epigenetic molecular changes and clinicopathological variables, including patient outcome. The present study was initiated based on the differential gene expression of the KiSS-1 gene in bladder cancer in two cDNA expression analyses. The first set of data were obtained by comparing each of nine bladder cancer cell lines against their pool, representing an optimal approach for comparing the expression levels of KiSS-1 of each of the cell lines, because each of them was considered in the reference. Interestingly, the two bladder cancer cells derived from low-stage (RT4) or low-grade (5637) tumors showed the highest ratios of KiSS-1 and 6q16.2-21. These observations were then followed by the analyses of a second set of data obtained by the study of expression profiles of normal urothelium and clinical tumor samples representative of different stages of the disease. We observed that tumors displayed lower transcript levels of KiSS-1 gene as compared to normal urothelium. A minimum of 75% enrichment of tumor cells was granted by macrodissection of the primary clinical samples before RNA extraction. Based on the lack of expression of KiSS-1 in stromal elements, such as fibroblasts, we believe that the expression ratios of KiSS-1 obtained in the cDNA expression profiling account specifically for the tumor cells.
The loss of KiSS-1 expression in bladder tumors as compared to their paired normal urothelium may support an involvement of this gene in bladder cancer progression. We have observed a highly positive messenger expression of KiSS-1 in superficial tumors including all pT1 lesions. This observation together with the finding that many of the invasive tumors already show partial or complete loss in KiSS-1 expression, and that primary tumors from all patients developing distant metastasis showed a KiSS-1 loss, support that KiSS-1 might have a tumor suppressor role and be a late event in the progression of the disease. Based on these results, it cannot be discarded that KiSS-1 might be related to the development of distant metastasis, as per the metastasis suppressor role already reported in melanoma, 4,5 and suggested also in other human cancers such as breast cancer 7 or thyroid tumors. 9 Moreover, this differential expression was associated with histopathological criteria and, more interestingly with clinical outcome.
KiSS-1 gene maps to 1q32-q41, 8 a region frequently deleted in certain tumors, including melanoma. In the present study, it is interesting to note that not only KiSS-1 was found differentially expressed, but also clones representing the regulatory chromosomal counterpart in chromosome 6, had similar changes. 27 We observed that ratios of KiSS-1 correlated with those of 6q16.2-21 in the bladder cancer cell lines and the tumor samples under cDNA analysis. This is an interesting observation, offering mechanistic support for the involvement of KiSS-1 in bladder cancer progression and suggesting a potential tumor suppressor role for KiSS-1. Moreover, aberrations, deletions, and loss of heterozygosity in chromosome 6 have been reported to be frequent in late-stage bladder tumors. 28-30 The lack of suitable antibodies can slow down the performance of more extensive clinical studies for KiSS-1 at the microanatomical level by immunohistochemistry. In this regard, the analysis by in situ hybridization has allowed us to circumvent this deficiency to study clinical material. KiSS-1 expression has been evaluated by Northern blot analysis in several tissues, including heart, brain, liver, lung, skeletal muscle, kidney, pancreas, and placenta. 6 To our knowledge, this is the first report of the expression of KiSS-1 in the urothelium.
The mechanism by which KiSS-1 is involved in the invasive/metastatic phenotype has not been completely elucidated. Biologically, the inactivation of the key regulatory RB1 and TP53 pathways has been shown to be necessary for bladder cancer development and progression. 31-33 We evaluated whether KiSS-1 expression was related to molecular targets of these known pathways, including p53, p21, and RB. 29-34 Based on the believed involvement of KiSS-1 to regulate events downstream of cell-matrix adhesion, independent or not to cytoskeleton reorganization, 7,9 we incorporated to the analysis data set related to adhesion proteins already studied in this cohort of patients, including moesin, zyxin, and E-cadherin. We observed that the loss of KiSS-1 expression was present in those patients that had also lost membrane E-cadherin expression, a gene described to display a metastasis suppressor role in several tumors, including breast 35 and bladder cancer. 36 This observation may indicate a cooperative involvement of KiSS-1 and E-cadherin in the suppression of metastatic potential of bladder tumors. KiSS-1 might function as an upstream regulator of adhesion molecules such as E-cadherin. 14 The association found between expression of KiSS-1 and the inactive hyperphosphorylated pRB products (total pRB), critical in the G1/S transition, 34 led us to investigate the variation of KiSS-1 messenger expression along the cell cycle. We generated cytospins containing enriched subpopulations of the distinct cell-cycle phases of the bladder cancer cell lines under investigation. However, there was no association between KiSS-1 expression and cell-cycle phases in the bladder cancer cell lines analyzed. To our knowledge, this is the first report on expression of KiSS-1 along cell cycle in bladder cancer cells. The expression levels of KiSS-1 in T24 that we have observed by cDNA microarray analysis and in situ hybridization are consistent with a report evaluating its mRNA expression in T24, one of the bladder cancer cell lines that we have incorporated in our study. 37
Overall, gene expression profiling identified the potential involvement of KiSS-1, a novel molecular target in bladder cancer. The loss of its messenger expression was related to tumor stage and grade when validated in human tissue microarrays containing primary bladder tumors. It was also shown to be associated with pRB and adhesion networks, suggesting a mechanistic support for its involvement in the progression of the disease. The potential tumor suppressor role of KiSS-1 in bladder cancer suggested in our results requires further investigation. Most importantly, KiSS-1 was shown to be of clinical relevance by the predictive nature of its loss of expression with the outcome of patients with bladder cancer.
Acknowledgments
We thank Geoffrey Childs, Thomas Harris, Aldo Massimi, and Thomas Belbin from the Albert Einstein College of Medicine, for their assistance in cDNA technology; and Minglan Lu and Elizabeth Charytonowizc for their contribution in the preparation of the tissue microarrays.
Footnotes
Address reprint requests to Marta Sanchez-Carbayo, Ph.D., Division of Molecular Pathology, Memorial Sloan-Kettering Cancer Center, 1275 York Ave., New York, NY 10021. E-mail: sanchezm@mskcc.org.
References
- 1.: Cancer Facts and Figures 2000.: American Cancer Society 2000,
- 2.Fidler IJ: Critical factors in the biology of human cancer metastasis. Cancer Res 1990, 50:6130-6138 [PubMed] [Google Scholar]
- 3.Welch DR, Chen P, Miele ME, McGary CT, Bower JM, Weissman BE, Standbridge EJ: Microcell-mediated transfer of chromosome 6 into metastatic human C8161 melanoma cells suppresses metastasis but does not inhibit tumorigenicity. Oncogene 1994, 9:255-262 [PubMed] [Google Scholar]
- 4.Miele ME, Robertson G, Lee JH, Coleman A, McGarry CT, Fisher PB, Lugo TG, Welch DR: Metastasis suppressed, but tumorigenicity and local invasiveness unaffected in the human melanoma cell line MelJuSo after introduction of human chromosomes 1 or 6. Mol Carcinog 1996, 15:284-299 [DOI] [PubMed] [Google Scholar]
- 5.Lee JH, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, Welch DR: KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst 1996, 88:1731-1737 [DOI] [PubMed] [Google Scholar]
- 6.Lee JH, Welch DR: Identification of highly expressed genes in metastasis-suppressed chromosome 6/human malignant melanoma hybrid cells using substractive hybridization and differential display. Int J Cancer 1997, 71:1035-1044 [DOI] [PubMed] [Google Scholar]
- 7.Lee JH, Welch DR: Suppression of metastasis in human breast carcinoma MDA-MB-435 cells after transfection with the metastasis suppressor gene, KiSS-1. Cancer Res 1997, 57:2384-2387 [PubMed] [Google Scholar]
- 8.West A, Votja PJ, Welch DR, Weissman BE: Chromosome localization and genomic structure of the KiSS-1 metastasis suppressor gene. Genomics 1998, 54:145-148 [DOI] [PubMed] [Google Scholar]
- 9.Ringel MD, Hardy E, Bernet VJ, Burch HB, Schuppert F, Burman KD, Saji M: Metastin receptor is overexpressed in papillary thyroid cancer and activated MAP kinase in thyroid cancer cells. J Clin Endocrinol Metab 2002, 87:2399-2402 [DOI] [PubMed] [Google Scholar]
- 10.Yan C, Wang H, Boyd DD: KiSS-1 represses 92-kDa type IV collagenase expression by down-regulating NF-kappa B binding to the promoter as a consequence of Ikappa Balpha-induced block of p65/p50 nuclear translocation. J Biol Chem 2001, 276:1164-1172 [DOI] [PubMed] [Google Scholar]
- 11.Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, Ishibashi Y, Watanabe T, Asada M, Yamada T, Suenaga M, Kitada C, Usuki S, Kurokawa T, Onda H, Nishimura O, Fujino M: Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature 2001, 411:613-617 [DOI] [PubMed] [Google Scholar]
- 12.Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, Brezillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, Blanpain C, Schiffmann SN, Vassart G, Parmentier M: The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem 2001, 276:34631-34636 [DOI] [PubMed] [Google Scholar]
- 13.Hori A, Honda S, Asada M, Ohtaki T, Oda K, Watanabe T, Shintani Y, Yamada T, Suenaga M, Kitada C, Onda H, Kurokawa T, Nishimura O, Fujino M: Metastin suppresses the motility and growth of CHO cells transfected with its receptor. Biochem Biophys Res Commun 2001, 286:958-963 [DOI] [PubMed] [Google Scholar]
- 14.Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, Szekeres PG, Sarau HM, Chambers JK, Murdock P, Steplewski K, Shabon U, Miller JE, Middleton SE, Darker JG, Larminie CG, Wilson S, Bergsma DJ, Emson P, Faull R, Philpott KL, Harrison DC: AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem 2001, 276:28969-28975 [DOI] [PubMed] [Google Scholar]
- 15.Cheung VG, Morley M, Aguilar F, Massimi A, Kucherlapati R, Childs G: Making and reading microarrays. Nat Genet 1999, 21:S15-S19 [DOI] [PubMed] [Google Scholar]
- 16.Stears RL, Getts RC, Gullans SR: A novel, sensitive detection system for high-density microarrays using dendrimer technology. Physiol Genomics 2000, 3:93-99 [DOI] [PubMed] [Google Scholar]
- 17.Wang E, Miller LD, Ohnmacht GA, Liu ET, Marincola FM: High-fidelity mRNA amplification for gene profiling. Nature Biotechnol 2000, 18:457-459 [DOI] [PubMed] [Google Scholar]
- 18.Eisen MB, Spellman PT, Brown PO, Botstein D: Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA 1998, 95:14863-14868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Felsenstein J: Confidence limits on phylogenies: an approach using the bootstrap. Evolution 1985, 39:783-791 [DOI] [PubMed] [Google Scholar]
- 20.Hoos A, Urist MJ, Stojadinovic A, Mastorides S, Dudas ME, Leung DHY, Kuo D, Brennan MF, Lewis JJ, Cordon-Cardo C: Validation of tissue microarrays for immunohistochemical profiling of cancer specimens using the example of human fibroblastic tumors. Am J Pathol 2001, 158:1245-1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Joint Committee on Cancer: Staging of cancer at genitourinary sites. American Joint Committee on Cancer: Manual for Staging of Cancer ed 3 1988:pp 194-195 J. B. Lippincott Co., Philadelphia
- 22.Mostofi FK: Histological Typing of Urinary Bladder Tumors. 1973. World Health Organization, Geneva
- 23.Capodieci P, Magi-Galluzi C, Moreira G, Jr, Zeheb R, Loda M: Automated in situ hybridization: diagnostic and research applications. Diagn Mol Pathol 1998, 7:69-75 [DOI] [PubMed] [Google Scholar]
- 24.Dawson-Saunders B, Trapp RG: Basic and Clinical Biostatistics ed 2 1994. Appleton & Lange, Norwalk
- 25.Golub TR, Slonim DK, Tamayo P, Huard C, Gaasenbeek M, Mesirov JP, Coller H, Loh ML, Downing JR, Caligiuri MA, Bloomfield CD, Lander ES: Molecular classification of cancer: class discovery and class prediction by gene expression monitoring. Science 1999, 286:531-537 [DOI] [PubMed] [Google Scholar]
- 26.Ross DT, Scherf U, Eisen MB, Perou CM, Rees C, Spellman P, Iyer V, Jeffrey SS, Van de Rijn M, Waltham M, Pergamenschikov A, Lee JC, Lashkari D, Shalon D, Myers TG, Weinstein JN, Botstein D, Brown PO: Systematic variation in gene expression patterns in human cancer cell lines. Nat Genet 2000, 24:227-235 [DOI] [PubMed] [Google Scholar]
- 27.Shirasakii F, Takata M, Hatta N, Takehara K: Loss of expression of the metastasis suppressor gene KiSS-1 during melanoma progression and its association with LOH of chromosome 6q16.3q23. Cancer Res 2001, 61:7422-7425 [PubMed] [Google Scholar]
- 28.Simon R, Burger H, Semjonov A, Hertle L, Terpe HJ, Becker W: Patterns of chromosomal imbalances in muscle invasive bladder cancer. Int J Oncol 2000, 17:1025-1029 [DOI] [PubMed] [Google Scholar]
- 29.Koo SH, Kwon KC, Ihm CH, Jeon YM, Park JW, Sul CK: Detection of genetic alterations by comparative genomic hybridization and cytogenetics analysis. Cancer Genet Cytogenet 1999, 110:87-93 [DOI] [PubMed] [Google Scholar]
- 30.Simon R, Burger H, Brinkschmidt C, Bocker W, Hertle L, Terpe HJ: Chromosomal aberrations associated with invasion in papillary superficial bladder cancer. J Pathol 1998, 185:345-351 [DOI] [PubMed] [Google Scholar]
- 31.Dalbagni G, Presti J, Reuter V, Fair WR, Cordon-Cardo C: Genetic alterations in bladder cancer. Lancet 1993, 324:469-471 [DOI] [PubMed] [Google Scholar]
- 32.Reznikoff CA, Belair C, Savelieva E, Zhai Y, Pfeifer K, Yeager T, Thompson KJ, DeVries S, Bindley C, Newton MA: Long-term genome stability and minimal genotypic and phenotypic alterations in HPV16 E7-, but not E6-, immortalized human uroepithelial cells. Genes Dev 1994, 8:2227-2240 [DOI] [PubMed] [Google Scholar]
- 33.Markl ID, Jones PA: Presence and location of TP53 mutation determines pattern of CDKN2A/ARF pathway inactivation in bladder cancer. Cancer Res 1998, 58:5348-5353 [PubMed] [Google Scholar]
- 34.Lu ML, Wikman F, Orntoft TF, Charytonowicz E, Rabbani F, Zhang Z, Dalbagni G, Pohar KS, Yu G, Cordon-Cardo C: Impact of alterations affecting the p53 pathway in bladder cancer on clinical outcome, assessed by conventional and array-based methods. Clin Cancer Res 2002, 8:171-179 [PubMed] [Google Scholar]
- 35.Debies MT, Welch DR: Genetic basis of human breast cancer metastasis. J Mammary Gland Biol Neoplasia 2001, 6:441-451 [DOI] [PubMed] [Google Scholar]
- 36.Giroldi LA, Bringuier PP, Shimazui T, Jansen K, Schalken JA: Changes in cadherin-catenin complexes in the progression of human bladder carcinoma. Int J Cancer 1999, 82:70-76 [DOI] [PubMed] [Google Scholar]
- 37.Seraj MJ, Harding MA, Gildea JJ, Welch DR, Theodorescu D: The relationship of BRMS1 and RhoGDI2 gene expression to metastatic potential in lineage related human bladder cancer cell lines. Clin Exp Metastasis 2000, 18:519-525 [DOI] [PubMed] [Google Scholar]