Abstract
Lipids accumulate in Bruch’s membrane (BrM), a specialized vascular intima of the eye, and in extracellular lesions associated with aging and age-related maculopathy (ARM). We tested the hypothesis that ARM and atherosclerotic cardiovascular disease share molecules and mechanisms pertaining to extracellular lipid accumulation by localizing cholesterol and apolipoprotein B (apo B) in BrM, basal deposits, and drusen. Human donor eyes were preserved <4 hours postmortem and cryosectioned. Sections were stained with traditional lipid stains and filipin for esterified and unesterified cholesterol or probed with antibodies to apo B, apo E, and apo C-III. Normal adult retinal pigment epithelium (RPE) was subjected to RT-PCR and Western blot analysis for apolipoprotein mRNA and protein. Esterified and unesterified cholesterol was present in all drusen and basal deposits of ARM and normal eyes. Both apo B and apo E but not apo C-III were found in BrM, drusen, and basal deposits. Fewer macular drusen were stained by traditional lipid stains and apolipoprotein antibodies than peripheral drusen. RPE contained apo B and apo E mRNA and protein. Finding cholesterol and apo B in sub-RPE deposits links ARM with important molecules and mechanisms in atherosclerosis initiation and progression. The combination of apo B mRNA and protein in RPE raises the possibility that intraocular assembly of apo B-containing lipoproteins is a pathway involved in forming cholesterol-enriched lesions in ARM.
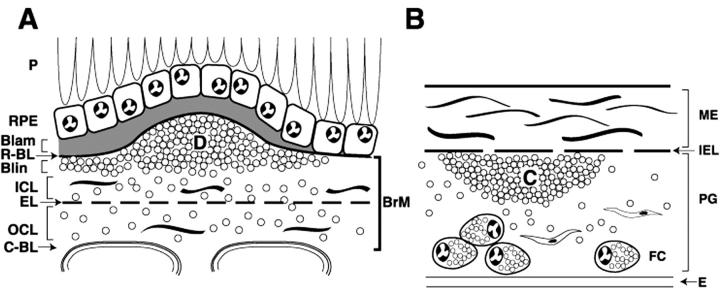
Age-related maculopathy (ARM) is the leading cause of new, untreatable vision loss in the elderly in Western societies. 1-3 As shown in Figure 1A ▶ , ARM involves the retinal pigment epithelium (RPE, cells dedicated to sustaining photoreceptor health), the choriocapillaris (the blood supply to photoreceptors and the RPE), and Bruch’s membrane (BrM, a thin vascular intima between the RPE and choriocapillaris). 4,5 Early ARM is characterized by moderate vision loss associated with characteristic extracellular lesions. Lesions between the RPE basal lamina and BrM can be focal (drusen) or diffuse (basal linear deposits). A diffuse lesion between the RPE and its basal lamina is basal laminar deposit. The term “sub-RPE deposits” is used for the combination of drusen and basal deposits and “basal deposits” for the combination of basal laminar deposit and basal linear deposit. Late ARM is characterized by severe vision loss associated with extensive RPE atrophy with or without the sequelae of choroidal neovascularization, ie, in-growth of choriocapillaries through BrM and under the RPE in the plane of drusen and basal linear deposits. Because the causes of ARM are obscure, recent studies have sought molecules present in the affected tissues and characteristic lesions to identify biochemical pathways perturbed by disease. 6 An important but incompletely characterized component of BrM and sub-RPE deposits is lipids. Normal BrM accumulates lipids with age, and the accumulation of esterified and unesterified cholesterol (EC and UC)-containing particles is especially prominent in the macula. 7-10 Drusen and basal deposits in aged eyes without ARM contain lipids, including cholesterol, 9-13 and current evidence suggests that individual sub-RPE deposits are preferentially enriched in either neutral lipids or polar lipids. 13 The source of lipids and mechanisms of deposition are unknown. Analyses of BrM/choroid lipid composition have implicated both local cells and plasma. 8,9
Figure 1.
Schematic cross-sections of Bruch’s membrane (BrM) from an eye with ARM (A) and atherosclerotic arterial intima (B). Endothelium and vascular lumina (choriocapillary, A; arterial, B) are at the bottom. Drawings are not at scale. For reference, the thickness of normal BrM and intima is 4 to 6 μm and 100 to 300 μm, respectively. Small circles in BrM (A) and PG layer (B) indicate esterified cholesterol-containing particles. A: P, photoreceptors; RPE, retinal pigment epithelium; R-BL, RPE basal lamina; Blam, basal laminar deposit; Blin, basal linear deposit; D, druse; ICL, inner collagenous layer; EL, elastic layer; OCL, outer collagenous layer; C-BL, choriocapillaris basal lamina. In normal eyes, BrM (thick bracket at right edge of panel A) consists of R-BL, ICL, EL, OCL, and C-Cl. Blam and Blin together comprise basal deposits, and basal deposits and drusen together comprise sub-RPE deposits. B: ME, musculoelastic layer; IEL, internal elastic layer; C, lipid-rich core; PG, proteoglycan layer; FC, foam cells; E, endothelium.
Atherosclerotic cardiovascular disease (CVD), the leading cause of death in Western societies, is also characterized by extracellular lipid deposition in a vessel wall. As shown in Figure 1B ▶ , an atherosclerotic lesion in the intima (inner wall) of large arteries contains a lipid-rich core encapsulated by connective tissue containing smooth muscle cells and foam cells (cholesterol-enriched macrophages). 14,15 According to the response-to-retention hypothesis, 16,17 retention of plasma lipoproteins in the intima is the key event initiating atherosclerosis. Lipoproteins contain neutral lipids (EC and triglyceride) surrounded by a surface of apolipoproteins, UC, and phospholipid. The most atherogenic plasma lipoproteins contain apolipoprotein B (apo B). 18 Retained apo B-containing lipoproteins directly or indirectly evoke subsequent deleterious events in the intima, including modifications resulting in extracellular cholesterol accumulation, 19-21 monocyte entry, macrophage foam cell formation, cytokine production, inflammation, and immune response. 22-24 Excess cholesterol within the core extracellular matrix contributes to a structurally unstable lesion, resulting in deadly complications of neovascularization, rupture, thrombosis, and hemorrhage. 25 Reduced apo B binding to intimal proteoglycans markedly attenuates atherosclerosis in mice. 26
Apo B is a large and intensively studied protein with globular and α-helical amphipathic domains and β-sheets. 27 Apo B-100 (4536 residues) is produced in the liver, where it is assembled into very low density lipoproteins before secretion into plasma. Apo B-48 (2152 residues) is produced in the small intestine as an essential protein of dietary chylomicrons. Apo B-100 and apo B-48 are products of the same gene, with apo B48 arising from post-transcriptional editing of apo B-100 mRNA. Successful transfer of lipids to nascent apo B regulates whether apo B is secreted in a mature lipoprotein particle or degraded via the ubiquitin-proteasome system. 28-30
Patients with CVD history or symptoms have increased risk for ARM 31,32 (G. McGwin, C. Owsley, C.A. Curcio, R.J. Crain, submitted), and CVD and ARM have common risk factors (age, smoking, and hypertension). 33,34 Here we test the hypothesis that CVD and ARM share common molecules and mechanisms pertaining to extracellular lipid accumulation in the vessel wall, beginning with cholesterol and apo B. We stained macular and peripheral sections of ARM and age-matched control eyes with routine lipid stains and filipin for EC and UC. 9 We examined apo B as well as apo E and apo C-III, two other components of atherogenic lipoproteins. 18 Apo E is also an established constituent of sub-RPE deposits. 35,36 Finally, we assessed the potential for RPE lipoprotein synthesis using reverse transcriptase polymerase chain reaction (RT-PCR) and Western blot analysis. We found cholesterol and apo B in sub-RPE lesions and evidence for intraocular synthesis of apo B.
Materials and Methods
Human Tissue
Eyes were obtained from non-diabetic donors. For histochemistry and immunohistochemistry, eyes were preserved within 4 hours of death by immersion in 4% paraformaldehyde/0.1 mol/L phosphate buffer (pH 7.4) for 6 to 16 hours after removal of the cornea and lens. Eyes were stored at 4°C in 1% paraformaldehyde until used. Eyes were examined internally with a stereo-dissecting microscope (SMZ-U, Nikon, Melville, NY) and using epi- and trans-illumination to detect drusen and pigmentary disturbances. 37 Normal eyes lacked grossly visible drusen and RPE changes in the macula. For Western blot analysis and RT-PCR, normal eyes were obtained within 6 hours of death.
Cryosections and Routine Stains
The retina/RPE/choroid was removed from the sclera, and samples were cut with a razor blade. Macular and peripheral samples were 7-mm wide (nasal to temporal). Macular samples included the fovea and the temporal half of the optic nerve head. Peripheral samples included the temporal equator and ora serrata. Samples were infiltrated with successive solutions of 10%, 20%, and 30% sucrose in phosphate buffer, 4:1 30% sucrose:Histoprep (Fisher Scientific, Fair Lawn, NJ) and 2:1 30% sucrose:Histoprep solution for 30 minutes each, 38 and then frozen in liquid nitrogen. Samples were sectioned at 10 μm. Sections were collected on gelatin-subbed slides, dried at 50 to 55°C for at least 2 hours, and stored at −20°C until used. For histopathological evaluation, one slide containing the foveal center (identified by yellow pigment and thin inner retinal layers) was stained with hematoxylin (Gill’s formulation no. 3, Fisher Scientific). Representative cryosections from each macula and periphery were stained with periodic acid Schiff and hematoxylin.
Histopathological and Clinical Evaluation
ARM cases were defined using semi-quantitative evaluation of foveal sections. 37 Non-exudative ARM eyes had either one druse >63 μm or RPE clumping, anterior migration, or atrophy with at least one druse or a continuous layer of basal deposits. Late exudative ARM eyes had choroidal neovascularization and/or fibrovascular scars with basal deposits or drusen. Clinical records, when available, were reviewed to eliminate eyes with other macular chorioretinal diseases. Clinical and histopathological characteristics of ARM eyes are summarized in Table 1 ▶ . The macula and periphery of these eyes contained basal deposits and drusen (small, medium, and large). Seven eyes had RPE atrophy, and three eyes had choroidal neovascularization. Normal eyes lacked significant macular pathology, and the periphery contained patchy or continuous thin diffuse deposits, small drusen, and some medium drusen.
Table 1.
Eyes Used for Immunohistochemistry and Histochemistry
| ARM eyes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Donor | Age | Gender | Clinical information | Histopathology | Experimental series | |||||
| VA | RE | History | Basal deposits | Drusen | RPE | CNV | ||||
| 1 | 73 | M | 3 | Normal | ARM | M | A | I, II, III, IV | ||
| 2 | 74 | M | 2 | Normal | ARM | 2 | M | Cl | I, III | |
| 3 | 78 | F | 4 | Normal | ARM/GA | 3 | S | A | I, II, III, IV | |
| 4 | 81 | F | — | — | — | L | I, II, III, IV | |||
| 5 | 81 | M | 3 | Normal | ARM | 2 | L | A | I, III | |
| 6 | 81 | M | — | — | — | 3 | L | Cl | I, III | |
| 7 | 82 | F | 4 | Normal | ARM | 2 | M | Cl | Y | II, III |
| 8 | 85 | F | 3 | Normal | ARM | 3 | A | I, II, III, IV | ||
| 9 | 85 | M | — | — | — | M | I, II, III, IV | |||
| 10 | 86 | F | — | — | — | 2 | L | A | Y | I, III |
| 11 | 88 | F | 5 | Normal | ARM/CNV | 2 | S | A | Y | II, III |
| 12 | 95 | F | 3 | Hyperopic | ARM | 2 | L | A | I, III | |
Normal eyes
Eyes used for histochemistry: lipid (n = 12; 61 to 92 yrs), Filipin (n = 5; 63 to 87 yrs).
Eyes used for apolipoprotein immunohistochemistry: apo B (n = 12; 61 to 92 yrs), apo E and C-III (n = 7; 63 to 87 yrs).
Clinical information: dash, indicates no records available; histopathology, blank indicates normal or minimal; Y, yes.
VA, visual acuity (corrected); 1, 20/20 or better; 2, 20/25 to 20/40; 3, 20/50 to 20/200; 4, 20/300 to 20/1600; 5, hand motion, light perception.
RE, refractive error; normal, −2 to +2D; hyperopic, >+2D to +4D.
Basal deposits: 2, <1/2 RPE height; 3, >1/2 RPE height; largest drusen; S<63 μm, 63<M<125 μm, L>125 μm.
GA, geographic atrophy; RPE, retinal pigment epithelium; Cl, clumping; A, atrophy; CNV, choroidal neovascularization.
Experimental series: I, lipid histochemistry; II, filipin histochemistry; III, apo B localization; IV, apo E and C-III localization.
Lipid Histochemistry
Cryosections were stained with bromine sudan black B (BSBB) and oil red O (ORO). BSBB and ORO bind EC, fatty acids, and triglycerides. BSBB also binds UC and other compounds. 39 Lipids were extracted from control slides with 1% HCl in 2:1 chloroform:methanol for 30 minutes. Filipin was used to bind UC and EC as described. 9 In brief, for EC detection, native UC was extracted from cryosections with 70% ethanol, native EC was hydrolyzed with cholesterol esterase (Roche) at a concentration of 1.65 units/ml in 0.1 mol/L potassium phosphate buffer (pH 7.4) for 3 hours at 37°C, and newly generated UC was stained with filipin (5 mg filipin, Sigma, dissolved in 1 ml dimethylformamide and diluted in 100 ml phosphate-buffered saline (PBS, 0.15 mol/L NaCl; 0.01 mol/L Na2HPO4, pH 7.4) for 30 minutes. Control sections were incubated in potassium phosphate buffer for 3 hours. All sections were stained with Mayer’s hematoxylin for 20 minutes at room temperature. For UC detection, cryosections were hydrated in potassium PB for 30 minutes before filipin labeling and counterstaining.
Indirect Immunofluorescence
Indirect immunofluorescence was used to demonstrate apolipoproteins. Cryosections were treated with acetone (5 minutes), dried at 50 to 55°C (10 minutes), rehydrated (5 minutes) with PBS, blocked with 20% donkey serum (Jackson Immunoresearch) in PBS for 2 hours, and then incubated with a primary antibody (see below) for 1.5 hours and a secondary antibody for 1 hour, both at 1:100 dilutions. Between incubations, sections were washed three times with 2% donkey serum in PBS for 5 minutes each. Slides probed with non-immune serum or irrelevant primary antibodies served as negative controls.
Evaluation of Labeling
Sections were examined using a Nikon Optiphot2 fluorescence microscope, a 20X plan apochromat and 40X plan fluor (oil) objectives, and three filter sets (excitation-barrier): 365 to 420 nm, 546 to 590 nm, and 480 to 520 nm, for filipin, rhodamine, and tissue autofluorescence, respectively. Sections stained with rhodamine-conjugated secondary antibodies were examined with the 546- to 590-nm filter set to identify labeling accounted for by autofluorescence. Basal deposits and drusen were recorded as present or absent. Basal deposits were graded as none, patchy, continuous layer (1/2 typical RPE height), or continuous layer (>1/2 typical RPE height). Drusen were defined as focal deposits of debris that raised the RPE by half its typical height. Labeling in BrM and in basal deposits was recorded as present if any label was detected. For drusen, labeling patterns, sizes (as indicated in Table 1 ▶ ), and numbers were documented. The contents of some large drusen were absent. Drusen with partial contents were evaluated and included in the total. The percentage of labeled drusen was determined for the macula and periphery of ARM eyes. The Fisher’s exact test was used to evaluate the significance of differences between the two regions.
Photography
Images were captured with a SensiCam imaging system (Cooke, Auburn Hills, MI) and IPLab imaging software (Scanalytics, Fairfax, VA). All images of experimental and control sections were exposed at matched times (0.05 seconds for filipin, 0.20 seconds for rhodamine). Digitized images were composited using Adobe Photoshop 6.0 (Aldus).
RT-PCR
Total RNA from human RPE was isolated using RNeasy Mini Kit (Qiagen). The RNase-Free DNase Set (Qiagen) was used during RNA preparation. Using a silica-based filter-binding isolation kit and increased speed and time of centrifugation during preparation, 6 to 11 μg of melanin-free total RNA were isolated from each donor eye. Total RNA with an optical density ratio (260 nm/280 nm) of 1.6 to 1.9 was used. RT-PCR was performed with the SuperScript One-Step RT-PCR with Platinum Taq (Invitrogen). PolyA+ RNA from HepG2 cells was purchased (Stratagene). The sequences of the oligonucleotide primers used for RT-PCR are the following: apo B sense 5′-TAG ACA CCA ACT TCT TCC ACG-3′; apo B antisense 5′-GGC GAC CTC AGT AAT TTT CTT G-3′; ApoC-III sense 5′-TGC TCC AGG AAC AGA GGT GC-3′; apo C-III antisense 5′-GTA GGA GAG CAC TGA GAA TAC T-3′; apo E sense 5′-ACT GGC ACT GGG TCG CTT T-3′; and apo E antisense 5′-GTT GTT CCT CCA GTT CCG ATT-3′. To distinguish between amplification of mRNA and genomic DNA, all primers were designed to span intron boundaries, based on human mRNA sequences published at NCBI GenBank (accession numbers: NM 000384 for apo B, K0039 for apo E, and NM 000040 for apo C-III). Reverse transcription was carried out at 50°C for 30 minutes, and the cDNA was denatured for 2 minutes at 94°C. The reaction was amplified through 30 to 40 cycles, each consisting of 30 seconds at 94°C (denaturing), 30 seconds at 55 to 68°C (annealing), and 1 minute at 72°C (extension). The reaction was incubated for 10 minutes at 72°C. The expected sizes of the RT-PCR product for apo B, apo C-III, and apo E are 614 bp, 439 bp, and 163 bp, respectively. RT-PCR products were separated on a 1.5% agarose gel with ethidium bromide. DNA bands were visualized by ultraviolet trans-illumination. The RT-PCR products of apo B were excised for restriction endonuclease analysis using SacI and StuI enzymes.
Sequencing
The primers 5′-GAG AAA CTG ACT GCT CTC AC-3′(sense) and 5′-ATG ATA GTG CTC ATC AAG ACT T-3′(antisense) were used to amplify a 234-bp apo B cDNA fragment that contains the editing site of ApoB-48. Sequence analysis was performed at the Genomics Core Facility of the Heflin Center for Human Genetics at UAB, using the BigDye Terminator v3.0 Cycle Sequencing Ready Reaction kit and an Applied Biosystems 3100 Genetic Analyzer.
Electrophoresis and Western Blot Analysis
RPE extracts were prepared as described. 40 In brief, the neural retina was removed, 0.5 ml of an extraction cocktail (200 mmol/L Tris-base, pH 6.8, 40% glycerol, 4% sodium dodecyl sulfate (SDS), 5% 2-mercaptoethanol, 0.05% bromophenol blue) was added to the eyecup, and the RPE was gently scraped off BrM with a rubber policeman. The human hepatoma HepG2 cells (a gift from Dr. Nassrin Dashti, University of Alabama at Birmingham) were seeded and maintained as described. 41 When cells were approximately 80% confluent, maintenance medium was removed, cells were rinsed with PBS, and 10% SDS containing protease inhibitor cocktail (Sigma no. P8340) was added. 42 After incubation at 37°C for 15 minutes, cells were sheared six times using a 1-ml syringe fitted on a 18- or 21-gauge needle, following which 50 to 100 μg of dithiothreitol was added. Extract protein content was determined by the Lowry method (Biorad). Samples were electrophoresed at 150 V by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) on a 4% stacking gel and a 5% to 15% gradient separating gel under reducing conditions. Proteins were electrotransferred onto a nitrocellulose membrane for 2 hours at 100 V. The membrane was blocked with 4% nonfat dry milk/TBST (MT; 20 mmol/L Tris-HCl, 150 mmol/L NaCl, pH 7.5, 0.05% Tween-20) and immunoblotted with the primary antibody diluted in MT, both at room temperature for 1 hour. The membrane was washed with TBST three times for 5 minutes each, followed by incubation with a secondary antibody conjugated to horseradish peroxidase for 1 hour at room temperature and an additional wash with MT. Dilutions of primary and secondary antibodies were 1:1000 and 1:2000 respectively. Immunoblotted proteins were detected by chemiluminescence (ECL, Amersham Pharmacia Biotech). Exposure times varied between 5 seconds and 10 minutes, depending on the protein examined. Blots were scanned on a flatbed scanner (Umax Powerlook), and the optical densities of protein bands (in negative image) were measured using IPLab.
Antibodies
Primary antibodies against human apolipoproteins included affinity purified polyclonal goat anti-apo B (Biodesign, K45253G), anti-apo E (Calbiochem, 178479), anti-apo C-III (Academy Bio-medical, Inc., 33A-G2–3a) and monoclonal mouse anti-apo B (1D1; gift from Dr. Ross Milne, University of Ottawa). Polyclonal antibodies were characterized by Western blot analysis of normal human plasma (see above). Major immunoreactive bands were observed at the appropriate positions (apo B-100, 512 kd; apo E, 35 kd; and apo C-III, 9 kd). Fainter bands at lower molecular weights for apo B and higher molecular weights for apo E likely represented proteolytic degradation products or aggregates, respectively. Secondary antibodies were rhodamine or horseradish peroxidase conjugated and included donkey anti-goat IgG and goat anti-mouse IgG (Jackson Immunoresearch).
Results
Staining results were obtained for at least 5 eyes in four experimental series (Table 1) ▶ . Lipids were localized in 12 normal and 12 ARM eyes. Co-localization of EC, UC, and apo B was examined in 5 normal eyes with sub-RPE deposits and 5 ARM eyes. Apo B localization was examined in 12 normal and 12 ARM eyes. Apo E and C-III localization was examined in 6 normal and 7 ARM eyes.
Lipid Histochemistry
To examine the distribution of lipids in sub-RPE deposits, cryosections were stained with BSBB and ORO. In all normal and ARM eyes, macular BrM stained with both BSBB and ORO, while peripheral BrM of fewer eyes stained with ORO than BSBB (Figure 2,A and E ▶ ; Table 2 ▶ ).
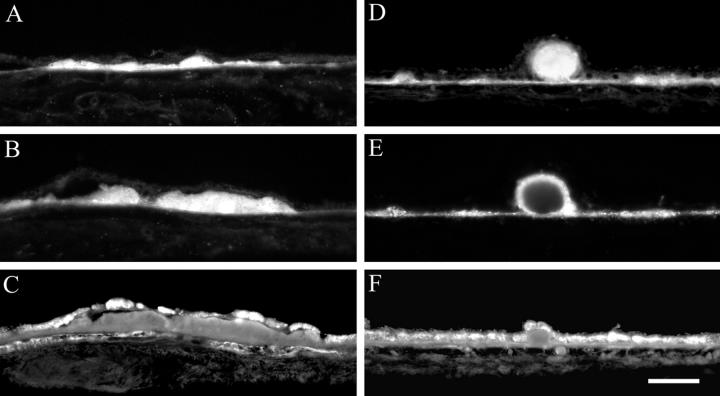
Figure 2.
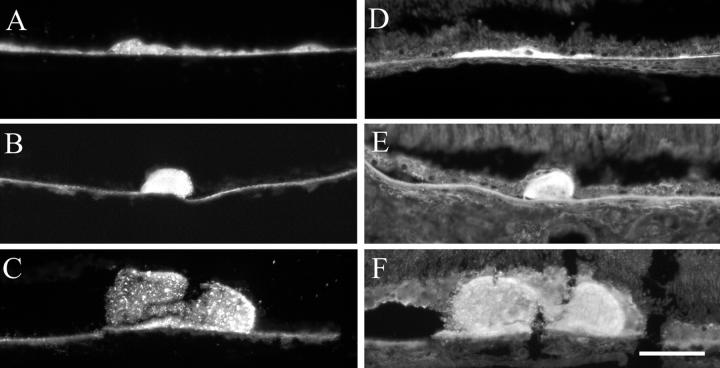
Lipid histochemistry of macula in eyes with ARM. Cryosections were stained with bromine sudan black B (A–D) and oil red O (E–H). Bruch’s membrane (A and E). Basal deposits (B and F). Drusen (C and G), Control sections (D and H) were lipid-extracted before staining. 79-year-old man (A, C, E, and F). 77-year-old man (B). 86-year-old woman (D). 95-year-old woman (G). 85-year-old woman (H). Bar, 0.05 mm.
Table 2.
Lipid and Filipin Histochemistry in the Macula and Periphery of Normal and ARM Eyes
| Bruch’s membrane | Basal deposits | Drusen | ||
|---|---|---|---|---|
| Normal eyes (n) | ||||
| BSBB | Macula (12) | 12 | — | — |
| Periphery (12) | 11 | 12 | 96/96 (100%) | |
| ORO | Macula (12) | 10 | — | — |
| Periphery (12) | 5 | 10 | 108/118 (92%) | |
| EC | Macula (5) | 5 | — | — |
| Periphery (5) | 5 | 5 | 30/30 (100%) | |
| UC | Macula (5) | 5 | — | |
| Periphery (5) | 5 | 5 | 35/35 (100%) | |
| ARM eyes (n) | ||||
| BSBB | Macula (10) | 10 | 8 | 29/39 (74%)* |
| Periphery (10) | 10 | 9 | 21/21 (100%)* | |
| ORO | Macula (10) | 10 | 9 | 27/45 (60%)† |
| Periphery (10) | 6 | 8 | 39/42 (93)† | |
| EC | Macula (5) | 5 | 5 | 6/6 (100%) |
| Periphery (5) | 5 | 5 | 19/19 (100%) | |
| UC | Macula (5) | 5 | 5 | 8/8 (100%) |
| Periphery (5) | 5 | 5 | 26/26 (100%) |
BSBB, bromine sudan black B; ORO, oil red O; EC, esterified cholesterol; UC, unesterified cholesterol; dash indicates lesions were absent. For Bruch’s membrane and basal deposits: numbers indicate eyes with stain. For drusen, numbers indicate the ratio of stained to total drusen pooled across eyes.
*Difference between macula and periphery significant (p = 0.0106).
†Difference between macula and periphery significant (p = 0.0003).
Normal Eyes
Basal deposits in the periphery of most eyes stained with both BSBB and ORO (Table 2) ▶ . In thick, continuous basal deposits lipids were present in the outer half of basal deposits adjacent to BrM and absent from the inner half of basal deposits adjacent to the RPE. Almost all drusen (92%) contained lipids (Table 2) ▶ , with different labeling patterns.
ARM Eyes
Deposits in most eyes stained with BSBB and ORO (Figure 2, B and F) ▶ . Most drusen contained lipids (Table 2) ▶ , with non-uniform labeling patterns (Figure 2, C and G) ▶ . The percent drusen labeled with ORO and BSBB was significantly lower in the macula (BSBB, 60%; ORO, 74%) than the periphery (BSBB, 93%; ORO, 100%) (BSBB, P = 0.0106; ORO, P = 0.0003). Control sections were negative (Figure 2, D and H) ▶ .
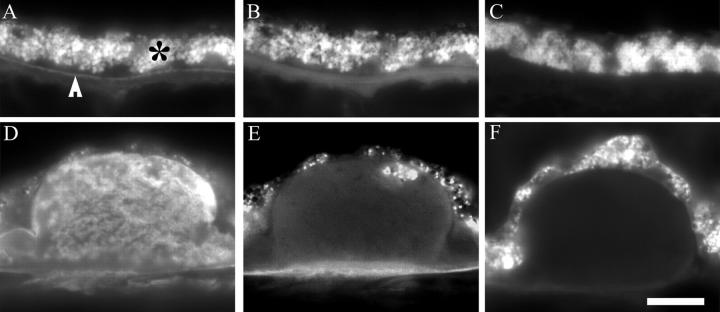
To examine the distribution of EC and UC in sub-RPE deposits, cryosections were stained with filipin with and without prior extraction and hydrolysis. Under ultraviolet excitation, RPE autofluorescence appears dimly orange or brown, and filipin appears bright blue. Cellular membranes throughout the retina and choroid were labeled for UC (Figure 3,D–F) ▶ . Figure 3 ▶ depicts thin basal deposits (Figure 3, A and D) ▶ and drusen with EC and UC from the macula. Drusen of all sizes in the macula (Figure 3, B, C, E, and F) ▶ and periphery (not shown) contained EC and UC. The morphology of fluorescent material ranged from homogeneous to clumped and could differ for EC and UC within the same druse (Figure 3, C and F) ▶ . EC and UC were present in basal deposits of all normal and ARM eyes, and 100% of drusen contained EC and UC in normal and ARM eyes (Table 2) ▶ .
Figure 3.
Esterified and unesterified cholesterol in macular basal deposits and drusen of ARM eyes. Cryosections from ARM eyes were stained with filipin. Esterified cholesterol (A–C), unesterified cholesterol (D–F). A and D: Thin basal deposit, 73-year-old man. B and E: Small druse, 73-year-old man. C and F: Medium druse, 85-year-old man. Bar, 0.05 mm.
Apolipoprotein Immunofluorescence
Apo B immunofluorescence was detected using a rhodamine-conjugated secondary antibody, and the specificity of labeling was confirmed by examining each section with filter sets for rhodamine and tissue autofluorescence (Figure 4, A–C) ▶ . Figure 4A ▶ shows specific apo B immunoreactivity in BrM (compare to control section in Figure 4C ▶ ). Apo B immunoreactivity was more prominent in inner BrM (Figure 4A ▶ , arrow), unlike autofluorescence, which was diffusely distributed throughout BrM (Figure 4B) ▶ . Specific labeling in drusen was detected in the same manner (Figure 4, D–F) ▶ .
Figure 4.
Apo B immunofluorescence and autofluorescence in cryosections of normal and ARM eyes. Sections were probed with polyclonal anti-apo B (A, B, D, and E) or non-immune serum (C and F). Images were obtained with a 40X plan fluor oil objective and either a rhodamine filter set (A, C, D and F) or an autofluorescence filter set (B and E). Exposure times were 0.1 second in panels A–C and 0.07 seconds in panels D–F. A–C: Normal, macula, 87-year-old man. D–F, ARM, periphery, 73-year-old-man. Bar in F, 0.15 mm. A: Apo B immunoreactivity is present in Bruch’s membrane (BrM), especially on the inner aspect (arrowhead). RPE is autofluorescent (*). B: In the same section as A, autofluorescence in BrM is distributed differently from the specific fluorescence in A. C: No fluorescence is detected in BrM of a control sections at the same exposure time as A. D: Apo B immunoreactivity is present in a druse. E: In the same section as D, autofluorescence is present in RPE and BrM and within the druse, in a different pattern from D. F: No fluorescence is detected in a druse in a control section at the same exposure time as D.
Apo B in Normal Eyes
By these methods, apo B was detectable in macular (4 of 12) and peripheral (9 of 12) BrM of normal eyes (Table 3) ▶ . Apo B immunoreactivity was clearly visible in the plasma of the choriocapillaris (Figure 5A ▶ , small arrow). Drusen immunofluorescence intensity and labeling pattern varied, and drusen often contained discrete areas devoid of label. Most drusen contained apo B immunoreactivity (Table 3) ▶ .
Table 3.
Apolipoprotein Immunoreactivity in the Macula and Periphery of Normal and ARM Eyes
| Bruch’s membrane | Basal deposits | Drusen | ||
|---|---|---|---|---|
| Normal eyes (n) | ||||
| Apo B | Macula (12) | 4 | — | — |
| Periphery (12) | 9 | 12 | 90/101 (89%) | |
| Apo E | Macula (7) | 7 | — | — |
| Periphery (6) | 6 | 6 | 26/26 (100%) | |
| Apo C-III | Macula (7) | 0 | — | — |
| Periphery (6) | 0 | 1 | 0/22 (0%) | |
| ARM eyes (n) | ||||
| Apo B | Macula (5) | 4 | 2 | 16/29 (55%)* |
| Periphery (5) | 4 | 2 | 11/11 (100%)* | |
| Apo E | Macula (7) | 7 | 6 | 16/27 (60%)† |
| Periphery (5) | 5 | 5 | 15/17 (88%)† | |
| Apo C-III | Macula (7) | 0 | 0 | 0/13 (0%) |
| Periphery (5) | 0 | 1 | 0/10 (0%) |
Dash indicates lesions were absent. For Bruch’s membrane and basal deposits, numbers indicate eyes with the detectable protein. For drusen, numbers indicate ratio of drusen with protein to total drusen counted across eyes.
*Difference between macula and periphery significant (p = 0.0074).
†Difference between macula and periphery significant (p = 0.0496).
Figure 5.
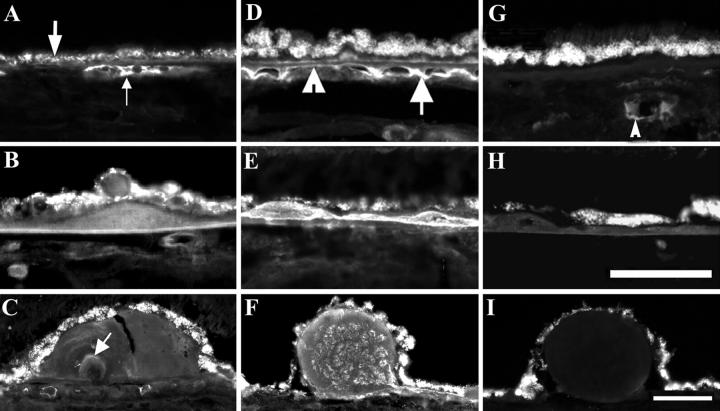
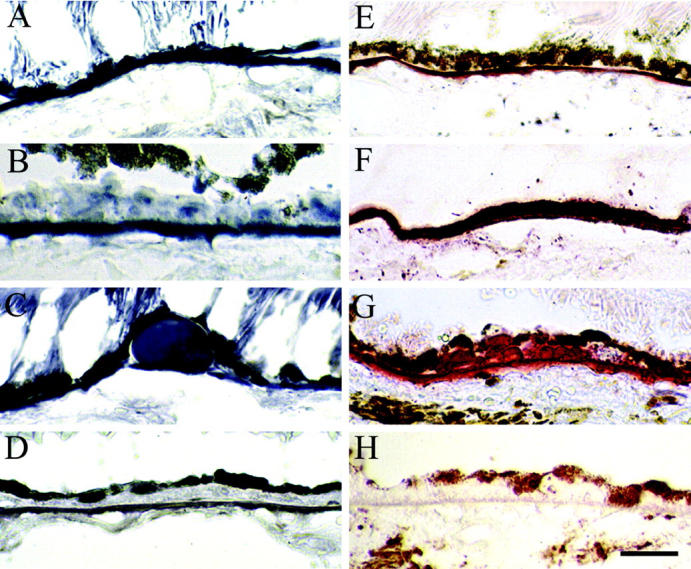
Indirect immunofluorescent localization of apo B, apo E, and apo C-III in cryosections of normal (A and G) and ARM (B–F, H, and I) eyes. Bruch’s membrane (BrM; A, D, and G), basal deposits (BD, B, E, and H), drusen (C, F, and I). Apo B, apo E, and apo C-III immunoreactivity is seen in the left, middle, and right columns respectively. A: Apo B is present in the vessels of the choroid (small arrow), and RPE is autofluorescent (large arrow); periphery of 73-year-old man. B: Apo B in BD; periphery, 78-year-old woman. C: Apo B in druse, white arrow points to apo B material within druse; macula, 81-year-old woman. D: Apo E was found in BrM (arrowhead) and intercapillary pillars (arrow); macula, 88-year-old woman. E: Apo E seen in thin BD; periphery, 85-year-old man. F: Apo E is heterogeneously present in druse; macula, 88-year-old woman. G: Apo C-III is absent from BrM, but present in vessels of the choroid (arrow); periphery, 73-year-old man. H: Apo C-III is absent from BD; periphery, 85-year-old man. I: Apo C-III is absent from druse; macula, 88-year-old woman. RPE, retinal pigment epithelium. Bar in H, 0.05 mm, applies to panels A–B, D–E, G–H; bar in I, 0.08 mm, applies to panels C, F, and I.
Apo B in ARM Eyes
Apo B immunoreactivity was detected in macular and peripheral BrM in association with apo B-positive sub-RPE deposits (Figure 5B ▶ , Table 3 ▶ ). Apo B was localized throughout thin basal deposits (Figure 5B) ▶ and in the outer or inner portion of thick basal deposits (not shown). Fifty-five percent of macular drusen and 100% of peripheral drusen contained apo B immunoreactivity (Table 3 ▶ , Figure 5C ▶ ), a statistically significant difference (P = 0.0074). Figure 5C ▶ illustrates a medium druse with whorls of apo B-positive material within apo B-negative amorphous material. To determine whether other components of atherogenic lipoproteins are also present in sub-RPE deposits, sections were probed with antibodies to apo E and C-III.
Apo E in Normal Eyes
Apo E immunoreactivity was present in macular and peripheral BrM, in basal deposits, and in drusen (Table 3) ▶ . The pattern of apo E immunoreactivity in drusen varied from homogeneous in small drusen to heterogeneous in large drusen.
Apo E in ARM Eyes
Apo E immunoreactivity was present in one or two layers of BrM (Figure 5D ▶ , arrowhead) and in basal deposits. Apo E was localized throughout thin basal deposits (Figure 5D) ▶ and in the outer or inner portion of thick basal deposits. Drusen in the macula and periphery contained apo E (Table 3 ▶ and Figure 5F ▶ ). Sixty percent of macular drusen and 88% of peripheral drusen were apo E-positive, a statistically significant difference (P = 0.0496). Apo E immunoreactivity was also seen in the intercapillary pillars (Figure 5D ▶ , arrow), plasma (Figure 5D ▶ , small arrow), and intima of some retinal vessels (not shown).
Apo C-III in Normal and ARM Eyes
Apo C-III immunoreactivity was absent from BrM, basal deposits, and drusen of normal and ARM eyes (Figure 5, G–I ▶ ; Table 3 ▶ ) but was occasionally seen in plasma (Figure 5G ▶ , arrow).
Co-Localization of Cholesterol and Apo B
To determine whether apo B is present in cholesterol-containing sub-RPE deposits of normal and ARM eyes, the same lesions were examined in adjacent cryosections processed for filipin histochemistry and apo B immunohistochemistry. UC was detected in peripheral basal deposits and drusen of normal eyes (Figure 6,A and D) ▶ . Filipin staining of an adjacent section pretreated with cholesterol esterase revealed EC in the same lesions (Figure 6, B and E) ▶ . The same lesion in an adjacent section is also apo B-immunoreactive (Figure 6, C and F) ▶ . The extent of apo B, EC, and UC labeling in most lesions overlapped completely and in some overlapped partially. Partial overlap is illustrated in Figure 6 ▶ , in which a homogeneously apo B-positive druse (Figure 6F) ▶ also contains EC (Figure 6E) ▶ superficially and UC throughout (Figure 6D) ▶ . Of 56 lesions examined, only two lesions in the macula of ARM eyes contained cholesterol and were devoid of apo B immunoreactivity (Table 4) ▶ .
Figure 6.
Co-localization of apo B, EC, and UC in sub-RPE deposits of normal and ARM eyes. Panels A–C represent the same basal deposit and D–F represent the same druse, on the basis of consistent location across adjacent cryosections. Basal deposits with apo B immunoreactivity (A) also contain EC (B) and UC (C). Drusen with apo B immunoreactivity (D) also contain EC (E) and UC (F). The UC-rich druse in F contains EC on the superficial rim only (E). RPE autofluorescence (A and D) is not visible at wavelengths used to visualize filipin (B, C, E, and F). A–C, Periphery, normal, 87-year-old woman. D–F, Periphery; ARM, 81-year-old woman. EC, esterified cholesterol; UC, unesterified cholesterol; RPE, retinal pigment epithelium. Bar, 0.05 mm.
Table 4.
Co-Localization of Apo B, Esterified, and Unesterified Cholesterol
| Degree of co-localization | ||||
|---|---|---|---|---|
| Complete | Partial | None | ||
| Normal | Macula (n = 0) | — | — | — |
| Periphery (n = 23) | 23 | 0 | 0 | |
| ARM | Macula (n = 12) | 6 | 4 | 2 |
| Periphery (n = 11) | 11 | 0 | 0 | |
n = the total number of sub-RPE lesions examined on a per lesion basis.
RT-PCR
To determine whether mRNA for apo B, apo E, or apo C-III is expressed in human RPE, we performed RT-PCR analysis of total RNA obtained from RPE of donor eyes (Figure 7A) ▶ . HepG2 mRNA served as a positive control. As shown in Figure 7A ▶ , the RT-PCR products of apo B and apo E were consistent with the predicted sizes, ie, 614 bp for apo B (lanes 2 and 3) and 163 bp for apo E (lanes 4 and 5) in both RPE (lanes 2 and 4) and HepG2 (lanes 3 and 5). We amplified 439 bp of apo C-III from the mRNA of HepG2 cells but not from human RPE (not shown). All RT-PCR results were confirmed from at least three donors (age 65 to 85 years). From the 614-bp apo B RT-PCR product, the digestion products resulting from restriction endonuclease analyses were 249 bp and 365 bp (SacI) and 318 bp and 296 bp (StuI), consistent with expected sizes (data not shown). These data indicate that mRNA transcripts of apo B and apo E but not apo C-III are present in human RPE.
Figure 7.

Apolipoprotein mRNA and protein in native human RPE and HepG2 cells. A: RT-PCR analysis of apo B and apo E mRNA transcript in human RPE. The total RNA was isolated from human RPE. The mRNA of HepG2 cells was used as positive control. RT-PCR was carried out with primers specific for human apo B and apo E, and resolved by electrophoresis on 1.5% agarose gel. The expected sizes of the RT-PCR product for apo B and apo E are 614 bp and 163 bp. RT-PCR amplification products representing: lane 1, 100 bp DNA molecular weight ladder; lanes 2 and 3, apo B mRNA (614 bp); lanes 4 and 5, apo E mRNA (163 bp); lanes 2 and 4, human RPE; and lanes 3 and 5, HepG2. B: Partial nucleic acid sequence and deduced amino acid sequence of human RPE apo B. The result shows that the apo B RT-PCR fragment contains a CAA condon, encoding Gln-2153 in apo B-100, not TAA, the stop codon in apo B-48. An asterisk shows the putative editing site of apo B-48. C: Western blot of human RPE extract and HepG2 cells. Detergent-extracted proteins from freshly isolated human RPE (65-year-old male, lane 1, 60 μg and lane 3, 9 μg) and HepG2 cells (lane 2, 6 μg and lane 4, 6 μg) were separated by SDS-PAGE and then transferred to nitrocellulose. Lanes were probed with the following primary antibodies, each at 1:1000 dilutions: monoclonal anti-human apo B (1D1) (lanes 1 and 2) and apo E (lanes 2 and 3). Primary antibodies were detected with HRP-conjugated secondary antibodies at 1:2000 dilution (see Methods). Position and vendor estimated standard molecular weights are indicated at the left. RPE, retinal pigment epithelium; HRP, horseradish peroxidase-conjugated.
Partial Sequence of Human RPE ApoB cDNA
To determine whether apo B mRNA from human RPE is from apo B-100 or apo B-48, the 234-bp RT-PCR products of apo B were sequenced from both 5′- and 3′-ends. This fragment contains a CAA codon, which encodes Gln-2153 in ApoB-100, not a TAA stop codon, the RNA-editing site of apo B-48 (Figure 7B) ▶ . Therefore, RPE cells express mRNA for apo B-100, not ApoB-48.
Western Blot Analysis of Human RPE Extracts
To determine whether human RPE contains apolipoproteins, detergent-extracted RPE isolated from three normal eyes (donor age, 55 to 92) were subjected to Western blot analysis and probed with the same antibodies used for immunohistochemistry. A monoclonal antibody to the N-terminus of apo B (1D1) was also used and gave the best results (Figure 7C) ▶ . In this analysis, both apo B and apo E were detected in RPE, indicated by prominent bands at 512 kd and 35 kd in lanes 1 and 3, respectively. Apo B and E were also detected in HepG2 cells (lane 2 and 4). When normalized for total protein, the density of the apo B band in the HepG2 cells was 40-fold higher than the apo B band detected in RPE. Apo E density in RPE, however, was 2.5-fold higher than apo E in HepG2. There was no detectable signal with the antibody against apo C-III (not shown). These results indicate that apo B and E protein, but not apo C-III, are detectable in native human RPE.
Discussion
Understanding the composition, source, and formation of ARM-associated sub-RPE deposits has been challenging, because of limited access to high quality human eye tissue, heterogeneity of lesions, overlap between age-related and disease-related deposits, interdependence of the ocular layers, and lack of a short-lived animal model for early ARM. Our study is apparently the first systematic examination of lesion lipid and apolipoprotein components that included both macula and periphery and the first census of drusen labeling. Demonstration of cholesterol and apo B in sub-RPE deposits link ARM with well-studied molecules and mechanisms of importance in CVD initiation and progression. Demonstration of apo B mRNA and protein in RPE implicates lipoprotein assembly as a pathway for formation of cholesterol-enriched lesions.
We demonstrated that EC and UC are ubiquitous components of macular and peripheral drusen in ARM eyes, as they are in BrM and age-related deposits in normal eyes. 9,10 Previous results indicated that some macular drusen did not bind ORO and that basal deposits preferentially bound either ORO or BSBB. 13 Our results with filipin, a specific marker for EC and UC, indicate that this variability was probably due to the lesser sensitivity of traditional lipid stains, without excluding between-lesion differences in non-cholesterol lipid components. It will be interesting to determine whether sub-RPE deposits exhibit a progression in the physical forms of cholesterol like the atherosclerotic intima. Maturation of the lipid-rich core is associated with pooling of EC, aggregation of UC-rich liposomes, and formation of UC crystals 19,43,44 that together contribute to plaque instability. 25 Neither we nor others have observed cholesterol crystals in sub-RPE deposits. However, we observed different morphologies of filipin fluorescence, consistent with multiple physical forms of cholesterol (see Figure 3, A and C ▶ ; Figure 5, E and F ▶ ). Although it is possible that this variability is introduced by hydrolyzing EC during histochemical staining, pooling of neutral lipid is also seen in drusen stained with oil red O. 10 In addition, distinctive EC labeling along the superficial rim of round drusen (Figure 5E) ▶ is a consistent finding in our material (Figure 7E ▶ of ref. 9 ). A cholesterol-accumulating sublayer of BrM is an apparent precursor to basal linear deposit, thought to provide a cleavage plane for dissecting choroidal vessels. 9,45 Therefore, cholesterol may play a role in promoting mechanical instability in ARM as well as atherosclerosis.
We detected apo B in a high proportion of drusen and basal deposits, in contrast to a previous report of inconsistent apo B immunoreactivity in drusen obtained with other antibodies. 46 Co-localization of apo B and cholesterol in sub-RPE deposits implicates apo B-containing lipoproteins as potential contributors to lesion cholesterol. This process is not specific to ARM, because a cholesterol-rich basal laminar deposit in dominant late-onset retinal degeneration also contains intense apo B immunoreactivity. 47 We compared apo B localization to that of apo E, because apo E is a component of sub-RPE deposits, 35,36,46 apo E mRNA transcripts are found in RPE and retina, and the apo E4 polymorphism is the only confirmed genetic association for ARM. 35,48,49 Apo E is also a component of very low density lipoprotein, 18 and it is localized in atherosclerotic intima. 50-52 Relative to apo E, apo B immunoreactivity was found in as many drusen but in fewer basal deposits. Further, unlike the bilaminar labeling pattern of apo E in BrM, 36 apo B immunoreactivity, where present, was more prominent in inner BrM. Differences in the labeling intensity and pattern of apo B and apo E may reflect differences in antibody sensitivity or in the number of epitopes available for binding on the two apolipoproteins. Apo C-III, the most abundant apo C in plasma and another component of atherogenic lipoproteins, was detected in plasma retained in our sections but not in BrM or sub-RPE deposits.
Significantly more peripheral drusen bound traditional histochemical stains and exhibited apolipoprotein immunoreactivity than macular drusen, suggesting that lipids other than EC and UC and apolipoproteins are diluted to undetectable levels by other components in macular drusen. Alternatively, since under identical cryo-preparation protocols some large macular drusen had only partial contents and all peripheral drusen were intact, these differences may reflect fragility of macular drusen. Drusen vary greatly in their uptake of angiographic dyes and in the risk conferred for neovascularization, with large, soft (sloping sides), or confluent drusen thought to be the most dangerous. 53-56 The expectation that compositional differences in drusen underlie these properties has not been supported by recent surveys concluding that all drusen contain similar proteins. 6,46 Our enumeration of individual, labeled drusen raises the possibility that quantitative differences in the proportions of characteristic protein and lipid constituents separate low-risk from high-risk drusen. Testing this hypothesis will require direct quantification of key proteins and lipids in macular and peripheral drusen of eyes with ARM.
A new finding is the demonstration of apo B-100 mRNA and protein in human RPE. Previous attempts to identify apo B mRNA in human RPE by RT-PCR gave inconsistent results, 46 and apo B has not appeared among RPE-expressed genes identified by other methods. 57-59 This may be due to low abundance of apo B mRNA and to difficulties in obtaining high quality RNA from native human RPE. These include variable postmortem delay to processing, potential contamination with blood, and the presence of melanin, an RT-PCR inhibitor. 60,61 Notably, we also found apo B protein in RPE, at low levels consistent with its rapid degradation in non-secreting cells. Finding both apo B-100 mRNA and protein in RPE is more convincing than finding either alone. While contamination or uptake cannot be excluded, the simplest explanation is that the RPE synthesizes apo B. Because apo B is essential for assembling large (>75 nm) triglyceride- and EC-containing lipoproteins in liver, intestine, and heart, 62 our data raise the intriguing possibility that the RPE assembles and secretes apo B-containing lipoproteins. If so, then perhaps the numerous 80 to 100 nm diameter EC-containing particles that accumulate with age in BrM 9 are native lipoproteins. It will be important to test this novel hypothesis by demonstrating synthesis and assembly of an apo B-containing lipoprotein by RPE in vitro.
Placing apo B in the principal lesions of ARM is significant because this molecule has a well-documented role in disease initiation and progression for CVD. According to the response-to-retention hypothesis, atherogenic apo-B containing lipoproteins from plasma enter arterial intima and bind directly to proteoglycans (biglycan and versican, inter alia) or indirectly via bridging molecules. Trapped lipoprotein particles are modified, leading to accumulated extracellular cholesterol and provoking massive responses from surrounding cells (macrophages, smooth muscle cells, and endothelial cells). Superficially, the RPE/BrM complex appears to provide an appropriate environment for a similar progression. BrM can be conceptualized as a specialized vascular intima that exhibits physiological thickening in the macula during adulthood. 9 BrM and sub-RPE deposits contain glycosoaminoglycans 63-66 and advanced glycation end-products that could retain and modify lipoproteins, respectively. 67,68 Proteins associated with inflammation and complement activation are present in drusen. 69,70 ARM-associated choroidal neovascular membranes include macrophages and smooth muscle actin-positive cells. 71,72 However, existing data indicate that an ocular response-to-retention scenario, if present, will differ importantly from that in the intima. For example, apo B should appear in normal BrM before the onset of ARM, but in our study apo B was not found in BrM of every normal eye. Further, the proteoglycans that bind apo-B-containing lipoproteins in intima have not been reported yet in BrM and drusen. Finally, an intima-like response-to-retention scenario predicts that the risk for ARM should be enhanced by hypercholesterolemia. In fact, epidemiological studies to date indicate little or no association between ARM and hypercholesterolemia 73,74 (G. McGwin, C. Owsley, C.A. Curcio, R.J. Crain, submitted), possibly due to methodological limitations. Our data suggest that apo B may derive from an intraocular source without ruling out the possibility of a plasma source. If an intraocular lipoprotein (see above) were the major source of cholesterol in BrM and sub-RPE deposits rather than plasma lipoproteins, then dissociation of risk factors pertaining to the pre-retention stage in ARM and CVD may be expected.
The concept that ARM may involve a retained lipoprotein, mechanisms of retention, and cellular responses specific to the environment of the eye is novel. It is useful as a framework for generating testable new ideas about mechanisms involved in different stages of a complex, multifactorial disease. Determining if an apo-B containing lipoprotein is produced within the eye will be the first step in testing this hypothesis.
Acknowledgments
We thank Drs. W.A. Bradley, N. Dashti, and G. McGwin, Jr., for helpful discussion, T. Bailey and D. Fisher for assistance, the Alabama Eye Bank for timely retrieval of donor eyes, and the donor families for their generosity.
Footnotes
Address reprint requests to Christine A. Curcio, Ph.D., Department of Ophthalmology, 700 South 18th Street, Room H020, University of Alabama at Birmingham, Birmingham AL 35294-0009. E-mail: curcio@.uab.edu.
Supported by NIH grants T32-EY07033 (G.M.), EY13258 (C.G), and EY06109 (C.A.C.), the International Retinal Research Foundation (G.M. and C.A.C.), and an unrestricted award from Research to Prevent Blindness, Inc. (R.P.B.) to the Department of Ophthalmology, University of Alabama at Birmingham. C.A.C. holds a Lew R. Wasserman Merit Award from RPB.
References
- 1.Klein R, Klein BEK, Linton KLP: Prevalence of age-related maculopathy. Ophthalmology 1992, 99:933-943 [DOI] [PubMed] [Google Scholar]
- 2.Mitchell P, Smith W, Attebo K, Wang JJ: Prevalence of age-related maculopathy in Australia: The Blue Mountains Eye Study. Ophthalmology 1995, 102:1450-1460 [DOI] [PubMed] [Google Scholar]
- 3.Vingerling JR, Dielemans I, Hofman A, Grobbee DE, Hijmering M, Kramer CFL, de Jong PTVM: The prevalence of age-related maculopathy in the Rotterdam study. Ophthalmology 1995, 102:205-210 [DOI] [PubMed] [Google Scholar]
- 4.Sarks SH: Ageing and degeneration in the macular region: a clinico-pathological study. Br J Ophthalmol 1976, 60:324-341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green WR, Enger C: Age-related macular degeneration histopathologic studies: The 1992 Lorenz E. Zimmerman Lecture. Ophthalmology 1993, 100:1519-1535 [DOI] [PubMed] [Google Scholar]
- 6.Hageman GS, Luthert PJ, Chong NHC, Johnson LV, Anderson DH, Mullins RF: An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res 2001, 20:705-732 [DOI] [PubMed] [Google Scholar]
- 7.Pauleikhoff D, Harper CA, Marshall J, Bird AC: Aging changes in Bruch’s membrane: a histochemical and morphological study. Ophthalmology 1990, 97:171-178 [PubMed] [Google Scholar]
- 8.Holz FG, Sheraidah G, Pauleikhoff D, Bird AC: Analysis of lipid deposits extracted from human macular and peripheral Bruch’s membrane. Arch Ophthalmol 1994, 112:402-406 [DOI] [PubMed] [Google Scholar]
- 9.Curcio CA, Millican CL, Bailey T, Kruth HS: Accumulation of cholesterol with age in human Bruch’s membrane. Invest Ophthalmol Vis Sci 2001, 42:265-274 [PubMed] [Google Scholar]
- 10.Haimovici R, Gantz DL, Rumelt S, Freddo TF, Small DM: The lipid composition of drusen, Bruch’s membrane, and sclera by hot stage polarizing microscopy. Invest Ophthalmol Vis Sci 2001, 42:1592-1599 [PubMed] [Google Scholar]
- 11.Wolter JR, Falls HF: Bilateral confluent drusen. Arch Ophthalmol 1962, 68:219-226 [DOI] [PubMed] [Google Scholar]
- 12.Farkas TG, Sylvester V, Archer D, Altona M: The histochemistry of drusen. Am J Ophthalmol 1971, 71:1206-1215 [DOI] [PubMed] [Google Scholar]
- 13.Pauleikhoff D, Zuels S, Sheraidah GS, Marshall J, Wessing A, Bird AC: Correlation between biochemical composition and fluorescein binding of deposits in Bruch’s membrane. Ophthalmology 1992, 99:1548-1553 [DOI] [PubMed] [Google Scholar]
- 14.Stary HC, Blankenhorn DH, Chandler AB, Glagov S, Insull WI, Jr, Richardson M, Rosenfeld ME, Schaffer SA, Schwartz CJ, Wagner WD, Wissler RW: A definition of the intima of human arteries and of its atherosclerosis-prone regions. Circulation 1992, 85:391-405 [DOI] [PubMed] [Google Scholar]
- 15.Stary H, Chandler AB, Glagov S, Guyton JR, Insull W, Jr, Rosenfeld ME, Schaffer SA, Schwartz CJ, Wagner W, Wissler R: A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. Circulation 1994, 89:2462-2478 [DOI] [PubMed] [Google Scholar]
- 16.Williams KJ, Tabas I: The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol 1995, 15:551-561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams KJ, Tabas I: The response-to-retention hypothesis of atherogenesis reinforced. Curr Opin Lipidol 1998, 9:471-474 [DOI] [PubMed] [Google Scholar]
- 18.Havel RJ, Kane JP: Introduction: structure and metabolism of plasma lipoproteins. Scriver CR Beaudet AL Sly WS Valle D eds. The Metabolic and Molecular Basis of Inherited Disease. 2001:pp 2707-2716 McGraw-Hill, New York
- 19.Kruth HS: The fate of lipoprotein cholesterol entering the arterial wall. Curr Opin Lipidol 1997, 8:246-252 [DOI] [PubMed] [Google Scholar]
- 20.Tabas I: Nonoxidative modifications of lipoproteins in atherogenesis. Annu Rev Nutr 1999, 19:123-139 [DOI] [PubMed] [Google Scholar]
- 21.Oorni K, Pentikainen MO, Ala-Korpela M, Kovanen PT: Aggregation, fusion, and vesicle formation of modified low density lipoprotein particles: molecular mechanisms and effects on matrix interactions. J Lipid Res 2000, 41:1703-1714 [PubMed] [Google Scholar]
- 22.Ross R: Atherosclerosis: an inflammatory disease. N Engl J Med 1999, 340:115-126 [DOI] [PubMed] [Google Scholar]
- 23.Glass CK, Witztum JL: Atherosclerosis: the road ahead. Cell 2001, 104:503-516 [DOI] [PubMed] [Google Scholar]
- 24.Libby P, Ridker PM, Maseri A: Inflammation and atherosclerosis. Circulation 2002, 105:1135-1143 [DOI] [PubMed] [Google Scholar]
- 25.Davies MJ: Stability and instability: two faces of coronary atherosclerosis: The Paul Dudley White Lecture 1995. Circulation 1996, 94:2013-2020 [DOI] [PubMed] [Google Scholar]
- 26.Skålen K, Gustafsson M, Rydberg EK, Hultén LM, Wiklund O, Innerarity TL, Borén J: Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nat Med 2002, 417:750-754 [DOI] [PubMed] [Google Scholar]
- 27.Segrest JP, Jones MK, De Loof H, Dashti N: Structure of apolipoprotein B-100 in low density lipoproteins. J Lipid Res 2001, 42:1346-1367 [PubMed] [Google Scholar]
- 28.Yao Z, Tran K, McLeod R: Intracellular degradation of newly synthesized apolipoprotein B. J Lipid Res 1997, 38:1937-1953 [PubMed] [Google Scholar]
- 29.Chan L, Chang BH, Liao W, Oka K, Lau PP: Apolipoprotein B: from editosome to proteasome. Recent Prog Horm Res 2000, 55:93-125 [PubMed] [Google Scholar]
- 30.Fisher EA, Ginsberg HN: Complexity in the secretory pathway: the assembly and secretion of apolipoprotein B-containing lipoproteins. J Biol Chem 2002, 277:17377-17380 [DOI] [PubMed] [Google Scholar]
- 31.Vingerling JR, Dielemans I, Bots ML, Hofman A, Grobbee DE, de Jong PTVM: Age-related macular degeneration is associated with atherosclerosis: The Rotterdam Study. Am J Epidemiol 1995, 142:404-409 [DOI] [PubMed] [Google Scholar]
- 32.Chaine G, Hullo A, Sahel J, Soubrane G, Espinasse-Berrod M-A, Schutz D, Bourgignon C, Harpey C, Brault Y, Coste M, Moccatti D, Bourgeois H: Case-control study of the risk factors for age-related macular degeneration. Br J Ophthalmol 1998, 82:996-1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith W, Assink J, Klein R, Mitchell P, Klaver CCW, Klein BEK, Hofman A, Jensen S, Wang JJ, de Jong PTVM: Risk factors for age-related macular degeneration: pooled findings from three continents. Ophthalmology 2001, 108:697-704 [DOI] [PubMed] [Google Scholar]
- 34.: Group A-REDSR: Risk factors associated with age-related macular degeneration: a case-control study in the Age-Related Eye Disease Study: Age-Related Eye Disease Study Report Number 3. Ophthalmology 2000, 107:2224-2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klaver CCW, Kliffen M, Van Duijn CM, Hofman A, Cruts M, Grobbee DE, Van Broeckhoven C, De Jong PTVM: Genetic association of apolipoprotein E with age-related macular degeneration. Am J Hum Genet 1998, 63:200-206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson DH, Ozaki S, Nealon M, Neitz J, Mullins RF, Hageman GS, Johnson LV: Local cellular sources of apolipoprotein E in the human retina and retinal pigmented epithelium: implications for the process of drusen formation. Am J Ophthalmol 2001, 131:767-781 [DOI] [PubMed] [Google Scholar]
- 37.Curcio CA, Medeiros NE, Millican CL: The Alabama Age-Related Macular Degeneration Grading System for donor eyes. Invest Ophthalmol Vis Sci 1998, 39:1085-1096 [PubMed] [Google Scholar]
- 38.Barthel LK, Raymond PA: Improved method for obtaining 3-μm cryosections for immunocytochemistry. J Histochem Cytochem 1990, 38:1383-1388 [DOI] [PubMed] [Google Scholar]
- 39.Adams C, Bayliss O: Lipid histochemistry. Glick D Rosenbaum R eds. Techniques of Biochemical and Biophysical Morphology. 1975:pp 100-156 John Wiley and Sons, New York
- 40.Mamballikalathil I, Mann C, Guidry C: Tractional force generation by porcine Müller cells: stimulation by retinal pigment epithelial cell-secreted growth factor. Invest Ophthalmol Vis Sci 2000, 41:529-536 [PubMed] [Google Scholar]
- 41.Chung BH, Dashti N: Lipolytic remnants of human VLDL produced in vitro: effect of HDL levels in the lipolysis mixtures on the apoCs to apoE ratio and metabolic properties of VLDL core remnants. J Lipid Res 2000, 41:285-297 [PubMed] [Google Scholar]
- 42.Chang CC, Sakashita N, Ornvold K, Lee O, Chang ET, Dong R, Lin S, Lee CY, Strom SC, Kashyap R, Fung JJ, Farese RV, Jr, Patoiseau JF, Delhon A, Chang TY: Immunological quantitation and localization of ACAT-1 and ACAT-2 in human liver and small intestine. J Biol Chem 2000, 275:28083-28092 [DOI] [PubMed] [Google Scholar]
- 43.Small DH: Progression and regression of atherosclerotic lesions: insights from lipid physical biochemistry. Arteriosclerosis 1988, 8:103-129 [DOI] [PubMed] [Google Scholar]
- 44.Guyton JR, Klemp KF: Development of the atherosclerotic core region: chemical and ultrastructural analysis of microdissected atherosclerotic lesions from human aorta. Arterioscler Thromb 1994, 14:1305-1314 [DOI] [PubMed] [Google Scholar]
- 45.Curcio CA, Millican CL: Basal linear deposit and large drusen are specific for early age-related maculopathy. Arch Ophthalmol 1999, 117:329-339 [DOI] [PubMed] [Google Scholar]
- 46.Mullins RF, Russell SR, Anderson DH, Hageman GS: Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. EMBO J 2000, 14:835-846 [PubMed] [Google Scholar]
- 47.Milam AH, Curcio CA, Cideciyan AV, Saxena S, John SK, Kruth HS, Malek G, Heckenlively JR, Weleber RG, Jacobson SG: Dominant late-onset retinal degeneration with regional variation of sub-RPE deposits, retinal function, and photoreceptor degeneration. Ophthalmology 2000, 107:2256-2266 [DOI] [PubMed] [Google Scholar]
- 48.Souied EH, Benlian P, Amouyel P, Feingold J, Lagarde J-P, Munnich A, Kaplan J, Coscas G, Soubrane G: The ε4 allele of the apolipoprotein E gene as a potential protective factor for exudative age-related macular degeneration. Am J Ophthalmol 1998, 125:353-359 [DOI] [PubMed] [Google Scholar]
- 49.Schmidt S, Saunders AM, De La Paz MA, Postel EA, Heinis RM, Agarwal A, Scott WK, Gilbert JR, McDowell JG, Bazyk A, Gass DM, Haines JL, Pericak-Vance MA: Association of the apolipoprotein E gene with age-related macular degeneration: possible effect modification by family history, age, and gender. Mol Vis 2000, 6:287-293 [PubMed] [Google Scholar]
- 50.O’Brien KD, Olin KL, Alpers CE, Chiu W, Ferguson M, Hudkins K, Wight T, Chait A: Comparison of apolipoproteins and proteoglycan deposits in human coronary atherosclerotic plaques: colocalization of biglycan with apolipoproteins. Circulation 1998, 98:519-527 [DOI] [PubMed] [Google Scholar]
- 51.Rapp JH, Lespine A, Hamilton RL, Colyvas N, Chaumeton AH, Tweedie-Hardman J, Kotite L, Kunitake ST, Havel RJ, Kane JP: Triglyceride-rich lipoproteins isolated by selected-affinity anti-apolipoprotein B immunosorption from human atherosclerotic plaque. Arterioscler Thromb 1994, 14:1767-1774 [DOI] [PubMed] [Google Scholar]
- 52.Chung BH, Tallis G, Yalamoori V, Anantharamaiah GM, Segrest JP: Liposome-like particles isolated from human atherosclerotic plaques are structurally and compositionally similar to surface remnants of triglyceride-rich lipoproteins. Arterioscler Thromb 1994, 14:622-635 [DOI] [PubMed] [Google Scholar]
- 53.Bird AC, Marshall J: Retinal pigment epithelial detachments in the elderly. Trans Ophthalmol Soc UK 1986, 105:674-682 [PubMed] [Google Scholar]
- 54.Pauleikhoff D, Barondes MJ, Minassian D, Chisholm IH, Bird AC: Drusen as risk factors in age-related macular disease. Am J Ophthalmol 1990, 109:38-43 [DOI] [PubMed] [Google Scholar]
- 55.Bressler NM, Silva JC, Bressler SB, Fine SL, Green WR: Clinicopathological correlation of drusen and retinal pigment epithelial abnormalities in age-related macular degeneration. Retina 1994, 14:130-142 [PubMed] [Google Scholar]
- 56.Arnold JJ, Quaranta M, Soubrane G, Sarks SH, Coscas G: Indocyanine green angiography of drusen. Am J Ophthalmol 1997, 124:344-356 [DOI] [PubMed] [Google Scholar]
- 57.Buraczynska M, Mears AJ, Zareparsi S, Farjo R, Filippova E, Yuan Y, MacNee SP, Hughes B, Swaroop A: Gene expression profile of native human retinal pigment epithelium. Invest Ophthalmol Vis Sci 2002, 43:603-607 [PubMed] [Google Scholar]
- 58.Sharon D, Blackshaw S, Cepko CL, Dryja TP: Profile of the genes expressed in the human peripheral retina, macula, and retinal pigment epithelium determined through serial analysis of gene expression (SAGE). Proc Natl Acad Sci USA 2002, 99:315-320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wistow G, Bernstein SL, Wyatt MK, Farris RN, Behal A, Touchman JW, Bouffard G, Smith D, Peterson K: Expressed sequence tag analysis of human RPE/choroid for the NEIBank Project: over 6000 non-redundant transcripts, novel genes, and splice variants. Mol Vis 2002, 8:205-220 [PubMed] [Google Scholar]
- 60.Giambernardi TA, Rodeck U, Klebe RJ: Bovine serum albumin reverses inhibition of RT-PCR by melanin. Biotechniques 1998, 25:564-566 [DOI] [PubMed] [Google Scholar]
- 61.Eckhart L, Bach J, Ban J, Tschachler E: Melanin binds reversibly to thermostable DNA polymerase and inhibits its activity. Biochem Biophys Res Commun 2000, 271:726-730 [DOI] [PubMed] [Google Scholar]
- 62.Bjorkegren J, Veniant M, Kim SK, Withycombe SK, Wood PA, Hellerstein MK, Neese RA, Young SG: Lipoprotein secretion and triglyceride stores in the heart. J Biol Chem 2001, 276:38511-38517 [DOI] [PubMed] [Google Scholar]
- 63.Hewitt TA, Nakasawa K, Newsome DA: Analysis of newly synthesized Bruch’s membrane proteoglycans. Invest Ophthalmol Vis Sci 1989, 30:478-486 [PubMed] [Google Scholar]
- 64.Call TW, Hollyfield JG: Sulfated proteoglycans in Bruch’s membrane of the human eye: localization and characterization using cupromeronic blue. Exp Eye Res 1990, 51:451-462 [DOI] [PubMed] [Google Scholar]
- 65.Lin WL, Essner E, McCarthy KJ, Couchman JR: Ultrastructural immunocytochemical localization of chondroitin sulfate proteoglycan in Bruch’s membrane of the rat. Invest Ophthalmol Vis Sci 1992, 33:2072-2075 [PubMed] [Google Scholar]
- 66.Kliffen M, Mooy CM, Luider TM, Huijmans JGM, Kerkvliet S, de Jong PTVM: Identification of glycosaminoglycans in age-related macular deposits. Arch Ophthalmol 1996, 114:1009-1014 [DOI] [PubMed] [Google Scholar]
- 67.Ishibashi T, Murata T, Hangai M, Nagai R, Horiuchi S, Lopez PF, Hinton D, Ryan S: Advanced glycation end products in age-related macular degeneration. Arch Ophthalmol 1998, 116:1629-1629 [DOI] [PubMed] [Google Scholar]
- 68.Handa JT, Verzijl N, Matsunaga H, Aotaki-Keen A, Lutty GA, te Koppele JM, Miyata T, Hjelmeland LM: Increase in the advanced glycation end product pentosidine in Bruch’s membrane with age. Invest Ophthalmol Vis Sci 1999, 40:775-779 [PubMed] [Google Scholar]
- 69.Johnson LV, Ozaki S, Staples MK, Erickson PA, Anderson DH: A potential role for immune complex pathogenesis in drusen formation. Exp Eye Res 2000, 70:441-449 [DOI] [PubMed] [Google Scholar]
- 70.Johnson LV, Leitner WP, Staples MK, Anderson DH: Complement activation and inflammatory processes in drusen formation and age-related macular degeneration. Exp Eye Res 2001, 73:887-896 [DOI] [PubMed] [Google Scholar]
- 71.Grossniklaus HE, Cingle KA, Yoon YD, Ketkar N, L’Hernault N, Brown S: Correlation of histologic 2-dimensional reconstructional and confocal scanning laser microscopic imaging of choroidal neovascularization in eyes with age-related maculopathy. Arch Ophthalmol 2000, 118:625-629 [DOI] [PubMed] [Google Scholar]
- 72.Lopez PF, Sippy BD, Lambert HM, Thach AB, Hinton DR: Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidal neovascular membranes. Invest Ophthalmol Vis Sci 1996, 37:855-868 [PubMed] [Google Scholar]
- 73.Klein R, Klein BEK, Franke T: The relationship of cardiovascular disease and its risk factors to age-related maculopathy. Ophthalmology 1993, 100:406-414 [DOI] [PubMed] [Google Scholar]
- 74.Klein R, Klein BEK, Jenson SC: The relation of cardiovascular disease and its risk factors to the 5-year incidence of age-related maculopathy: The Beaver Dam Eye Study. Ophthalmology 1997, 104:1804-1812 [DOI] [PubMed] [Google Scholar]