Abstract
Chimerism on the parenchymal level has been shown for several human allografts, including liver, heart, and kidney, with the integrated recipient-derived cells most likely originating from multipotent bone marrow precursors. We investigated whether chimerism also occurs within epithelial structures of the lung. For this purpose archival tissue biopsies from seven explanted human lung allografts were obtained. We performed laser microdissection of the target structures with subsequent short tandem repeat analysis to detect chimerism within the isolated cells. We found integration of recipient-derived cells in the bronchial epithelium, in type II pneumocytes and in seromucous glands lying adjacent to larger bronchi in all lung allografts studied. Quantitative analysis revealed that the epithelial structures displaying signs of chronic injury, such as squamous metaplasia, showed a markedly higher degree of chimerism (24% versus 9.5%). We therefore conclude that in human lungs, epithelial chimerism occurs at least within bronchi, type II pneumocytes, and seromucous peribronchial glands. Although a bone marrow origin of immigrating host-derived stem cells has been suggested by previous studies in rodents, analysis of lung biopsies from bone marrow-transplanted patients (n = 3) could not prove such delineation in this study. The observation of an enhanced integration of recipient cells into chronically damaged epithelial structures suggests that extrapulmonary precursor cells are able to contribute to pulmonary regeneration.
A continuously growing number of studies demonstrates that adult cells are able to display far more differentiating plasticity than previously thought. First investigated by performing transplantation studies in rodents, injected bone marrow-derived hematopoietic stem cells have been shown to differentiate into adult parenchymal cells of a wide range of tissue types including liver, kidney, heart, intestine, skin, and brain 1-8 as well as into most mesenchymal tissues including muscle, bone, and cartilage. 9,10 Furthermore, not only can bone marrow-derived precursors give rise to many tissues, but cells originating from muscle or brain seem to have the ability to turn back into bone marrow. 11,12 Therefore, bone marrow-derived stem cells or stem cells in general are likely to be able to cross the boundaries of the original embryonic cell layers in the adult and may have the capacity to help restoring damaged tissue in a variety of organs.
To determine whether such plasticity occurs also in humans, gender-mismatched allografts were analyzed for chimerism using Y-chromosome in situ hybridization. In fact, chimerism within the graft was detected in all solid organ transplants that have been analyzed. An integration of recipient-derived cells into the parenchyma was observed for hepatocytes and cholangiocytes in the liver, 13-15 for tubular epithelial and endothelial cells in the kidney, 8,16 and for cardiomyocytes in the heart. 17 In turn, also an integration of donor-derived hepatic cells could be detected thus leading to the conclusion that integrated cells at least in the liver are most likely of bone marrow origin. 14
In the murine lung, engraftment of bone marrow-derived cells could be found on the mesenchymal level as fibroblast-like cells contributing to the stroma 9 and on the epithelial level as bronchial epithelial cells or type II pneumocytes within the alveolar wall. 6 The integrated cells exhibited both the morphological and molecular phenotype of the airway epithelium, with the type II pneumocytes expressing surfactant B mRNA. Recently, marrow-derived type I pneumocytes were also detected within the alveolar epithelium of mice after bleomycin-induced lung injury. 18
In this study we investigated whether in human lung allografts recipient-derived cells are integrated into the parenchyma and if so, whether these cells are of bone-marrow origin. For this purpose, we studied both tissue from lung allografts that had been explanted because of organ failure as well as lung tissue from patients who had received therapeutic bone marrow transplantation. To have access to all available specimens without being limited to certain gender-mismatch constellations, we combined laser-assisted microdissection of the cells of interest with subsequent genotyping by short tandem repeat (STR)-polymerase chain reaction (PCR) to determine whether cells are donor- or recipient-derived.
Materials and Methods
Patient Material
Formalin-fixed, paraffin-embedded archival lung tissues were obtained from the files of the Institute of Pathology of the Hannover Medical School. We selected specimens from a total of 10 patients for this study, 7 of them had received lung transplantation for various reasons and the graft had been removed later because of organ failure. The other three patients had undergone therapeutic bone marrow transplantation and bioptic or surgical lung specimens were obtained from these patients after transplantation (Table 1) ▶ . One male patient who had received a female liver allograft was also selected for control purposes.
Table 1.
Clinical and Histopathological Data Regarding the Selected Cases
| Type of transplantation | Patient | Age | Reason for transplantation | Time from transplantation to removal/surgery | Histopathological findings in lung specimens |
|---|---|---|---|---|---|
| Lung transplantation | 1 | 31 | Cystic fibrosis | 7 years | Chronic rejection |
| 2 | 47 | NA | 6 years | Chronic rejection | |
| 3 | 43 | PPH | 30 days | Diffuse alveolar damage | |
| 4 | 42 | AAD | 210 days | Bronchlolitis obliterans | |
| 5 | 51 | AAD | 32 days | Bronchiolitis obliterans | |
| 6 | 26 | Cystic fibrosis | 20 days | Interstitial fibrosing pneumonia | |
| 7 | 25 | Cystic fibrosis | 4 days | Diffuse alveolar damage | |
| Bone marrow transplantation | 8 | 13 | Agranulocytosis | 246 days | Aspergillosis |
| 9 | 5 | Aplastic anemia | 75 days | Aspergillosis | |
| 10 | 57 | CLL | 313 days | Interstitial pneumonia |
Abbreviations: PPH, primary pulmonary hypertension; AAD, alpha-1-antitrypsin deficiency; CLL, chronic lymphocytic leukemia; NA, not available.
Immunohistochemical Staining
For reliable identification of the target structure, ie, bronchial epithelial cells, pneumocytes, and mucous glands, we performed a double-immunostaining procedure before microdissection. In a first staining reaction the epithelial cells were labeled with the commercially available pan-cytokeratin antibody KL-1 (Immunotech, Hamburg, Germany) using diaminobenzidine as the first chromogen. This led to an intense brown staining of all of the epithelial structures that were under investigation. To avoid false-positive results because of infiltrating leukocytes within epithelia we performed a second immunohistochemical staining reaction on the same tissue sections now using a mixture of the antibodies recognizing leukocyte common antigen (LCA) and CD68 (both DAKO, Glostrup, Denmark) at equal parts. In this second reaction Vector VIP (Vector Laboratories, Burlingame, CA) was the visualizing substrate giving a dark-blue to violet color that provided sufficient contrast to the brown color of the diaminobenzidine chromogen (Figure 1, A and B) ▶ .
Figure 1.
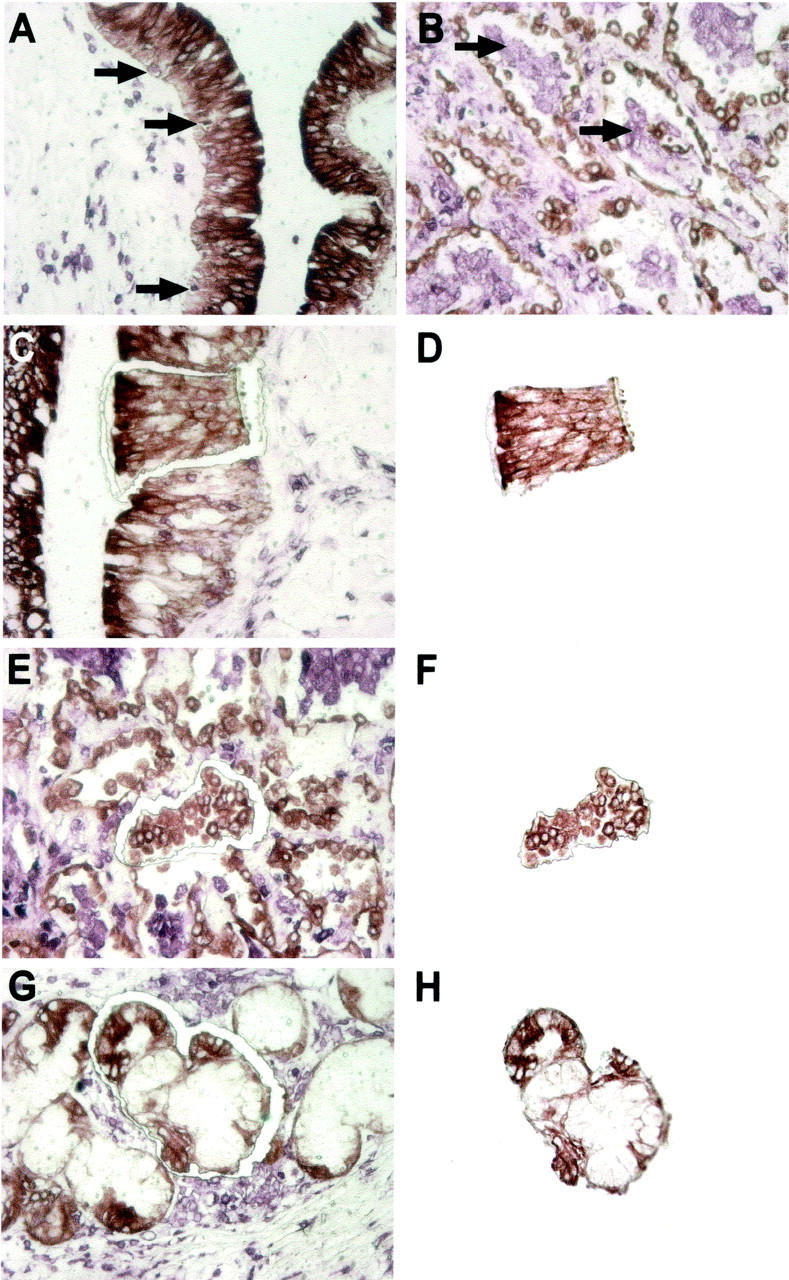
Double immunostaining and laser microdissection of epithelial cells. Bronchial epithelial cells (A) and hyperplastic type II pneumocytes (B) show dense cytoplasmic staining for pan-cytokeratin antibody KL-1 (diaminobenzidine, brown). On the same tissue sections leukocytes as well as foamy macrophages within the air spaces are labeled with anti-LCA plus anti-CD68 (VIP, blue) making them clearly detectable even within the epithelium (arrows). For microdissection of bronchial epithelium, groups of cells were first cut out with the laser beam (C) and then catapulted into the lid of a reaction tube (D). E and F: Microdissection and catapulting of a small group of type II pneumocytes lining the alveolar septae and partly protruding into the lumina. G and H: Microdissection and catapulting of a small acinus or seromucous glands lying near to a large bronchus. The dissected tissue pieces shown in D, F, and H contain ∼30 to 50 cells each. The glands are stained brown and the dense surrounding lymphocytic infiltrate is marked blue. Original magnifications, ×400.
The whole immunoperoxidase-staining procedure was performed with the R.T.U. Vectastain Universal Elite ABC-Kit (Vector Laboratories, Burlingame, CA) according to the manufacturer’s instructions. No predigestion steps for antigen retrieval were necessary; all primary antibodies were incubated at a dilution of 1:100.
Laser Microdissection
Laser-based microdissection of bronchial epithelium, pneumocytes, and mucous glands was performed using the PALM Laser MicroBeam system (P.A.L.M., Bernried, Germany), essentially as described. 19 Clusters of cells were isolated and pooled together in samples containing ∼500 to 1000 cells for qualitative and at least 2000 cells for quantitative analysis. Infiltrating leukocytes were either avoided by the laser beam or laser-ablated (Figure 1; C to H) ▶ .
Short Tandem Repeat PCR
For detection of recipient-derived cells within the isolated epithelial structures, we used a PCR assay that analyzes one highly polymorphic STR marker located within the human β-actin-related pseudogene, H-β-Ac-psi-2 (ACTBP2). This marker is commonly known as SE33 and contains a tetranucleotide repeat that displays considerable polymorphism and a fairly high heterozygosity rate of up to 93%. 20,21 To increase sensitivity in amplifying partially degraded DNA from formalin-fixed tissue, we designed new primers. Compared to the primers usually used to analyze the SE33 locus, they reduce the length of the PCR products by 85 bp to fragments ranging from 140 to 236 bp in length (forward primer: 5′-AGAGAGAGAAAGGAAGGAAGG-3′; reverse primer: 5′-CTACCGCTATAGTAACTTGC-3′). The amplification reaction was performed in a final volume of 25 μl containing 200 nmol/L of each primer, 0.5 U Hot Start Taq Polymerase (Qiagen, Hilden, Germany), 1.5 mmol/L MgCl2, 250 nmol/L of dNTP, and up to 10 μl of DNA lysate. The forward primer is labeled with 6-FAM at the 5′ end. The reaction mixture was preheated at 95°C for 10 minutes, followed by 35 cycles at 95°C for 30 seconds, 56°C for 30 seconds, and 72°C for 1 minute, with a final elongation step at 72°C for 10 minutes.
The PCR products were analyzed using a capillary electrophoresis instrumentation (ABI310; Applied Biosystems, Darmstadt, Germany). One μl of the PCR product was mixed with 0.3 μl of size standard (GeneScan350, Applied Biosystems) and 12 μl of formamide. This mixture was heated for 2 minutes at 90°C and immediately chilled on ice. The samples were placed in the sample holder and the electrophoresis was started as described in detail by the manufacturer. We routinely used an injection time of 5 seconds. Electropherograms displaying size and intensity of the PCR products were created using the software package supplied with the instrument (GeneScan310 Analysis).
To be able to detect recipient-derived cells within the microdissected tissue, first the allelotypes of both donor and recipient had to be determined. We identified the recipient’s alleles by analyzing tissue from the originally explanted lung, whereas a mixture of recipient and donor could be obtained by examining tissue from the explanted lung allograft without microdissection. By subtracting the already known recipient allelotype from the PCR results of the mixed tissue, the donor’s alleles could also be identified. In nontransplanted tissues never more than two alleles were detected (data not shown).
Quantitative Analysis
For quantitative evaluation of the degree of chimerism that was found in the microdissected samples, we constructed a calibration curve, essentially as described for the determination of mixed chimerism in recipients of bone marrow transplantation. 22 Because of fragmentation of the DNA isolated from formalin-fixed tissue, PCR analysis might show an impaired and unequal amplification efficiency compared to DNA isolated from fresh tissue. To minimize the distortion of quantification because of these effects, we used for evaluation only the peak height values corresponding to the shortest alleles and/or the alleles lying closest together for donor and recipient. Furthermore, every PCR was repeated five times.
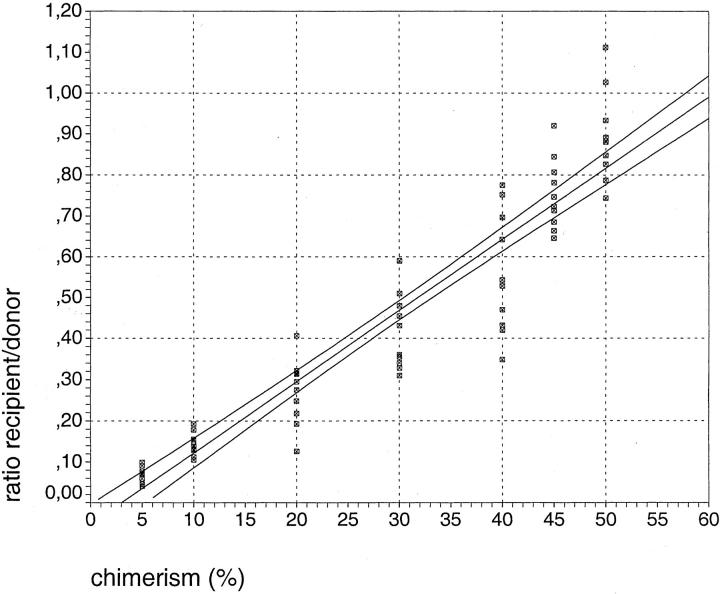
For the generation of a calibration curve, we mixed archival DNA from lung tissue of two different individuals in varying ratios from 5 to 50%. For each PCR ∼2000 cells were used and the analysis was performed 10 times for every mixture. We chose one allele from each individual according to the above-mentioned criteria and determined the ratio of the peak height of the minor to the major fraction. A standard curve was calculated from the mean values obtained for each defined mixture (Figure 2) ▶ .
Figure 2.
Calibration curve for quantitative STR analysis. DNA from formalin-fixed lung tissue from two individuals was mixed in defined ratios to construct a calibration curve. This calibration curve relates the extent of chimerism and the measured peak ratios after STR-PCR. Every measurement was performed 10 times and each individual measurement is represented by one square. For the quantitative determination of the chimerism in an unknown sample, the peak ratio for the alleles most similar in DNA length was calculated. Subsequently, using the calibration curve the extent of chimerism was determined. The calibration curve is shown together with the 95% confidence interval represented by the two lines paralleling the calibration curve (see also Materials and Methods).
We determined the percentage of chimerism within the different samples as follows: first the ratio of peak height recipient versus peak height donor was calculated. Then, starting from the y axis, the corresponding percentage value was determined on the x axis. The results are presented as percentage ± a confidence interval of 95%.
Y-Chromosome Hybridization
For the detection of Y-chromosome-positive cells in transplanted organs we followed a modified version of the CISH protocol described previously 23 with a Y-chromosome-specific centromere probe (Appligene-Oncor, Illkirch, France) in combination with standard immunohistochemistry for cytokeratin (KL-1; Beckman-Coulter, Immunotech). Briefly, a 5-μm-thick tissue slide of the formalin-fixed and paraffin-embedded liver biopsy was deparaffinized, graded in alcohol and incubated with a KL-1 antibody. For detection BCIP/NBT/INT (DAKO) was used. After microscopic verification of the immunohistochemical reaction, an overnight incubation with a DNA probe for the centromeric region of the Y-chromosome followed. The detection of the CISH signals was done with a tyramide-induced amplification and staining with AEC+ (DAKO).
Results
Comparison of STR Analysis after Microdissection and Y-Chromosome Hybridization for the Detection of Chimerism
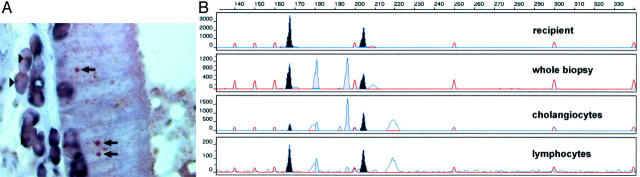
To ensure that our newly developed method (STR genotyping after laser microdissection) and the more frequently used sex chromosome in situ hybridization both give concordant results, we compared directly these different methodological approaches. We restricted this comparison to the analysis of female grafts in male recipients thereby looking for the presence of Y-chromosome-positive parenchymal cells. (In the case of a male graft in a female recipient, one has to look for Y-chromosome-negative parenchymal cells, which is much less reliable.) Figure 3A ▶ demonstrates the presence of distinct Y-chromosome signals in several nuclei of cholangiocytes. The genotyping of laser-microdissected cholangiocytes revealed all four alleles (two from the donor and two from the recipient) confirming the establishment of a chimeric cholangiocyte population in this transplanted liver. (Unfortunately, biopsies from female lungs in male recipients were not available. Therefore, we had to perform the comparison in a different organ.) A lymphocytic infiltrate dissected from the stroma of this organ revealed, as expected, predominantly the recipient’s genotype (Figure 3B ▶ , bottom).
Figure 3.
Comparison of Y-chromosome hybridization and STR-PCR after microdissection. A: Detection of Y-chromosome-positive cholangiocytes in a female liver transplanted into a male recipient (arrows). One to five percent of cholangiocytes were positive for the Y-chromosome. Y-chromosome-positive infiltrating leukocytes (immunohistochemically labeled with anti-LCA and anti-CD68) are also clearly discernible (arrowheads). Leukocytes are stained by blue cytoplasmic dye. No nuclear counterstain, which could mask Y-chromosome hybridization signals, was performed. B: Genotyping of the whole section and laser-microdissected cholangiocytes. A dissected leukocyte infiltrate displayed, as expected, predominantly the recipient’s genotype whereas the laser-microdissected cholangiocytes show clear chimerism.
Bronchial Chimerism in Lung Allografts
To answer the question whether chimerism of epithelial cells occurs in human lung allografts, different types of lung epithelial cells (bronchial epithelium, type II pneumocytes, seromucous glands) were isolated by laser-assisted microdissection and the genotype (ie, donor’s or recipient’s origin) was determined subsequently by PCR as described previously. 15 Formalin-fixed and paraffin-embedded biopsies from seven lung allografts were retrieved from the archives of the Institute of Pathology. All lung allografts analyzed were removed because of organ failure. Compared to trans-bronchial biopsies that normally contain only very limited amounts of parenchyma, these surgical specimens provided enough tissue to examine bronchial epithelium, pneumocytes, and also the seromucous glands lying adjacent to the large bronchi. Where possible we examined the larger bronchi separately, otherwise we collected all bronchial epithelium that was available from one lung specimen into one sample. Each microdissected tissue contained ∼500 to 1000 cells, which was sufficient for qualitative analysis of chimerism. The immunohistochemical double staining provided distinct labeling of the epithelial structures and at the same time specific identification of infiltrating leukocytes lying within the epithelium (Figure 1) ▶ . In cases with marked inflammatory reaction, this was particularly important to avoid false-positive results because of infiltrating leukocytes (which predominantly are of recipient’s origin).
We could detect recipient-derived parenchymal cells within bronchi of all seven lung allografts under study. The establishment of this intragraft in situ chimerism is a very early event (already detectable after 4 days in case 7, and persisting for up to 7 years in case 1).
Chimerism of Type II Pneumocytes and Seromucous Glands
In cases in which sufficient material was available, we also studied alveolar lining cells and seromucous glands lying adjacent to large bronchi. Both cellular compartments are also intensely labeled with the KL-1 antibody, which made it possible to perform microdissection of these structures on the same tissue sections from which bronchial epithelial cells had already been isolated.
As the alveolar lining cells are normally very flat and difficult to define morphologically, the isolation of pneumocytes was only possible in cases in which marked type II hyperplasia occurred as an unspecific sign of alveolar damage. In these cases, type II pneumocytes are lining the alveolar septae as cuboid epithelia protruding into the lumina (Figure 1, E and F) ▶ , facilitating identification and microdissection.
Seromucous glands and type II pneumocytes were analyzed only in a subset of cases, because not every lung was suitable for this purpose. Chimerism of the target structures was detectable in all four cases in which pneumocytes could be obtained as well as in all four cases in which seromucous glands could be isolated.
Quantitative Analysis of in Situ Microchimerism
To analyze the in situ microchimerism quantitatively and to correlate the extent of chimerism to histomorphological findings, we developed a quantitative genotyping assay (see Figure 2 ▶ and Materials and Methods). In comparison to the strictly qualitative assay, for quantitative PCR more cells have to be used per sample. In addition, every measurement was performed five times to get reliable quantitative results. Therefore, more cells had to be collected and not all lung specimens could be analyzed.
Nevertheless, in a second set of experiments, bronchial epithelium (n = 4), type II pneumocytes (n = 3), and glands (n = 4) were dissected from those biopsies in which enough cells were available. All together, the degree of chimerism was similar in all three different cellular compartments ranging from 6 to 26% in the bronchi (5.7 ± 2.5% to 25.5 ± 0.9%), from 9 to 20% in the pneumocytes (9.1 ± 2.3% to 20.0 ± 1.5%), and from 9 to 24% in the seromucous glands (9.1 ± 2.3% to 24.2 ± 1.3%).
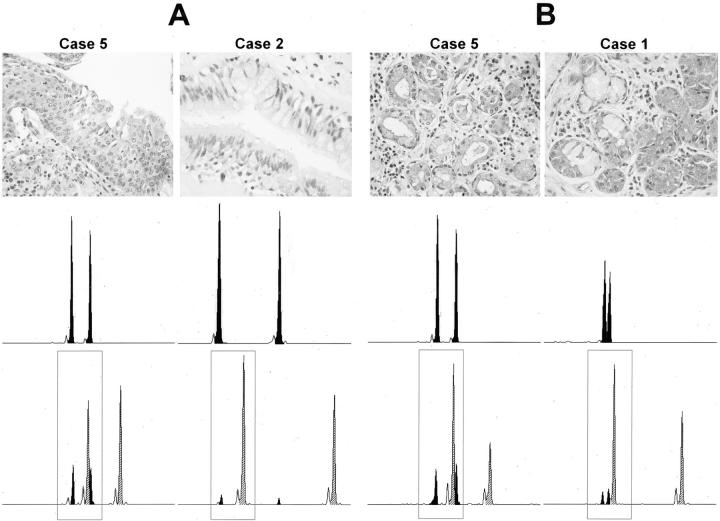
Interestingly, the degree of chimerism seemed to be related to the extent of injury. Although the number of cases was too small for sound statistical analysis, the two lungs with the highest proportion of recipient cells within the bronchi (cases 4 and 5) also suffered from severe chronic and acute bronchitis that had led to marked squamous metaplasia of the bronchial epithelial cells (Figure 4A) ▶ . Also loss of goblet cells and vanishing of ciliae on the luminal surface of the airway epithelium was apparent in these cases. In the two cases where none of these features of injury were detectable, chimerism reached a degree of only 9.5% or less (Table 2) ▶ . Similar observations were made concerning the peribronchial glands. In case 5 the seromucous glands surrounding the damaged bronchi were also affected by chronic inflammation and thus displayed histological signs of marked dysplasia. Accordingly, in this case the highest extent of gland chimerism was found (24% versus 14% or less in cases without inflammation).
Figure 4.
Increased chimerism in chronically injured epithelium. A: The chimerism in the bronchial epithelium observed in case 5 with squamous metaplasia is compared to case 2 without metaplasia. The higher degree of chimerism in case 5 is obvious from the electropherogram at the bottom. The top electropherogram represents pure recipient’s genotype from the explanted lungs. B: Comparison of the chimerism in peribronchial glands in case 5, in which the glands display reactive epithelial changes, with peribronchial gland chimerism in case 1, which exhibits glands with inflammatory infiltrates but a quite normal morphology of ducts and acini. Top: The morphological appearance of the bronchial and glandular epithelium, respectively. The top electropherograms show the recipient’s allelotype for each case. The bottom electropherograms display representative results obtained for the different cases after laser microdissection. Within the frames are the alleles selected for quantitative evaluation. Black peaks, recipient; hatched peaks, donor; small empty peaks, so called “stutter bands” caused by sliding of the Taq polymerase along the repetitive sequence during the amplification process.
Table 2.
Quantitative Analysis
| Case no. | Percentage of recipient-derived cells | ||
|---|---|---|---|
| Bronchi | Pneumocytes | Glands | |
| 1 | 8.0 ± 2.2 | 9.1 ± 2.3 | |
| 9.5 ± 2.5 | |||
| 8.5 ± 2.1 | |||
| 5.7 ± 2.5 | |||
| 2 | 5.7 ± 2.5 | 14.1 ± 2.1 | |
| 9.5 ± 2.5 | |||
| 3 | 9.1 ± 2.3 | 12.2 ± 2.1 | |
| 4 | 24.2 ± 1.3 | 20.0 ± 1.5 | |
| 24.1 ± 1.3 | |||
| 9.5 ± 2.5 | |||
| 5 | 25.5 ± 0.9 | 24.2 ± 1.3 | |
| 6 | 12.0 ± 2.0 | ||
To validate these observations, two bronchi from the same lung specimen, but displaying remarkably different degrees of damage, were analyzed separately using the quantitative chimerism assay. In fact, the bronchus with extended squamous metaplasia exhibited 24% chimerism (24.2 ± 1.3%), whereas the other one with mild changes and preserved columnar arrangement of the epithelium contained only 9.5% (±2.5%) of recipient-derived parenchymal cells (Table 2 ▶ , case 4).
Concerning the type II pneumocytes, no histological differences could be observed between the specimens available for analysis. Therefore, no correlation between histology and extent of chimerism could be analyzed for this cellular compartment.
Lung Specimens of Bone Marrow-Transplanted Patients
Several lines of evidence support the hypothesis that the parenchymal cells of recipient’s origin found in transplanted organs are bone marrow derived. 1,4,6,7 Therefore, we analyzed lung biopsies from three recipients of bone marrow transplantation searching for parenchymal cells with the genotype of the bone marrow donor. In all three specimens no donor-derived DNA could be detected (see Figure 5B ▶ ).
Figure 5.
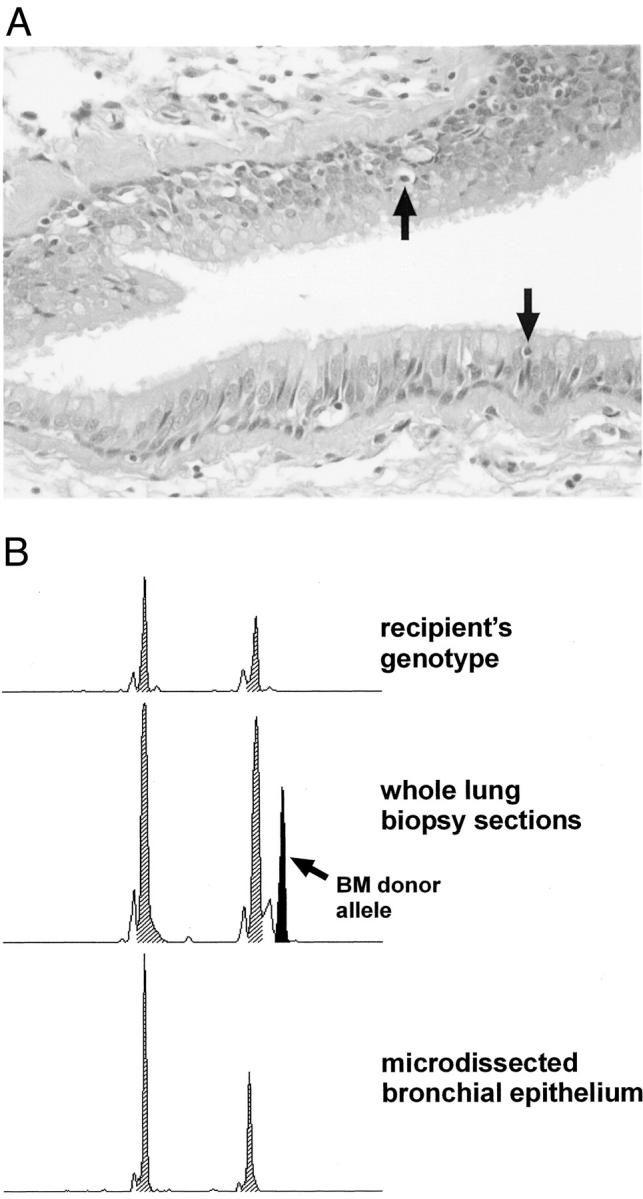
Analysis of bronchi from recipients of bone marrow transplantation. A: Representative bronchial epithelium from one patient who had received therapeutic bone marrow transplantation. Note the infiltration of single lymphocytes into the epithelial layer (arrows). B: Electropherograms demonstrating the absence of chimerism within microdissected bronchi.
These negative results also provide an excellent negative control excluding the possibility of contamination of samples by cellular fragments lying underneath the dissected cells. These negative results also rule out infiltrating leukocytes as a source of false-positive results, because all three lung specimens from bone marrow recipients showed inflammatory reaction toward the bronchi (Figure 5A) ▶ . The careful microdissection process, supported by immunohistochemical labeling of target and unwanted bystander cells, ensures the recovery of pure cell preparations for the genotyping.
Discussion
In this study we report for the first time that recipient-derived cells are integrated into pulmonary epithelia of human lung allografts after transplantation. Immunohistochemically labeled epithelial structures were isolated by laser-assisted microdissection from lung transplants that had been removed because of organ failure. By subsequent use of STR-PCR for the identification of recipient cells within the graft, we detected chimerism in bronchial epithelium, hyperplasia type II pneumocytes lining the alveolar septae and seromucous glands surrounding larger bronchi.
As already demonstrated in our previous study, 15 the simultaneous immunohistochemical labeling of the cells of interest (cytokeratin-positive epithelial cells) and infiltrating leukocytes (CD68+ and LCA+) excluded reliably false-positive results because of contaminating leukocytes of recipient’s origin. It also facilitated the identification and microdissection of epithelial cells in a pure and homogeneous form (Figure 1) ▶ . Furthermore, the modified quantitative chimerism assay proved to be sufficiently reproducible for archival material when performing several replicates per measurement. For a given sample only peaks with similar size were compared to reduce distortion of the quantification because of differences in PCR product size.
Analysis of more than 100 of nontransplantation samples with the same STR assay for the purpose of identification never did exhibit more than two alleles. Also the absence of chimerism in the analysis of lung specimens from three bone marrow-transplanted patients (Figure 5 ▶ and below) demonstrates the specificity of the methodology that obviously does not show false-positive results. Therefore, the unknown donor-derived alleles could be extrapolated from the additionally occurring STR-PCR products in the transplant that did not match the alleles identified in the recipient’s own lung (see “Materials and Methods”).
We found recipient-derived cells in the bronchi of all seven lungs under study as well as within pneumocytes and the glandular epithelium of all of the selected cases that were investigated for these cellular compartments. Our results differ from those obtained by a previous study in 1991, 24 which could not detect chimerism within the bronchial and alveolar epithelium. Yousem and Sonmez-Alpan 24 used sex chromosome in situ hybridization. With the exception of one case, they analyzed male grafts in female recipients thereby looking for the absence of Y-chromosome in parenchymal cells in the transplanted organ. In this sex-mismatch constellation it is very difficult to discriminate between true female cells and male cells artificially negative for the Y-chromosome because of tangential nuclear sectioning. Moreover, in that study only trans-bronchial biopsy specimens were investigated that in most cases contain only very limited amounts of suitable lung tissue and that are at the same time considerably damaged by the sampling procedure.
Our control experiments illustrated in Figure 3 ▶ demonstrate that our newly developed methodology and the more widely used Y-chromosome hybridization provide identical results. Therefore, the different results may be primarily because of case selection or suitability of biopsies for molecular studies.
The survival times of the examined grafts ranged from 4 days to 7 years, demonstrating that the engraftment both takes place very early after transplantation and persists for a long time. In this respect, the results obtained in this study for parenchymal chimerism of the lung are consistent with those found for other transplanted organs such as liver, heart, and kidney 8,14,17 where chimerism occurred very early, persisted very long, and was detected in all specimens. Also the engraftment rates are quite similar ranging from 2 to 30%.
As suggested by previous studies, which demonstrated the engraftment of bone marrow-derived stem cells into the lung parenchyma in mouse models of hematopoietic stem cell transplantation, 6,18 we expected to find this engraftment also in the lungs of human recipients of bone marrow transplantation. There are two possible explanations why we could not find donor-derived cells in any of the samples that we obtained from three patients in this study. First, under normal conditions the engraftment rate for bronchial epithelium described in mouse model systems is quite low (∼4%). 6 This may be too low for detection using the chimerism assay described herein. 25,26 Second, the lung tissue that was available from recipients of bone marrow transplantation was limited concerning size and quality, which certainly impaired the detection sensitivity. For the same reason, only a few bronchial epithelia and no pneumocytes or glands could be analyzed. Taken together, our negative results cannot rule out the bone marrow origin of the engrafted cells in human transplants, which was suggested by studies in rodents. 6,18
The quantitative chimerism analysis of the lung allografts revealed that the two cases that displayed histological signs of severe chronic injury both of the bronchi as well as of peribronchial glands also exhibited increased engraftment rates of recipient cells within these cellular compartments. This may suggest that chimerism after transplantation is increased by cellular damage with elevated cell turnover. We have made a similar observation in our previous work on hepatic chimerism, where engraftment seemed to be remarkably higher in liver transplants with recurrent hepatitis. 15 Further support for this hypothesis comes from studies on rodents. After hematopoietic stem cell transplantation and consecutive induction of extensive liver damage by toxic agents, up to 50% of the liver was repopulated by bone marrow-derived hepatocytes. 3,4 Similar effects could be observed for alveolar epithelium after bleomycin-induced lung injury. Also, injected bone marrow cells were even able to restore two-thirds of infarcted myocardium. Whether the integration of parenchymal cells originating from outside the respective organ also takes place under physiological conditions has recently been questioned. 27 In this study we provide evidence that at least in the case of severe organ damage extrapulmonary stem cells are recruited to the lung. If nonpulmonary cells can substantially contribute to lung regeneration, this may create new therapeutic options with great potential.
Acknowledgments
We thank Britta Hasemeier for preparing Figure 3 ▶ and Holly Sundberg for critically reading and editing the manuscript.
Footnotes
Address reprint requests to Hans Kreipe, M.D., Institute of Pathology, Medizinische Hochschule Hannover, Carl-Neuberg-Str. 1, D-30625 Hannover, Germany. E-mail: kreipe.hans@mh-hannover.de.
Supported by grant SFB 265 C11 from the Deutsche Forschungsgemeinschaft.
References
- 1.Eglitis MA, Mezey E: Hematopoietic cells differentiate into both microglia and macroglia in the brains of adult mice. Proc Natl Acad Sci USA 1997, 94:4080-4085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kopen GC, Prockop DJ, Phinney DG: Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci USA 1999, 96:10711-10716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP: Bone marrow as a potential source of hepatic oval cells. Science 1999, 284:1168-1170 [DOI] [PubMed] [Google Scholar]
- 4.Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M: Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med 2000, 6:1229-1234 [DOI] [PubMed] [Google Scholar]
- 5.Theise ND, Badve S, Saxena R, Henegariu O, Sell S, Crawford JM, Krause DS: Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology 2000, 31:235-240 [DOI] [PubMed] [Google Scholar]
- 6.Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ: Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 2001, 105:369-377 [DOI] [PubMed] [Google Scholar]
- 7.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P: Bone marrow cells regenerate infarcted myocardium. Nature 2001, 410:701-705 [DOI] [PubMed] [Google Scholar]
- 8.Poulsom R, Forbes SJ, Hodivala-Dilke K, Ryan E, Wyles S, Navaratnarasah S, Jeffery R, Hunt T, Alison M, Cook T, Pusey C, Wright NA: Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol 2001, 195:229-235 [DOI] [PubMed] [Google Scholar]
- 9.Pereira RF, Halford KW, O’Hara MD, Leeper DB, Sokolov BP, Pollard MD, Bagasra O, Prockop DJ: Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc Natl Acad Sci USA 1995, 92:4857-4861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F: Muscle regeneration by bone marrow-derived myogenic progenitors. Science 1998, 279:1528-1530 [DOI] [PubMed] [Google Scholar]
- 11.Bjornson CR, Rietze RL, Reynolds BA, Magli MC, Vescovi AL: Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science 1999, 283:534-537 [DOI] [PubMed] [Google Scholar]
- 12.Jackson KA, Mi T, Goodell MA: Hematopoietic potential of stem cells isolated from murine skeletal muscle. Proc Natl Acad Sci USA 1999, 96:14482-14486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, Novelli M, Prentice G, Williamson J, Wright NA: Hepatocytes from non-hepatic adult stem cells. Nature 2000, 406:257. [DOI] [PubMed] [Google Scholar]
- 14.Theise ND, Nimmakayalu M, Gardner R, Illei PB, Morgan G, Teperman L, Henegariu O, Krause DS: Liver from bone marrow in humans. Hepatology 2000, 32:11-16 [DOI] [PubMed] [Google Scholar]
- 15.Kleeberger W, Rothamel T, Glockner S, Flemming P, Lehmann U, Kreipe H: High frequency of epithelial chimerism in liver transplants demonstrated by microdissection and STR-analysis. Hepatology 2002, 35:110-116 [DOI] [PubMed] [Google Scholar]
- 16.Lagaaij EL, Cramer-Knijnenburg GF, van Kemenade FJ, van Es LA, Bruijn JA, van Krieken JH: Endothelial cell chimerism after renal transplantation and vascular rejection. Lancet 2001, 357:33-37 [DOI] [PubMed] [Google Scholar]
- 17.Quaini F, Urbanek K, Beltrami AP, Finato N, Beltrami CA, Nadal-Ginard B, Kajstura J, Leri A, Anversa P: Chimerism of the transplanted heart. N Engl J Med 2002, 346:5-15 [DOI] [PubMed] [Google Scholar]
- 18.Kotton DN, Ma BY, Cardoso WV, Sanderson EA, Summer RS, Williams MC, Fine A: Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development 2001, 128:5181-5188 [DOI] [PubMed] [Google Scholar]
- 19.Lehmann U, Glockner S, Kleeberger W, von Wasielewski HF, Kreipe H: Detection of gene amplification in archival breast cancer specimens by laser-assisted microdissection and quantitative real-time polymerase chain reaction. Am J Pathol 2000, 156:1855-1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polymeropoulos MH, Rath DS, Xiao H, Merril CR: Tetranucleotide repeat polymorphism at the human beta-actin related pseudogene H-beta-Ac-psi-2 (ACTBP2). Nucleic Acids Res 1992, 20:1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moller A, Brinkmann B: Locus ACTBP2 (SE33). Sequencing data reveal considerable polymorphism. Int J Legal Med 1994, 106:262-267 [DOI] [PubMed] [Google Scholar]
- 22.Scharf SJ, Smith AG, Hansen JA, McFarland C, Erlich HA: Quantitative determination of bone marrow transplant engraftment using fluorescent polymerase chain reaction primers for human identity markers. Blood 1995, 85:1954-1963 [PubMed] [Google Scholar]
- 23.Wilkens L, Bredt M, Flemming A, Mengel M, Klempnauer J, Kreipe H, Flemming P: Detection of chromosomal aberrations in well-differentiated hepatocellular carcinoma by bright-field in situ hybridization. Mod Pathol 2002, 15:470-475 [DOI] [PubMed] [Google Scholar]
- 24.Yousem SA, Sonmez-Alpan E: Use of a biotinylated DNA probe specific for the human Y chromosome in the evaluation of the allograft lung. Chest 1991, 99:275-279 [DOI] [PubMed] [Google Scholar]
- 25.Thiede C, Florek M, Bornhauser M, Ritter M, Mohr B, Brendel C, Ehninger G, Neubauer A: Rapid quantification of mixed chimerism using multiplex amplification of short tandem repeat markers and fluorescence detection. Bone Marrow Transplant 1999, 23:1055-1060 [DOI] [PubMed] [Google Scholar]
- 26.Nollet F, Billiet J, Selleslag D, Criel A: Standardisation of multiplex fluorescent short tandem repeat analysis for chimerism testing. Bone Marrow Transplant 2001, 28:511-518 [DOI] [PubMed] [Google Scholar]
- 27.Wagers AJ, Sherwood RI, Christensen JL, Weissman IL: Little evidence for developmental plasticity of adult hematopoietic stem cells. Science 2002, 297:2256-2259 [DOI] [PubMed] [Google Scholar]