Abstract
Within the human prostate epithelium four cell populations can be discriminated based on their expression of keratins (K). Basal cells express high levels of K5 and K14, as well as p63, whereas they have very low levels of androgen receptor, prostate-specific antigen (PSA), K8, and K18. Luminal secretory cells lack p63, K5, and K14 but express high levels of K8, K18, androgen receptor, and PSA. Additionally, cells have been identified with a keratin phenotype intermediate between basal and luminal cells that co-express high levels of K5 and K18 (K5/18) as well as hepatocyte growth factor receptor c-MET. Although intermediate cells have been proposed as precursor cells of prostate cancer, their biology is ill defined. Epithelial cells in proliferative inflammatory atrophy (PIA) appear to be cycling rapidly as indicated by expression of Ki-67, and morphological transitions have been identified between PIA and high-grade prostate intraepithelial neoplasia. Many of the atrophic epithelial luminal cells in PIA are candidates for intermediate cells based in part on weak expression of PSA and androgen receptor, high levels of K8/18, and lack of p63. The objective of this study was to further clarify the phenotype of the proposed intermediate cells in PIA and to quantitatively determine the level in which these intermediate cells preferentially occur in PIA lesions. Intermediate cells were immunohistochemically demonstrated using antibodies to K5, K14, K18, and c-MET. Using radical prostatectomy specimens (n = 15) the area fraction of intermediate cells in normally differentiated prostate epithelium and PIA were quantified by a grid point counting method. Atrophic luminal cells of PIA lesions expressed K5 in 39.2 ± 7.4% of cells compared to 2.4 ± 2.3% in normal epithelium (P < 0.00001). By contrast, K14 was only expressed in 3.0 ± 3.2% of the luminal cells. Previous studies have shown that virtually 100% of these atrophic luminal cells are strongly positive for K8/18. c-MET was present in 44.1 ± 14.1% of luminal cells in PIA but only in 2.1 ± 2.8% of luminal cells in normal epithelium (P < 0.00001). To unambiguously determine whether intermediate luminal cells in PIA show increased proliferative activity and decreased p27kip1 expression, double-staining immunofluorescence of Ki-67 and K5, as well as p27Kip1 and K5 was performed. Luminal cells in PIA often co-expressed K5 and Ki-67. Although p27Kip1 was strongly expressed in K5-negative differentiated cells in normal epithelium, p27Kip1 staining was absent in many of the K5-positive cells in the luminal compartment of PIA. We conclude that cells phenotypically intermediate between basal and secretory cells are enriched in PIA lesions. The finding of a large number of highly proliferating intermediate cells in PIA provides further support that these cells may serve as preferred target cells in prostate carcinogenesis.
The phenotypic identification and cell biological characterization of epithelial stem cells has gained increasing interest in the study of human malignancies. Within rapidly proliferating tissues such as bone marrow, intestinal tract, and squamous epithelia, cell turn-over is governed by stem cells. 1,2 Stem cells are generally characterized by a relatively undifferentiated state while they have high proliferative capacity and a long life-span. After mitosis, they give rise to an intermediate cell group that amplifies the divisions of the stem cells by undergoing additional rounds of cell division. The intermediate cells then mature toward terminally differentiated cells that have very limited or no proliferative capacity.
Within the prostate epithelium basal and luminal cells can be distinguished based on their localization, morphology, and their phenotypic characteristics. Luminal secretory cells express high levels of androgen-receptor, prostate-specific antigen (PSA), and keratins (K) 8 and 18 (K8++/18++). Basal cells specifically display high expression of p63, BCL-2, epidermal growth factor receptor, K5, and K14 (K5++/14++) while they contain low levels of androgen receptor, K8 and K18. 3,4 Two opposing stem cell models have been put forth in the development and maintenance of the prostate epithelium. Cell-kinetic studies in developing rats and analysis of proliferation in humans undergoing androgen-deprivation therapy have indicated that both basal and luminal cells are independently capable of self-renewal, suggesting distinct histogenic origins. 5-7 Another model proposes that a subgroup of basal cells represents a stem cell population of the entire prostate epithelium. Independent of androgens, these stem cells give rise to intermediate cells that transiently proliferate and translocate toward the luminal cell compartment. Terminal differentiation finally contributes to the development of a large cohort of androgen-dependent luminal cells. 8 Candidates for the amplifying cell population have been identified within the prostate epithelium that are characterized by an intermediate phenotype in between basal and luminal cells. Firstly, in addition to the vast majority of basal cells with the K5++/14++/18+ phenotype, cells highly expressing K5 without K14 (K5++/18+) have been identified within the basal cell compartment (intermediate basal cells). The second intermediate cell type is characterized by co-localization of K18 and the basal cell marker K5 (K5+/18++) in the luminal layer (intermediate luminal cells). 9-11 Although fully differentiated prostate epithelial cells expressing high levels of PSA and androgen receptor have not been produced as yet in vitro, human cell culture studies have indicated that basal cells (K5++/14++/18+) can be progenitors of the intermediate (K5/18) as well as luminal epithelium (K18++) that express low levels of PSA. 10,11 Although several phenotypic differences have been described in basal and luminal cells, only a few studies have focused on the protein expression profile in the intermediate cell population. Cells with a morphologically intermediate position within multilayered prostate epithelium lack expression of the cell cycle-dependent kinase inhibitor p27kip1. 12 Furthermore, we demonstrated that immunophenotypically intermediate cells within the luminal prostate epithelium highly express the hepatocyte growth factor receptor c-MET, which is involved in cell motility, morphogenesis, tissue regeneration, and malignant transformation. 13 As the intermediate cell population was suggested to represent amplifying cells modulating the expansion and development of the prostate epithelium, it is pivotal to determine whether intermediate cells reveal proliferative activity. Approximately 70% of all proliferative activity in the human prostate is encountered within the basal cell layer. 14 Interestingly, increased proliferation has been observed in atrophic glandular epithelium in the prostate. 15-18 As glandular atrophy is often associated with chronic inflammation, the term proliferative inflammatory atrophy (PIA) was proposed for these lesions. 18 In that study, there was evidence that the atrophic luminal cells had an intermediate phenotype in that many cells expressed bcl-2 (normally a basal cell marker, in the prostate), and virtually all of the cells expressed high levels of keratins 8/18, as shown by staining with the CAM 5.2 monoclonal antibody. 18 Many of the atrophic luminal cells also expressed low to moderate levels of androgen receptor, and low to moderate levels of PSA indicative of at least partial secretory luminal cell differentiation. 18 In the present study we provide quantitative evidence that intermediate cells are indeed highly enriched in PIA lesions by analysis of various keratins and c-MET expression. In addition, we use tyramide-enhanced double-label immunofluorescence to unambiguously demonstrate that many of the intermediate luminal cells express the proliferation marker Ki-67 but not the cell cycle-dependent kinase inhibitor p27kip1.
Materials and Methods
Patient Samples
All patient samples were obtained from archival tissue blocks obtained from radical prostatectomy specimens from patients with clinically localized prostate cancer. All specimens were fixed in 10% neutral buffered formalin before routine processing into paraffin blocks. All tissue samples were obtained with appropriate informed consent. Representative prostate sections were selected that contained PIA in routine hematoxylin and eosin (H&E)-stained sections. Staining for K5 and K14 were performed on samples from 14 patients from the Johns Hopkins Hospital. Patient ages ranged from 49 to 66 years. Preoperative PSA ranged from 1.3 to 9.5 ng/ml. Gleason score ranged from 6 to 8. Pathological stage ranged from T2N0Mx to T3aN0Mx. Staining for c-MET was performed on samples from 15 patients from University Medical Center ‘St. Radboud,’ Nijmegen, The Netherlands. Patient ages ranged from 49 to 66 years. Preoperative PSA ranged from 0.8 to 21 ng/ml. Gleason score ranged from 5 to 8. Pathological stage ranged from pT2N0Mx to pT3bN0Mx.
Immunohistochemistry
Single Label Staining for c-MET
Sections (4 μm) of paraffin-embedded tissue were dewaxed and rehydrated using xylene and ethanol, respectively. After immersion in 10 mmol/L of citrate buffer (pH 6.0) the slides were subjected to microwave irradiation (600 W) for 4 minutes. Endogenous peroxidase activity was quenched for 30 minutes with 40% methanol containing 0.6% H2O2. After preincubation with 10% normal swine serum for 10 minutes, the slides were incubated with a dilution of 1:250 rabbit anti-human c-MET antibody (C-28; Santa Cruz Biotechnology, Santa Cruz, CA) for 1 hour at 37°C. The slides were washed in phosphate-buffered saline (PBS) for 3 × 5 minutes, followed by incubation with biotinylated donkey anti-rabbit (1:200) antibody for 30 minutes, and conjugation with 1:100 avidin-biotin-peroxidase complex (Vectastain; Vector Laboratories, Burlingame, CA) for 30 minutes. Chromogenic development resulted from incubation of slides with 0.76% (w/v) 3,3′-diaminobenzidine tetrahydrochloride containing 0.00075% (v/v) H2O2 for 10 minutes, and was enhanced after exposure to a dilution of 0.5% CuSO4/0.9% NaCl for 5 minutes. All slides were counterstained with hematoxylin. Antibodies were diluted in PBS containing 5% bovine serum albumin and incubations were performed in the dark at room temperature unless stated otherwise.
Single Label Staining for Keratin 5
Dewaxed and rehydrated tissue sections (4 μm) were immersed in Antigen Unmasking Solution (Vector, Laboratories) and steamed at 98°C (Black and Decker, Towson, MD) for 14 minutes. Slides were preincubated with PBS containing Tween-20 (PBST) for 5 minutes, and were quenched for endogenous peroxidase using 3% hydrogen peroxide in dH2O for 7 minutes. After rinsing twice in PBST using a wash bottle, slides were incubated with a dilution of 1:15,000 of rabbit anti-mouse keratin 5 (no. AF138; Covance Research Products, Denver, PA) for 45 minutes at room temperature. After rinsing twice in PBST, slides were incubated with the universal biotinylated secondary antibody (Vectastain Elite kit, Vector Laboratories, Burlingame, CA), diluted 1:1000 in PBST with normal serum added for 30 minutes. Avidin-biotin complex-horseradish peroxidase (ABC-HRP) (Vectastain Elite kit) was applied for 30 minutes. Slides were then rinsed twice and 3,3′-diaminobenzidine tetrahydrochloride substrate (Vector Laboratories) was applied for 5 minutes. Slides were then rinsed twice and counterstained with hematoxylin.
Single Label Staining for Keratin 14
Sections were subjected to antigen retrieval (steaming) and quenching of peroxidase as for keratin 5. Slides were rinsed twice with PBS and were incubated in protein blocker (ChemMate Secondary Detection kit; Ventana Medical Systems, Tucson, AZ) for 5 minutes. After rinsing twice, sections were incubated with the mouse monoclonal anti-human keratin 14 antibody (1:100: Innogenex) for 45 minutes at room temperature. Slides were rinsed twice and then incubated for 30 minutes in 3Ab-AB2 biotinylated secondary antibody. Slides were rinsed twice and then incubated in ABC-HRP for 30 minutes. Slides were rinsed twice and incubated with 3,3′-diaminobenzidine tetrahydrochloride substrate according to ChemMate instructions for 5 minutes. Slides were then rinsed twice and counterstained with hematoxylin.
Immunofluorescence Double Labeling for Ki-67/Keratin 5 and p27Kip1/Keratin 5
Sections from specimens obtained from the Johns Hopkins Hospital were subjected to antigen retrieval (steaming) and endogenous peroxide block as above for keratin 5. Slides were treated with the DAKO Biotin Blocking System (DAKO, Carpinteria, CA) as per the manufacturer’s protocol. After rinsing twice in PBST, slides were treated with Protein Block no. 2 from the DAKO Catalyzed Signal Amplification (CSA) for 10 minutes. Slides were rinsed twice and reacted with either a 1:5000 dilution of mouse monoclonal antibody anti-p27kip1 (Transduction Labs, San Diego, CA) or a 1:100 dilution of mouse anti-human Ki-67 (Zymed Laboratories, South San Francisco, CA) in antibody dilution buffer (Ventana Medical Systems, Tucson, AZ) containing 1:15,000 diluted rabbit polyclonal anti-keratin 5 (cker5; BabCO, Richmond, CA) overnight at 4°C. After washing in PBST for 5 minutes, slides were incubated with the biotinylated secondary anti-mouse antibody (for detection of Ki-67 or p27kip1) from the CSA kit for 5 minutes. After rinsing twice, slides were incubated in strepavidin-peroxidase (CSA kit) for 15 minutes at room temperature, followed by rinsing and incubation with biotinyl tyramide (CSA kit) diluted 1:10 in protein block for 15 minutes. Slides were incubated with strepavidin Alexa Fluor 568 diluted 1:100 (to localize Ki-67 or p27Kip1) mixed with goat anti-rabbit antibody conjugated directly to Alexa Fluor 488 (Molecular Probes, Eugene, OR) diluted 1:50 (to localize keratin 5) in Dulbecco’s PBS. Slides were rinsed twice and stained with DAPI (Sigma Chemical Co., St. Louis, MO) diluted 1:3000 in dH2O. Slides were mounted with Gelmount (Biomeda, Foster City, CA).
Fluorescence Microscopy
Slides were imaged with a Zeiss Axioskop epifluorescence microscope equipped with short arc mercury lamp illumination (Carl Zeiss Inc., Thornwood, NY) and appropriate fluorescence excitation/emission filters. Fluorescent images were captured with a cooled charge-coupled device camera (Micro MAX digital camera; Princeton Instruments, Trenton, NJ) using IPLab Software (Scanalytics Inc., Fairfax, VA).
Quantification and Statistical Analysis
Expression of K5, K14, and c-MET was quantified within the luminal cell compartment of normal appearing, secretory epithelium and PIA. To obtain an area fraction of epithelial cell staining, a quantitative stereological point counting method was used using a microscope objective eyepiece equipped with a Weibel type I stereological graticule. 19-22 The grid consists of a series of short lines arranged in rows with the ends of the lines forming an equilateral triangular network. A total of 90 luminal cells were scored in each slide using an Olympus BX-40 light microscope as previously reported. 23,24 In this method, the region of interest is delineated on the slide with an ink pen. Within this area, a field is selected at random to start counting, and then one traverses each additional microscopic field within the area systematically. The observer scores only the cells on which the grid point lands, and thus the method is considered nonbiased. Using a ×20 objective, each field encompasses ∼0.9 mm squared. Thus, in each field up to many individual glands are sampled (between 5 to 25 glands are generally present encompassing hundreds to more than 1000 epithelial cells). Because the total number of grid tips in this field is 30, at least four of these fields are scored. Because the grid points often land on stroma or lumen, ∼30 fields are scored to obtain 90 cells. Because many of these lesions do not encompass 30 separate fields, the same fields can be sampled more than once because the grid points will not land on the same spots on subsequent samplings. In previous studies using this approach, we have shown that counting as few as 30 events in standard slides of human prostate tissue can yield highly statistically significant data for immunohistochemistry counting and that there is high intra- and interobserver reproducibility. 23,25 The percentages of positive cells in PIA and normal epithelium were statistically analyzed using a Student’s t-test. A probability value (P) of 0.05 or less was considered significant.
Results
Morphology of PIA Lesions
PIA lesions encompass simple atrophy and the various forms of postatrophic hyperplasia. 18 The significance of including both simple atrophy and postatrophic hyperplasia in the definition of PIA is that these lesions often occur together, both usually have associated chronic and/or acute inflammation, and both have an increased proliferative fraction. 17,18 In terms of the requirement for inflammatory cells in PIA, the vast majority of all focal atrophy lesions contain at least some increase in chronic and/or acute inflammation. Also, because the amount of inflammation from field to field within a given atrophy lesion can be highly variable we now submit that to refer to a lesion as PIA does not require easily recognizable inflammation; essentially all forms of focal glandular atrophy are considered PIA.
The lesions were generally composed of a two-layered epithelium consisting of flat basal cells and cubical to low-columnar luminal cells; this is opposed to high-columnar cells that are characteristic of normal prostate epithelium (Figure 1, A and C) ▶ . Luminal cells in PIA had scant basophilic cytoplasm and generally contained small round to oval nuclei localized in the center of the cell with inconspicuous to slightly enlarged nucleoli (Figure 1B) ▶ , whereas corresponding cells in normal epithelium had abundant pale cytoplasm, and basally localized nuclei without enlarged nucleoli. In contrast to normal epithelium, the epithelial lining in PIA generally did not form abundant papillary protrusions but was either rounded, or at times rather angular. The periglandular stroma in PIA lesions often showed mononuclear inflammatory infiltrates mainly composed of lymphocytes, macrophages, and occasionally plasma cells. At times, mononuclear cells, identifiable as macrophages and/or polymorphonuclear neutrophils were prominent both intraluminally and within the epithelium as well. Corpora amylacea were often found in the lumina of PIA lesions.
Figure 1.
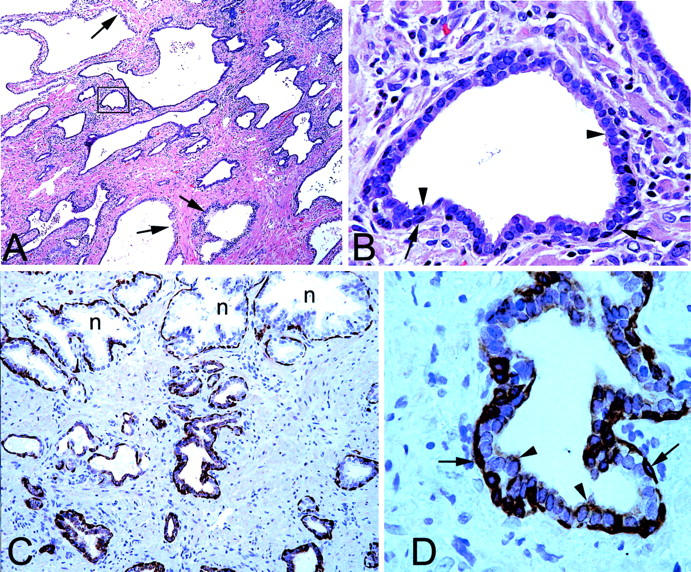
Morphological and K5 phenotype of PIA. A: Low-power image showing region of simple atrophy. Arrows indicate normal appearing epithelium with abundant clear cytoplasm. Note basophilic appearance of the majority of the acini. H&E stain. B: High-power image of area indicated in boxed region in A. Note two cell layers consisting of cuboidal and basophilic luminal cells (arrowheads) and darker underlying basal cells (arrows). H&E. C: Medium-power view of normal appearing epithelium (n) and PIA stained with anti-K5 antibodies. Note strong staining of basal layer in all epithelia, lack of staining of luminal cells in normal acini, and weak but positive staining in many of the luminal cells in atrophic acini. D: Higher power view of an atrophic acinus from C. Arrowheads indicate luminal secretory cells and arrows indicate basal cells. Original magnifications: ×40 (A); ×400 (B, D); ×100 (C).
Enrichment of Intermediate Cells in PIA Lesions
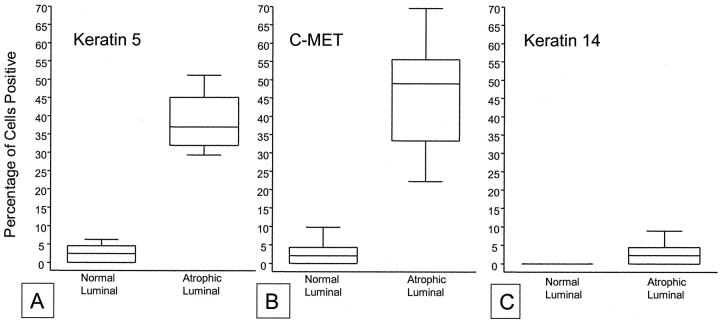
Intermediate cells in the luminal prostate epithelium are characterized by co-expression of K5 and K18, while K14 is absent (Table 1) ▶ . 11 In normal appearing prostate epithelium K5 and K14 were mainly localized within the basal cell compartment. A number of the K5-positive basal cells, however, were negative for K14. Within the normal appearing prostate epithelium, quantitative assessment by stereological grid point counting revealed that a mean of 2.4 ± 2.3% of luminal cells expressed K5, indicating sporadic existence of intermediate cells, while K14 was not observed in the luminal cell layer. In marked contrast to normal appearing epithelium, K5 was localized in 39.2 ± 7.4% of the luminal cells in PIA lesions (Figure 1, C and D) ▶ . The increase of K5 expression in PIA lesions was statistically significant (P < 0.00001). In 3.0 ± 3.1% of luminal cells in PIA lesions, K14 was identified (P = 0.003). Previously, we have shown that intermediate cells localized within the luminal prostate epithelium highly express the c-MET receptor. 13 In the present study, in normal appearing prostate epithelium, c-MET was localized in 2.1 ± 2.8% of luminal cells compared with 44.1 ± 14.1% in PIA lesions. This striking increase of c-MET expression in PIA was statistically significant (P < 0.00001, Figure 2 ▶ ). The results for staining for K5, K14, and c-MET in PIA versus normal prostate tissue are illustrated graphically in Figure 3 ▶ . It is clear that there is no overlap between the percentage of luminal cells staining for K5 or c-MET in PIA versus normal appearing prostate epithelium.
Table 1.
Selected Protein Expression Identifies at Least Four Different Prostate Epithelial Cell Types
| Protein Expression | Prostate epithelial cell types | |||
|---|---|---|---|---|
| Basal | Basal intermediate | Secretory intermediate | Mature secretory | |
| p63 | ++ | ++ | − | − |
| K14* | ++ | − | − | − |
| K5* | ++ | ++ | ++ | − |
| C-Met* | +/− | +/− | +/− | − |
| K8 | + | + | + | ++ |
| K18 | + | + | + | ++ |
| AR | −/+ | −/+ | +/− | ++ |
*Indicates data presented in this study. AR, nuclear androgen receptor; ++, strong nearly homogeneous staining; +, weak nearly homogeneous staining; +/−, weak to strong heterogeneous staining; −/+, weak heterogeneous staining; −, no staining.
Figure 2.
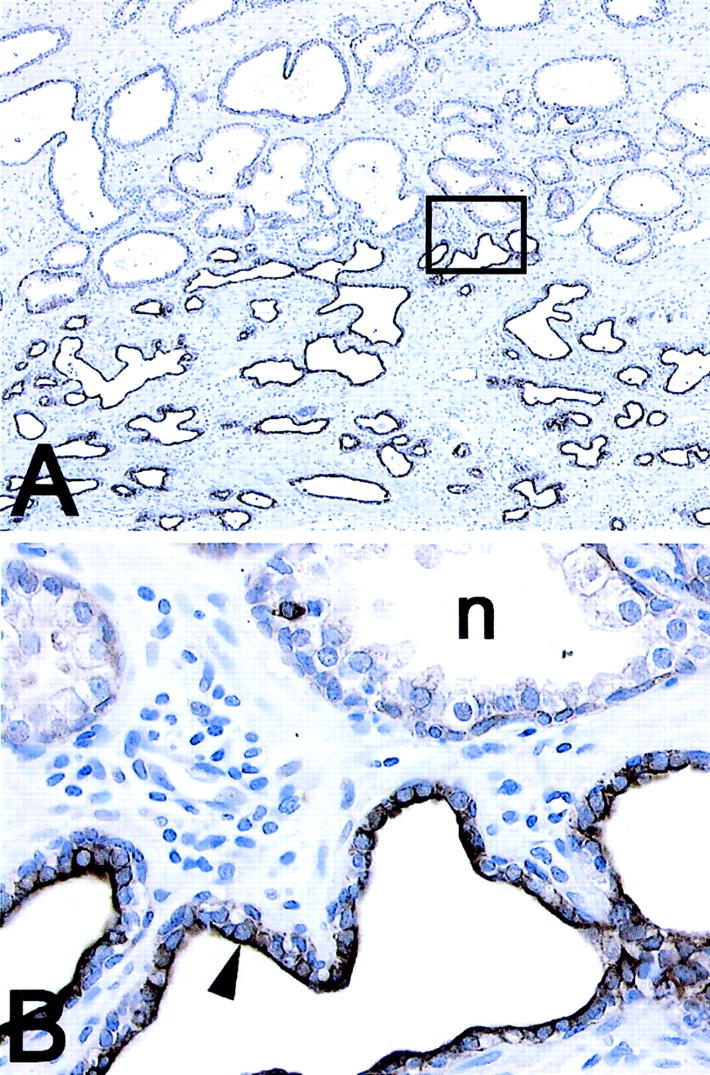
c-MET expression in PIA. A: Low-power image showing region of simple atrophy (bottom) and normal appearing epithelium (top). Note strong staining in atrophic epithelium for c-MET. B: High-power image of area indicated in boxed region in A. n, Normal acinus. Arrowhead indicates atrophic luminal epithelial cell. Original magnifications: ×50 (A); ×400 (B).
Figure 3.
Box plot of stereological grid point counting data from immunohistochemical staining results for K5, K14, and c-MET in PIA versus normal prostate luminal cells. Box extends from first to third quartiles, median is indicated by the line within the box. Whiskers include all remaining data points. A, K5; B, c-MET; C, K14. Note complete lack of overlap for both K5 and c-MET and lack of difference for K14.
Proliferative Activity of Intermediate Cells
Based on the high expression of Ki-67 as well as topoisomerase IIα and proliferating cell nuclear antigen, PIA is considered an actively proliferating lesion. 17,18 Although the cells that stained positive for Ki-67 were generally localized to the luminal aspect of the atrophic glands 17,18 it remained unclear whether these cells also expressed K5, indicative of intermediate differentiation. To unambiguously determine in a qualitative manner whether intermediate luminal cells (those luminal cells expressing K5) in PIA lesions specifically show proliferative activity, we performed co-localization studies of K5 with Ki-67. Although we did not perform grid point counting, the majority of the Ki-67-positive cells in PIA were in the luminal layer (as previously demonstrated) and these cells generally co-expressed K5 (Figure 4, A and B) ▶ .
Figure 4.
Immunofluorescence double labeling of PIA. A: PIA acinus stained for K5 (green), Ki-67 (red), and nuclei (blue). L, lumen; S, stroma. Note near continuous basal layer showing strong K5 staining and weaker but positive K5 staining in the majority of luminal cells. Arrow indicates a single Ki-67-positive cell that is clearly localized in the luminal compartment and is co-expressing K5. B: High-power view from another area of PIA showing Ki-67-positive luminal cell. C: Normal appearing prostate acinus stained for p27Kip1 (red), K5 (green), and nuclei (blue). Note strong staining for p27Kip1 in the vast majority of columnar luminal secretory cells. D: Area of PIA from same specimen as in C. Note absence of p27Kip1 staining in a group of K5-positive luminal cells (arrows). Original magnifications: ×400 (A, C, D); ×1000 (B).
In a previous study we found that luminal cells in PIA also often stained negative for p27Kip1, which was different from normal appearing prostate luminal cells, of which the vast majority stain strongly for p27Kip1 within their nuclei. 18 To determine whether the luminal epithelial cells that express K5 in PIA show decreased nuclear p27Kip1 expression we next performed co-localization studies of K5 and p27Kip1. Although the majority of luminal secretory cells in normal appearing nonatrophic epithelium (K5-negative) expressed p27kip1 (Figure 4C) ▶ , there were often groups of luminal cells revealing K5 expression in PIA that lacked expression of p27kip1 (Figure 4D) ▶ .
Discussion
Human prostatic epithelial cells with a phenotype intermediate between basal and secretory cells (intermediate cells) have gained increasing interest in the study of normal epithelial differentiation and malignant transformation because they have been postulated to be the cell type of origin for prostate carcinogenesis. 3,9,11,12 However, analysis of the biological characteristics of intermediate cells has been hampered by lack of techniques to identify this specific cell group and by the relatively low number of intermediate cells within the normal appearing prostate epithelium. Distinction of intermediate cells in the normal prostate epithelium warrants the application of immunohistochemical techniques, as neither cellular morphology nor topographic localization are specific for this cell population. Identification of intermediate secretory cells requires demonstration of K5 in the luminal cell layer, whereas the identification of intermediate basal cells requires the absence of K14 in the basal cell layer. 11 Recently we were able to better characterize these populations by using a fluorescent triple-staining technique for the identification of K5, K14, and K18 using directly labeled antibodies on frozen tissues and cell cultures. 11 In the present study we developed a method based on catalyzed deposition of biotinyl tyramide for fluorescent double staining for the analysis of intermediate cell characteristics on formaldehyde-fixed, paraffin-embedded prostate tissues which enables broad use and optimal histopathological analysis of prostate tissue. Using both standard immunohistochemistry for light microscopy and this dual labeling technique, we now report that intermediate luminal cells are enriched in areas of atrophic prostate epithelium referred to as PIA. In addition, a subset of secretory intermediate cells in PIA (K5+/18++) expressed the proliferation marker Ki-67 while many of these cells lacked expression of the cell cycle-dependent kinase inhibitor p27kip1. These observations provide strong evidence that intermediate cells have proliferative capacity and support their putative role as amplifying cells in the prostate epithelium.
Isaacs and Coffey 8 proposed a stem cell model in which the stem cell population gives rise to amplifying cells that by transient proliferation modulates development of the entire epithelium. During expansion this cell group putatively undergoes translocation from the basal toward the luminal cell compartment while gradually shifting its gene expression profile from stem cells toward terminally differentiated cells. In vitro basal cells characterized as K5++/14++/18+ are indeed progenitor cells of intermediate (K5/18) as well as more differentiated cells (K18++) that lack expression of the basal keratins. 10,11,26 Intermediate amplifying cells are believed to be androgen-independent for their survival. An increase of proliferative activity in the luminal cell layer during androgen deprivation might therefore represent selection of transiently proliferating, intermediate cells rather than marking a distinct luminal stem cell population. 4-7 Furthermore, intermediate cells highly express the receptor c-MET. 13 Activation of c-MET is involved in complex cell biological processes such as morphogenesis and tissue regeneration in multiple tissues including prostatic epithelium. 27,28 Both dynamic processes are essentially associated with coordinated cell proliferation, migration, and differentiation corresponding to the postulated role of intermediate cells in the prostate epithelium.
Glandular atrophy has been put forth as a regenerative lesion of the human prostate epithelium. Although proliferation in the respective epithelium is highly increased, 16-18 apoptotic activity maintains at normal levels. 17,18 Furthermore, glandular atrophy is often accompanied by local chronic, and at times acute, inflammation and was therefore termed PIA. 18 As currently shown, the increased proliferative activity in PIA lesions represents an enrichment of intermediate cells, identified by K5 and c-MET expression, within the luminal epithelium. Although further phenotypic studies of intermediate cells are ongoing, the paradoxical expression of several basal cell markers within the luminal compartment of PIA might be explained by selection of intermediate cells. 18 The sparse expression of K14 and lack of p63 staining in the luminal layer of PIA is further evidence that the luminal cells in PIA are not simply basal cells. 29 The quantitative aspects of our current findings add utility for those who wish to study focal atrophic lesions in the human prostate. Based on the present findings, we propose that a lesion or even an individual acinus/duct can be defined as PIA if the majority of luminal cells are atrophic in appearance by H&E (cuboidal and basophilic) and if >10% of the luminal cells express basal cell-specific keratins—the latter can be defined by either K5, as in the present study, or using the more commonly used antibody 34βE12, which recognizes K1, K5, K10, and K14.
Interestingly, in the original immunophenotypic and morphological descriptive study of PIA, we indicated that the luminal cells in PIA lesions lacked expression of basal-specific keratins, as indicated by lack of staining for the 34βE12 antibody. 18 In that set of experiments we used protease treatment for antigen unmasking. In the present study, we found that keratin 5, which is generally specific for basal cells, was indeed positive in many of the luminal cells in PIA, as was staining for 34βE12 (data not shown). A major difference in these studies is that in the present study we used steam heat in citrate buffer, without protease treatment, for antigen retrieval. Because PIA cells generally lacked expression of K14 in the luminal layer, even when using steam-only antigen retrieval, we propose that having K5 without its natural partner K14, renders the K5 susceptible to protease digestion in the luminal cells in PIA. This is consistent with studies in which the transfection of individual keratin genes into cells lacking an appropriate dimeric partner resulted in instability of the resultant polypeptides. 30,31 This K5 protease susceptibility would explain why we did not see any staining for 34βE12 in the luminal cells in PIA in the previous study. 18 Indeed, in preliminary studies we find that steam heating followed by protease digestion will primarily remove K5 staining in luminal cells in PIA but does not affect staining in the basal cells (WR Gage, AM De Marzo, unpublished observations).
In this study, we document that luminal cells within PIA lesions, which appear to be regenerating, overexpress the receptor c-MET. Interestingly, levels of c-MET are also raised in ulcerative colitis, acute pancreatitis, and obstructive cholangiopathy. 32-34 In these inflammatory lesions c-MET might be involved in cell proliferation, migration, and differentiation as part of a regenerative process. The receptor c-MET is generally considered proto-oncogenic as co-transfection of HGF and c-MET in NIH3T3 cells gives rise to an aggressive and malignant phenotype. 35 Furthermore, c-MET is overexpressed in several human malignancies such as pancreas, kidney, as well as prostate carcinoma. 35-39 In agreement with the stem cell model of Isaacs and Coffey, 8 high expression of c-MET together with their high proliferative activity support the concept of intermediate cells as putative progenitor cells for prostate carcinogenesis.
This stem cell model was recently updated in which it was proposed that there are at least two type types of intermediate, transiently amplifying cells. Basal intermediate cells were hypothesized to give rise to benign prostatic hyperplasia (BPH), and secretory intermediate cells were postulated to give rise to prostate intraepithelial neoplasia and carcinoma. 3 Although luminal secretory intermediate cells represent a minority of the normally differentiated luminal epithelium, in this study we provide further evidence, including a quantitative assessment, that they are enriched in PIA lesions. We also postulated that the basal cells, by virtue of expression of high levels of genome protective enzymes such as glutathione S-transferase Pi, are protected from undergoing neoplastic transformation. 3 Existence of chronic stress or persisting release of toxic agents by chronic inflammatory cells might subject the luminal intermediate cells in PIA to undergo genetic damage. Although many of the luminal cells in PIA appear to have induced expression of GSTP1, not all of the cells have. It has, therefore, been proposed that those luminal cells in PIA that lack GSTP1 expression may be targets for genome damage and hence neoplastic transformation. 18 Interestingly, changes of copy numbers of chromosome 8 in PIA, as occurring in prostate intraepithelial neoplasia and adenocarcinoma, have been documented. 40,41
In conclusion, we demonstrate that intermediate cells have proliferative activity supporting their histogenic role as amplifying cells in a hierarchic stem cell model of the prostate epithelium. The intermediate cell population is highly enriched in PIA lesions and might thus be susceptible to genetic damage. Thus, the present results provide additional evidence that luminal epithelial cells in PIA are potential targets for neoplastic transformation. Further molecular and phenotypic characterization of intermediate cells, as well as the development of appropriate animal models and cell culture systems, will be required to provide more definitive evidence as to whether intermediate cell populations in prostate epithelium are the true targets of carcinogenesis.
Footnotes
Address reprint requests to Angelo M. De Marzo, M.D., Ph.D., Department of Pathology, Division of Genitourinary Pathology, Bunting/Blaustein Cancer Research Building, Room 153, 1650 Orleans St., Baltimore, MD 21231-1000. E-mail: ademarz@jhmi.edu.
This work was supported the Public Health Service (grants NIH/NCI K08CA78588 and R01CA84997 to A. M. D.).
References
- 1.Potten CS, Morris RJ: Epithelial stem cells in vivo. J Cell Sci 1988, 10:S45-S62 [DOI] [PubMed] [Google Scholar]
- 2.Potten CS, Loeffler M: Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development 1990, 110:1001-1020 [DOI] [PubMed] [Google Scholar]
- 3.De Marzo AM, Nelson WG, Meeker AK, Coffey DS: Stem cell features of benign and malignant prostate epithelial cells. J Urol 1998, 160:2381-2392 [DOI] [PubMed] [Google Scholar]
- 4.van Leenders GJ, Schalken JA: Stem cell differentiation within the human prostate epithelium: implications for prostate carcinogenesis. BJU Int 2001, 88(Suppl 2):35-50 [DOI] [PubMed] [Google Scholar]
- 5.Evans GS, Chandler JA: Cell proliferation studies in rat prostate. I. The proliferative role of basal and secretory epithelial cells during normal growth. Prostate 1987, 10:163-178 [DOI] [PubMed] [Google Scholar]
- 6.English HF, Santen RJ, Isaacs JT: Response of glandular versus basal rat ventral prostatic epithelial cells to androgen withdrawal and replacement. Prostate 1987, 11:229-242 [DOI] [PubMed] [Google Scholar]
- 7.van der Kwast TH, Tetu B, Suburu ER, Gomez J, Lemay M, Labrie F: Cycling activity of benign prostatic epithelial cells during long-term androgen blockade: evidence for self-renewal of luminal cells. J Pathol 1998, 186:406-409 [DOI] [PubMed] [Google Scholar]
- 8.Isaacs JT, Coffey DS: Etiology and disease process of benign prostatic hyperplasia. Prostate 1989, 2:S33-S50 [DOI] [PubMed] [Google Scholar]
- 9.Verhagen AP, Ramaekers FC, Aalders TW, Schaafsma HE, Debruyne FM, Schalken JA: Colocalization of basal and luminal cell-type cytokeratins in human prostate cancer. Cancer Res 1992, 52:6182-6187 [PubMed] [Google Scholar]
- 10.Robinson EJ, Neal DE, Collins AT: Basal cells are progenitors of luminal cells in primary cultures of differentiating human prostatic epithelium. Prostate 1998, 37:149-160 [DOI] [PubMed] [Google Scholar]
- 11.van Leenders G, Dijkman H, Hulsbergen-van de Kaa C, Ruiter D, Schalken J: Demonstration of intermediate cells during human prostate epithelial differentiation in situ and in vitro using triple-staining confocal scanning microscopy. Lab Invest 2000, 80:1251-1258 [DOI] [PubMed] [Google Scholar]
- 12.De Marzo AM, Meeker AK, Epstein JI, Coffey DS: Prostate stem cell compartments: expression of the cell cycle inhibitor p27Kip1 in normal, hyperplastic, and neoplastic cells. Am J Pathol 1998, 153:911-919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Leenders GJ, Van Balken B, Aslders T, Hulsbergen-van de Kaa C, Ruiter D, Schalken J: Intermediate cells in normal and malignant prostate epithelium express c-MET: implications for prostate cancer invasion. Prostate 2002, 51:98-107 [DOI] [PubMed] [Google Scholar]
- 14.Bonkhoff H, Stein U, Remberger K: The proliferative function of basal cells in the normal and hyperplastic human prostate. Prostate 1994, 24:114-118 [DOI] [PubMed] [Google Scholar]
- 15.Liavag I: Atrophy and regeneration in the pathogenesis of prostatic carcinoma. Acta Pathol Microbiol Scand 1968, 73:338-350 [DOI] [PubMed] [Google Scholar]
- 16.Feneley MR, Young MP, Chinyama C, Kirby RS, Parkinson MC: Ki-67 expression in early prostate cancer and associated pathological lesions. J Clin Pathol 1996, 49:741-748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruska KM, Sauvageot J, Epstein JI: Histology and cellular kinetics of prostatic atrophy. Am J Surg Pathol 1998, 22:1073-1077 [DOI] [PubMed] [Google Scholar]
- 18.De Marzo AM, Marchi VL, Epstein JI, Nelson WG: Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. Am J Pathol 1999, 155:1985-1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lennox B: Observations on the accuracy of point counting including a description of a new graticule. J Clin Pathol 1975, 28:99-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wright SG, Tomkins AM: Quantitative histology in giardiasis. J Clin Pathol 1978, 31:712-716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corazza GR, Frazzoni M, Dixon MF, Gasbarrini G: Quantitative assessment of the mucosal architecture of jejunal biopsy specimens: a comparison between linear measurement, stereology, and computer aided microscopy. J Clin Pathol 1985, 38:765-770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sangster G, Crocker J, Jenkins R, Leyland MJ: Cells which contain S-100 protein in Hodgkin’s disease: a quantitative study. J Clin Pathol 1985, 38:1021-1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parsons JK, Nelson CP, Gage WR, Nelson WG, Kensler TW, De Marzo AM: GSTA1 expression in normal, preneoplastic, and neoplastic human prostate tissue. Prostate 2001, 49:30-37 [DOI] [PubMed] [Google Scholar]
- 24.Zha S, Gage WR, Sauvageot J, Saria EA, Putzi MJ, Ewing CM, Faith DA, Nelson WG, De Marzo AM, Isaacs WB: Cycloocygenase-2 is up-regulated in proliferative inflammatory atrophy of the prostate, but not in prostate carcinoma. Cancer Res 2001, 61:8617-8623 [PubMed] [Google Scholar]
- 25.Putzi MJ, Zhong H, Gage WR, Mouton PR, Semenza GL, Rubin MA, Simons JW, De Marzo AM: Hif-1a expression in primary and metastatic adenocarcinoma of the prostate. Prostate (in press)
- 26.Hudson DL, O’Hare M, Watt FM, Masters JR: Proliferative heterogeneity in the human prostate: evidence for epithelial stem cells. Lab Invest 2000, 80:1243-1250 [DOI] [PubMed] [Google Scholar]
- 27.Brinkmann V, Foroutan H, Sachs M, Weidner KM, Birchmeier W: Hepatocyte growth factor/scatter factor induces a variety of tissue-specific morphogenic programs in epithelial cells. J Cell Biol 1995, 131:1573-1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gmyrek GA, Walburg M, Webb CP, Yu HM, You X, Vaughan ED, Vande Woude GF, Knudsen BS: Normal and malignant prostate epithelial cells differ in their response to hepatocyte growth factor/scatter factor. Am J Pathol 2001, 159:579-590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parsons JK, Gage WR, Nelson WG, De Marzo AM: p63 protein expression is rare in prostate adenocarcinoma: implications for cancer diagnosis and carcinogenesis. Urology 2001, 58:619-624 [DOI] [PubMed] [Google Scholar]
- 30.Kulesh DA, Oshima RG: Cloning of the human keratin 18 gene and its expression in nonepithelial mouse cells. Mol Cell Biol 1988, 8:1540-1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kulesh DA, Cecena G, Darmon YM, Vasseur M, Oshima RG: Posttranslational regulation of keratins: degradation of mouse and human keratins 18 and 8. Mol Cell Biol 1989, 9:1553-1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Otte JM, Kiehne K, Schmitz F, Folsch UR, Herzig KH: C-met protooncogene expression and its regulation by cytokines in the regenerating pancreas and in pancreatic cancer cells. Scand J Gastroenterol 2000, 35:90-95 [DOI] [PubMed] [Google Scholar]
- 33.Liu Z, Sakamoto T, Ezure T, Yokomuro S, Murase N, Michalopoulos G, Demetris AJ: Interleukin-6, hepatocyte growth factor, and their receptors in biliary epithelial cells during a type I ductular reaction in mice: interactions between the periductal inflammatory and stromal cells and the biliary epithelium. Hepatology 1998, 28:1260-1268 [DOI] [PubMed] [Google Scholar]
- 34.Kitamura S, Kondo S, Shinomura Y, Isozaki K, Kanayama S, Higashimoto Y, Minami T, Kiyohara T, Yasunaga Y, Ishikawa H, Ohtani T, Ishiguro S, Matsuzawa Y: Expression of hepatocyte growth factor and c-met in ulcerative colitis. Inflamm Res 2000, 49:320-324 [DOI] [PubMed] [Google Scholar]
- 35.Rong S, Segal S, Anver M, Resau JH, Vande Woude GF: Invasiveness and metastasis of NIH 3T3 cells induced by Met-hepatocyte growth factor/scatter factor autocrine stimulation. Proc Natl Acad Sci USA 1994, 91:4731-4735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Natali PG, Prat M, Nicotra MR, Bigotti A, Olivero M, Comoglio PM, Di Renzo MF: Overexpression of the met/HGF receptor in renal cell carcinomas. Int J Cancer 1996, 69:212-217 [DOI] [PubMed] [Google Scholar]
- 37.Humphrey PA, Zhu X, Zarnegar R, Swanson PE, Ratliff TL, Vollmer RT, Day ML: Hepatocyte growth factor and its receptor (c-MET) in prostatic carcinoma. Am J Pathol 1995, 147:386-396 [PMC free article] [PubMed] [Google Scholar]
- 38.Pisters LL, Troncoso P, Zhau HE, Li W, von Eschenbach AC, Chung LW: c-met proto-oncogene expression in benign and malignant human prostate tissues. J Urol 1995, 154:293-298 [PubMed] [Google Scholar]
- 39.Ebert M, Yokoyama M, Friess H, Buchler MW, Korc M: Coexpression of the c-met proto-oncogene and hepatocyte growth factor in human pancreatic cancer. Cancer Res 1994, 54:5775-5778 [PubMed] [Google Scholar]
- 40.Macoska JA, Trybus TM, Wojno KJ: 8p22 loss concurrent with 8c gain is associated with poor outcome in prostate cancer. Urology 2000, 55:776-782 [DOI] [PubMed] [Google Scholar]
- 41.Shah R, Mucci NR, Amin A, Macoska JA, Rubin MA: Postatrophic hyperplasia of the prostate gland: neoplastic precursor or innocent bystander? Am J Pathol 2001, 158:1767-1773 [DOI] [PMC free article] [PubMed] [Google Scholar]