Abstract
Currently, the etiology of the serious developmental anomaly congenital diaphragmatic hernia (CDH) is unknown. We have used an animal model of CDH to address this issue. We characterized four separate teratogens that produced diaphragmatic defects in embryonic rats that are similar to those in infants with CDH. We then tested the hypothesis that all these agents share the common mechanism of perturbing the retinoid-signaling pathway. Specifically, inhibition of retinal dehydrogenase-2 (RALDH2), a key enzyme necessary for the production of retinoic acid and that is expressed in the developing diaphragm, was assayed by measuring retinoic acid production in cytosolic extracts from an oligodendrocyte cell line. The following compounds all induce posterolateral defects in the rat diaphragm; nitrofen, 4-biphenyl carboxylic acid, bisdiamine, and SB-210661. Importantly, we demonstrate that they all share the common mechanism of inhibiting RALDH2. These data provide an important component of mounting evidence suggesting that the retinoid system warrants consideration in future studies of the etiology of CDH.
Congenital diaphragmatic hernia (CDH) is a serious developmental disorder occurring in ∼1 in 3000 live births in which the diaphragm muscle fails to form completely, resulting in a hole in the diaphragm and incomplete separation of the thoracic and abdominal cavities. Lung hypoplasia and pulmonary hypertension are major pathological consequences that account for much of the morbidity and mortality of this problem.1,2
An animal model of CDH was developed, resulting from toxicological studies that showed that nitrofen, a herbicide, although relatively harmless to adult rodents, caused developmental anomalies in the lungs, hearts, diaphragms, and skeletal tissues of fetuses in pregnant rats.3,4 Diaphragmatic defects resulting from a single 100-mg dose of nitrofen administered to pregnant rats on day 8 of gestation are very similar to those documented in human CDH, with respect to the size and location of the defect and the accompanying intrusion of the abdominal viscera into the thoracic cavity. Further, the associated developmental defects observed with nitrofen-induced CDH such as skeletal and cardiac malformations are similar to those seen in a subpopulation of infants with CDH.5-7
Data derived from studies of the nitrofen model suggest that the pathogenesis of CDH is linked to a malformation of the primordial diaphragm, the pleuroperitoneal fold.8 However, the etiology of CDH is completely unknown. Further, despite the fact that the nitrofen model of CDH has been used since the 1970’s, a clear understanding of the mechanisms underlying the herbicide’s teratogenicity is lacking. Given the striking similarities between the pathologies observed in CDH in the nitrofen-induced rat model and in infants with CDH, the possibility of a common underlying etiology certainly has to be considered. Therefore, we sought to delineate the biochemical mechanisms underlying the actions of nitrofen.
There are several pieces of data that provide a rationale for examining the role of the retinoic acid system in the etiology of CDH. Past studies examining the effects of vitamin A-deficient diets in rodents during pregnancy demonstrated that some of the offspring had diaphragmatic hernias.9,10 In 1994, Mendelsohn and colleagues11 published data showing that in a subset of double-retinoic acid receptor subtype knockouts, fetuses had diaphragmatic hernias. Major and colleagues12 provided preliminary evidence supporting a role of vitamin A as a factor in human CDH. In a small study of human mothers and infants born with or without CDH, it was reported that the retinol levels in the maternal and infant plasma were abnormal when CDH was present. More recently, a direct interaction of nitrofen and the retinoid system arose from studies using transgenic mice with a lacZ reporter linked to a retinoid response element (RARE). The expression of the transgene was markedly reduced in response to nitrofen exposure.13
In this study, we take the next step by determining the specific stage in the retinoid cascade affected by nitrofen. Specifically, we test the hypothesis that nitrofen acts to inhibit retinal dehydrogenase-2 (RALDH2) and thus the formation of retinoic acid from retinaldehyde. Further, we characterize three other compounds that induce diaphragmatic defects. Past reports have indicated that 4-biphenyl carboxylic acid (BPCA),14 bisdiamine [N,N′-octamethylenebis (dichloroacetamide)]15,16 and SB-21066117 induce diaphragmatic defects. BPCA is a breakdown product of a thromboxane-A2 receptor antagonist, bisdiamine is a spermatogenesis inhibitor, and SB-210661 is a benzofuranyl urea derivative developed for inhibiting 5-lipoxygenase. We demonstrate that these compounds induce diaphragmatic defects characteristic of CDH. Importantly, they all share, along with nitrofen, the same common mechanism of inhibiting RALDH2 in a dose-dependent manner.
Materials and Methods
Animals
Timed pregnant Sprague-Dawley rats were used following procedures approved by the Animal Welfare Committee at the University of Alberta. The morning on which a sperm plug was observed in the breeding cage was designated as embryonic day (E) 0.
Chemicals
SB-210661 was generously provided by Dr. H. M. Solomon (GlaxoSmithKline Pharmaceuticals, King of Prussia, PA). Bisdiamine [N,N′-octamethylenebis (dichloroacetamide)] was purchased from ACROS Organics (Fisher Scientific, Pittsburgh, PA) and 4-biphenyl carboxylic acid from Sigma (St. Louis, MO). Nitrofen was obtained from the United States Environmental Protection Agency (Bethesda, MD) and China National Chemical Construction Jiangsu Company (Nanjing, China). The dosages and timings of administration for each chemical are listed in Table 1▶ . Each compound was dissolved in 1 ml of olive oil using sonication. On the appropriate day(s) of gestation (Table 1)▶ , pregnant rats were anesthetized with halothane temporarily (10 minutes) and the drug solutions were delivered via gavage feed. Rats were returned to the original cage and housed in the lab for further dosing where specified.
Table 1.
Dosages and Days of Administration of Teratogens and Corresponding Incidence and Location of Diaphragmatic Defects
| Compound/dose | Days of administration | Hernias/number of fetuses | Location of hernia (left/right/bilateral) |
|---|---|---|---|
| Nitrofen | |||
| 100 mg | E8 | 266/507 | 153L/86R/27B |
| BPCA | |||
| 100 mg | E8 | 0/10 | — |
| 50 mg/kg | E8–10 | 0/8 | — |
| 75 mg/kg | E8–10 | 0/12 | — |
| 50 mg/kg | E7–14 | 22/62 | 4L/16R/2B |
| SB-210661 | |||
| 100 mg | E8 | 0/13 | — |
| 50 mg/kg | E7–14 | 23/30 | 1L/14R/8B |
| 75 mg/kg | E7–14 | 11/13 | 5L/6R/0B |
| 100 mg/kg | E7–14 | 26/26 | 0L/0R/26B |
| Bisdiamine | |||
| 100 mg | E8 | 0/12 | — |
| 50 mg/kg | E8–9 | 2/10 | 1L/1R/0B |
| 100 mg/kg | E8–9 | 1/14 | 1L/0R/0B |
| 75 mg/kg | E10–11 | 7/18 | 3L/3R/1B |
| 100 mg/kg | E10–11 | 6/17 | 0L/0R/6B |
| 50 mg/kg | E11–12 | 16/16 | 0L/6R/10B |
| 100 mg/kg | E11–12 | 2/17 | 0L/2R/0B |
Cesarean Section and Diaphragm Isolation
On E18, rats were anesthetized and cesarean sections performed to deliver the fetuses. The fetuses were euthanized, decapitated, and placed into 4% paraformaldehyde for 1 to 2 days of fixation. Using a dissecting microscope, the diaphragms were then exposed and removed for subsequent assessment of defects. In some cases, immunolabeling for neural cell adhesion molecule was performed to delineate the orientation of myotubes and/or phrenic nerve intramuscular branches.18 Photographs of the diaphragms were taken with a Nikon 990 digital camera mounted on a Leica research microscope.
Retinal Dehydrogenase Assay
To measure inhibitory effects on retinoic acid synthesis we used the RALDH2 isolated from an oligodendrocyte cell line.19,20 Trypsinized oligodendrocyte cells were collected on ice, spun down, and triturated in an equal volume of 10 mmol/L of phosphate buffer, pH 7.4, with 30 mmol/L of NaCl containing 1 mmol/L of phenylmethyl sulfonyl fluoride, 1 μmol/L of leupeptin, 1% aprotinin, and 1 μmol/L of pepstatin as protease inhibitors. The homogenate was centrifuged for 15 minutes at 13,000 × g to obtain a supernatant containing the cytoplasmic proteins. Protein concentrations in these extracts were determined with the bicinchoninic acid protein assay (Sigma). Isoelectric focusing (IEF) of native proteins was performed in an Isobox IEF apparatus (Hoefer Scientific/Pharmacia, Freiburg, Germany) with agarose gels using agarose-coated polyester film (GEL-Fix; Serva, Heidelberg, Germany), silanized glass plates and 1-mm-thick plastic spacers. The gel solution contained 0.8% agarose (11402, Serva), 2% sorbitol (Merck, Darmstadt, Germany), and 3% ampholytes pI 4 to 7 (42948, Serva). Electrode wicks were soaked in 0.5 mol/L of acetic acid and 0.5 mol/L of NaOH for anode and cathode, respectively. Samples were loaded with 10 μg of protein per lane. Running conditions were: 10 minutes at 1 W; removal of the sample mask; 5 minutes, 5 W; 45 minutes, 15 W; all at 1200 V maximum. Internal protein standards with pI 3.6, 4.6, 5.1, 6.6, 8.2, 8.6, and 8.8 (Sigma) marked pH positions in the gel. Lanes with pI markers were fixed and stained with Coomassie G250. After IEF, parallel lanes of the gel were cut into 16 or 24 consecutive slices 2.25 mm apart. These IEF fractions were distributed into the wells of microtiter plates, where proteins were eluted and assayed for retinoic acid (RA) synthesis from 50 nmol/L all-trans retinaldehyde in the presence of 0.6 mg/ml dithiothreitol and 0.8 mg/ml NAD+.21 After 4 hours of incubation at 37°C in darkness, 50 μl/well of reaction products were removed and tested with RA-sensitive reporter cells. The reporter cell line22 consists of F9 teratocarcinoma cells transfected with the β-galactosidase gene under the control of the RA-responsive element from the RA receptor β. The cells were grown in CO2-buffered Dulbecco’s modified Eagle’s medium, supplemented with 10% fetal calf serum, penicillin/streptomycin (Sigma, P3539), and 0.8 g/L geneticin (Life Technologies, Inc., Grand Island, NY). For the RA assay, reporter cells were plated into poly-l-lysine-coated 96-well microtiter plates, grown to confluence, and cultured for ∼12 hours with 150 μl of cell culture medium plus 50 μl of supernatant from the enzyme reaction. The RA-dependent induction of β-galactosidase was then visualized with the X-Gal staining procedure.20 To measure the efficacy of enzyme inhibitors, IEF fractions were collected in medium that contained 0.1 μmol/L, 1 μmol/L, 10 μmol/L, or 100 μmol/L of SB-210661, bisdiamine, nitrofen, or BPCA. After 15 minutes of incubation at room temperature the NAD+/RAL solution was added for the aldehyde dehydrogenase reaction as described above. To ascertain that enzyme inhibitors did not interfere with RA detection, the reporter cells were incubated with medium containing inhibitors plus 0.1 nmol/L or 10 pmol/L of all-trans RA. Experimental procedures were identical to those when RA production was determined. Aldehyde dehydrogenase activity was also measured in cytosolic extracts without previous IEF separation. In this procedure cytosolic extracts containing 10 μg of protein were dissolved in 50 μl of medium with enzyme inhibitors and then processed as described. We chose concentrations of 1 pmol/L to 1 mmol/L for nitrofen and BPCA, 1 nmol/L to 1 mmol/L for SB-210661, and 0.1 pmol/L to 1 mmol/L for bisdiamine. Three independent experiments were performed.
Immunohistochemistry
Paraffin-embedded embryos were cut with a microtome at 7 μm transversely and mounted. Slides were dewaxed, rehydrated, then incubated with 0.3% hydrogen peroxide in methanol for 30 minutes, and 0.4% Triton X-100/phosphate-buffered saline (PBS) for 1 hour. A blocking step was performed with 5% normal goat serum in 0.4% Triton X-100/PBS, followed by incubation with the primary antibody, rabbit anti-RALDH2 (1:1500; P. McCaffery, Waltham, MA) overnight at 4°C (antibody omitted for control sections). The slides were incubated in a biotinylated goat anti-rabbit IgG (Vector Laboratories, Burlingame, CA) secondary antibody at a dilution of 1:200 for 1 hour then washed in PBS. Slides were incubated with 1:100 avidin-biotinylated peroxidase complex (ABC kit PK4000, Vector). The antigen labeling was visualized by a 3,3-diaminobenzidine tetrahydrochloride product intensified by nickel (0.1 mol/L Tris buffer containing 0.04% 3,3-diaminobenzidine tetrahydrochloride with 0.04% hydrogen peroxide and 0.6% nickel ammonium sulfate). The slides were then counterstained with eosin and dehydrated in ethanol before being coverslipped.
Results
Diaphragmatic Defects
Fetuses removed from pregnant rats treated with nitrofen and 4-biphenyl carboxylic acid showed no outward abnormalities at the doses administered. A significant fraction (∼50%) of the fetal rats from animals given high doses of SB-210661 (100 mg/kg/day, E7 to E14) or bisdiamine (100 mg/day on E10 to E11 or E11 to E12) displayed head abnormalities including blunt snouts and some skin defects (loose skin covering the trunk). Internal malformations were not systematically examined, although lung hypoplasia and retarded overall growth was evident in the majority of fetuses exposed to the teratogens regardless of CDH status.
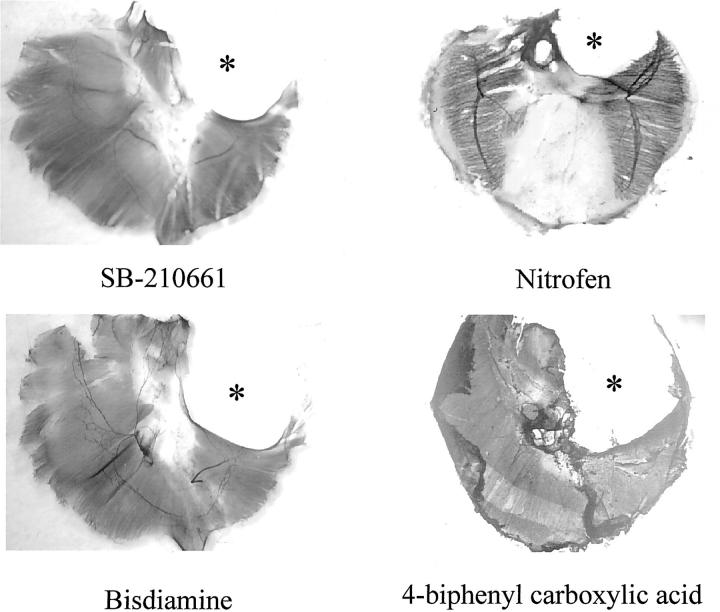
Figure 1▶ shows representative examples of diaphragm defects induced by the four compounds. The number of diaphragmatic defects produced by each of the compounds and data regarding the location of the diaphragmatic defects are contained in Table 1▶ . As reported previously,18 a single dose of nitrofen administered on E8 results in ∼50% of the embryos having hernias, with a predominance of left-sided defects. Past studies have also demonstrated that single doses of nitrofen administered beyond E11 induced solely right-sided defects. None of the other compounds induced diaphragmatic defects after a single administration on E8. Repeated doses were required.
Figure 1.
Photomicrographs of whole diaphragms labeled for neural cell adhesion molecule to demarcate myotubes. Representative examples of left-sided diaphragmatic defects (*) induced by each of the four compounds. The posterolateral portion of the diaphragm was affected in all cases.
Administration of 4-biphenyl carboxylic acid at a dosage of 50 mg/kg/day from E7 to E14 induced predominantly right-sided diaphragmatic defects. SB-210661, administered at a dose level of 100 mg/kg/day from E7 to E14 caused 100% large bilateral defects; with ∼80% of the diaphragm tissue missing in some cases. Doses of 50 and 75 mg/kg/day from E7 to E14 induced defects of more moderate size in 74% and 85% of fetuses, respectively. Bisdiamine had a different dose dependency. A dose of 100 mg/day administered on E10 and E11 induced large bilateral defects. The same dosage administered on E11 and E12 induced only right-sided defects. Overall, the rank order of efficacy in producing diaphragmatic defects was bisdiamine > SB-210661 > nitrofen > BPCA.
Measurements of Retinal Dehydrogenase Inhibition
Using the immortalized oligodendrocyte cell line OLN93 as a source of retinal dehydrogenase we investigated the inhibitory effect of nitrofen, bisdiamine, BPCA, and SB-210661 on this enzyme. Because RA synthesis was assessed by means of β-galactosidase expression in a bioassay we tested whether enzyme inhibitors interfered with RA detection in this system. At a concentration of 0.1 μmol/L none of the inhibitors had a significant effect on RA detection (Table 2)▶ , nor did 100 μmol/L of bisdiamine and nitrofen. SB-210661 reduced the reporter cell response at 100 μmol/L to a small degree. At this concentration BPCA interfered with the zymography assay but not enough to render the RALDH inhibition data invalid (because BPCA inhibited RA synthesis completely and at much lower concentrations).
Table 2.
Interference of Enzyme Inhibitor with Retinal Dehydrogenase Detection Assay
| Inhibitor | Bisdiamine | Nitrofen | SB-210661 | BPCA |
|---|---|---|---|---|
| 0.1 μmol/L | 0 ± 0 | 0 ± 0 | 0.3 ± 0.5 | 9.8 ± 24.1 |
| 100 μmol/L | 7.5 ± 10.6 | 0 ± 0 | 17.5 ± 17.7 | 48.0 ± 7.4 |
Complete inhibition of RA detection = 100, no inhibition = 0 (mean ± SD).
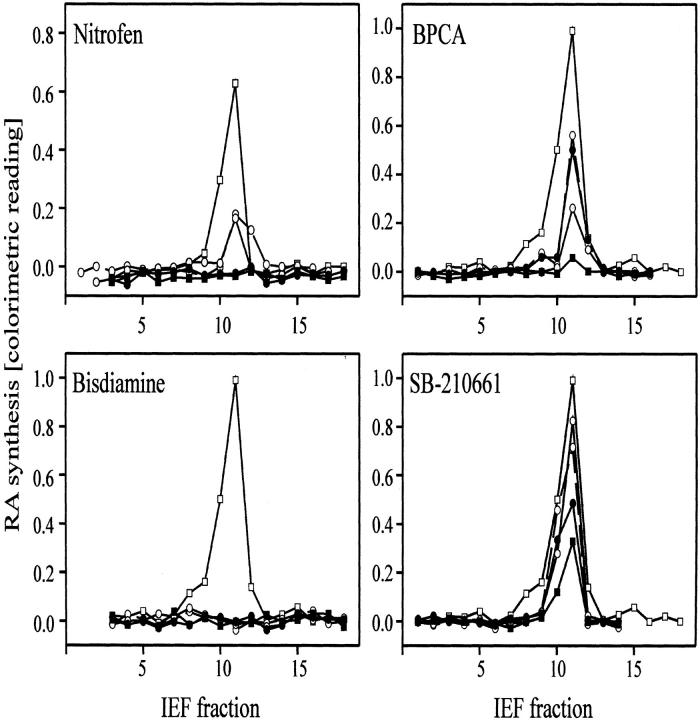
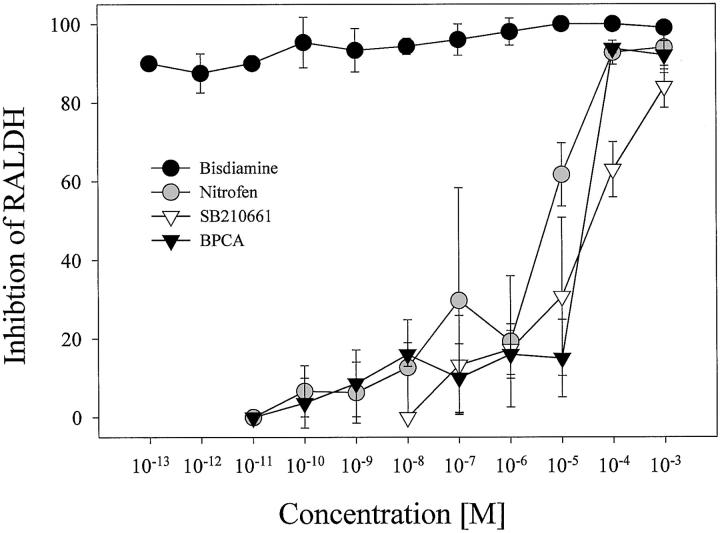
In zymography assays, IEF fractions were incubated with 0.1, 1, 10, and 100 μmol/L of nitrofen, BPCA, bisdiamine, SB-210661, or no enzyme inhibitor (Figure 2)▶ . All compounds reduced RA synthesis in a dose-dependent manner. Complete inhibition of the aldehyde dehydrogenase activity was found with all concentrations of bisdiamine, 10 μmol/L of nitrofen or higher, and 100 μmol/L of BPCA. Because only one peak of RA-synthesizing activity was detected we then used cytosol homogenates without previous separation by IEF to determine dose-response functions for a broader range of concentrations (Figure 3)▶ . The ED50 for suppression of RALDH activity were 10 to 100 μmol/L for BPCA and SB-210661, ∼10 μmol/L for nitrofen, and less than 1 pmol/L for bisdiamine. In accordance with the zymography assays and the rank order of efficacy in producing diaphragmatic defects, bisdiamine, which suppressed RALDH activity at all tested concentrations, was the most potent inhibitor, followed by nitrofen, BPCA, and SB-210661.
Figure 2.
IEF fractions of cytosolic extracts from OLN93 cells exhibit RALDH activity. One peak of enzyme activity was detected in fractions with pI 5.0 to 5.6. Protein fractions were incubated with 0.1 μmol/L (-○-), 1 μmol/L (-░⃞-), 10 μmol/L (-•-), or 100 μmol/L (-▪-) of each of the four compounds or no enzyme inhibitor (-□-).
Figure 3.
Dose-dependent effect of enzyme inhibitors on RALDH activity. Relative inhibition on RA synthesis is plotted against concentration for each of the four compounds. Error bars indicate SD of three independent experiments.
RALDH2 Expression
Immunolabeling for RALDH2 was performed to demonstrate its expression in the developing diaphragm, the pleuroperitoneal fold. As shown in Figure 4▶ there is intense labeling within the pleuroperitoneal fold at E13, the age when the structure is well defined. As reported previously,23 there is also RALDH2 expression in the developing lung.
Figure 4.
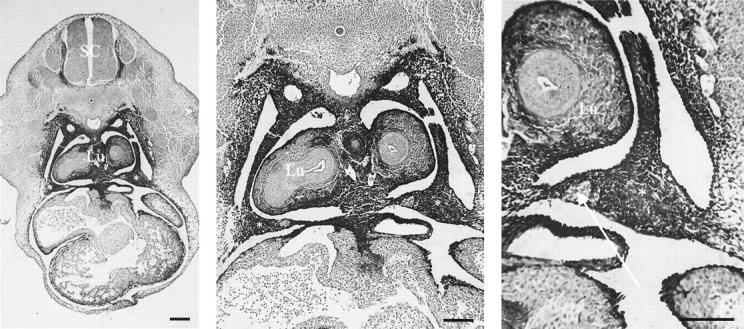
Immunolabeling for RALDH2 in the primordial diaphragm, the pleuroperitoneal fold at E13. There is intense RALDH2 expression (intense black 3,3-diaminobenzidine tetrahydrochloride deposits) in bilateral pleuroperitoneal folds (*), as shown at different magnifications. Lu, lung; SC, spinal cord. Arrow, phrenic nerve. Scale bars, 100 μm.
Discussion
We have demonstrated that nitrofen, bisdiamine, SB-210661, and 4-biphenyl carboxylic acid all induce diaphragmatic defects similar to those observed in infants with CDH. Importantly, all of these compounds inhibit RALDH2, a key enzyme responsible for the generation of retinoic acid from retinal. These data, in conjunction with that from past studies, support the hypothesis that perturbations of the retinoid system could be involved in the etiology of CDH.
Each of the four compounds induced diaphragmatic defects in the posterolateral corners of the diaphragm, similar to what is observed in infants with CDH. The differences in the number of treatments required for each compound is likely because of the length of time that each remains in the body before degradation or elimination. Nitrofen persists at elevated levels for at least 3 days,24 which would explain why a single large dose is all that is needed to induce diaphragmatic hernias. Conversely, biphenyl carboxylic acid may be much more labile (the parent compound, AH23848, has a plasma half-life of 1 to 1.5 hours), so that supplemental doses are necessary to produce sufficiently high concentrations to be effective. SB-210661 and biphenyl carboxylic acid may have a comparable half-life within embryonic tissues because the same dosages administered throughout the identical period of gestation produce similar fetal consequences.
In addition to inducing diaphragmatic defects, all four compounds can produce cardiac defects. These include maldevelopment of the outflow tracts of the heart and septal defects. The cardiac defects may result from problems with neural crest proliferation and differentiation in the fetuses.14,15,17,25,26 There is no strong evidence, however, that diaphragm embryogenesis has any dependence on neural crest development. It may be that these compounds also interfere with the proliferation or differentiation of mesenchymal cells. We are currently examining the hypothesis that a defect in the formation of the mesenchymal substratum of the primordial diaphragm, the pleuroperitoneal fold, is associated with teratogen-induced CDH.8
Recent studies using mice with a lacZ reporter gene linked to the activation of RARE demonstrated that nitrofen was inhibiting some aspect of retinoid function.13 The nitrofen-induced suppression of RARE was reversed by the addition of retinoic acid supplementation. Further, binding studies suggested that nitrofen was acting upstream of the binding of retinoic acid to its receptors. The preceding step in the retinoid cascade is the NAD-dependent oxidation of all-trans-retinal to all-trans-retinoic acid by RALDH2.27 One of the CDH-inducing teratogens, bisdiamine, is a known inhibitor of alcohol dehydrogenase. Thus, we hypothesized that the compounds were acting by interfering with that aspect of retinoid function. The results demonstrating that all four CDH-inducing compounds inhibit RALDH2 in a dose-dependent manner strongly supports the hypothesis. Further, the most potent inhibitor of RALDH2, bisdiamine, was also the most effective at inducing diaphragmatic defects in embryonic rats. The other CDH-inducing compounds had similar dose-response curves for inhibition of RALDH2 activity. The concentration of nitrofen present in the embryo in response to gavage feed of 100 mg has been estimated to be in the μmol range. Similarly, exposure of fetal mouse embryos maintained in culture to ∼15 μmol of nitrofen produced a pronounced suppression or RARE-lacZ activation.13 The data in Figure 3▶ demonstrates that the ED50 for nitrofen suppression of RALDH2 activity is within a similar range.
Collectively, the data support the hypothesis that the primordial diaphragm tissue, the pleuroperitoneal fold, is dependent on retinoid-mediated signaling for its proper formation. Retinoic acid receptors function as transcriptional activators that modulate the expression of developmentally regulated genes by binding as a ligand/receptor complex to DNA sequences designated as retinoic acid response elements.28 Consistent with this notion is the fact that the cervical mesenchymal tissues and developing diaphragmatic tissue strongly express proteins associated with the metabolism and binding of retinoids.28-30 Further, retinoic acid has been shown to be involved in regulating extracellular matrix formation, mesenchymal cell migration, and establishment of dorsoventral polarity, all of which could be key components of early diaphragm embryogenesis.31,32 However, a determination of the specific cells responsible for the embryological origins of the pleuroperitoneal fold will be necessary before ascertaining the role of retinoid signaling and its perturbation by CDH-inducing teratogens.
In ∼60 to 65% of cases in infants with CDH there are no obvious associated anomalies other than the diaphragm defect.33,34 The reason that the diaphragm might be particularly susceptible to perturbations of the retinoid system is unclear. Immunolabeling for RALDH2 and HPLC analyses demonstrate that there are gradients of retinoic acid levels within the cervical mesenchyme.31,32 Further, the levels of retinoic acid and associated activation of RAREs necessary for regulating retinoid-mediated transcription varies among genes in a dose-dependent manner.31 Thus, it is conceivable that the safety margin for retinoic acid-mediated regulation of primordial diaphragm development is relatively low and thus more susceptible to perturbations compared with other tissues. Heart development is also particularly susceptible, as cardiac anomalies are observed in the rat model of nitrofen-induced CDH5,6 and are the most commonly associated defect in infants with CDH.33 RALDH2 is expressed in the developing lung28 and its suppression by nitrofen could explain some of the abnormalities of lung development observed in the nitrofen model of CDH.35-38
One could formulate several hypotheses regarding retinoid system malfunction and the etiology of CDH in humans. Transient deficiencies or imbalances in the levels of retinoid metabolites, binding proteins, nuclear receptors, or retinoid-metabolizing enzymes within the developing primordial diaphragm at the time of initial malformation of the pleuroperitoneal fold (∼4 to 5 weeks of gestation) could account for diaphragmatic defects. These defects, in theory, could be because of acute dietary deficiencies, impaired placental transport, spontaneous malregulation within the retinoid metabolic cascade, teratogenic-mediated insults, or chromosomal defects. Evidence for the latter arises from several references to an association of CDH with chromosome 15q defects.39-43 A search of available databases found that some of the genes on chromosome 15 in the region of the deletion or translocation (15q24-26) encode for cellular retinoic acid-binding protein-1 (CRABP-1).
Acknowledgments
We thank Monika Klemeit for valuable help with the zymography assays, Dr. Christiane Richter-Landsberg for the OLN93 cells, and Dr. Peter McCaffery for the RA reporter cells.
Footnotes
Address reprint requests to Dr. John J. Greer, Ph.D., University of Alberta, Department of Physiology, 513 HMRC, Edmonton, AB, Canada T6G 2S2. E-mail: john.greer@ualberta.ca.
Suported by the Canadian Institutes of Health (CIHR), the March of Dimes, and the Deutsche Forschungsgemeinschaft (SFB542 to J. M.).
Jörg Mey and Randal P. Babiuk contributed equally to the study.
J. J. G. is a senior scholar of the Alberta Heritage Foundation for Medical Research and R. P. B. and R. C. received studentships from Alberta Lung Association Studentship and University of Alberta Perinatal Research Centre, respectively.
References
- 1.Harrison MR, Adzick NS, Estes JM, Howell LJ: A prospective study of the outcome for fetuses with diaphragmatic hernia. JAMA 1994, 271:382-384 [PubMed] [Google Scholar]
- 2.Karamanoukian HL, Glick PL, Wilcox DT, O’Toole SJ, Rossman JE, Azizkhan RG: Pathophysiology of congenital diaphragmatic hernia. XI: Anatomic and biochemical characterization of the heart in the fetal lamb CDH model. J Pediatr Surg 1995, 30:925-928 [DOI] [PubMed] [Google Scholar]
- 3.Ambrose AM, Larson PS, Borzelleca JF, Smith RBJ, Hennigar GRJ: Toxicologic studies on 2,4-dichlorophenyl-p-nitrophenyl ether. Toxicol Appl Pharmacol 1997, 19:263-275 [DOI] [PubMed] [Google Scholar]
- 4.Costlow RD, Manson JM: The heart and diaphragm: target organs in the neonatal death induced by nitrofen (2,4-dichlorophenyl-p-nitrophenyl ether). Toxicology 1981, 20:209-227 [DOI] [PubMed] [Google Scholar]
- 5.Migliazza L, Xia H, Diez-Pardo JA, Tovar JA: Skeletal malformations associated with congenital diaphragmatic hernia: experimental and human studies. J Pediatr Surg 1999, 34:1624-1629 [DOI] [PubMed] [Google Scholar]
- 6.Migliazza L, Otten C, Xia H, Rodriguez JI, Diez-Pardo JA, Tovar JA: Cardiovascular malformations in congenital diaphragmatic hernia: human and experimental studies. J Pediatr Surg 1999, 34:1352-1358 [DOI] [PubMed] [Google Scholar]
- 7.Migliazza L, Xia H, Alvarez JI, Arnaiz A, Diez-Pardo JA, Alfonso LF, Tovar JA: Heart hypoplasia in experimental congenital diaphragmatic hernia. J Pediatr Surg 1999, 34:706-710 [DOI] [PubMed] [Google Scholar]
- 8.Greer JJ, Allan DW, Babiuk RP, Lemke RP: Recent advances in understanding the pathogenesis of nitrofen-induced congenital diaphragmatic hernia. Pediatr Pulmonol 2000, 29:394-399 [DOI] [PubMed] [Google Scholar]
- 9.Wilson JG, Roth B, Warkany J: An analysis of the syndrome of malformations induced by maternal Vitamin A deficiency. Effects of restoration of vitamin A at various times during gestation. Am J Anat 1953, 92:189-217 [DOI] [PubMed] [Google Scholar]
- 10.Anderson DH: Incidence of congenital diaphragmatic hernia in the young of rats bred on a diet deficient in vitamin A. Am J Dis Child 1941, 62:888-889 [Google Scholar]
- 11.Mendelsohn C, Lohnes D, Decimo D, Lufkin T, LeMeur M, Chambon P, Mark M: Function of the retinoic acid receptors (RARs) during development (II). Multiple abnormalities at various stages of organogenesis in RAR double mutants. Development 1994, 120:2749-2771 [DOI] [PubMed] [Google Scholar]
- 12.Major D, Cadenas M, Fournier L, Leclerc S, Lefebvre M, Cloutier R: Retinol status of newborn infants with congenital diaphragmatic hernia. Pediatr Surg Int 1998, 13:547-549 [DOI] [PubMed] [Google Scholar]
- 13.Chen M, MacGowan A, Ward S, Bavik C, Greer JJ: Activation of the retinoic acid response element is inhibited in an animal model of congenital diaphragmatic hernia. Biol Neonate (in press) [DOI] [PubMed]
- 14.Sutherland MF, Parkinson MM, Hallett P: Teratogenicity of three substituted 4-biphenyls in the rat as a result of the chemical breakdown and possible metabolism of a thromboxane A2-receptor blocker. Teratology 1989, 39:537-545 [DOI] [PubMed] [Google Scholar]
- 15.Taleporos P, Salgo MP, Oster G: Teratogenic action of bis(dichloroacetyl)diamine on rats: patterns of malformations produced in high incidence at time-limited periods of development. Teratology 1978, 18:5-15 [DOI] [PubMed] [Google Scholar]
- 16.Tasaka H, Takenaka H, Okamoto N, Koga Y, Hamada M: Diaphragmatic hernia induced in rat fetuses by administration of bisdiamine. Congenital Anomalies 1992, 32:347-355 [Google Scholar]
- 17.Solomon HM, Wier PJ, Johnson CM, Posobiec LM, Rendemonti JE, Rumberger DF: Benzofuranyl ureas with potent cardiovascular teratogenicity in rats. Teratology 2000, 61:211-221 [DOI] [PubMed] [Google Scholar]
- 18.Allan DW, Greer JJ: Pathogenesis of nitrofen-induced congenital diaphragmatic hernia in fetal rats. J Appl Physiol 1997, 83:338-347 [DOI] [PubMed] [Google Scholar]
- 19.Richter-Landsberg C, Heinrich M: OLN-93: a new permanent oligodendroglia cell line derived from primary rat brain glial cultures. J Neurosci Res 1998, 45:161-173 [DOI] [PubMed] [Google Scholar]
- 20.Mey J, Hammelmann S: Retinoic acid synthesis by oligodendrocytes in vitro. Cell Tissue Res 2000, 302:49-58 [DOI] [PubMed] [Google Scholar]
- 21.McCaffery P, Dräger UC: A sensitive bioassay for enzymes that synthesize retinoic acid. Brain Res Brain Res Protoc 1997, 1:232-236 [DOI] [PubMed] [Google Scholar]
- 22.Wagner M, Han B, Jessell TM: Regional differences in retinoid release from embryonic neuronal tissue detected by an in vitro reporter assay. Development 1992, 116:55-66 [DOI] [PubMed] [Google Scholar]
- 23.Malpel S, Mendelsohn C, Cardoso WV: Regulation of retinoic acid signaling during lung morphogenesis. Development 2000, 127:3057-3067 [DOI] [PubMed] [Google Scholar]
- 24.Manson JM: Mechanism of nitrofen teratogenesis. Environ Health Perspect 1986, 70:137-147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choy M, Oltjen SJ, Moon AJ, Armstrong MT, Armstrong PB: Bisdiamine inhibits extracellular matrix formation and cell proliferation of atrioventricular mesenchyme from developing chick heart valves. Teratology 1999, 59:148-155 [DOI] [PubMed] [Google Scholar]
- 26.Yu J, Gonzalez S, Rodriguez JI, Diez-Pardo JA, Tovar JA: Neural crest-derived defects in experimental congenital diaphragmatic hernia. Pediatr Surg Int 2001, 17:294-298 [DOI] [PubMed] [Google Scholar]
- 27.Niederreither K, Subbarayan V, Dolle P, Chambon P: Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat Genet 1999, 21:444-448 [DOI] [PubMed] [Google Scholar]
- 28.Giguere V: Retinoic acid receptors and cellular retinoid binding proteins: complex interplay in retinoid signaling. Endocr Rev 1994, 15:61-79 [DOI] [PubMed] [Google Scholar]
- 29.Bavik CO, Ward SJ, Ong DE: Identification of a mechanism to localize generation of retinoic acid in rat embryos. Mech Dev 1997, 69:155-167 [DOI] [PubMed] [Google Scholar]
- 30.Berggren K, McCaffery P, Drager UC, Forehand CJ: Differential distribution of retinoic acid synthesis in the chicken embryo as determined by immunolocalization of the retinoic acid synthetic enzyme, RALDH-2. Dev Biol 1999, 210:288-304 [DOI] [PubMed] [Google Scholar]
- 31.Ross SA, McCaffery PJ, Drager UC, DeLuca LM: Retinoids in embryonal development. Physiol Rev 2000, 80:1022-1054 [DOI] [PubMed] [Google Scholar]
- 32.Yan M, Sinning AR: Retinoic acid administration is associated with changes in the extracellular matrix and cardiac mesenchyme within the endocardial cushion. Anat Rec 2001, 263:53-61 [DOI] [PubMed] [Google Scholar]
- 33.Fauza DO, Wilson JM: Congenital diaphragmatic hernia and associated anomalies: their incidence, identification, and impact on prognosis. J Pediatr Surg 1994, 29:1113-1117 [DOI] [PubMed] [Google Scholar]
- 34.Losty PD, Vanamo K, Rintala RJ, Donahoe PK, Schnitzer JJ, Lloyd DA: Congenital diaphragmatic hernia—does the side of the defect influence the incidence of associated malformations? J Pediatr Surg 1998, 33:507-510 [DOI] [PubMed] [Google Scholar]
- 35.Nakao Y, Ueki R: Congenital diaphragmatic hernia induced by nitrofen in mice and rats: characteristics as animal model and pathogenetic relationship between diaphragmatic hernia and lung hypoplasia. Congenital Anomalies 1987, 27:397-417 [Google Scholar]
- 36.Wickman DS, Siebert JR, Benjamin DR: Nitrofen-induced congenital diaphragmatic defects in CD1 mice. Teratology 1993, 47:119-125 [DOI] [PubMed] [Google Scholar]
- 37.Guilbert TW, Gebb SA, Shannon JM: Lung hypoplasia in the nitrofen model of congenital diaphragmatic hernia occurs early in development. Am J Physiol 2000, 279:L1159-L1171 [DOI] [PubMed] [Google Scholar]
- 38.Acosta JM, Chai Y, Meara JG, Bringas PJ, Anderson KD, Warburton D: Prenatal exposure to nitrofen induces Fryns phenotype in mice. Ann Plast Surg 2001, 46:635-640 [DOI] [PubMed] [Google Scholar]
- 39.Kristoffersson U, Heim S, Mandahl N, Sundkvist L, Szelest J, Hagerstrand L: Monosomy and trisomy of 15q24—-qter in a family with a translocation t(6;15)(p25;q24). Clin Genet 1987, 32:169-171 [DOI] [PubMed] [Google Scholar]
- 40.Smith SA, Martin KE, Dodd KL, Young LD: Severe microphthalmia, diaphragmatic hernia and Fallot’s tetralogy associated with a chromosome 1;15 translocation. Clin Dysmorphol 2000, 3:287-291 [PubMed] [Google Scholar]
- 41.Bettelheim D, Hengstschlager M, Drahonsky R, Eppel W, Bernaschek G: Two cases of prenatally diagnosed diaphragmatic hernia accompanied by the same undescribed chromosomal deletion (15q24 de novo). Clin Genet 1998, 53:319-320 [DOI] [PubMed] [Google Scholar]
- 42.Aviram-Goldring A, Daniely M, Frydman M, Shneyour Y, Cohen H, Barkai G: Congenital diaphragmatic hernia in a family segregating a reciprocal translocation t(5;15)(p15.3;q24). Am J Med Genet 2000, 90:120-122 [DOI] [PubMed] [Google Scholar]
- 43.Schlembach D, Zenker M, Trautmann U, Ulmer R, Beinder E: Deletion 15q24-26 in prenatally detected diaphragmatic hernia: increasing evidence of a candidate region for diaphragmatic development. Prenat Diagn 2001, 21:289-292 [DOI] [PubMed] [Google Scholar]