Abstract
Ghrelin, a growth hormone-releasing hormone produced by gastroenteropancreatic endocrine cells, hypothalamus, and pituitary, was recently identified in medullary thyroid carcinomas and derived cell lines. However, no data exist on its expression in either normal or neoplastic thyroid follicular cells. We analyzed ghrelin expression by immunohistochemistry, in situ hybridization, and reverse transcriptase-polymerase chain reaction in 15 fetal, 4 infant, and 10 adult thyroids, and in 54 tumors of follicular origin. We also analyzed the effects of ghrelin on cell proliferation in N-PAP and ARO thyroid carcinoma cell lines. Ghrelin-binding sites were investigated using reverse transcriptase-polymerase chain reaction to detect its growth hormone secretagogue receptor (GHS-R) mRNA and an in situ-binding localization procedure. Strong ghrelin immunoreactivity was found in fetal but not in infant or adult thyroids. Ghrelin protein and mRNA were present, in variable amounts, in benign and malignant tumors. Normal thyroids, thyroid tumors, and cell lines showed ghrelin binding sites by binding localization, in the absence of the specific GHS receptor mRNA (with the exception of one normal thyroid). Moreover, ghrelin induced dose-dependent inhibition of growth in cell lines. In conclusion, ghrelin is expressed in fetal but not in adult thyroid, and is re-expressed in tumors; the presence of ghrelin receptors other than GHS-R in normal and neoplastic adult thyroid is suggested; ghrelin inhibits cell proliferation of thyroid carcinoma cell lines in vitro.
Ghrelin is a novel gastrointestinal hormone, recently identified in the rat and human stomach, 1 with complex functions related to growth hormone (GH) release, gastrointestinal motility, control of food intake, and regulation of insulin secretion. 1-8 Subsequently, ghrelin was identified in the pituitary gland, arcuate nucleus of the hypothalamus, kidney, placenta, and endocrine pancreas. 1,9-12 Ghrelin production in certain tumors has also been reported, specifically pituitary adenomas, gastrointestinal carcinoids, and endocrine tumors of the pancreas. 12-14 The actions of ghrelin are mediated by specific receptors shared by synthetic compounds of the GH secretagogue family. 1,15 Two types of receptors are currently known, referred to as GH secretagogue receptor (GHS-R) types 1a and 1b. These receptors are widely distributed in central (brain, pituitary) and peripheral organs, 1,2,16,17 and mediate GH release-related functions. They also have several other activities that are currently incompletely understood. The latter include growth control activities in tumor cells, as detected in thyroid, breast, prostate, and lung tumors. 18-21
In the stomach, ghrelin is localized in the neuroendocrine cell compartment of the oxyntic mucosa, produced by X-like cells that appear to be the main source of circulating ghrelin. 9 The gastric antrum and small intestine contain very few ghrelin-producing cells and the large intestine is virtually devoid of ghrelin. 9,14 This apparent foregut-restricted ghrelin expression prompted an analysis of other foregut endodermal derivatives. The thyroid gland is of endodermal origin and therefore a possible source of ghrelin, although no ghrelin immunoreactivity has been reported so far in normal rat and human thyroid. Recently, Kanamoto and colleagues 22 reported substantial production of ghrelin in a medullary thyroid carcinoma cell line. In this study, reverse transcriptase-polymerase chain reaction (RT-PCR) analyses demonstrated prepro-ghrelin gene transcripts in a medullary thyroid carcinoma cell line and in total normal human thyroid tissue. The latter finding was not paralleled by ghrelin immunoreactivity of follicular cells and suggested that probably the minor C cell population of the thyroid is responsible for the RT-PCR signal.
As for the ghrelin receptor, by means of a binding assay, we previously demonstrated GH secretagogue binding sites in the thyroid gland, in thyroid carcinomas of follicular origin, and in several thyroid carcinoma cell lines. 16,18 In these cell lines, synthetic GH secretagogues determined a biological effect, resulting in a significant inhibition of cell proliferation. However, no data were available on the possible expression and biological function of the natural GHS-receptor ligand, ghrelin, in normal thyroid, follicular-derived thyroid carcinomas, and related cell lines.
The aim of the present study was therefore to investigate ghrelin and GHS receptor expression in the human fetal and adult thyroid and in a series of thyroid carcinomas of follicular origin, by means of immunohistochemistry (IHC), in situ hybridization and RT-PCR analysis. In addition, ghrelin activity on cell proliferation was investigated in vitro using cell lines originating from follicular-derived thyroid tumors. We here show that fetal (but not adult) thyrocytes and a percentage of follicular-derived tumors contain large amounts of immunoreactive ghrelin and its specific mRNA. Unexpectedly, RT-PCR receptor analysis showed that mRNAs for both ghrelin/GHS receptor types 1a and 1b were absent in all of the thyroid tumors and cell lines investigated as well as in all but one normal adult thyroid. However, biotinylated ghrelin bound to the majority of both normal and neoplastic tissues, and cell lines. The presence of functional ghrelin receptors was also supported by in vitro studies in which ghrelin had a specific, dose-dependent anti-proliferative effect in follicular-derived thyroid carcinoma cell lines.
Materials and Methods
Tissue Samples
Fifteen fetal thyroids (from 8 to 38 weeks of gestation, from voluntary or elective abortions, or autopsies for intrauterine fetal deaths), and 4 infant and 10 adult thyroid glands (age of patients from 10 to 83 years, resected for hyperplastic goiter or papillary carcinoma) were collected from the files of the Departments of Pathology, Universities of Turin and Genoa. In addition, we collected 54 follicular-derived tumors of the thyroid for which formalin-fixed, paraffin-embedded tissue blocks were available for both conventional histology and IHC. Of these, in 33 cases fresh frozen material was available for mRNA studies. All cases were classified according to the World Health Organization or Armed Forces Institute of Pathology classification schemes of thyroid tumors. 23,24 The follicular origin was confirmed by a negative calcitonin reaction and positive thyroglobulin immunostaining in all cases, except for anaplastic carcinoma.
Cell Lines and Cell Growth Studies
N-PAP and ARO, two cell lines of human thyroid carcinoma of follicular origin, were used. N-PAP was obtained from a papillary carcinoma and ARO from an undifferentiated carcinoma of the thyroid (kind gifts of Professor A. Fusco, University of Naples). The two cell lines were grown as a monolayer in RPMI medium (Gibco, Paisley, Scotland) with 10% fetal calf serum (Gibco) at 37°C in a 5% CO2-humidified atmosphere.
To assay the effect of ghrelin on cell proliferation, cells were seeded in quadruplicate in 48-multiwell plates (104 cells/well) in standard culture medium and allowed to adhere for 24 hours. Ghrelin (10 nmol/L to 1 μmol/L) was then added to the medium and cells were treated for up to 96 hours. Experiments were repeated three times. After 48 and 96 hours of treatment, respectively, cells were fixed in 2.5% glutaraldehyde, stained with 0.1% crystal violet in 20% methanol and solubilized in 10% acetic acid. Cell growth was evaluated by measuring absorbance at 590 nm in a microplate reader (Multiskan Bichromatic; Labsystem Oy, Helsinki, Finland). A calibration curve was set up with known numbers of cells and a linear correlation between absorbance and cell counts was established up to 1 × 105 cells. Statistical analysis was performed by analysis of variance (cutoff for significance, P = 0.05).
To test for peptide toxicity in the different cell lines, cell viability was tested in control and treated cells by means of trypan blue staining.
To evaluate ghrelin secretion by N-PAP and ARO cell lines, ghrelin levels (in ng/L) were measured in the culture media of both cell lines in duplicate, after extraction in reverse-phase C18 columns, by radioimmunometric assay (Phoenix Pharmaceutical, Inc, Belmont, CA). Sensitivity of the assay was 30 pg/tube. Intra-assay coefficients of variation range was 0.3 to 10.7%.
Immunohistochemistry (IHC)
Sections serial to those used for conventional histology were collected onto poly-l-lysine-coated slides and stained for ghrelin using a standard manual immunoperoxidase procedure with streptavidin-peroxidase (LSAB2 kit; DAKO, Glostrup, Denmark) and diaminobenzidine as the final reaction product. Ghrelin antibody was an anti-human ghrelin polyclonal serum (amino acids 24 to 51, code H-031-30; Phoenix Pharmaceuticals, Inc.), diluted 1/15,000, incubated for 1 hour at room temperature, with no previous antigen retrieval and revealed applying catalyzed reporter deposition (CARD) amplification with slight modifications. 25 Negative control reactions forghrelin included omission of the primary antibody and preabsorption with a 100-fold excess of the antigen, as described elsewhere. 14 The positive control for ghrelin were the endocrine cells of the oxyntic mucosa of the stomach. The follicular origin of the tumors was evaluated by IHC with polyclonal anti-calcitonin (diluted 1/80, DAKO) and anti-thyroglobulin (diluted 1/3000; Biogenex, San Ramon CA) antibodies. Selected cases were also stained with a double-immunohistochemical procedure using the same antibodies that react with ghrelin and thyroglobulin. This procedure was based on an immunoalkaline phosphatase (LSAB plus kit, DAKO)/fast red (BioGenex) reaction for ghrelin, followed by immunoperoxidase staining of thyroglobulin, using an anti-rabbit secondary antibody and the EnVision kit (DAKO). Ghrelin protein expression was also evaluated in the two cell lines applying the same ghrelin antibody at a working dilution of 1/400, and visualized in immunofluorescence with an anti-rabbit fluorescein isothiocyanate-conjugated antibody (diluted 1/100; Sigma Aldrich, Steinheim, Germany).
In Situ Hybridization
Selected cases of fetal thyroid (12 cases), infant and adult normal thyroid (9 cases), and tumors (38 cases) were studied by means of a nonradioactive in situ hybridization procedure to confirm immunohistochemical findings and to analyze ghrelin mRNA localization. Silane-coated slides were hybridized overnight at a working dilution of 33 nmol/L of an equimolar mixture of two 45-mer anti-sense probes 1 corresponding to nucleotides 90 to 134 and 421 to 465 of prepro-ghrelin sequence. Probes were digoxigenin-labeled with the Boehringer labeling kit (Mannheim, Germany) following the manufacturer’s instructions. Prehybridization treatments included a microwave passage (5 minutes at 800 W in citrate buffer pH 6.0) and proteinase K digestion (1 μg/ml) for 4 minutes. Hybridization products were revealed applying the DAKO GenPoint kit (DAKO) as described elsewhere 26 with minor modifications, including 1/5 dilution of tyramide solution and hot washing in phosphate-buffered saline (PBS) after tyramide incubation. 25 Positive controls were represented by sections of normal oxyntic mucosa of the stomach. Hybridization with an unrelated probe, as well as omission of the specific probe and RNase digestion, served as negative controls, to test both the specificity of the anti-sense probes and possible tyramide-based background.
RT-PCR for Ghrelin and GHS-R
In 33 cases of follicular-derived thyroid tumors, as well as in 5 adult total thyroid tissue, RT-PCR was performed to reveal mRNAs for both ghrelin and its receptor. GHS-R type 1a and 1b mRNA expression was also evaluated in N-PAP and ARO cell lines. Total RNA extraction and complementary DNA transcription was performed as described elsewhere. 26 The primers for ghrelin were synthesized according to the sequence reported by Gualillo and colleagues, 11 and the sequences were 5′-TGAGCCCTGAACACCAGAGAG-3′ for the forward primer and 5′-AAAGCCAGATGAGCGCTTCTA-3′ for the reverse primer. Those for GHS-receptor 1a and 1b were synthesized according to Korbonits and colleagues 13 and used for RT-PCR using the same conditions described by these authors. The following sequences were used: 5′-TCGTGGGTGCCTCGCT-3′ as the forward primer for both GHS-R 1a and GHS-R 1b, 5′-CACCACTACAGCCAGCATTTTC-3′ for the GHS-R 1a reverse primer and 5′-GCTGAGACCCACCCAGCA-3′ for the GHS-R 1b reverse primer. The expected size of the amplicons was 327 bp, 65 bp, and 66 bp for ghrelin, GHS-R 1a and GHS-R 1b, respectively. β2-microglobulin amplification served as a control of the RNA quality (see details in Papotti and colleagues 26 ). Positive controls included normal gastric mucosa for ghrelin and pituitary tissue for GHS-R 1a and 1b amplifications. Negative controls consisted of omission of cDNA in the PCR mixture and of the reverse transcriptase enzyme during retrotranscription.
To further test the RT-PCR product specificity, Southern blot analysis was performed using the probe sequence previously published by Korbonits and colleagues. 13 The following sequences were used: 5′-AGGGACCAGAACCACAAGCAAACCG-3′ for ghrelin and 5′-TCCGGTTCAACGCCCCCTTTG-3′ for GHSR-1 probes. Membranes were hybridized overnight at 42°C with 25 pmol of digoxigenin-labeled ghrelin and GHS-R oligonucleotide probes. The membranes were then washed with 2× standard saline citrate-0.1% sodium dodecyl sulfate for 5 minutes at 42°C and 0.5× standard saline citrate-0.1% sodium dodecyl sulfate at 42°C for 10 minutes. Digoxigenin-labeled specific hybridization was visualized using an immunological detection system (Boehringer Mannheim, Mannheim, Germany) using anti-digoxigenin antibodies conjugated with alkaline phosphatase. Detection was performed using the chemiluminescent substrate disodium 3-(4-methxyspiro-1,2-dioxetane-3,2-(5-chloro) tricyclo (3.3.1.) decan-4-yl) phenylphosphatase (Boehringer Mannheim), according to the manufacturer’s instructions. All blots were exposed to X-ray films with intensifying screens at room temperature for 3 or 5 hours.
Nonradioactive in Situ Localization of Ghrelin Binding
Full-length ghrelin (Tocris Cookson Ltd., Bristol, UK) was labeled with biotin following a standard technique, 27 with minor modifications. In particular, to minimize the steric hindrance between avidin (streptavidin) and the biotinylated protein, an aminocaproyl spacer arm (a modified activated biotin, caproylamidobiotin-N-hydroxy succimide ester) was introduced between the biotin and the activated carboxyl group of the protein (B002-6; Società Prodotti Antibiotici SpA, Milan, Italy). Ghrelin (0.5 mg/ml) was dialyzed against 0.1 mol/L of sodium bicarbonate buffer, pH 8.5, and subsequently incubated for 4 hours at room temperature under continuous slow rotation with B002-6 dissolved in dimethyl sulfoxide (1 mg/ml). The solution was dialyzed overnight at 4°C against PBS.
Frozen serial sections of 16 thyroid tumors and 3 normal thyroid tissues were collected onto poly-l-lysine-coated slides and stored for 1 week at −80°C. One section was stained with hematoxylin and eosin to assess the quality of tissue preservation. Then the sections were thawed at 37°C for 4 minutes and quenched in PBS. Endogenous peroxidase activity was blocked in 6% H2O2 for 8 minutes, after which the sections were incubated with biotinylated ghrelin at a concentration of 10−9 mol/L for 2 hours at room temperature. Sections were then fixed in −20°C methanol for 5 minutes and in −20°C acetone for 5 seconds. Bound biotinylated ghrelin was visualized after the streptavidin/tyramide procedure described for ghrelin IHC. Control experiments included competition assays in parallel sections with unlabeled full-length ghrelin at a concentration of 10−6 mol/L for 20 minutes at room temperature, before incubation with labeled ghrelin, as described, as well as omission of the biotinylated peptide. Pituitary tissue served as positive control. For in vitro binding experiments, N-PAP and ARO cell lines were incubated for 2 hours at 4°C with 10−9 mol/L biotinylated ghrelin, then fixed in −20°C methanol for 5 minutes and in −20°C acetone for 5 seconds, and the reaction visualized with the streptavidin/tyramide procedure described above. Control experiments included a competition assay using preincubation for 10 minutes at 4°C with 10−7 mol/L unlabeled ghrelin as well as with an unrelated peptide (10−7 mol/L oxytocin) (Sigma).
Results
Ghrelin Expression in Nonneoplastic Thyroid Tissues
Fetal thyroid (from 8 to 38 weeks of gestational age) produced ghrelin (Table 1) ▶ in the follicular cells, as shown by IHC using a specific antibody that recognizes the C-terminal portion of both acylated and nonacylated ghrelin (Figure 1) ▶ . Immunostaining was completely abolished in parallel sections by pretreatment of the antibody with excess ghrelin. The extent of ghrelin protein expression was much lower in the late fetal period, in which ghrelin immunoreactivity was confined to the apical portion of the follicular cells.
Table 1.
Ghrelin Expression in Fetal and Infant Thyroid Tissue
| No. | Sex | Age | Cause of death/associated disease | Ghrelin | |
|---|---|---|---|---|---|
| IHC | In situ hybridization | ||||
| 7122 | M/F* | 8 weeks† | Voluntary abortion | +++ | +++ |
| 13099 | na | 10 weeks† | Voluntary abortion | +++ | +++ |
| 6286 | na | 11 weeks† | Voluntary abortion | ++ | ++ |
| 6120 | na | 11 weeks† | Voluntary abortion | ++ | + |
| A36 | na | 12 weeks† | Voluntary abortion | +++ | na |
| 2542 | M | 13 weeks† | IUFD (placental abruption) | +++ | na |
| 867 | F | 14 weeks† | Elective abortion | ++ | + |
| 7727 | F | 16 weeks† | IUFD (osteogenesis imperfecta) | + | − |
| 6433 | M | 18 weeks† | Elective abortion | +++ | ++ |
| 11370 | M | 19 weeks† | Elective abortion | + | + |
| 11372 | F | 20 weeks† | Elective abortion | ++ | + |
| 6081 | M | 22 weeks† | IUFD (placental failure) | ++ | na |
| A3 | M | 25 weeks† | Elective abortion | ++ | + |
| A42 | M | 36 weeks† | IUFD (placental failure) | ++ | ++ |
| A5 | F | 38 weeks† | IUFD (hypoxia) | + | + |
| 2332 | M | 10 years | Follicular adenoma | − | − |
| 350380 | F | 10 years | Papillary carcinoma | − | − |
| 6549 | F | 11 years | Nodular goiter | − | − |
| 5509 | F | 13 years | Papillary carcinoma | − | − |
M, male; F, female; *, twins; na, not available;
†, gestational age; IUFD, intrauterine fetal death; IHC, immunohistochemistry; +, positive in less than 25% of tumor cells; ++, positive in 25 to 50% of tumor cells; +++, positive in more than 50% of tumor cells.
Figure 1.
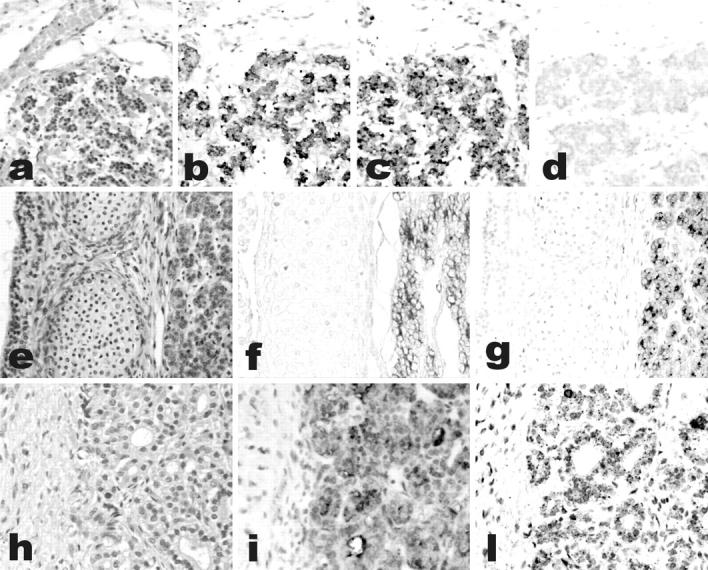
Ghrelin expression in the human fetal thyroid. Thyroids from the early fetal period (a–d, gestational age 10 weeks; e–g, gestational age 12 weeks) show co-expression of thyroglobulin (b and f) and ghrelin (c and g) in the same cell population, as detected by IHC. Staining is abolished by preabsorption with full-length ghrelin peptide (d). Ghrelin expression is weaker in late fetal thyroid (h–l, gestational age 28 weeks), as shown by both IHC (i) and in situ hybridization (l). a, e, h: H&E; b–d, f, g, i: immunoperoxidase; l: in situ hybridization. Original magnifications, ×200.
Immunoreactive ghrelin was not found in infant (Table 1) ▶ or in adult (Table 2) ▶ thyroid follicular cells, or in the setting of multinodular goiter or in peritumoral follicles. Three cases of Hashimoto’s thyroiditis in adult patients were also tested for ghrelin protein expression, and showed no immunoreactivity (data not shown). Double-immunohistochemical reactions confirmed thyroglobulin expression in ghrelin-producing cells. No ghrelin immunoreactivity was ever observed in other structures of the thyroid, including blood vessel and the capsule.
Table 2.
Ghrelin and GHS-R Type 1a and 1b Expression in Normal Thyroid and Follicular-Derived Tumors
| Diagnosis (total cases) | M/F | Mean age (years) | Ghrelin | GHS-R1a RT-PCR | GHS-R1b RT-PCR | NR-ISGB | ||
|---|---|---|---|---|---|---|---|---|
| ICC | In situ hybridization | RT-PCR | ||||||
| NT (10) | 4/6 | 51 | 0/10 | 0/5 | 3/5 | 0/5 | 1/5 | 2/3 |
| FA (18) | 8/10 | 47.3 | 13/18 | 5/11 | 9/12 | 0/12 | 0/12 | 6/6 |
| FC (10) | 3/7 | 52.3 | 9/10 | 6/6 | 4/6 | 0/6 | 0/6 | 1/1 |
| PTC (12) | 5/7 | 39.8 | 9/12 | 6/9 | 5/10 | 0/10 | 0/10 | 5/5 |
| PDC (8) | 2/6 | 57.5 | 8/8 | 8/8 | 1/1 | 0/1 | 0/1 | 0/2 |
| AC (6) | 2/4 | 58.6 | 3/6 | 3/4 | 2/4 | 0/4 | 0/4 | 2/2 |
M, male; F, female; ICC, immunocytochemistry; NR-ISGB, nonradioactive in situ localization of ghrelin binding; NT, normal thyroid; FA, follicular adenoma; PTC, papillary thyroid carcinoma; FCC, follicular carcinoma; PDC, poorly differentiated carcinoma; AC, anaplastic carcinoma.
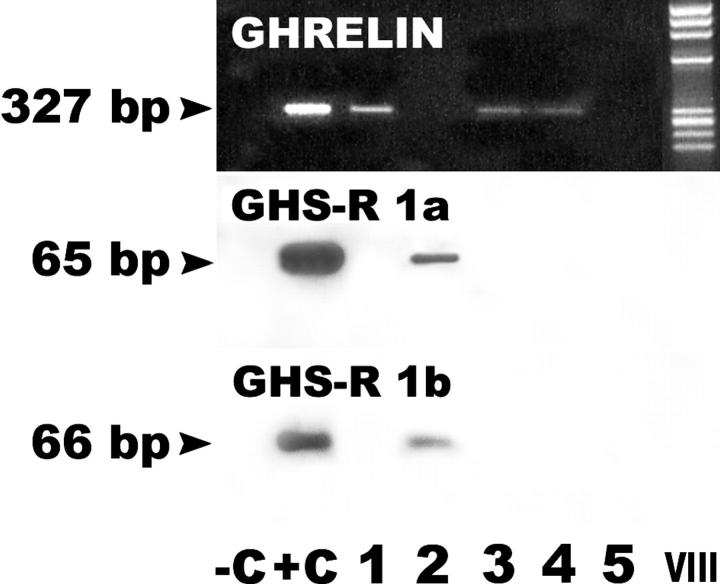
mRNA analysis by means of in situ hybridization confirmed the immunohistochemical findings in fetal, infant, and adult thyroid tissues (Tables 1 and 2) ▶ ▶ . No signal was detected in gastric mucosa or fetal thyroid tissue in the absence of the tyramide amplification step. The more sensitive RT-PCR technique revealed ghrelin transcripts detectable in three of five cases of adult normal thyroid (Table 2 ▶ , Figure 2 ▶ ).
Figure 2.
Ghrelin and GHS-R 1a and 1b mRNA expression in normal thyroid tissue. A specific PCR product (expected size 327 bp) for ghrelin is obtained from total mRNA extracted from whole normal thyroid tissue in three samples (lanes 1, 3, and 4). A single sample (lane 2) shows the presence of the specific PCR amplification product for both GHS-R 1a and 1b (expected size 65 and 66 bp for GHSR 1a and 1b, respectively). −C, negative control, consisting in omission of cDNA in the PCR mixture; +C, positive controls were normal gastric mucosa for ghrelin and pituitary gland for GHS-R 1a and 1b mRNAs; VIII, high molecular weight marker VIII (Boehringer Mannheim, Germany).
Ghrelin Expression in Thyroid Tumors and Cell Lines
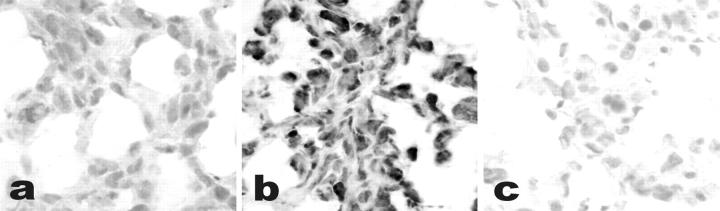
Moderate to strong ghrelin immunoreactivity was observed in 42 of 54 cases, having either a focal (15 cases, <25% of tumor cells) or a diffuse (27 cases) pattern of reactivity (Figure 3) ▶ . The focal pattern was mainly recognized in conventional follicular adenomas (six of eight cases). Interestingly, all five cases of benign tumors with oxyphilic features (oncocytic adenomas) were positive for ghrelin. Malignant tumors expressed ghrelin in 9 of 19 follicular, 9 of 12 papillary, 8 of 8 poorly differentiated, and 3 of 6 anaplastic carcinomas (Table 2) ▶ . Ghrelin protein expression was also demonstrated by immunofluorescence in both N-PAP and ARO cell lines (Figure 4) ▶ . Moreover, ghrelin protein was demonstrated by radioimmunometric assay in N-PAP (49 ng/L) but not in ARO cell culture media.
Figure 3.
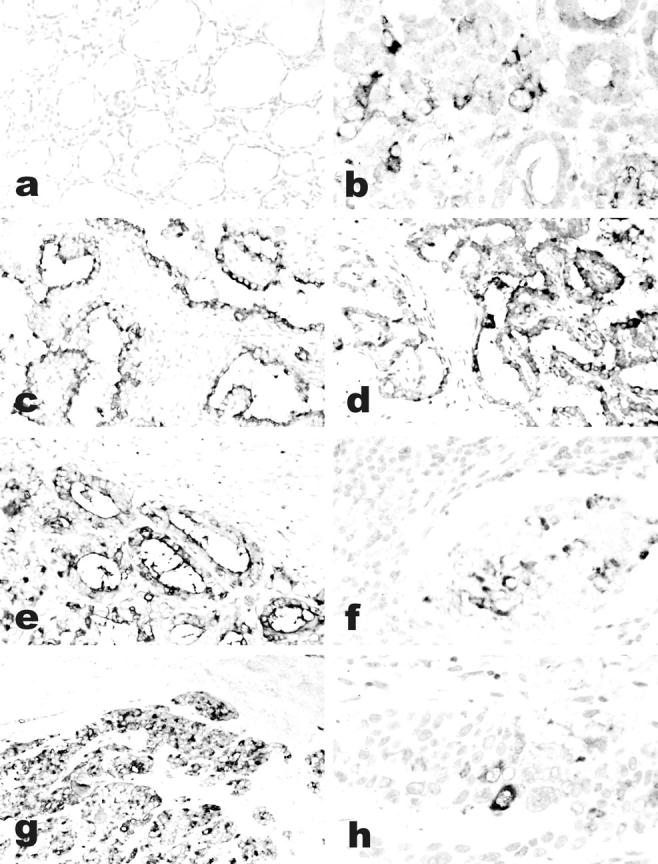
Ghrelin expression in thyroid tumors. Immunoreactive ghrelin is absent in peritumoral thyroid tissue (a), focally present in a follicular adenoma (b) and in an anaplastic carcinoma (h), and strongly expressed in classic (c) and follicular variant (e) papillary, and in poorly differentiated (g) carcinomas. Immunohistochemical data are paralleled by a specific in situ hybridization signal for ghrelin mRNA (d, same area as c). Lymph node metastases retain the ghrelin expression capacity of the primary sites (f, same case as e). a–h: Immunoperoxidase. Original magnifications: ×200 (a, c–e, g); ×400 (b, f, h).
Figure 4.
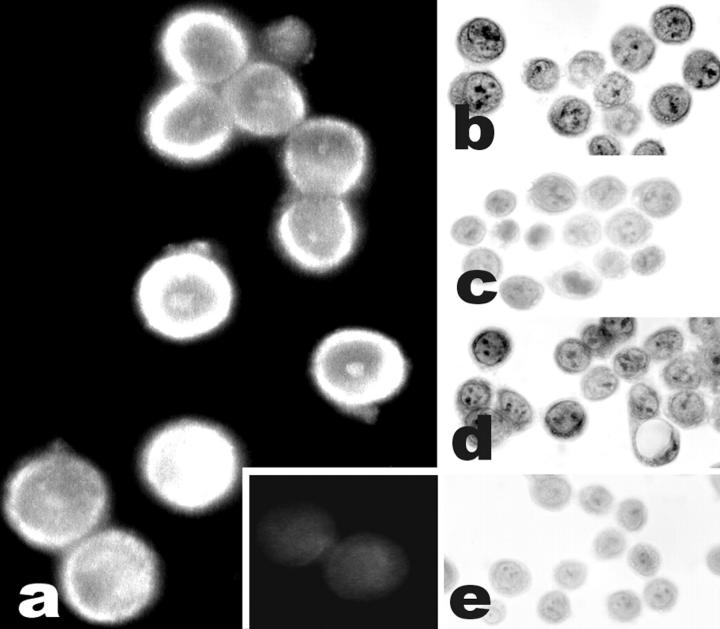
Ghrelin expression in ARO cell line. A strong immunofluorescence signal is present (a) as compared to a negative reaction in a control section stained omitting the primary antibody (a, inset). The same cell line shows ghrelin binding sites, as demonstrated by nonradioactive binding localization (b), which is not prevented by competition with an unrelated, unlabeled peptide (oxytocin) (d) but is abolished by competition with an excess of unbiotinylated ghrelin (c) or with omission of the biotinylated peptide (e). Original magnifications: ×1000 (a); ×400 (b–e).
All of the above cases were also analyzed for ghrelin mRNA expression by means of either in situ hybridization or RT-PCR, or both. Ghrelin mRNA was detected in 42 of 54 tumors, considering both methods (28 of 38 and 21 of 33 by means of in situ hybridization and RT-PCR, respectively). Comparing the results of IHC and mRNA analysis, 5 of 33 cases had discordant protein and mRNA (as detected by RT-PCR) ghrelin expression, four of them being positive by IHC and negative by RT-PCR. Comparing in situ hybridization and IHC, five cases showed discordant results, being four positive for IHC only, and the remaining one positive by in situ hybridization only. Incidentally, all of the latter cases had focal (<25% of tumor cells) patterns of reactivity for both in situ hybridization and IHC.
Ghrelin Effect on Thyroid Carcinoma Cell Line Proliferation in Vitro
The proliferation of N-PAP and ARO cells was evaluated under both basal conditions and ghrelin treatment.
Ghrelin determined a dose-dependent, although modest, inhibition of cell growth in both cell lines tested (Figure 5) ▶ . Reduction of cell growth was significant at any time point in N-PAP cells using 1 μmol/L of ghrelin (P = 0.005, treated cells versus controls), with a maximum 25% decrease in cell number. At 100 nmol/L, ghrelin still determined a significant inhibition in N-PAP cells after 96 hours of treatment (−22%, P = 0.01), whereas 10 nmol/L of ghrelin did not affect cell proliferation at any time point. In ARO cells, the growth inhibition was significant using 1 μmol/L of ghrelin only (P = 0.01 at 48 hours and P = 0.005 at 96 hours of treatment, in treated cells versus controls).
Figure 5.
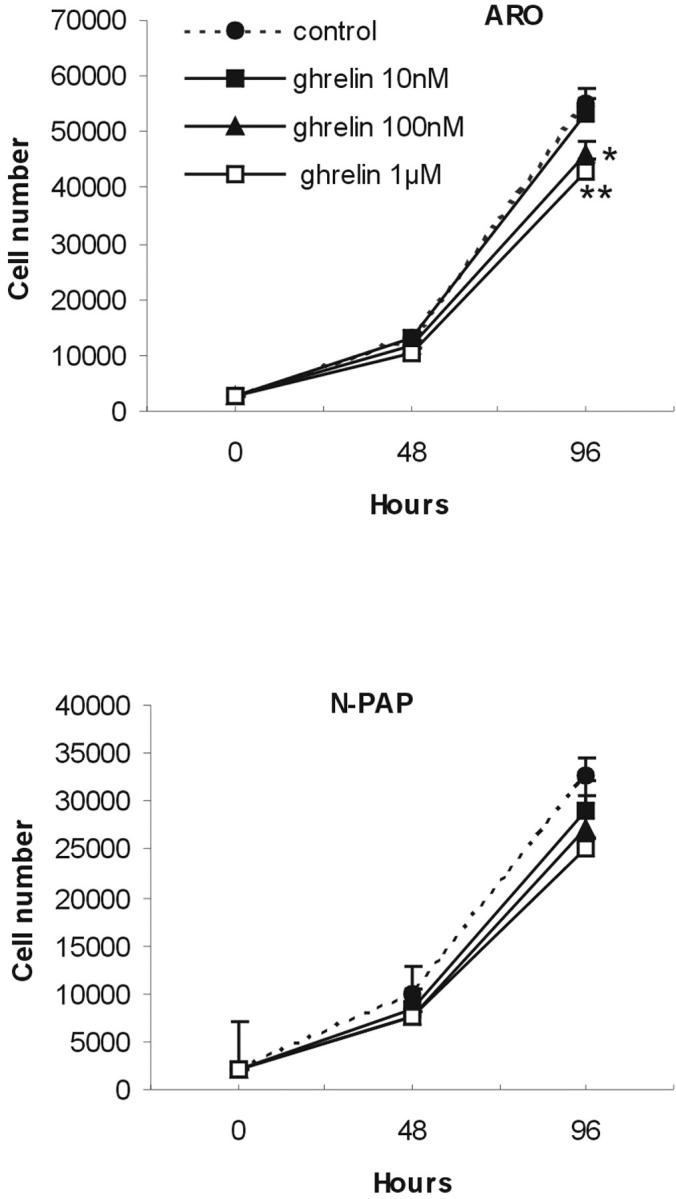
Ghrelin effect on ARO and N-PAP thyroid carcinoma cell line proliferation. Ghrelin treatment determined a dose-dependent inhibition of cell proliferation in ARO and N-PAP cells. At 10 nmol/L, ghrelin was ineffective in both cell lines, whereas 100 nmol/L of ghrelin significantly reduced N-PAP cell proliferation at 96 hours (*, P = 0.01, treated versus control). Ghrelin (1 μmol/L) significantly inhibited ARO and N-PAP cell growth at any time point (*, P = 0.01; **, P = 0.005, treated versus control). Values are mean ± SD of three different experiments with cells seeded in quadruplicate.
Study of peptide toxicity performed using trypan blue staining revealed that ghrelin was not toxic. No significant difference in ARO or N-PAP cell viability was observed in untreated versus ghrelin-treated cells, at any peptide concentration and at any time point tested (percentage of viable ARO cells at 48 and 96 hours of treatment, respectively, 96% and 95% in untreated cells, 94% and 93% in the presence of 1 μmol/L of ghrelin; percentage of viable N-PAP cells at 48 and 96 hours of treatment, respectively, 98% and 96% in untreated cells, 97% and 96% in the presence of 1 μmol/L of ghrelin).
GHS Receptor Expression and Ghrelin Binding Sites in Normal and Neoplastic Thyroid Tissues
RT-PCR was used to analyze ghrelin receptor (GHS-R 1a and 1b) in adult normal thyroid tissues and in 33 tumors, as well as in the two thyroid carcinoma cell lines. None of the tumors or cell lines expressed the specific mRNA. Nontumoral thyroid specimens tested in parallel were also negative with the single exception of GHS-R 1a and 1b mRNA expression in a single case of adult thyroid gland specimen (incidentally, a peritumoral sample of a papillary carcinoma, which was negative as stated above).
As opposed to GHS-R mRNA findings, a large percentage of both normal (2 of 3 cases) and neoplastic (14 of 16 cases) thyroid specimens (Figure 6) ▶ , as well as the two cell lines (Figure 4) ▶ , bound ghrelin according to a newly designed nonradioactive in situ technique. Specificity of the signal was confirmed using an excess of unlabeled ghrelin as a competitor, which abolished any staining determined by the labeled peptide. In cell culture experiments, additional negative controls included competition with an unrelated peptide (oxytocin), which did not affect biotinylated ghrelin-binding capacity.
Figure 6.
a: Ghrelin-binding sites on frozen sections of anaplastic carcinoma. Nonradioactive in situ localization of ghrelin binding reveals specific ghrelin (10−9 mol/L) binding sites (b), which are not displaced by excess cold ghrelin (10−7 mol/L) (c).
Discussion
In this study, we provide the first evidence that ghrelin, a recently identified gastric hormone, is expressed in the thyroid in fetus but not in adult, and in a high percentage of thyroid tumors of follicular origin. Moreover, we report that autocrine interactions between ghrelin and putative receptors other than the GHS-R may be active in the thyroid, because ghrelin-binding sites were detected in both normal and neoplastic thyroid tissues, in the absence of GHS-R 1 mRNA expression. The functionality of these possible alternative ghrelin receptors is also supported by the evidence of a specific, although modest, anti-proliferative effect of ghrelin in two different follicular-derived thyroid carcinoma cell lines.
Immunoreactive ghrelin was present in most follicular cells and co-localized with thyroglobulin in primordial cords of the embryonic thyroid, as early as week 8 of gestation, and was detected in decreasing amounts from week 20 of pregnancy to the late fetal period. The intensity of ghrelin immunoreactivity was similar to that of the oxyntic mucosa of the stomach, indicating that, in fetal life, the thyroid may be an additional important source of circulating ghrelin. The cells producing ghrelin in the fetal thyroid were follicular cells, as confirmed by the co-localization of ghrelin with thyroglobulin. In infant and adult thyroid glands, no immunoreactivity was observed in the follicular compartment. These data are in agreement with the previous study of Kanamoto and colleagues 22 on ghrelin expression in parafollicular cells and tumors, in which immunohistochemical evaluation of normal thyroid tissue showed no reactivity. The finding of ghrelin mRNA in whole thyroid tissue mRNA extracts observed in RT-PCR experiments by Kanamoto and colleagues 22 and confirmed by the present study, could be explained by a residual minimal transcription of ghrelin gene in follicular cells, although this hypothesis could not be confirmed by in situ techniques such as in situ hybridization, that are known to be less sensitive than PCR. In addition, the minor C cell population could be responsible for ghrelin mRNA PCR detection in whole thyroid tissue, as already originally suggested by Kanamoto and colleagues, 22 but we have no evidence to confirm this hypothesis in the current study.
Ghrelin expression was also observed in a high proportion of follicular-derived tumors of the thyroid. Medullary carcinomas were not tested here, because they have already been reported to express ghrelin 22 as mentioned above. We demonstrated that neoplastic transformation enables follicular cells to (re-) express ghrelin, from the earliest phases, because adenomas contain ghrelin in three-quarters of cases. Malignant tumors retain the capacity to produce ghrelin irrespective of their grade of differentiation. Similarly, ghrelin was present in the thyroid follicular-derived tumor cell lines, and the anti-proliferative effect of the peptide was evident in both undifferentiated (ARO) and differentiated (N-PAP) tumor cell lines. No apparent differences in ghrelin expression were observed in thyroid tumors, despite tumor grade and differentiation, thus suggesting the absence of a prominent role for ghrelin in tumor progression mechanisms.
It is not known whether the mechanism mediating ghrelin activities in fetal thyroid and tumors is the result of a direct effect on specific receptors, or if it follows a more complex pathway via GH release. To address this point, the demonstration of specific receptors for ghrelin in thyroid cells is a crucial step. In the present case series, GHS-R types 1a and 1b mRNAs were not detected in any thyroid tumor, although specific binding sites for synthetic ghrelin analogs, such as hexarelin, were reported in a percentage of thyroid tumors in a previous report, 18 and specific ghrelin-binding sites have been demonstrated using either radioactive (G Muccioli and M Papotti, unpublished observation) or nonradioactive methods, as in the current study. It can be speculated that, at the peripheral level, GHS/ghrelin receptors other than the known GHS receptor identified in central organs (pituitary and hypothalamus) by Howard and co-workers 15 exist, representing either subtypes of the GHS-R itself or novel receptors, yet to be cloned. Discrepancies between the presence of binding sites for ghrelin and the identification of the specific GHS-R mRNA have already been reported in other tumors. 19 Unfortunately, no fresh frozen material of fetal thyroid origin was available to evaluate both GHS-R mRNA and ghrelin-binding site status during gland development.
The effect of ghrelin on cell proliferation of thyroid neoplastic cells confirms the data previously reported in thyroid carcinoma cell lines using synthetic GHS. 18 Similarly, in neoplastic cells from tissues other than the thyroid, both ghrelin and GHS were able to inhibit cell proliferation in vitro. 19-21 The relatively high concentration of ghrelin (100 nmol/L to 1 μmol/L) required to exert an anti-proliferative effect in the studied thyroid carcinoma cell lines is in agreement with what was previously observed in lung carcinoma or breast cancer cell lines using either ghrelin or synthetic GHS. 19,21 These data are at variance with recent findings on lower dose-dependent ghrelin effects on cell growth in prostate cancer cell lines, 20 even if, however, different cancer cell lines were analyzed, and possible cell line-dependent or different culture conditions could be responsible for these discrepancies.
Because the anaplastic thyroid carcinoma cell line (ARO) required higher ghrelin concentrations (1 μmol/L) for inhibition of cell proliferation compared to the papillary carcinoma-derived cell line (N-PAP), we speculate that more undifferentiated cells may express a lower-affinity or modified GHS receptor. These in vitro evidences allow us to suggest that, besides their endocrine effect, synthetic and natural GH secretagogues may act as negative growth factors in neoplastic conditions, possibly through a receptor different from GHS-R1a. Therefore, such alternative binding sites for ghrelin could depend either on the central/peripheral location (as suggested above) or on the normal/neoplastic cell phenotype.
In conclusion, we have shown that: 1) follicular cells of the developing thyroid are an additional source of ghrelin in fetal life; 2) the adult thyroid is devoid of immunoreactive ghrelin, but most thyroid tumors of follicular origin express ghrelin mRNA and protein; 3) hypothetical receptor types other than GHS-R, the only currently known ghrelin receptor, could mediate ghrelin functions in thyroid tumors; and 4) ghrelin possesses anti-proliferative properties in thyroid carcinoma cell lines suggesting that autocrine circuits may be operating in the growth control of follicular tumors.
Acknowledgments
We thank Prof. G. Bussolati for his suggestions; Dr. Enza Ferrero for carefully proofreading the manuscript; Drs. C. Pecchioni, A. Funaro, P. Gugliotta, and T. Marrocco for skillful technical assistance; and Prof. A. Fusco (University of Naples) for the gift of thyroid carcinoma cell lines.
Footnotes
Address reprint requests to Mauro Papotti, M.D., Department of Pathology, University of Turin, Via Santena 7, I-10126 Turin, Italy. E-mail: mauro.papotti@unito.it.
Partially supported by grants from the Italian Ministry of University and Research (Rome), Associazione Italiana per la Ricerca sul Cancro (Milan), and the Fondazione per lo Studio delle Malattie e Endocrine e Metaboliche (Turin).
References
- 1.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K: Ghrelin is a growth hormone-releasing acylated peptide from stomach. Nature 1999, 402:656-660 [DOI] [PubMed] [Google Scholar]
- 2.Dieguez C, Casanueva FF: Ghrelin: a step forward in the understanding of somatotroph cell function and growth regulation. Eur J Endocrinol 2000, 142:413-417 [DOI] [PubMed] [Google Scholar]
- 3.Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, Kennedy AR, Roberts GH, Morgan DG, Ghatei MA, Bloom SR: The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology 2000, 141:4325-4328 [DOI] [PubMed] [Google Scholar]
- 4.Arvat E, Maccario M, Di Vito L, Broglio F, Benso A, Gottero C, Papotti M, Muccioli G, Dieguez C, Casanueva FF, Deghenghi R, Camanni F, Ghigo E: Endocrine activities of ghrelin, a natural growth hormone secretagogue (GHS), in humans: comparison and interactions with hexarelin, a nonnatural peptidyl GHS, and GH-releasing hormone. J Clin Endocrinol Metab 2001, 86:1169-1174 [DOI] [PubMed] [Google Scholar]
- 5.Broglio F, Arvat E, Benso A, Gottero C, Muccioli G, Papotti M, van der Lely AJ, Deghenghi R, Ghigo E: Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J Clin Endocrinol Metab 2001, 86:5083-5086 [DOI] [PubMed] [Google Scholar]
- 6.Egido EM, Rodriguez-Gallardo J, Silvestre RA, Marco J: Inhibitory effect of ghrelin on insulin and pancreatic somatostatin secretion. Eur J Endocrinol 2002, 146:241-244 [DOI] [PubMed] [Google Scholar]
- 7.Date Y, Nakazato M, Hashiguchi S, Dezaki K, Mondal MS, Hosoda H, Kojima M, Kangawa K, Arima T, Matsuo H, Yada T, Matsukura S: Ghrelin is present in pancreatic alpha-cells of humans and rats and stimulates insulin secretion. Diabetes 2002, 51:124-129 [DOI] [PubMed] [Google Scholar]
- 8.Lee HM, Wang G, Englander EW, Kojima M, Greeley GH, JR: Ghrelin, a novel gastrointestinal endocrine peptide that stimulates insulin secretion: enteric distribution, ontogeny, influence of endocrine, and dietary manipulations. Endocrinology 2002, 143:185-190 [DOI] [PubMed] [Google Scholar]
- 9.Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M: Ghrelin, a novel growth-hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 2000, 141:4255-4261 [DOI] [PubMed] [Google Scholar]
- 10.Mori K, Yoshimoto A, Takaya K, Hosoda K, Ariyasu H, Yahata K, Mukoyama M, Sugawara A, Hosoda H, Kojima M, Kangawa K, Nakao K: Kidney produces a novel acylated peptide, ghrelin. FEBS Lett 2000, 486:213-216 [DOI] [PubMed] [Google Scholar]
- 11.Gualillo O, Caminos J, Blanco M, Garcia-Caballero T, Kojima M, Kangawa K, Dieguez C, Casanueva F: Ghrelin, a novel placental-derived hormone. Endocrinology 2001, 142:788-794 [DOI] [PubMed] [Google Scholar]
- 12.Volante M, Allìa E, Gugliotta P, Deghenghi R, Muccioli G, Ghigo E, Papotti M: Expression of ghrelin and of GH secretagogue receptor by pancreatic islet cells and related endocrine tumors. J Clin Endocrinol Metab 2002, 87:1300-1308 [DOI] [PubMed] [Google Scholar]
- 13.Korbonits M, Bustin SA, Kojima M, Jordan S, Adams EF, Lowe DG, Kangawa K, Grossman AB: The expression of the growth hormone secretagogue receptor ligand ghrelin in normal and abnormal human pituitary and other neuroendocrine tumors. J Clin Endocrinol Metab 2001, 86:881-887 [DOI] [PubMed] [Google Scholar]
- 14.Papotti M, Cassoni P, Volante M, Deghenghi R, Muccioli G, Ghigo E: Ghrelin-producing endocrine tumors of the stomach and intestine. J Clin Endocrinol Metabol 2001, 86:5052-5059 [DOI] [PubMed] [Google Scholar]
- 15.Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, Hamelin M, Hreniuk DL, Palyha OC, Anderson J, Paress PS, Diaz C, Chou M, Liu KK, McKee KK, Pong SS, Chaung LY, Elbrecht A, Dashkevicz M, Heavens R, Rigby M, Sirinathsinghji DJ, Dean DC, Melillo DG, Van de Ploeg LH, Patchett AA, Nargund R, Griffin PR, De Martino JA, Gupta SK, Schaeffer JM, Smith RG, Van der Ploeg LHT: A receptor in pituitary and hypothalamus that functions in growth hormone release. Science 1996, 273:974-977 [DOI] [PubMed] [Google Scholar]
- 16.Papotti M, Ghe C, Cassoni P, Catapano F, Deghenghi R, Ghigo E, Muccioli G: Growth hormone secretagogue binding sites in peripheral human tissues. J Clin Endocrinol Metab 2000, 85:3803-3807 [DOI] [PubMed] [Google Scholar]
- 17.Muccioli G, Ghe C, Ghigo MC, Papotti M, Arvat E, Boghen MF, Nilsson MH, Deghenghi R, Ong H, Ghigo E: Specific receptors for synthetic GH secretagogues in the human brain and pituitary gland. J Endocrinol 1998, 157:99-106 [DOI] [PubMed] [Google Scholar]
- 18.Cassoni P, Papotti M, Catapano F, Ghe C, Deghenghi R, Ghigo E, Muccioli G: Specific binding sites for synthetic growth hormone secretagogues in non tumoral and neoplastic human thyroid tissue. J Endocrinol 2000, 165:139-146 [DOI] [PubMed] [Google Scholar]
- 19.Cassoni P, Papotti M, Ghe C, Catapano F, Sapino A, Graziani A, Deghenghi R, Reissmann T, Ghigo E: Identification, characterization and biological activity of specific receptors for natural (ghrelin) and synthetic growth hormone secretagogues in human breast carcinomas and cell lines. J Clin Endocrinol Metab 2001, 86:1738-1745 [DOI] [PubMed] [Google Scholar]
- 20.Jeffery PL, Herington AC, Chopin LK: Expression and action of the growth hormone releasing peptide ghrelin and its receptor in prostate cancer cell lines. J Endocrinol 2002, 172:R7-R11 [DOI] [PubMed] [Google Scholar]
- 21.Ghe C, Cassoni P, Catapano F, Marrocco T, Deghenghi R, Ghigo E, Muccioli G, Papotti M: The antiproliferative effect of synthetic peptidyl GH secretagogues in human CALU-1 lung carcinoma cells. Endocrinology 2002, 143:484-491 [DOI] [PubMed] [Google Scholar]
- 22.Kanamoto N, Akamizu T, Hosoda H, Hataya Y, Ariyasu H, Takaya K, Hosoda K, Saijo M, Moriyama K, Shimatsu A, Kojima M, Kangawa K, Nakao K: Substantial production of ghrelin by a human medullary thyroid carcinoma cell line. J Clin Endocrinol Metab 2001, 86:4984-4990 [DOI] [PubMed] [Google Scholar]
- 23.Hedinger CE: Histological typing of thyroid tumors. International Histological Classification of Tumours, 1988, vol 11 Springer Verlag, Berlin
- 24.Rosai J, Carcangiu ML, DeLellis RA: Tumors of the thyroid gland, Atlas of Tumor Pathology, series 3, 1990, vol 5 Armed Forces Institute of Pathology, Washington DC
- 25.Volante M, Pecchioni C, Bussolati G: Post-incubation heating significantly improves tyramide signal amplification. J Histochem Cytochem 2000, 48:1583-1585 [DOI] [PubMed] [Google Scholar]
- 26.Papotti M, Croce S, Macri L, Funaro A, Pecchioni C, Schindler M, Bussolati G: Correlative immunohistochemical and reverse transcriptase-polymerase chain reaction analysis of somatostatin receptor type 2 in neuroendocrine tumors of the lung. Diagn Mol Pathol 2000, 9:47-57 [DOI] [PubMed] [Google Scholar]
- 27.Mouton CA, Pang D, Natraj CV, Shafer JA: A reagent for covalently attaching biotin to protein via a cleavable connection. Arch Biochem Biophys 1982, 218:101-108 [DOI] [PubMed] [Google Scholar]