Abstract
Here, we provide the first study of prolactin (PRL) and prolactin receptor (PRLR) expression during the nonseasonal murine hair cycle, which is, in contrast to sheep, comparable with the human scalp and report that both PRL and PRLR are stringently restricted to the hair follicle epithelium and are strongly hair cycle-dependent. In addition we show that PRL exerts functional effects on anagen hair follicles in murine skin organ culture by down-regulation of proliferation in follicular keratinocytes. In telogen follicles, PRL-like immunoreactivity was detected in outer root sheath (ORS) keratinocytes. During early anagen (III to IV), the developing inner root sheath (IRS) and the surrounding ORS were positive for PRL. In later anagen stages, PRL could be detected in the proximal IRS and the inner layer of the ORS. The regressing (catagen) follicle showed a strong expression of PRL in the proximal ORS. In early anagen, PRLR immunoreactivity occurred in the distal part of the ORS around the developing IRS, and subsequently to a restricted area of the more distal ORS during later anagen stages and during early catagen. The dermal papilla (DP) stayed negative for both PRL and PRLR throughout the cycle. Telogen follicles showed only a very weak PRLR staining of ORS keratinocytes. The long-form PRLR transcript was shown by real-time polymerase chain reaction to be transiently down-regulated during early anagen, whereas PRL transcripts were up-regulated during mid anagen. Addition of PRL (400 ng/ml) to anagen hair follicles in murine skin organ culture for 72 hours induced premature catagen development in vitro along with a decline in the number of proliferating hair bulb keratinocytes. These data support the intriguing concept that PRL is generated locally in the hair follicle epithelium and acts directly in an autocrine or paracrine manner to modulate the hair cycle.
Hair follicles are unusual in that they undergo lifelong cycles of growth and regression. Active hair growth (anagen) is accompanied by hair shaft elongation, melanogenesis, and by massive keratinocyte proliferation, whereas hair follicle regression (catagen) is characterized by terminal differentiation and apoptosis, resulting in the resting stage (telogen) and in hair shaft shedding (exogen). The molecular mechanisms that are responsible for this tightly controlled process are still not clear, but in the last decade a large, yet limited number of growth factors, cytokines, neuropeptides, neurotransmitters, and hormones have been shown to play important regulatory roles. 1-3 A particularly intriguing issue in this context is the search for the set of locally generated hormones and neurotrophines that are involved in that growth control 4,5 beyond the well-recognized effects of locally metabolized steroid hormones. 6
It has recently been recognized that prolactin (PRL) is expressed in numerous extrapituitary sites (such as the placenta, mammary gland, brain, and lymphocytes 7 ). Furthermore PRL has an amazingly versatile repertoire of bioregulatory functions beyond lactation, which includes immune response, osmoregulation, angiogenesis, development and hair growth modulation. 7-11
On the basis of earlier observations that implicated PRL in the hair growth regulation in diverse species, 10-14 we had previously hypothesized that both intracutaneously generated and systemically delivered PRL might serve as a hair growth modulator. 8 Subsequently, prolactin receptor (PRLR) knockout mice were shown to have hair cycle abnormalities. 15 In addition, in mammals with seasonally dependent cycles of pelage replacement, the increasing PRL during spring was shown to reactivate telogen follicles and induce anagen. 16 In cashmere goats, PRL and melatonin have been shown to stimulate hair shaft elongation in vitro. 17 In contrast, Wiltshire sheep show increased PRL levels after experimentally increased photoperiods associated with a short-term inhibitory effect on growing anagen follicles. 18,19 This is consistent with previous observations that shortening of the photoperiod accompanied by reduced PRL plasma levels results in initiation of fiber growth of the winter fur. 11,13,20 Thus, systemic PRL levels seem to play a dual role during the seasonal dependent hair growth cycle by operating to induce both transitional phases: catagen and proanagen. 10,18,19
In humans with their seasonally independent hair cycles, 2 hyperprolactinemia is associated with androgenetic alopecia, amenorrhea, infertility, and hirsutism. 21-23 PRL may increase adrenal androgen production, and can attenuate 5-α-reductase activity both in vivo and in vitro thus increasing dihydro testosterone (DTH) synthesis. 24 However, hair loss may also be a side-effect of treatment with the PRL inhibitor bromocriptine. 25-27
The PRLR is a single-pass membrane-bound protein that belongs to the cytokine receptor family and transduces its signal by binding Janus kinases (JAKs) and activating signal transducers and activators of transcription (Stat) proteins. 28,29 Several isoforms of PRLR arise from alternative initiation sites of transcription and gene splicing. In mice, one long and three short forms of the PRLR have been described. 29 All four receptors have been shown to bind the ligand, but only the long form of the PRLR is able to transduce a signal via the JAK/Stat pathway. 28,29
The PRLR is related structurally and functionally to the growth hormone (GH) receptor. 30 However, murine GH does not bind to lactogen receptors, in contrast to primate GHs. 30 On the other hand, murine placental lactogens are potent agonists of the PRLR. 31 And although PRL is incapable of binding to the receptors for GH, it has somatotrophic activity in rodents. 32 PRLRs have been shown to be expressed in epidermal keratinocytes in humans, 33 in the wool follicles of sheep, 34,35 and anagen hair follicles of mice 15 suggesting that PRL operates directly on the skin.
Recently, we showed that disruption of the PRLR gene in mice results in hair cycle perturbations and slightly longer and coarser hair. 15 The knockout mice exhibit advanced hair replacement cycles. However, PRLR deletion occurs throughout the animal and is accompanied by reduced estrogen and progesterone and elevated PRL blood levels. 36 Hence, it remained unclear whether these hair growth alterations reflect PRLR-mediated signaling in murine hair follicles or whether they are the indirect results of systemic changes in the level of other hormones and cytokines.
To further clarify the influence of PRL on hair follicle growth independent of seasonal coat changes and systemic hormone interactions, we investigated the expression of PRL, PRLR, and the PRLR ligand placental lactogen 1 (PL1) during the depilation-induced murine hair cycle by immunohistochemistry and real-time polymerase chain reaction (PCR). We also adopted a functional approach to test the direct effect of PRL on anagen VI hair follicles in murine skin organ culture.
Materials and Methods
Animals
Syngenic, female C57BL/6 mice (6 to 9 weeks of age) in the telogen stage of the hair cycle, or pregnant mothers, were purchased from Charles River (Sulzfeld, Germany). The mice were housed in community cages at the animal facilities of the Universitätsklinikum, Hamburg, under a 12-hour light:12 hour dark photoperiod and were fed mouse chow and water ad libitum.
Hair-Cycle Induction and Skin Harvesting
Anagen was induced in the back skin of mice in the telogen phase of the hair cycle (identified by their homogeneously pink back skin color) by applying a liquid 1:1 melted wax/rosin mixture under anesthesia as previously described. 37 After hardening, the wax/rosin mixture was peeled off the skin, plucking out all telogen hair shafts, which induces the homogeneous development of anagen follicles that are morphologically indistinguishable from spontaneous anagen follicles. At 0, 1, 3, 5, 8, 12, 17, 19, 20, and 25 days after depilation, mice were sacrificed and their back skin was harvested perpendicular to the paravertebral line to obtain longitudinal hair follicle sections. Skin samples were frozen in liquid nitrogen as previously described. 38
Immunohistochemistry
Prolactin
Cryosections from murine back skin (days 0 to 34 of the depilation-induced hair cycle) were fixed in acetone, washed in Tris-buffered saline, and incubated for 20 minutes at room temperature first with avidin, followed by biotin (ABC Kit; Vector Laboratories, Burlingame, CA). The samples were blocked with 10% goat serum and 3% bovine serum albumin for 20 minutes and incubated with rabbit anti-sheep PRL antiserum (AgResearch, Hamilton, New Zealand) 1:700 overnight at 4°C. After three washes in Tris-buffered saline, biotinylated goat anti-rabbit secondary antibody (Jackson ImmunoResearch, Hamburg, Germany) 1:200 was applied for 45 minutes. Washes and incubation with Vectastain reagent (ABC kit, Vector Laboratories) for 30 minutes followed. AEC+ was used as substrate (DAKO, Hamburg, Germany) and sections were counterstained with hematoxylin and mounted using Kaiser’s glycerol gelatin. Sections from murine pituitary glands were taken as positive control. Incubation of cryosections with preimmune rabbit serum (AgResearch) served as negative control.
Prolactin Receptor
Cryosections were treated the same way as for the anti-PRL staining. A blocking solution of 10% goat serum and 3% bovine serum albumin was applied overnight at 4°C followed by incubation with rabbit anti-sheep PRLR anti-serum (AgResearch), 1:200 for 1 hour. Biotin-labeled goat anti-rabbit IgG (Jackson ImmunoResearch) 1:200 was used as secondary antibody and AEC+ as substrate. Tissue sections from murine mammary gland and thymus served as a positive control and incubation of murine skin sections with the preimmune serum in place of the primary antibody served as negative control.
RNA Extraction
Total RNA was isolated from 0.2 to 0.5 g of each frozen murine back skin sample by grinding to powder under liquid nitrogen in a freezer mill (SPEX 7700; Glen Creston Ltd., Middlesex, UK), and extracting with TRIzol reagent (Life Technologies, Inc., Rockville, MD) according to the manufacturer’s instructions. RNA concentration was measured by spectrophotometry at 260 nm and RNA integrity was verified by agarose gel electrophoresis.
Real-Time Polymerase Chain Reaction
Expression of PRL and PRLR mRNA in skin was detected by real-time PCR. First strand cDNA was generated from 0.25 μg of each RNA preparation by reverse transcription with the Superscript Preamplification System (Life Technologies, Inc.) using oligo-dT primers according to the manufacturer’s instructions.
Oligonucleotide primers were designed using Primer Express software (Applied Biosystems, Foster City, CA) for murine glyceraldehyde-3-phosphate dehydrogenase (GAPDH), PRL, PL1, and PRLR long form, and synthesized as custom primers (Life Technologies, Inc.). Sequences of these primer sets are shown in Table 1 ▶ . PCR reactions in 20-μl volumes were assembled using the SYBR Green PCR Master Mix (Applied Biosystems), containing a passive reference dye to correct for well-to-well variation. Reactions were run on an Applied Biosystems 7700 thermocycler, as prescribed by the manufacturer. PCR consisted of an initial denaturing step at 94°C for 3 minutes, followed by 40 cycles of annealing at 55°C for 45 seconds, 72°C extension for 30 seconds, and 94°C denaturation for 30 seconds. The identities of PCR products were confirmed by DNA sequencing (DNA Sequencing Facility, University of Waikato, Hamilton, New Zealand). The relative concentration of mRNA of the target genes (PRL, PL1, PRLR-long form) was measured as the number of cycles of PCR required to exceed threshold fluorescence, normalized against that of an internal standard gene (GAPDH), according to the quantitation procedures recommended by Applied Biosystems.
Table 1.
PCR Primers
| Target gene | Sequence | Amplicon size, bp |
|---|---|---|
| PRL forward | 5′-CTCTCAGGCCATCTTGGAGAA-3′ | |
| PRL reverse | 5′-GGCTGACCCCTGGCTGTT-3′ | 68 |
| PL1 forward | 5′-CTTGAGGTGCCGAGTTGTCTT-3′ | |
| PL1 reverse | 5′-GGAAAGCATTACAAGTCTGGTTCA-3′ | 99 |
| PRLR long-form forward | 5′-ATAAAAGGATTTGATACTCATCTGCTAGAG-3′ | |
| PRLR long-form reverse | 5′-TGTCATCCACTTCCAAGAACTCC-3′ | 133 |
| GAPDH forward | 5′-TGCACCACCAACTGCTTAG-3′ | |
| GAPDH reverse | 5′-GGATGCAGGGATGATGTTC-3′ | 177 |
Murine Skin Organ Culture
C57BL/6 mice (6 to 8 weeks old) were depilated as described above. At day 0 after depilation for the anagen development study and at day 16 after depilation for the catagen experiments, 4 -μm punch biopsies from dorsal back skin were prepared that contained only hair follicles in synchronized late anagen VI. Six skin punches per treatment from two different mice (each experiment) were placed on gelatin sponges in 6-well plates containing Dulbecco’s modified Eagle medium supplemented with fetal calf serum, l-glutamine and antibiotic/anti-mycotic mixture. The skin samples were cultured for 72 hours at 5% CO2 with the addition of two concentrations (200 and 400 ng/ml) of ovine PRL (Sigma, Chemie, Deisenhofen, Germany) The medium was changed at 0, 24, and 48 hours. Normal PRL levels in mice vary between nonpregnant females (30 to 80 ng/ml), pregnant females (150 to 600 ng/ml), and males (5 to 20 ng/ml). 39,40 After culturing, the tissue was fixed in 4% paraformaldehyde, embedded in paraffin, and stained with hematoxylin and eosin (H&E) for quantitative histomorphometry.
Quantitative Histomorphometry and Statistical Analysis
H&E-stained paraffin sections were screened for longitudinal hair follicles. At least 20 follicles per biopsy punch (n = 6) were counted and the hair-cycle stage of each follicle was assessed and classified by morphological criteria and assigned to their respective hair-cycle stages, following our previously published guidelines. 41 The hair-cycle score was assessed for the catagen induction study and calculated as described. 42 Statistical significance was calculated using the Mann-Whitney U-test.
Ki-67 Immunohistochemistry
To evaluate proliferating cells we used our established protocol for Ki-67 immunohistochemistry. 43,44 Cryosections from murine skin organ culture were preincubated with 10% goat serum, followed by incubation with rabbit anti-mouse Ki-67 antiserum 1:100 (Dianova, Hamburg, Germany). To detect Ki-67 immunoreactivity rhodamine-conjugated goat anti-rabbit secondary antibody 1:200 (Jackson ImmunoResearch, Hamburg, Germany) was applied. Sections were then counterstained with 4,6-diamidino-2-phenylindole, 1:5000. Negative controls were made by omitting the primary antibody and positive controls were run by comparison with tissue sections from the back skin of mice in anagen VI stage of the depilation induced hair cycle. Sections were examined under a Zeiss Axioscope microscope. The number of positive cells for Ki-67 immunoreactivity was counted per hair bulb. At least 20 bulbi per biopsy punch (n = 6 per group) were counted and statistical significance was calculated by the Mann-Whitney U-test.
Results
PRL-Like Immunoreactivity Is Found in the Proximal Inner and Outer Root Sheath (ORS) Keratinocyte and Is Hair Cycle-Dependent
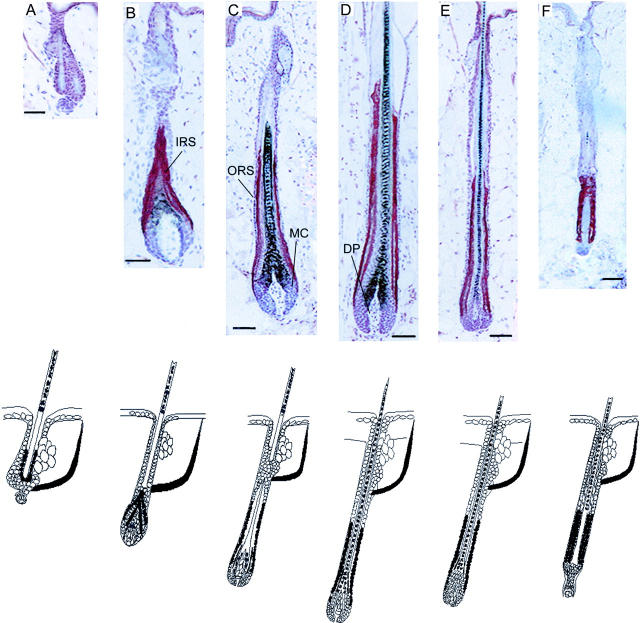
To explore the distribution of PRL protein in the murine hair follicle in relation to the hair cycle, we looked first for PRL expression during the depilation-induced hair cycle. In telogen, PRL-like immunoreactivity was weakly present in ORS keratinocytes (Figure 1A) ▶ .
Figure 1.
PRL-like immunoreactivity during the depilation-induced murine hair cycle was stained by the ABC method using AEC+ (red) as substrate and hematoxylin for counterstaining. A, Telogen; B, anagen III hair follicles; C, anagen IV; D, anagen VI; E, catagen III; and F, catagen VII. MC, germinal matrix cells. Bottom: Schematic representation of immunoreactivity patterns of PRL during the murine hair cycle. Black: PRL expression.
With the development of the inner root sheath (IRS) during early anagen (anagen III), PRL immunoreactivity could be detected in the inner layer of the proximal ORS and the IRS (Figure 1B) ▶ . During later anagen stages (IV to VI), PRL staining extended with hair shaft elongation and could be seen in a restricted area that included the inner layer of the proximal two-thirds of the ORS and lower IRS (Figure 1, C and D) ▶ . This immunoreactivity pattern continued in early catagen follicles (Figure 1E) ▶ . In later catagen stages (VI) after the catagen development had been initiated spontaneously the ORS up to the regressing IRS was strongly stained for PRL (Figure 1F) ▶ . When the hair follicle entered the next telogen phase, PRL expression again was restricted to ORS keratinocytes.
Throughout the hair cycle, the epidermis, individual cells in the dermis, and the arrector pili muscle were positive for PRL, whereas the hair follicle mesenchyme (the DP and connective tissue sheath) as well as the sebaceous gland stayed negative. The epidermis was partially positive during late anagen (Figure 1) ▶ .
PRLR Is Expressed in the ORS and Expression Increases in Anagen and Catagen Hair Follicles
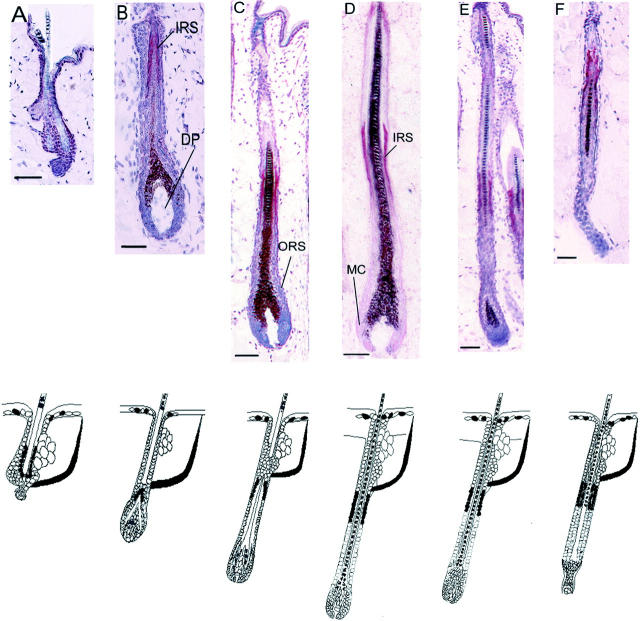
Like PRL, the follicular expression of PRLR-like immunoreactivity was restricted to the hair follicle epithelium and was hair cycle-dependent. In telogen follicles, only very few keratinocytes adjacent to the hair shaft showed PRLR immunoreactivity (Figure 2A) ▶ . Again, the DP, sebaceous gland, and fibroblasts were all negative throughout the cycle, the arrector pili muscles were positive, and the epidermis was slightly positive for PRLR. In early anagen (III to IV), PRLR-like immunoreactivity occurred only around the distal part of the developing IRS (Figure 2B) ▶ . During later anagen stages, a restricted region of the central ORS at a constant distance from the hair bulb became strongly PRLR-positive (Figure 2, C and D) ▶ . Early, spontaneously developed catagen hair follicles primarily showed the same expression pattern as anagen follicles (Figure 2E) ▶ . In late catagen, PRL-R immunoreactivity could still be detected in the ORS, but also in the corresponding area of the IRS (Figure 2F) ▶ . In new telogen follicles, PRLR staining was again seen in ORS keratinocytes.
Figure 2.
PRLR immunoreactivity during the depilation-induced murine hair cycle. A, Telogen; B, anagen III; C, anagen IV; D, anagen VI; E, catagen III; and F, catagen VII. MC, germinal matrix cells. Bottom: Schematic representation of immunoreactivity patterns of PRLR during the murine hair cycle. Black: PRLR expression.
Expression of PRLR Transcripts and Its Ligands Is Hair Cycle-Dependent, but Reciprocal during the Murine Hair Cycle
To determine whether the expression of PRLR transcripts was expressed hair cycle-dependent in the skin we performed PCR on samples collected throughout a depilation-induced murine hair cycle. In addition, we assayed for the RNA of the PRLR ligands PRL and PL1 as further indicators of local production in the skin and variation in their transcription levels.
Transcripts for PRL and PL1 were found in mouse skin samples (Figure 3, A and B) ▶ . PRL and PL exhibited similar expression patterns in the sense that both show a peak in early to mid anagen and decline subsequently. Although PRL transcription increases on day 5 and declines slowly, that of PL1 rose by more than 20-fold on day 3 and rapidly declined again thereafter. They were both up-regulated and declined back to minimum during catagen (Figure 3, A and B) ▶ . PL2 mRNA was not detectable in skin. Another member of this family of proteins, GH, was also detected and showed a similar pattern of rise and fall to that of PRL (data not shown). The most abundant PRLR mRNA was that of the long form, which declined directly after depilation (day 3), recovered by day 8 (anagen V), and returned to initial (telogen) levels by late anagen (anagen VI). Thereafter, PRLR transcripts remained at constant steady-state levels throughout catagen and telogen (Figure 3C) ▶ . Short-form variants of PRLR were extremely rare in skin: short form 1 was undetectable by PCR whereas products for short forms 2 and 3 were amplified at such high cycle numbers that no meaningful pattern of expression could be discerned (data not shown). Thus, the genes for PRLR, PRL, and PL1 are all expressed in murine skin and their transcription is regulated in a hair cycle-dependent manner.
Figure 3.
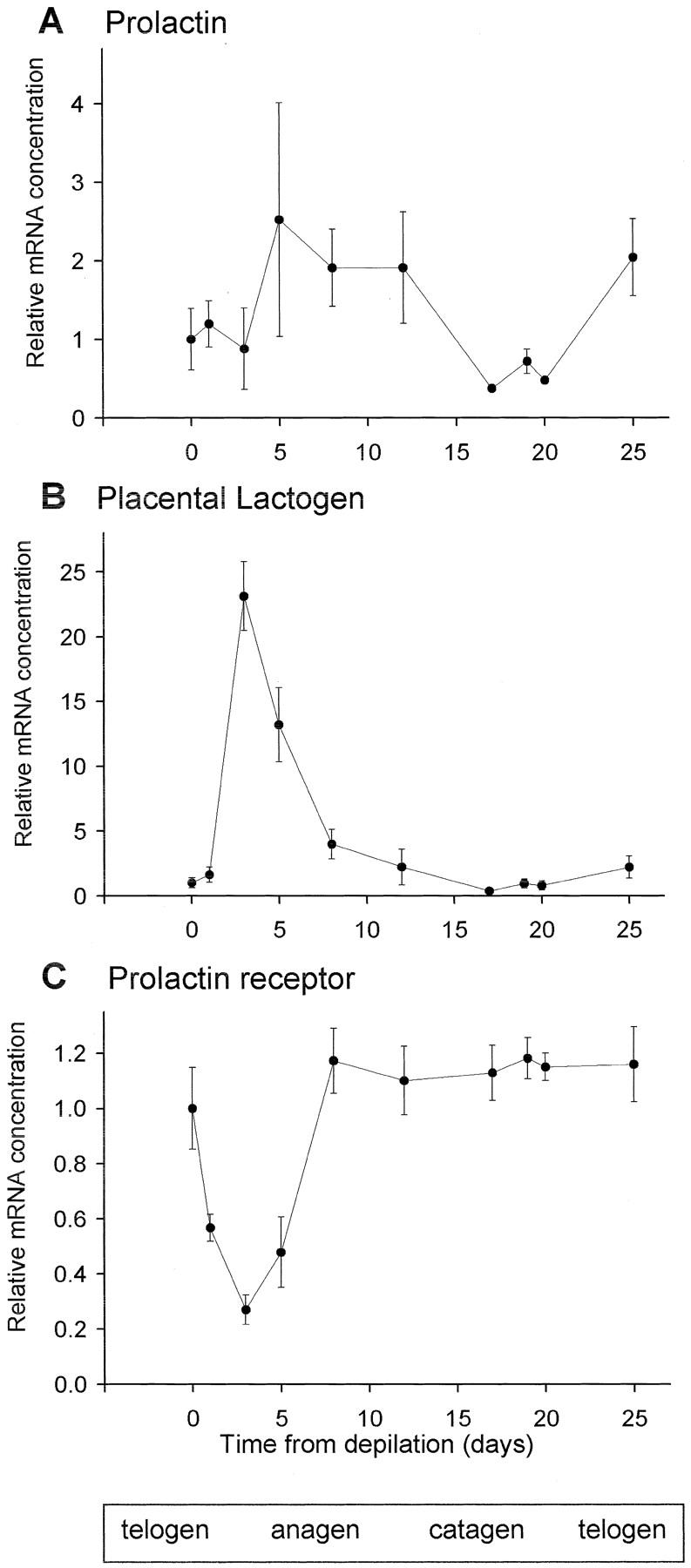
Real-time PCR analysis of expression of PRLRs and their ligands in murine skin. Amplification of product was measured in real time and the cycle number at which the reaction entered exponential phase growth was detected. These values are compared to show relative changes in specific mRNA concentration and normalized to the expression of GAPDH. Each data point is the mean of three mice, except for day 3 (n = 6) and day 5 (n = 8). Error bars are SEM. The major stages of the hair follicle growth cycle are indicated at the bottom.
PRL Induces Premature Catagen Development in Murine Skin Organ Culture
To investigate whether PRL exerts directly growth-modulating effects on the hair follicle, PRL was added to organ-cultured murine skin from C57BL/6 mice 16 days after anagen induction by depilation. The hair follicles in the biopsy samples were thus collected shortly before entry into apoptosis-driven hair follicle regression (catagen). Quantitative histomorphometry revealed that PRL was able to accelerate catagen development in organ-cultured mouse skin, ie, in the absence of systemic influences or functional nerves/vasculature. Although most control follicles were still in late anagen VI/catagen I after 72 hours (Figure 4A) ▶ , the PRL-treated follicles had already entered later catagen stages (Figures 4B and 5A) ▶ . Testing two concentrations of PRL (200 ng/ml and 400 ng/ml) we observed that the addition of 200 ng/ml of PRL resulted in an increase of regressing hair follicles (data not shown), but higher doses (400 ng/ml) were required to show a significant difference between control and test groups (Figure 4 ▶ and Figure 5, A and B ▶ ). We used in our experiments a concentration of 200 ng and 400 ng PRL, because physiological PRL levels in humans are unlikely to be associated with hair growth abnormalities. A supraphysiological dose of PRL was purposely chosen to imitate the increased PRL levels that are seen with effluvium-associated hyperprolactinemia, eg, in patients seen with prolactinoma. 7
Figure 4.
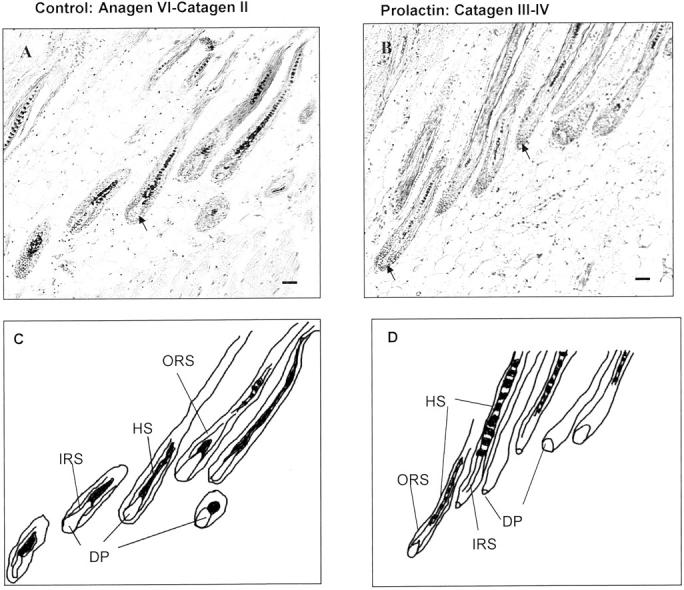
H&E staining for quantitative histomorphometry after 72 hours in culture. A: Control hair follicles in late anagen VI (arrow points to DP) and early catagen II. B: PRL-treated follicles in catagen III to IV (arrowhead points to DP). C: Schematic drawing of control hair follicles mostly in late anagen VI. D: Schematic drawing of PRL (400 ng/ml)-treated hair follicles in catagen III to IV.
Figure 5.
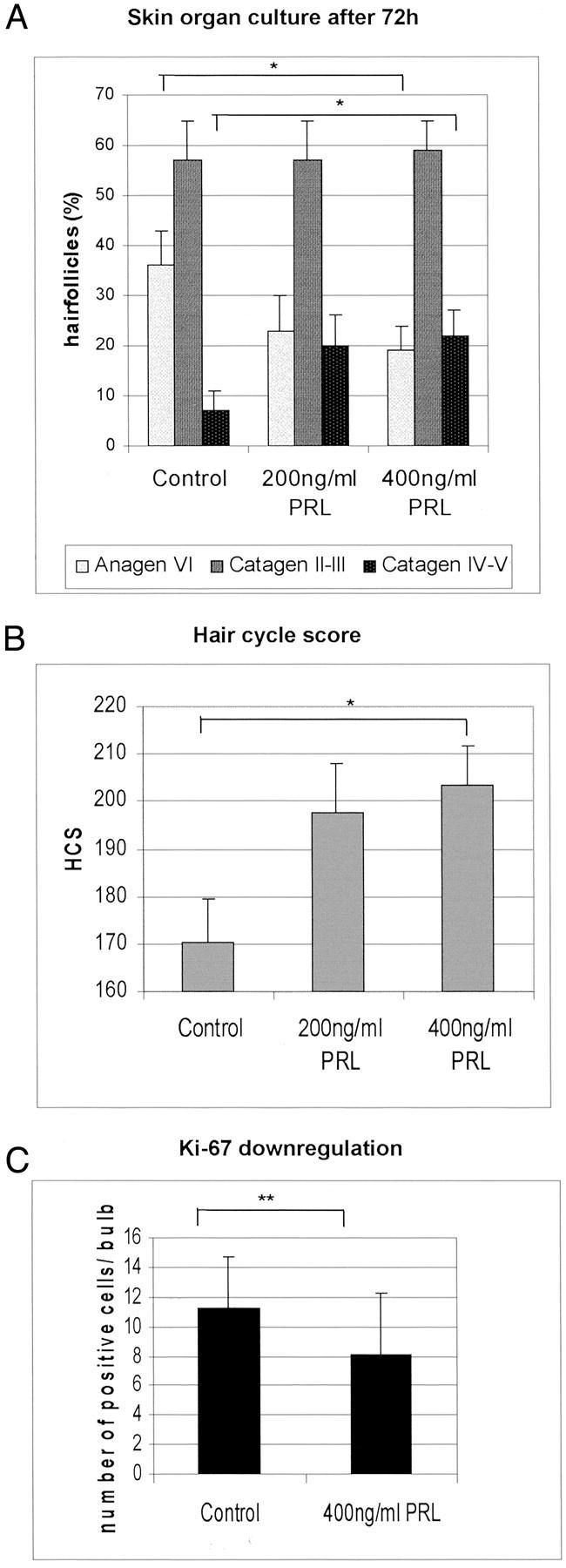
A: The percentage of hair follicles in defined hair-cycle stages were assessed by histomorphometry, and the statistical significance was calculated using the Mann-Whitney U-test: *, P <0.05; **, P <0.01. B: Calculation of the hair-cycle score: all hair follicles of each group were classified and each stage of the hair cycle has been scored as follows: anagen VI/catagen I = 100, catagen II to III = 200, catagen IV to VI = 300. The hair-cycle score indicates the mean of the stages of all hair follicles per group. C: Total mean number of Ki-67+ cells in the hair bulbs after 72 hours of skin organ culture. PRL-treated hair follicles show a down-regulation of proliferating cells.
In skin samples treated with 400 ng/ml of PRL, the number of late anagen/early catagen follicles was reduced and >20% of the follicles had already entered late stages of hair follicle regression (ie, catagen IV to V) compared to only 7% in the control group (Figure 4 ▶ and Figure 5, A and B ▶ ). This was confirmed by calculation of the hair-cycle score, which allows one to quantitatively assess and compare the full range of catagen stages between experimental groups. The hair-cycle score showed the highest values for the high-dose PRL-treated group (400 ng/ml) (Figure 5B) ▶ , and the lowest for control follicles, with 200 ng/ml of PRL-treated follicles occupying an intermediate position (Figure 5B) ▶ . These data revealed that catagen development in PRL-treated hair follicles was significantly advanced compared to follicles treated with vehicle only.
Down-Regulation of Proliferation in Murine Hair Follicles by PRL
After the observation that PRL-treated murine skin organ cultures displayed an acceleration of hair follicles entering catagen stages III to IV compared to the control group (Figure 5A) ▶ a complementary analysis of proliferating cells within the hair bulb was conducted. As Figure 5C ▶ shows, a significant down-regulation of Ki-67-positive cells in the matrix keratinocytes of hair bulbs occurred in the PRL-treated group compared to the control group (P < 0.01).
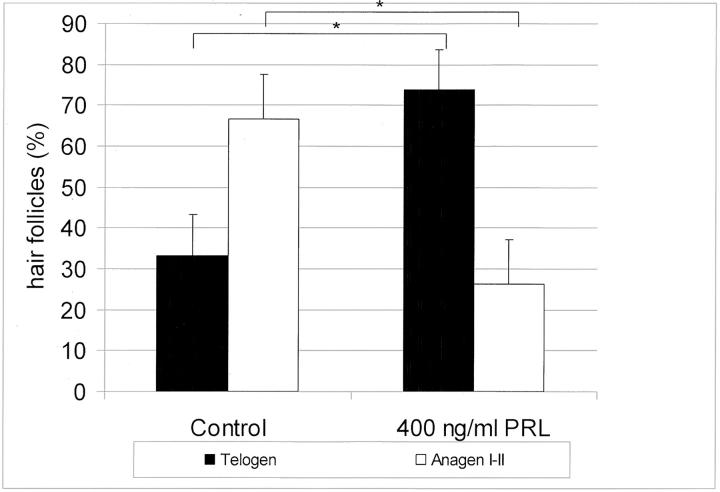
PRL Delays Anagen Development in Murine Skin Organ Culture
To investigate whether PRL exerts also directly growth-modulating effects on anagen development, PRL was added to organ-cultured murine skin from C57BL/6 mice directly (Figure 6) ▶ after anagen induction by depilation. Quantitative histomorphometry revealed that PRL was able to delay anagen development in organ-cultured mouse skin. Although most control follicles had already entered early anagen stages after 72 hours, the PRL-treated follicles were still in telogen (Figure 6) ▶ . Testing two concentrations of PRL (200 ng/ml and 400 ng/ml) we could again observe that addition of 200 ng/ml of PRL resulted in an increase of telogen hair follicles (data not shown), but higher doses (400 ng/ml) were required to show a significant difference between control and test groups (Figure 6) ▶ . Although 40% of PRL (400 ng/ml)-treated hair follicles of skin, harvested directly after depilation, were in early anagen, almost 60% of the follicles stayed in the resting phase (telogen). In contrast, 65% of control hair follicles had already entered anagen, whereas only 35% of control hair follicles were still in telogen.
Figure 6.
Quantitative histomorphometry of hair follicles after 72 hours in culture directly after depilation. The percentage of hair follicles in defined hair-cycle stages were assessed by histomorphometry, and the statistical significance was calculated using Mann-Whitney U-test: *, P <0.05; **, P <0.01.
Discussion
Our data show for the first time that PRL and its receptor are expressed in a hair cycle-dependent manner during the nonseasonal murine hair cycle and that PRL is able to induce premature catagen development in mice by direct effects on skin hair follicles. It had never before been shown that PRL expression in mature mammalian skin is developmentally regulated (namely, ie, hair cycle-dependent). Even in sheep, hair cycle-dependence has only been demonstrated for PRL receptor expression, but not its ligands. The hair cycle in sheep, in contrast to mice, is seasonally dependent and therefore not comparable with the human scalp, which is also primarily seasonally independent. Thus, our study also demonstrates for the first time the expression of PRL and its receptor in a seasonally independent hair cycle. In addition, we show in our functional in vitro essay that PRL is able to induce catagen by a direct action on the skin itself, rather than by up- or down-regulation of other hormones, eg, in ovaries or in the adrenal gland. This provides novel, important hints for further investigation on the effects of PRL on seasonally independent hair follicles (eg, human hair follicles) and supports the hypothesis that PRL acts as an autocrine or paracrine factor in hair follicles as it has already been shown in mammary tissue. 45,46
We further demonstrated that PRL and PRLR are located in the IRS and ORS keratinocytes of anagen and catagen hair follicles of mice. Although PRL protein expression in murine hair follicles has not been previously reported, other studies have shown that PRLR RNA is localized in the epidermis, skin glands, ORS, and DP of ovine anagen hair follicles. 34,35,47 In contrast to studies in sheep, we could detect the PRLR only in follicular epithelial cells and it was notably absent from the DP, connective tissue sheath, and sebaceous gland. These findings were partially consistent with those of Craven and colleagues, 15 who found PRLR staining in the epidermis, sebaceous gland, and ORS of the hair follicle, but not in the DP of murine anagen hair follicles. Our data show increasing expression of the PRL mRNA and protein during anagen corresponding to the increasing length of the IRS with a maximum of expression in the anagen VI-catagen transition.
In addition, the most abundant PRLR mRNA in the skin detected by PCR was that of the long form. This is in line with the study by Ouhtit and colleagues 47 who could detect a higher signal for the long form than for the short form of the PRLR in the skin of rats by in situ hybridization. Short form variants were rare or undetectable, suggesting that they play little, if any, role in modulating PRL signaling in murine skin.
Interestingly, PRLR long-form transcript steady-state levels substantially declined immediately after the start of follicle growth (anagen I) and recovered by day 8 (anagen V). These data confirm our immunohistochemical data that show increasing PRLR protein expression during anagen. It is reasonable to speculate that this down-regulation of PRLR transcripts immediately after anagen induction abrogates the potentially inhibitory action of PRL in early anagen. A subsequent increase of PRLR expression could demonstrate increasing inhibition of keratinocyte proliferation within the hair follicle by PRL, which becomes effective in late anagen.
PRL transcript levels showed a slight peak during early to mid anagen (day 5) and stayed relatively low during the entire hair cycle. The PRLR ligand placental lactogen I was up-regulated on day 3 (anagen III) and rapidly declines again thereafter. Thus, both PRL and placental lactogen I are produced locally in the skin, show a hair cycle-dependent expression, and indicate paracrine or autocrine roles for lactogenic hormones in spontaneous hair growth cycles. But we have to note here, that we cannot exclude with certainty an influence of wounding after depilation on PRL gene expression by PCR and that our current data await confirmation by examination of the spontaneous murine hair cycle. However, our data are supported by our previous findings, in which both anagen VI and catagen terminal hair follicles display the strongest immunoreactivity for PRL in normal, unmanipulated human scalp skin. 48 This is consistent with the concept that hair follicle entry into the cycle is associated with an up-regulation of PRL gene expression, as observed during the murine depilation-induced hair cycle (in which up-regulation of PRL transcription would be expected to precede the increase in immunoreactivity of the protein product).
The PRL gene has been recently been reported not to be expressed in human skin. 49 However, contrary to this report, we have most recently been able to show that even human skin and human scalp hair follicle do transcribe the PRL gene and that PRL also induces premature catagen development in organ-cultured human scalp hair follicles. 48 This is consistent with the concept that hair follicle entry into the cycle is associated with an up-regulation of PRL gene expression, as observed during the murine depilation-induced hair cycle (in which up-regulation of PRL transcription would be expected to precede the increase in immunoreactivity of the protein product). Our data are an important correction of the literature and support the relevance of the current findings from the murine system for human skin and human hair follicles.
Both PRL gene expression and PRL protein expression increased during anagen. The reciprocal expression of PRL and PRLR transcripts during the hair cycle may indicate a negative feedback loop. This hypothesis is supported by detection of abnormally high PRL levels in PRLR-null mutations. 36
Hair replacement was advanced in the knockout mice by 4 days in male mice compared to 4 weeks in female PRLR-null mutations. 15 Because the female PRLR knockout mice show also a decreased estradiol level in the blood compared to control mice it cannot be excluded that this advancement is because of the removal of estradiol, a powerful inhibitor of hair growth. 50 However, our functional data show that treatment of high-dose PRL results in premature catagen development in murine skin organ culture suggesting direct catagen-inductive activity of PRL. The catagen induction is accompanied by down-regulation of proliferation in hair follicle keratinocytes. PRL seems to function by inhibition of keratinocyte proliferation rather than by induction of terminal differentiation, because in serum-free keratinocyte cultures, PRL does not possess mitogenic activity and, although the PRL-receptor was expressed in differentiated keratinocytes, PRL did not affect differentiation markers. 33 It has recently been reported that several genes, involved in intracellular signaling, were down-regulated in PRL-induced telogen hair follicles compared to anagen follicles. 51 Thus, we cannot exclude that PRL acts in addition by regulation of genes, which are so far unknown to be involved in the regulation of hair follicle cycling. PRLR-null mutations have slightly longer and coarser hair compared to wild-type mice, which may be because of either a prolonged anagen phase or increased proliferation of follicular keratinocytes. Our data demonstrating down-regulation of keratinocyte proliferation by PRL suggest the second mechanism. In addition, our results are in line with previous studies, in which administration of PRL, either systemically or locally, in the skin have been shown to induce catagen in sheep. 10
In summary, PRL and its receptor are expressed in a hair cycle-dependent manner in murine skin and addition of PRL to murine anagen hair follicles induces catagen by down-regulation of proliferation in follicular keratinocytes. Disruption of the PRLR shortens the telogen phase of the murine hair cycle and advances the anagen phase of murine hair follicles. 15 These data support our hypothesis that PRL has a direct local inhibitory effect on nonseasonal hair follicles. Thus, PRLR ligands deserve to be explored as potential therapeutic agents for PRL-induced androgenetic alopecia in women.
Acknowledgments
We thank Gundula Pilnitz-Stolze, Marion Woodcock, and Silvia Wegerich for their excellent technical support.
Footnotes
Address reprint requests to Ralf Paus, M.D., Dept. of Dermatology, University Hospital Eppendorf, University of Hamburg, Martinistr.52, D-20246 Hamburg, Germany. E-mail: paus@uke.uni-hamburg.de.
Supported in part by grants from Cutech Srl, Padova (to R.P.).
References
- 1.Paus R, Cotsarelis G: The biology of hair follicles. N Engl J Med 1999, 341:491-497 [DOI] [PubMed] [Google Scholar]
- 2.Stenn KS, Paus R: Controls of hair follicle cycling. Physiol Rev 2001, 81:449-494 [DOI] [PubMed] [Google Scholar]
- 3.Cotsarelis G, Millar SE: Towards a molecular understanding of hair loss and its treatment. Trends Mol Med 2001, 7:293-301 [DOI] [PubMed] [Google Scholar]
- 4.Paus R, Botchkarev VA, Botchkareva NV, Mecklenburg L, Luger T, Slominski A: The skin POMC system (SPS). Leads and lessons from the hair follicle. Ann NY Acad Sci 1999, 885:350-363 [DOI] [PubMed] [Google Scholar]
- 5.Paus R, Muller-Rover S, Botchkarev VA: Chronobiology of the hair follicle: hunting the “hair cycle clock.” J Invest Dermatol Symp Proc 1999, 4:338-345 [DOI] [PubMed] [Google Scholar]
- 6.Hoffmann R: Enzymology of the hair follicle. Eur J Dermatol 2001, 11:296-300 [PubMed] [Google Scholar]
- 7.Freeman ME, Kanyicska B, Lerant A, Nagy G: Prolactin: structure, function, and regulation of secretion. Physiol Rev 2000, 80:1523-1631 [DOI] [PubMed] [Google Scholar]
- 8.Paus R: Does prolactin play a role in skin biology and pathology? Med Hypotheses 1991, 36:33-42 [DOI] [PubMed] [Google Scholar]
- 9.Thomas DG, Loudon ASI, Jabbour HN: Local infusion of prolactin stimulates early localised development of red deer (Cervus elaphus) summer coat. J Endocrinol 1994, 143:P44 [Google Scholar]
- 10.Pearson AJ, Ashby MG, Wildermoth JE, Craven AJ, Nixon AJ: Effect of exogenous prolactin on the hair growth cycle. Exp Dermatol 1999, 8:358-360 [PubMed] [Google Scholar]
- 11.Martinet L, Allain D, Weiner C: Role of prolactin in the photoperiodic control of moulting in the mink (Mustela vison). J Endocrinol 1984, 103:9-15 [DOI] [PubMed] [Google Scholar]
- 12.Duncan MJ, Goldman BD: Hormonal regulation of the annual pelage color cycle in the Dungarian hamster, Phodopus sungorus II. Role of prolactin. J Exp Zool 1984, 230:97-103 [DOI] [PubMed] [Google Scholar]
- 13.Rougeot J, Allain D, Martinet L: Photoperiodic and hormonal control of seasonal coat changes in mammals with special reference to sheep and mink. Acta Zoologica Fennica 1984, 171:13-18 [Google Scholar]
- 14.Rose J, Oldfield J, Stormshak F: Apparent role of melatonin and prolactin in initiating winter fur growth in mink. Gen Comp Endocrinol 1987, 65:212-215 [DOI] [PubMed] [Google Scholar]
- 15.Craven AJ, Ormandy CJ, Robertson FG, Wilkins RJ, Kelly PA, Nixon AJ, Pearson AJ: Prolactin signaling influences the timing mechanism of the hair follicle: analysis of hair growth cycles in prolactin receptor knockout mice. Endocrinology 2001, 142:2533-2539 [DOI] [PubMed] [Google Scholar]
- 16.Dicks P: The role of prolactin and melatonin in regulating the timing of spring moult in the cashmere goat. Laker JP Allain D eds. Hormonal Control of Fibre Growth and Shedding. 1994:pp 109-127 European Fine Fibre Network, Aberdeen European Fine Fibre Network Occasional Publication No. 2.
- 17.Ibraheem M, Galbraith H, Scaife J, Ewen S: Growth of secondary hair follicles of the cashmere goat in vitro and their response to prolactin and melatonin. J Anat 1994, 185:135-142 [PMC free article] [PubMed] [Google Scholar]
- 18.Pearson AJ, Parry AL, Ashby MG, Choy VJ, Wildermoth JE, Craven AJ: Inhibitory effect of increased photoperiod on wool follicle growth. J Endocrinol 1996, 148:157-166 [DOI] [PubMed] [Google Scholar]
- 19.Nixon AJ, Ford CA, Wildermoth JE, Craven AJ, Pearson AJ: Regulation of prolactin receptor expression in ovine skin in relation to circulating prolactin and wool follicle growth status. J Endocrinol 2002, 172:605-614 [DOI] [PubMed] [Google Scholar]
- 20.Allain D, Thébault RG, Rougeot J, Martinet L: Biology of fibre growth in mammals producing fine fibre and fur in relation to control by day length: relationship with other seasonal functions. Laker JP Allain D eds. Hormonal Control of Fibre Growth and Shedding. 1994:pp 23-40 European Fine Fibre Network, Aberdeen European Fine Fibre Network Occasional Publication No. 2.
- 21.Moltz L: Hormonal diagnosis in so-called androgenetic alopecia in the female. Geburtshilfe und Frauenheilkunde 1988, 48:203-214 [DOI] [PubMed] [Google Scholar]
- 22.Schmidt JB: Hormonal basis of male and female androgenic alopecia: clinical relevance. Skin Pharmacol 1994, 7:61-66 [DOI] [PubMed] [Google Scholar]
- 23.Schmidt JB, Lindmaier A, Trenz A, Schurz B, Spona J: Hormone studies in females with androgenic hair loss. Gynecol Obstet Invest 1991, 31:235-239 [DOI] [PubMed] [Google Scholar]
- 24.Serafini P, Lobo RA: Prolactin modulates peripheral androgen metabolism. Fertil Steril 1986, 45:41-46 [PubMed] [Google Scholar]
- 25.Fabre N, Montastruc JL, Rascol O: Alopecia: an adverse effect of bromocriptine. Clin Neuropharmacol 1993, 16:266-268 [PubMed] [Google Scholar]
- 26.Blum I, Leiba S: Increased hair loss as the side-effect of bromocriptine treatment. N Engl J Med 1980, 303:1418. [DOI] [PubMed] [Google Scholar]
- 27.Sinclair RD, Banfield CC, Dawber RPR: Handbook of Diseases of the Hair and Scalp. 1999. Blackwell Science, London
- 28.Kelly PA, Binart N, Freemark M, Lucas B, Goffin V, Bouchard B: Prolactin receptor signal transduction pathways and actions determined in prolactin receptor knockout mice. Biochem Soc Trans 2001, 29:48-52 [DOI] [PubMed] [Google Scholar]
- 29.Ormandy CJ, Binart N, Helloco C, Kelly PA: Mouse prolactin receptor gene: genomic organization reveals alternative promoter usage and generation of isoforms via alternative 3′-exon splicing. DNA Cell Biol 1998, 17:761-770 [DOI] [PubMed] [Google Scholar]
- 30.Goffin V, Shiverick KT, Kelly PA, Martial JA: Sequence-function relationships within the expanding family of prolactin, growth hormone, placental lactogen, and related proteins in mammals. Endocr Rev 1996, 17:385-410 [DOI] [PubMed] [Google Scholar]
- 31.MacLeod KR, Smith WC, Ogren L, Talamantes F: Recombinant mouse placental lactogen-I binds to lactogen receptors in mouse liver and ovary: partial characterization of the ovarian receptor. Endocrinology 1989, 125:2258-2266 [DOI] [PubMed] [Google Scholar]
- 32.Byatt JC, Staten NR, Salsgiver WJ, Kostelc JG, Collier RJ: Stimulation of food intake and weight gain in mature female rats by bovine prolactin and bovine growth hormone. Am J Physiol 1993, 264:E986-E992 [DOI] [PubMed] [Google Scholar]
- 33.Poumay Y, Jolivet G, Pittelkow MR, Herphelin F, De Potter IY, Mitev V, Houdebine LM: Human epidermal keratinocytes upregulate expression of the prolactin receptor after the onset of terminal differentiation, but do not respond to prolactin. Arch Biochem Biophys 1999, 364:247-253 [DOI] [PubMed] [Google Scholar]
- 34.Choy VJ, Nixon AJ, Pearson AJ: Localisation of receptors for prolactin in ovine skin. J Endocrinol 1995, 144:143-151 [DOI] [PubMed] [Google Scholar]
- 35.Choy VJ, Nixon AJ, Pearson AJ: Distribution of prolactin receptor immunoreactivity in ovine skin and during the wool follicle growth cycle. J Endocrinol 1997, 155:265-275 [DOI] [PubMed] [Google Scholar]
- 36.Clement-Lacroix P, Ormandy C, Lepescheux L, Ammann P, Damotte D, Goffin V, Bouchard B, Amling M, Gaillard-Kelly M, Binart N, Baron R, Kelly PA: Osteoblasts are a new target for prolactin: analysis of bone formation in prolactin receptor knockout mice. Endocrinology 1999, 140:96-105 [DOI] [PubMed] [Google Scholar]
- 37.Paus R, Hofmann U, Eichmueller S, Czarnetzki BM: Distribution and changing density of gamma-delta T cells in murine skin during the induced hair cycle. Br J Dermatol 1994, 130:281-289 [DOI] [PubMed] [Google Scholar]
- 38.Paus R, Muller-Rover S, Van Der Veen C, Maurer M, Eichmuller S, Ling G, Hofmann U, Foitzik K, Mecklenburg L, Handjiski B: A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J Invest Dermatol 1999, 113:523-532 [DOI] [PubMed] [Google Scholar]
- 39.Sinha YN: Molecular size variants of prolactin and growth hormone in mouse serum: strain differences and alterations of concentrations by physiological and pharmacological stimuli. Endocrinology 1980, 107:1959-1969 [DOI] [PubMed] [Google Scholar]
- 40.Gee DM, Flurkey K, Mobbs CV, Sinha YN, Finch CE: The regulation of luteinizing hormone and prolactin in C57BL/6J mice: effects of estradiol implant size, duration of ovariectomy, and aging. Endocrinology 1984, 114:685-693 [DOI] [PubMed] [Google Scholar]
- 41.Muller-Rover S, Handjiski B, van der Veen C, Eichmuller S, Foitzik K, McKay IA, Stenn KS, Paus R: A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol 2001, 117:3-15 [DOI] [PubMed] [Google Scholar]
- 42.Maurer M, Handjiski B, Paus R: Hair growth modulation by topical immunophilin ligands: induction of anagen, inhibition of massive catagen development, and relative protection from chemotherapy-induced alopecia. Am J Pathol 1997, 150:1433-1441 [PMC free article] [PubMed] [Google Scholar]
- 43.Lindner G, Botchkarev VA, Botchkareva NV, Ling G, van der Veen C, Paus R: Analysis of apoptosis during hair follicle regression (catagen). Am J Pathol 1997, 151:1601-1617 [PMC free article] [PubMed] [Google Scholar]
- 44.Foitzik K, Lindner G, Mueller-Roever S, Maurer M, Botchkareva N, Botchkarev V, Handjiski B, Metz M, Hibino T, Soma T, Dotto GP, Paus R: Control of murine hair follicle regression (catagen) by TGF-beta1 in vivo. EMBO J 2000, 14:752-760 [DOI] [PubMed] [Google Scholar]
- 45.Clevenger CV, Plank TL: Prolactin as an autocrine/paracrine factor in breast tissue. J Mammary Gland Biol Neoplasia 1997, 2:59-68 [DOI] [PubMed] [Google Scholar]
- 46.Ginsburg E, Vonderhaar BK: Prolactin synthesis and secretion by human breast cancer cells. Cancer Res 1995, 55:2591-2595 [PubMed] [Google Scholar]
- 47.Ouhtit A, Morel G, Kelly PA: Visualization of gene expression of short and long forms of prolactin receptor in the rat. Endocrinology 1993, 133:135-144 [DOI] [PubMed] [Google Scholar]
- 48.Krause K, Paus R, Nakamura H, Foitzik K: Expression of prolactin mRNA and protein in human skin and its role in catagen control of human hair follicles. Arch Dermatol Res 2002, 65:201294 [Google Scholar]
- 49.Slominski A, Malarkey WB, Wortsman J, Asa SL, Carlson A: Human skin expresses growth hormone but not the prolactin gene. J Lab Clin Med 2000, 136:476-481 [DOI] [PubMed] [Google Scholar]
- 50.Oh H-S, Smart RC: An estrogen receptor pathway regulates the telogen-anagen hair follicle transition and influences epidermal cell proliferation. Proc Natl Acad Sci USA 1996, 93:12525-12530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rufaut WR, Pearson AJ, Nixon AJ, Wheeler TT, Wilkins RJ: Identification of differentially expressed genes during a wool follicle growth cycle induced by prolactin. J Invest Dermatol 1999, 113:865-872 [DOI] [PubMed] [Google Scholar]