Abstract
Ultraviolet (UV) light is one of the major factors implicated in the pathogenesis of pterygium. The mechanism by which UV light induces this disease remains elusive. The aim of this study was to evaluate the effects of UVB irradiation on the expression of growth factors in cultured pterygium epithelial cells and to demonstrate their distribution within pterygium. We cultured pterygial epithelial cells from pterygium explants and these cells were exposed to 20 mJ/cm2 of UVB. Total RNA was extracted at 0, 6, and 12 hours after irradiation. 32P-labeled cDNA was synthesized and analyzed using microarray technology to determine the differential expression of 268 growth factor and cytokine related genes. Semiquantitative reverse transcriptase-polymerase chain reaction was used to corroborate this data. Conditioned media derived from cells exposed to UVB irradiation was analyzed for protein expression by enzyme-linked immunosorbent assay. Immunohistochemistry was used to evaluate the distribution of heparin-binding epidermal growth factor-like growth factor (HB-EGF) in pterygium tissue. Analysis of the hybridization signals revealed that the genes encoding HB-EGF, fibroblast growth factor 3, and cytotoxic trail ligand receptor were consistently elevated at 6 and 12 hours after UVB treatment. HB-EGF mRNA was elevated 6.8-fold at 6 hours after irradiation and was augmented in culture supernatants after the same treatment. Furthermore, HB-EGF reactivity was identified in the epithelium and vasculature of pterygium by immunohistochemistry. HB-EGF was present in normal limbal epithelium, although it was not induced in cultured limbal epithelial cells by UV irradiation. HB-EGF is a potent mitogen, localized in pterygium tissue, and significantly induced by UVB in pterygium-derived epithelial cells. We postulate that this growth factor is a major driving force in the development of pterygia and a means by which UV irradiation causes the pathogenesis of pterygium.
Pterygia are characterized by the encroachment of a wing of altered vascular tissue over the cornea. It is a disorder of epithelial hyperplasia and accompanied fibrovascular growth thought to originate at the corneal-conjunctival junction, where it is proposed that altered limbal stem cells migrate centripetally to encroach on the central cornea. 1 The origin of pterygia remains controversial, and the pathogenesis is poorly understood. Explanations for the shape and location of pterygia suggest that ultraviolet (UV) irradiation might be the key initiating event in the pathogenesis of this disease.
The role of UV light as an etiological agent for pterygia remains unproven, although there is strong epidemiological evidence for UV as a possible causative factor. Several studies have demonstrated a significant association between pterygia and UV exposure. 2-4 The UV insolation theory helps explain the link between pterygia and UV irradiation. 5 Peripheral light focusing of scattered light, especially light entering the anterior chamber laterally, by refraction in the anterior chamber results in a 20-fold concentration of light, including UV intensity, on the limbus; especially on the nasal aspect, where the majority of pterygia occur.
UV light has long been associated as the etiological agent for cutaneous malignancies such as melanoma, basal cell carcinoma, and squamous cell carcinoma. The mechanism by which UV light achieves this has been extensively studied and it seems that cytokines and growth factors play a central role in this process. 6-9 The proteins most commonly implicated in pathological growth disorders are growth factors. 10 Recent work pertaining to the effects of UV on the skin using microarray analysis in cultured epidermal keratocytes using a dose of UVB of 8 mJ/cm2 revealed that there was a peak of growth factor mRNA production at 4 to 8 hours after UVB exposure. 8 The growth factors up-regulated included five members of the interleukin (IL)-8 family, transforming growth factor-β, and heparin-binding epidermal growth factor-like growth factor (HB-EGF).
The effect of UV irradiation in the anterior segment of the eye has been shown to up-regulate IL-1, IL-6, IL-8, and tumor necrosis factor-α production in the cornea. 11 There is evidence to suggest that the pathogenesis of pterygia centers on UV light-altered limbal epithelial cells. These altered limbal cells form the pterygium epithelial cell (PEC) that we have previously isolated and cultured. 12 Recently using a similar model we demonstrated the up-regulation of two potent proinflammatory and angiogenic cytokines in UVB-exposed PEC. The increase of IL-6 and IL-8 was dose- and time course-dependent. 13
Although several reports have demonstrated the presence of growth factors in pterygia, 14-18 none have shown an altered expression of growth factors in response to UV light. In this study we investigated the role of UVB irradiation in the regulation of growth factor expression in PECs and in pterygium.
Materials and Methods
Donor Ocular Tissue and Cell Culture
PECs
Epithelial cells were cultured from explants of fresh pterygium tissue (1 to 2 mm2), as previously reported. 12 PEC were subsequently expanded in 75-cm2 tissue-culture flasks (Nunc, Roskilde, Denmark) in Eagle’s minimal essential medium (EMEM) (Trace Biosciences, Sydney, Australia) supplemented with 10% fetal bovine serum (Trace Biosciences) and 100 U/ml of penicillin and 100 mg/ml of streptomycin (Trace Biosciences). Cells were characterized by morphology and by flow cytometric analysis that revealed positive cytokeratin immunoreactivity in 98% of these cells, suggesting these cells comprised a pure population of epithelial cells.
Limbal Epithelium Cells (LECs)
A specimen of normal human corneal limbus was obtained from a patient undergoing ocular surgery at the Prince of Wales Hospital, Sydney, Australia. Consent for tissue donation was obtained from the patient before the procedure. Fresh tissue was cultured in six-well culture plates (Nunc). Initial explant culture medium was EpiLife (Chemicon, Temecula, CA) with growth supplement, Epilife Defined Growth Supplement (EDGS) (Chemicon) to promote epithelial cell growth. Once growth had been established, the cells were passaged and cultured on uncoated tissue-culture plastic in EMEM (Trace Biosciences) supplemented with 10% fetal bovine serum (Trace Biosciences), 100 of U/ml penicillin, and 100 μg/ml of streptomycin. These cells were also characterized morphologically and after four passages cells were characterized using flow cytometry. Using a standard direct immunofluorescence technique including a fluorescein isothiocyanate-conjugated C-11 anti-pancyto-keratin antibody (Sigma, St. Louis, MO) flow cytometric analysis revealed a pure population of cells with positive cytokeratin immunoreactivity in 93% of these cells. This also suggests these cells comprised a pure population of epithelial cells.
UVB Irradiation of Cultured Cells
Epithelial cells were counted and seeded at 1 × 106 cells per 150 mm × 25-mm culture dish (Corning Glass, Corning, NY). For each time point analyzed three plates were used. On reaching semiconfluence cells were washed three times with 20 ml of phosphate-buffered saline (PBS) and placed in EMEM under serum-free conditions for 24 hours. This medium was removed and cells were washed again and irradiated in 9.5 ml of PBS.
The cells were UV irradiated using FL20SE bulbs (Philips, Sydney, Australia). An IL-1400A UV energy detecting system (International Light, Newburyport, MA) was used to achieve a total dose of 20 mJ/cm2. Some cells were incubated in PBS for an equivalent time but were not irradiated. After irradiation, cells were placed in fresh serum-free media and kept at 37°C in a 5% CO2 atmosphere for various periods before harvesting total RNA. At specific time intervals cells were washed with PBS and placed in a denaturing solution (Clontech, Palo Alto, CA) for RNA isolation using a standard procedure according to the Atlas Pure Total RNA Labeling System User Manual protocol (Clontech).
RNA Isolation, cDNA Probe, and Array Hybridization
Total RNA was extracted from cells using an Atlas Pure Total RNA Isolation kit (Clontech). Cell lysates were processed precisely as outlined by the manufacturers. (Available online at www.clontech.com). Briefly, total RNA was extracted using phenol/chloroform, total RNA was subsequently treated with 1 U/μL of RNase-free DNase I (Clontech). RNA yield was evaluated by UV absorbance at 260 nm. RNA integrity was confirmed by electrophoresis through a 0.8% agarose, 2.2 mol/L formaldehyde gel. 19 Total RNA (25 μg per sample) was converted to 32P-labeled cDNA as specified in the Atlas Expression Array User Manual (Clontech). Each probe was purified using the gravity column provided.
Atlas human cytokine/receptor cDNA expression array membranes containing 268 cytokine- and growth-related cDNAs were treated as specified by the manufacturer. Briefly, array membranes were prepared by performing a 2-minute wash with deionized H2O. Prehybridization using 5 ml of prewarmed ExpressHyb (Clontech) containing 0.5 mg of heat-denatured sheared salmon testes DNA was performed at 68°C for 30 minutes. cDNA probe (1.0 × 106 dpm of each) was prepared and hybridized with the array membrane at 68°C for 16 hours with continuous agitation. Membranes were washed four times with 200 ml of 2× standard saline citrate (1× standard saline citrate: 0.15 mol/L NaCl and 0.015 mol/L sodium citrate), 1% sodium dodecyl sulfate at 68°C for 30 minutes, and once with 200 ml of 2× standard saline citrate at room temperature then analyzed in a phosphorimager.
Phosphorimager results were analyzed using AtlasImage 2.01 software (Clontech). This software automatically averages the two hybridization signals for a particular gene and compares this intensity to the same gene in the control array. A threefold difference in signal intensity between the test and control membranes was considered to represent a significant difference in gene expression after UVB exposure. To avoid any bias introduced by possible differential cDNA amounts loaded onto respective membranes the signal intensities were normalized with the AtlasImage 2.01 software (Clontech). The method used was the global normalization–median method on this double-spotted array. This method uses the median gene value of signal intensity over background for each array to calculate a normalization coefficient. A complete listing of genes included in the Atlas human cytokine/receptor cDNA expression array can be obtained at the Clontech web site (http://www.clontech.com/atlas/genelists/index.html).
Enzyme-Linked Immunosorbent Assay
For detection of soluble HB-EGF protein, 8 × 105 cells (both PECs and LECs) were cultured in triplicate on 150 × 25-mm culture dishes (Corning Glass). Once cells reached semiconfluence (80 to 90%) they were washed three times with 10 ml of PBS and placed in serum-free EMEM for 24 hours. The cells were exposed to UVB as outlined above. After irradiation cells were placed in 6 ml of serum-free EMEM and supernatants sampled at 0, 12, 24, 48, and 72 hours after irradiation.
Conditioned media and media alone was clarified by centrifugation and concentrated to 300 μl using Centriplus-10 concentrators (Amicon, Beverly, MA). In parallel 100 μl of a capture goat anti-human HB-EGF antibody (final concentration 1 μl/ml; R&D Systems, Minneapolis, MN) was placed in 96-well Immulon-4 plates (Dynatech, Chantilly, VA) and allowed to incubate overnight at room temperature. Plates were washed three times with PBS-Tween-20, and 300 μl of blocking buffer (PBS containing 1% bovine serum albumin, 5% sucrose, and 0.05% NaN3) was added and the plate incubated for 1 hour at room temperature. Each plate was washed a further three times and serial dilutions of human recombinant HB-EGF (R&D Systems) 0.05 to 3.2 ng/ml or concentrated PEC and LEC cell supernatants were added (100 μl/well) and incubated for 2 hours at room temperature. After washing the wells three times in PBS-Tween-20, 100 μl/well of 5 μg/ml of biotinylated goat anti-human HB-EGF antibody (R&D Systems) was added and the plate incubated for 2 hours at room temperature. Wells were washed three times and 100 μl/well of 1:1000 streptavidin-horseradish peroxidase (DAKO, Carpinteria, CA) in PBS was added and incubated for 20 minutes at room temperature. The plates were washed four times and developed with 40 μg/ml of 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) CABTS substrate (Sigma). The absorbance was read at 405 nm using a Spectramax Plus microplate reader (Molecular Devices, Sunnyvale, CA). The standard curve was linear between 0.05 and 3.2 ng/ml of HB-EGF. Absorbance values were converted to pg/ml values on the basis of the linear regression transformation of the standard curve.
Revere Transcriptase-Polymerase Chain Reaction (RT-PCR)
Total RNA from cultured PECs and LECs was extracted as above. RT-PCR was used to amplify a 750-bp HB-EGF complementary DNA (cDNA) fragment from these cell lines that corresponded to bases 282 to 1035 of the published human HB-EGF cDNA sequence. 20 RT-PCR was also performed to amplify the signal for GAPDH to standardize for equal loading in all lanes. Primer pairs were custom-designed (Life Technologies Inc., Gaithersburg, MD). The forward HB-EGF primer (24-mer) sequence was 5′-GGT GCT GAA GCT CTT TCT GGC TGC-3′ and the reverse HB-EGF primer (25-mer) was 5′-ATT ATG GGA GGC CCA ATC CTA GAC G-3′. 21 The forward GAPDH primer sequence was 5′-TGA TGA CAT CAA GAA GGT GGT GAA-3′ and the reverse GAPDH primer was 5′-TCC TTG GAG GCC ATG TGG GCC AT-3′. Amplification was performed using the Preamplification System for First-Strand cDNA (Life Technologies, Inc.) using a Perkin Elmer Cetus DNA thermal cycler according to the manufacturer’s instructions (Perkin Elmer, Branchburg, NJ). Total RNA (1 μg per 12 μl of reaction volume) was reverse-transcribed into cDNA using random primers. A 2-minute hot start at 95°C was performed. Denaturation was performed at 95°C for 30 seconds. Annealing temperature was 55°C for 30 seconds and extension was performed at 72°C for 30 seconds. The reaction was terminated with a 2-minute extension at 72°C. The signal was amplified for 31 cycles of PCR. PCR products were separated by electrophoresis in a 1.2% agarose gel (FMC, Rockland, ME) containing 50 ng/ml of ethidium bromide. PCR products were identified in relation to a 100-bp to 1500-bp ladder (Promega, Madison, WI). To verify that the amplified products were from mRNA and not genomic DNA contamination, reverse transcriptase was omitted from the reaction, resulting in no products after PCR amplification.
Immunohistochemical Analysis
Formalin-fixed, paraffin-embedded pterygia (n = 5), normal limbus (n = 2), and normal conjunctiva (n = 2) were cut (4 μm thick) and processed for immunohistochemistry as previously described. 22 After blocking specimens in swine serum, a goat anti-human HB-EGF antibody (R&D Systems) was added to each section overnight at 4°C. Sections were extensively washed in 0.05 mol/L of Tris-buffered saline (pH 7.6) before the addition of secondary swine anti-goat antibody complexed with horseradish peroxidase (DAKO) and the immunoreactivity was revealed by adding 3-amino-9-ethylcarbazole (Sigma). Control reactions were achieved by omitting the primary antibody. Sections were counterstained with hematoxylin.
Statistical Analysis—Enzyme-Linked Immunosorbent Assay
The enzyme-linked immunosorbent assay data presented are means ± SEM for triplicate values. Two sample t-tests were performed to determine significant differences among control versus treatment groups.
Results
cDNA Microarray Analysis in UVB-Irradiated PECs
We used a powerful and relatively novel approach to determine the effect of UVB exposure on differential gene expression in cultured PECs. The identity of individual genes corresponding to the spot pairs can be determined at http://www.clontech.com.
Analysis of the hybridization signals (Figure 1) ▶ revealed that nine genes were up-regulated and 36 genes were down-regulated within 6 hours after UVB treatment. Twelve hours after irradiation 7 genes were significantly induced and 42 were down-regulated. Among these prominent inducible genes were both growth factors and growth factor receptors (Tables 1 and 2) ▶ ▶ . Of the genes that were up-regulated by UVB irradiation there were only three consistently elevated at 6 and 12 hours after irradiation. These genes encode for HB-EGF, fibroblast growth factor-3, and cytotoxic ligand TRAIL receptor.
Figure 1.
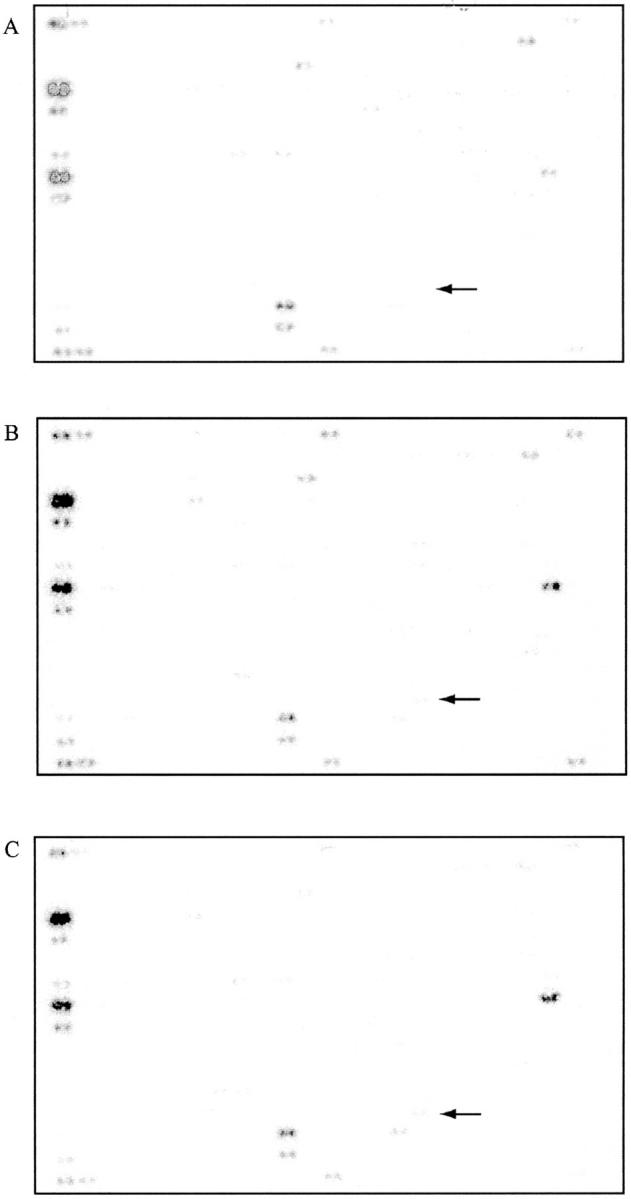
Microarrays for PEC. Nonirradiated PEC (A), 6 hours after UV exposure (B), and 12 hours after UV exposure (C). The differential gene expression for HB-EGF is highlighted by arrows.
Table 1.
Genes Up-Regulated in PECs 6 Hours after UVB Exposure Compared to Nonirradiated PECs
| Ratio | Protein/gene |
|---|---|
| 4.67 | Transforming growth factor beta receptor III precursor (TGF beta receptor III) |
| 4.43 | Endothelin 2 (ET2) |
| 4.29 | Heparin-binding EGF-like growth factor (HB-EGF) |
| 3.63 | Stromal cell-derived factor 1 receptor (SDF1 receptor) |
| 3.54 | G-protein-coupled receptor HM74 |
| 3.52 | Cytotoxic ligand TRAIL receptor |
| 3.15 | Fibroblast growth factor 3 (FGF3) |
| 3.15 | Endothelin receptor type A (EDNRA; ETA) |
| 3.03 | Wnt-13 |
Table 2.
Genes Up-Regulated in PECs 12 Hours after UVB Exposure Compared to Nonirradiated PECs
| Ratio | Protein/gene |
|---|---|
| 4.67 | Fibroblast growth factor 3 (FGF3) |
| 3.62 | Transforming growth factor-beta 3 (TGF-beta3) |
| 3.52 | Heparin-binding EGF-like growth factor (HB-EGF) |
| 3.42 | Small inducible cytokine A1 (SCYA1) |
| 3.33 | Cytotoxic ligand TRAIL receptor |
| 3.26 | Frizzled 5 |
| 3.07 | Leukemia inhibitory factor precursor (LIF) |
RT-PCR
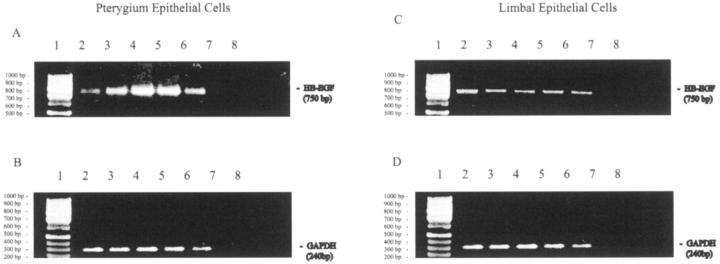
RT-PCR was used to semiquantitate and confirm the array data. Total RNA was extracted from LECs and PECs after UVB treatment. Although both cell lines constitutively expressed HB-EGF this growth factor was significantly induced in a time course-dependent manner in pterygium-derived epithelial cells (Figure 2A ▶ , lanes 3 to 6). Maximal induction occurred 6 hours after UVB (6.8-fold) (Figure 2A ▶ , lane 4) after the expression was normalized to GAPDH (Figure 2, B and D) ▶ . In contrast, there was no significant modulation of HB-EGF mRNA expression in LEC throughout the same time course (Figure 2C ▶ , lanes 2 to 6).
Figure 2.
RT-PCR analysis for HB-EGF (750 bp) mRNA in UVB stimulated PEC (A) and LEC (C). B and D represent RT-PCR for GAPDH (240 bp) in PEC and LEC, respectively. Equal amounts of total RNA were reverse-transcribed from both cell types in all lanes. Time points at 0, 2, 6, 12, and 24 hours after irradiation are represented by lanes 2 to 6, respectively. When no reverse-transcriptase enzyme and no gene-specific primers were included, no PCR product formed (lanes 7 and 8, respectively). A 100-bp ladder (Promega) was run in parallel (lane 1). These results are representative of triplicate experiments in three different cell lines.
Enzyme-Linked Immunosorbent Assay
Cell supernatants were concentrated and assayed for HB-EGF by enzyme-linked immunosorbent assay. Culture medium alone served as a control and contained no detectable HB-EGF. Likewise no detectable HB-EGF was found in LEC supernatants. However, 43 ± 2.2 pg/ml of HB-EGF was detected in culture medium from nonirradiated PECs, which was significantly increased to 120 ± 27.3 pg/ml (P < 0.05) 48 hours after UVB treatment.
Immunohistochemistry
HB-EGF was expressed by the epithelium in all pterygia specimens analyzed (Figure 3; A, C, and E) ▶ . HB-EGF was also associated with endothelial cells in pterygia. HB-EGF was detected in the epithelium of limbus (Figure 3G) ▶ , predominately in the superficial layers of the epithelium; however this staining was not as intense as that exhibited by pterygia. In contrast, little or no reactivity was detected in normal conjunctiva (Figure 3H) ▶ .
Figure 3.
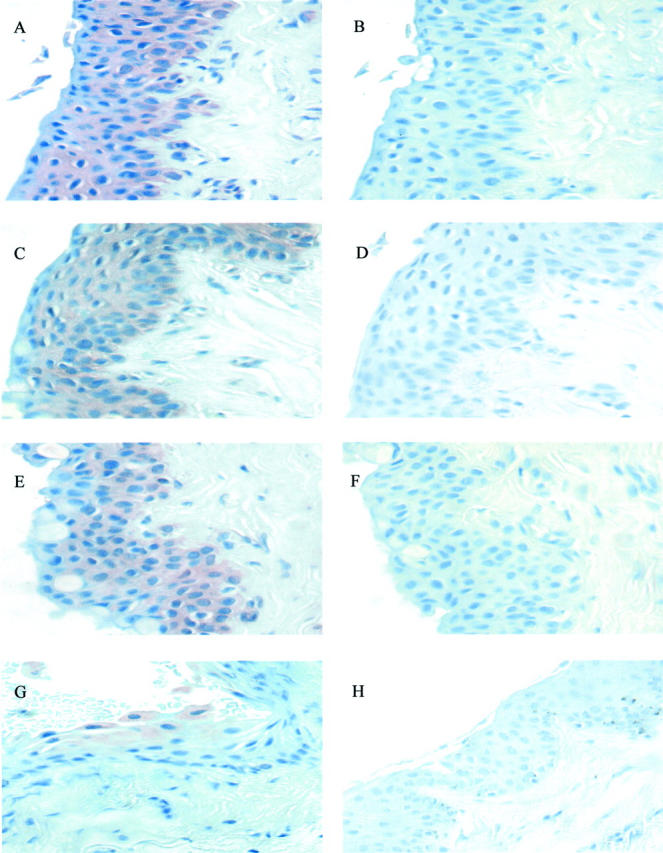
Expression of HB-EGF in pterygia. Pterygium tissue (A through F), normal limbus (G), and normal conjuntiva (H) were analyzed by immunohistochemistry to determine the expression of HB-EGF. Sections A, C, E, G, and H included the anti-HB-EGF antibody and sections B, D, and F were incubated without primary antibody and served as controls. All tissue sections were counterstained with hematoxylin. These data are representative of all pterygia examined. Original magnifications, ×600.
Discussion
This is the first study to evaluate the production of growth factors and cytokines in pterygium-derived epithelial cells after UV exposure using microarray analysis. Of the three factors that were consistently up-regulated by UVB irradiation two were growth factors. We focused our investigation on HB-EGF because of the recently documented presence of the receptor for this molecule in pterygium tissue 23,24 and the known physiological and pathophysiological actions of this protein in other tissues. HB-EGF was first identified as a 20- to 22-kd soluble glycoprotein that is structurally a member of the EGF family of growth factors. 20 The heparin-binding domain modulates the biological activity of this protein. Like other members of the EGF family, HB-EGF is synthesized as a transmembrane protein (proHB-EGF) and is cleaved at the plasma membrane to yield a soluble HB-EGF of 75 to 86 amino acids. 20,25 HB-EGF is a potent mitogen for fibroblasts, smooth muscle cells, and keratinocytes, as well as being an inducer of cell migration. 26-29 HB-EGF has a potential role in physiological wound healing. 30 As proHB-EGF has a growth inhibitory effect, 31,32 the processing of proHB-EGF means switching to the opposite activity with respect to growth. HB-EGF is present in normal skin and has been detected in skin cancers derived from the basal epithelial cell layer, including basal and squamous cell carcinomas of the skin as well as other malignancies. 33-36
Metalloproteases (MMP) are implicated in the cleavage of proHB-EGF and metalloprotease inhibitors (TIMP) prevent this process. 37 The presence of MMP and TIMP in pterygium is well documented. 38-42
The interaction between HB-EGF, MMP, and TIMP in pterygia is likely to result in the liberation of the soluble form of HB-EGF from pterygium epithelium. Given the mitogenic effects of HB-EGF and its ability to stimulate cell migration it is a likely candidate to promote the cellular growth and migration that is a characteristic factor of this lesion.
HB-EGF binds to the EGF receptor (EGFR), inducing phosphorylation and also binds to and stimulates another EGF receptor family member, HER4. 27,28 HB-EGF exerts its growth-promoting effects through EGFR. Stimulation of EGFR has been shown to induce a mitogenic response in many cell types. 43 Li and Tseng 44 have shown that fibroblasts in the conjunctiva, limbus, and cornea all express EGFR mRNA, but under normal circumstances it is not abundantly expressed. EGFR-1 is expressed diffusely on the cell surface of pterygium fibroblasts and is functionally active in these cells. 24 Fibroblast proliferation within the subepithelial region is obvious, 24 this also suggests that the proliferative force is highest adjacent to, and perhaps driven by the pterygium epithelium. EGFR has been demonstrated in pterygium epithelium and normal conjunctival epithelium. 23 Although there is a high expression of EGFR in pterygium compared to normal conjunctival controls, there is also a differential pattern of EGFR expression within the epithelium. It seems that there is EGFR expression throughout the full thickness of pterygium epithelium compared to a basally located expression within normal conjunctiva. 23 This corresponds to expression of HB-EGF within pterygia in this study.
HB-EGF is augmented in cutaneous thermal injuries and the pattern of its epidermal distribution also changes with this type of insult. 45 In normal skin HB-EGF is confined to the basal keratinocytes of the epidermis whereas there is a more panepithelial distribution after burn injuries. This is a similar pattern to that seen in pterygium and the possibility exists that HB-EGF may be up-regulated in response to cell injury after irradiation given the cytoprotective actions of HB-EGF in other tissues. 46-49
As well as strong staining for HB-EGF in the epithelium of pterygium, HB-EGF also localizes to the vasculature of pterygium. HB-EGF is a potent mitogen and migration factor for vascular smooth muscle cells and promotes neovascularization in the rabbit cornea. 50 HB-EGF seems to use VEGF as an intermediate to induce mitogenesis of vascular endothelial cells 50 because it is known to be present in increased amounts in pterygium. 14 Pterygium vasculature also exhibits intense immunoreactivity for MMP-7, 38 which is one of the main contenders postulated for converting proHB-EGF to HB-EGF. 51
Alternate theories of pterygium pathogenesis have focused on the pterygial fibroblast and the potential for this cell line to have a transformed phenotype given its capacity for anchorage-independent growth in culture. 52 HB-EGF is a potent growth factor capable of stimulating altered cell growth and anchorage independence. 53 Therefore, HB-EGF may stimulate pterygium fibroblast proliferation and growth in this disease.
We speculate that the up-regulation of HB-EGF in PECs after UVB irradiation potentates a role for this growth factor in the pathogenesis of pterygium. HB-EGF was detected in the epithelium of pterygium by immunohistochemistry and this coupled with the pattern of staining in normal limbal epithelium strengthens the case for limbal stem cells being the cell of origin for the pterygial epithelial cell. 5,54
Footnotes
Address reprint requests to Denis Wakefield, M.D., The University of New South Wales, Inflammation Research Unit, School of Pathology, PO Box 1, Kensington, Sydney, Australia, NSW, 2052. E-mail: d.wakefield@med.unsw.edu.au.
Supported by the National Health and Medical Research Council of Australia.
References
- 1.Coroneo MT, Di Girolamo N, Wakefield D: The pathogenesis of pterygia. Curr Opin Ophthalmol 1999, 10:282-288 [DOI] [PubMed] [Google Scholar]
- 2.Taylor HR, West SK, Rosenthal FS, Munoz B, Newland HS, Emmett EA: Corneal changes associated with chronic UV irradiation. Arch Ophthalmol 1989, 107:1481-1484 [DOI] [PubMed] [Google Scholar]
- 3.Threlfall TJ, English DR: Sun exposure and pterygium of the eye: a dose-response curve. Am J Ophthalmol 1999, 128:280-287 [DOI] [PubMed] [Google Scholar]
- 4.Panchapakesan J, Hourihan F, Mitchell P: Prevalence of pterygium and pinguecula: the Blue Mountains Eye Study. Aust N Z J Ophthalmol 1998, 26:S2-S5 [DOI] [PubMed] [Google Scholar]
- 5.Coroneo MT: Pterygium as an early indicator of ultraviolet insolation: a hypothesis. Br J Ophthalmol 1993, 77:734-739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takashima A, Bergstresser PR: Impact of UVB radiation on the epidermal cytokine network. Photochem Photobiol 1996, 63:397-400 [DOI] [PubMed] [Google Scholar]
- 7.Murakami T, Fujimoto M, Ohtsuki M, Nakagawa H: Expression profiling of cancer-related genes in human keratinocytes following non-lethal ultraviolet B irradiation. J Dermatol Sci 2001, 27:121-129 [DOI] [PubMed] [Google Scholar]
- 8.Li D, Turi TG, Schuck A, Freedberg IM, Khitrov G, Blumenberg M: Rays and arrays: the transcriptional program in the response of human epidermal keratinocytes to UVB illumination. EMBO J 2001, 15:2533-2535 [DOI] [PubMed] [Google Scholar]
- 9.Herrlich P, Sachsenmaier C, Radler-Pohl A, Gebel S, Blattner C, Rahmsdorf HJ: The mammalian UV response: mechanism of DNA damage induced gene expression. Adv Enzyme Regul 1994, 34:381-395 [DOI] [PubMed] [Google Scholar]
- 10.Nicola NN: Cytokines and Their Receptors. 1994. Oxford University Press, Oxford
- 11.Kennedy M, Kim KH, Harten B, Brown J, Planck S, Meshul C, Edelhauser H, Rosenbaum JT, Armstrong CA, Ansel JC: Ultraviolet irradiation induces the production of multiple cytokines by human corneal cells. Invest Ophthalmol Vis Sci 1997, 38:2483-2491 [PubMed] [Google Scholar]
- 12.Di Girolamo N, Tedla N, Kumar RK, McCluskey P, Lloyd A, Coroneo MT, Wakefield D: Culture and characterisation of epithelial cells from human pterygia. Br J Ophthalmol 1999, 83:1077-1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Girolamo N, Coroneo MT, Wakefield D: UVB-mediated induction of interleukin-6 and -8 in pterygia and cultured human pterygium epithelial cells. Invest Ophthalmol Vis Sci 2002, 43:3430-3437 [PubMed] [Google Scholar]
- 14.Lee DH, Cho HJ, Kim JT, Choi JS, Joo CK: Expression of vascular endothelial growth factor and inducible nitric oxide synthase in pterygia. Cornea 2001, 20:738-742 [DOI] [PubMed] [Google Scholar]
- 15.Kria L, Ohira A, Amemiya T: Immunohistochemical localization of basic fibroblast growth factor, platelet derived growth factor, transforming growth factor-beta and tumor necrosis factor-alpha in the pterygium. Acta Histochem 1996, 98:195-201 [DOI] [PubMed] [Google Scholar]
- 16.Kria L, Ohira A, Amemiya T: Growth factors in cultured pterygium fibroblasts: immunohistochemical and ELISA analysis. Graefes Arch Clin Exp Ophthalmol 1998, 236:702-708 [DOI] [PubMed] [Google Scholar]
- 17.Powers MR, Qu Z, O’Brien B, Wilson DJ, Thompson JE, Rosenbaum JT: Immunolocalization of bFGF in pterygia: association with mast cells. Cornea 1997, 16:545-549 [PubMed] [Google Scholar]
- 18.Nakagami T, Watanabe I, Murakami A, Okisaka S, Ebihara N: Expression of stem cell factor in pterygium. Jpn J Ophthalmol 2000, 44:193-197 [DOI] [PubMed] [Google Scholar]
- 19.Di Girolamo N, Lloyd A, McCluskey P, Filipic M, Wakefield D: Increased expression of matrix metalloproteinases in vivo in scleritis tissue and in vitro in cultured human scleral fibroblasts. Am J Pathol 1997, 150:653-666 [PMC free article] [PubMed] [Google Scholar]
- 20.Higashiyama S, Abraham JA, Miller J, Fiddes JC, Klagsbrun M: A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science 1991, 251:936-939 [DOI] [PubMed] [Google Scholar]
- 21.Umata T, Hirata M, Takahashi T, Ryu F, Shida S, Takahashi Y, Tsuneoka M, Miura Y, Masuda M, Horiguchi Y, Mekada E: A dual signaling cascade that regulates the ectodomain shedding of heparin-binding epidermal growth factor-like growth factor. J Biol Chem 2001, 276:30475-30482 [DOI] [PubMed] [Google Scholar]
- 22.Di Girolamo N, Tedla N, Lloyd A, Wakefield D: Expression of matrix metalloproteinases by human plasma cells and B lymphocytes. Eur J Immunol 1998, 28:1773-1784 [DOI] [PubMed] [Google Scholar]
- 23.Liu Z, Xie Y, Zhang M: Overexpression of type I growth factor receptors in pterygium. Chin Med J 2002, 115:418-421 [PubMed] [Google Scholar]
- 24.Maini R, Collison DJ, Maidment JM, Davies PD, Wormstone IM: Pterygial derived fibroblasts express functionally active histamine and epidermal growth factor receptors. Exp Eye Res 2002, 74:237-244 [DOI] [PubMed] [Google Scholar]
- 25.Goishi K, Higashiyama S, Klagsbrun M, Nakano N, Umata T, Ishikawa M, Mekada E, Taniguchi N: Phorbol ester induces the rapid processing of cell surface heparin-binding EGF-like growth factor: conversion from juxtacrine to paracrine growth factor activity. Mol Biol Cell 1995, 6:967-980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higashiyama S, Abraham JA, Klagsbrun M: Heparin-binding EGF-like growth factor synthesis by smooth muscle cells. Horm Res 1994, 42:9-13 [DOI] [PubMed] [Google Scholar]
- 27.Raab G, Klagsbrun M: Heparin-binding EGF-like growth factor. Biochim Biophys Acta 1997, 1333:F179-F199 [DOI] [PubMed] [Google Scholar]
- 28.Elenius K, Paul S, Allison G, Sun J, Klagsbrun M: Activation of HER4 by heparin-binding EGF-like growth factor stimulates chemotaxis but not proliferation. EMBO J 1997, 16:1268-1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higashiyama S, Iwamoto R, Goishi K, Raab G, Taniguchi N, Klagsbrun M, Mekada E: The membrane protein CD9/DRAP 27 potentiates the juxtacrine growth factor activity of the membrane-anchored heparin-binding EGF-like growth factor. J Cell Biol 1995, 128:929-938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marikovsky M, Breuing K, Liu PY, Eriksson E, Higashiyama S, Farber P, Abraham J, Klagsbrun M: Appearance of heparin-binding EGF-like growth factor in wound fluid as a response to injury. Proc Natl Acad Sci USA 1993, 90:3889-3893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyoshi E, Higashiyama S, Nakagawa T, Hayashi N, Taniguchi N: Membrane-anchored heparin-binding epidermal growth factor-like growth factor acts as a tumor survival factor in a hepatoma cell line. J Biol Chem 1997, 272:14349-14355 [DOI] [PubMed] [Google Scholar]
- 32.Iwamoto R, Handa K, Mekada E: Contact-dependent growth inhibition and apoptosis of epidermal growth factor (EGF) receptor-expressing cells by the membrane-anchored form of heparin-binding EGF-like growth factor. J Biol Chem 1999, 274:25906-25912 [DOI] [PubMed] [Google Scholar]
- 33.Downing MT, Brigstock DR, Luquette MH, Crissman-Combs M, Besner GE: Immunohistochemical localization of heparin-binding epidermal growth factor-like growth factor in normal skin and skin cancers. Histochem J 1997, 29:735-744 [DOI] [PubMed] [Google Scholar]
- 34.Narita T, Kawakami-Kimura N, Sato M, Matsuura N, Higashiyama S, Taniguchi N, Kannagi R: Alteration of integrins by heparin-binding EGF-like growth factor in human breast cancer cells. Oncology 1996, 53:374-381 [DOI] [PubMed] [Google Scholar]
- 35.Naef M, Yokoyama M, Friess H, Buchler MW, Korc M: Co-expression of heparin-binding EGF-like growth factor and related peptides in human gastric carcinoma. Int J Cancer 1996, 66:315-321 [DOI] [PubMed] [Google Scholar]
- 36.Kobrin MS, Funatomi H, Friess H, Buchler MW, Stathis P, Korc M: Induction and expression of heparin-binding EGF-like growth factor in human pancreatic cancer. Biochem Biophys Res Commun 1994, 202:1705-1709 [DOI] [PubMed] [Google Scholar]
- 37.Lanzrein M, Garred O, Olsnes S, Sandvig K: Diphtheria toxin endocytosis and membrane translocation are dependent on the intact membrane-anchored receptor (HB-EGF precursor): studies on the cell-associated receptor cleaved by a metalloprotease in phorbol-ester-treated cells. Biochem J 1995, 310:285-289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Girolamo N, Coroneo MT, Wakefield D: Active matrilysin (MMP-7) in human pterygia: potential role in angiogenesis. Invest Ophthalmol Vis Sci 2001, 42:1963-1968 [PubMed] [Google Scholar]
- 39.Di Girolamo N, McCluskey P, Lloyd A, Coroneo MT, Wakefield D: Expression of MMPs and TIMPs in human pterygia and cultured pterygium epithelial cells. Invest Ophthalmol Vis Sci 2000, 41:671-679 [PubMed] [Google Scholar]
- 40.Di Girolamo N, Wakefield D, Coroneo MT: Differential expression of matrix metalloproteinases and their tissue inhibitors at the advancing pterygium head. Invest Ophthalmol Vis Sci 2000, 41:4142-4149 [PubMed] [Google Scholar]
- 41.Dushku N, John MK, Schultz GS, Reid TW: Pterygia pathogenesis: corneal invasion by matrix metalloproteinase expressing altered limbal epithelial basal cells. Arch Ophthalmol 2001, 119:695-706 [DOI] [PubMed] [Google Scholar]
- 42.Solomon A, Li DQ, Lee SB, Tseng SC: Regulation of collagenase, stromelysin, and urokinase-type plasminogen activator in primary pterygium body fibroblasts by inflammatory cytokines. Invest Ophthalmol Vis Sci 2000, 41:2154-2163 [PubMed] [Google Scholar]
- 43.Carpenter G: The EGF receptor: a nexus for trafficking and signaling. Bioessays 2000, 22:697-707 [DOI] [PubMed] [Google Scholar]
- 44.Li DQ, Tseng SC: Three patterns of cytokine expression potentially involved in epithelial-fibroblast interactions of human ocular surface. J Cell Physiol 1995, 163:61-79 [DOI] [PubMed] [Google Scholar]
- 45.McCarthy DW, Downing MT, Brigstock DR, Luquette MH, Brown KD, Abad MS, Besner GE: Production of heparin-binding epidermal growth factor-like growth factor (HB-EGF) at sites of thermal injury in pediatric patients. J Invest Dermatol 1996, 106:49-56 [DOI] [PubMed] [Google Scholar]
- 46.Nguyen HT, Bride SH, Badawy AB, Adam RM, Lin J, Orsola A, Guthrie PD, Freeman MR, Peters CA: Heparin-binding EGF-like growth factor is up-regulated in the obstructed kidney in a cell- and region-specific manner and acts to inhibit apoptosis. Am J Pathol 2000, 156:889-898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pillai SB, Hinman CE, Luquette MH, Nowicki PT, Besner GE: Heparin-binding epidermal growth factor-like growth factor protects rat intestine from ischemia/reperfusion injury. J Surg Res 1999, 87:225-231 [DOI] [PubMed] [Google Scholar]
- 48.Takemura T, Kondo S, Homma T, Sakai M, Harris RC: The membrane-bound form of heparin-binding epidermal growth factor-like growth factor promotes survival of cultured renal epithelial cells. J Biol Chem 1997, 272:31036-31042 [DOI] [PubMed] [Google Scholar]
- 49.Michalsky MP, Kuhn A, Mehta V, Besner GE: Heparin-binding EGF-like growth factor decreases apoptosis in intestinal epithelial cells in vitro. J Pediatr Surg 2001, 36:1130-1135 [DOI] [PubMed] [Google Scholar]
- 50.Abramovitch R, Neeman M, Reich R, Stein I, Keshet E, Abraham J, Solomon A, Marikovsky M: Intercellular communication between vascular smooth muscle and endothelial cells mediated by heparin-binding epidermal growth factor-like growth factor and vascular endothelial growth factor. FEBS Lett 1998, 425:441-447 [DOI] [PubMed] [Google Scholar]
- 51.Yu WH, Woessner JF, Jr, McNeish JD, Stamenkovic I: CD44 anchors the assembly of matrilysin/MMP-7 with heparin-binding epidermal growth factor precursor and ErbB4 and regulates female reproductive organ remodeling. Genes Dev 2002, 16:307-323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen JK, Tsai RJ, Lin SS: Fibroblasts isolated from human pterygia exhibit transformed cell characteristics. In Vitro Cell Dev Biol Anim 1994, 30A:243-248 [DOI] [PubMed] [Google Scholar]
- 53.Harding PA, Davis-Fleischer KM, Crissman-Combs MA, Miller MT, Brigstock DR, Besner GE: Induction of anchorage independent growth by heparin-binding EGF-like growth factor (HB-EGF). Growth Factors 1999, 17:49-61 [DOI] [PubMed] [Google Scholar]
- 54.Dushku N, Reid TW: Immunohistochemical evidence that human pterygia originate from an invasion of vimentin-expressing altered limbal epithelial basal cells. Curr Eye Res 1994, 13:473-481 [DOI] [PubMed] [Google Scholar]