Idiopathic pulmonary fibrosis (IPF) is a common form of lung fibrotic disease whose causes remain an enigma. The disease is limited to the lung and cases are reported from around the world with no predilection by ethnicity or race. Although no specific genetic factors have been identified, there have been cases of reported inheritable forms of IPF. 1-3 IPF is progressive and characterized by increased fibroblastic proliferation and extracellular matrix remodeling. The result of these processes is the dramatic disruption of the lungs natural architecture. Until recently, the most common underlying theory as to the cause of IPF was that an inflammatory process injures the lung, causing runaway fibrosis and tissue destruction. This hypothesis has been challenged recently and several reports have implicated epithelial-mesenchymal signaling as playing an important role in IPF. 4,5 A better understanding of the molecular mechanisms underlying the causes and effects of IPF will be important in the drive to develop better therapeutics and treatments that have been lacking. In this issue of The American Journal of Pathology, Chilosi and colleagues 6 report that the Wnt signal transduction pathway is aberrantly activated in IPF. This observation is important considering the wealth of knowledge supporting a role for Wnt signaling in cellular proliferation and the implications of Wnt signaling in human disease.
Wnt Signaling and Human Disease
Vertebrate Wnt proteins are homologues of the Drosophila wingless gene and have been show to play important roles in regulating cell differentiation, proliferation, and polarity. 7-10 Wnt proteins are cysteine-rich secreted glycoproteins that signal through at least three known pathways. The best understood of these, commonly called the canonical pathway, involves binding of Wnt proteins to frizzled cell surface receptors and low-density lipoprotein cell surface co-receptors, inhibiting glycogen synthase kinase 3β (GSK-3β) phosphorylation of the cytoskeletal protein β-catenin. Hypophosphorylated β-catenin is then translocated to the nucleus where it binds to members of the LEF/TCF family of transcription factors. Binding of β-catenin converts LEF/TCF factors from repressors to activators, thereby switching on cell-specific gene transcription. The other two pathways that Wnt proteins can signal through either activate calmodulin kinase II and protein kinase C (known as the Wnt/Ca++ pathway) or jun N-terminal kinase (also known as the planar cell polarity pathway).
Several components of the Wnt pathway have been implicated in tumorigenesis in humans and mice. Wnt1 was first identified from a retroviral integration in mice that caused mammary tumors. 11,12 Overexpression of protein kinase CK2 in the mammary gland, which potentiates β-catenin-dependent Wnt signaling, also increases the incidence of mammary tumors in transgenic mice. 13,14 The tissue that has revealed the most extensive correlation between Wnt signaling and tumorigenesis is gut epithelia. Approximately 80% of all colon carcinomas contain mutations in the adenomatous polyposis coli (APC) tumor suppressor gene, which regulates Wnt signaling by binding to β-catenin and axin, directing the destruction of hypophosphorylated β-catenin. 15 These mutations in APC eliminate interaction with β-catenin, increasing its nuclear accumulation and LEF/TCF-regulated gene transcription. In addition, several reports have described mutations in β-catenin itself in some colon tumors and these mutations occur in or near the GSK-3β phosphorylation sites. 15,16 Chilosi and colleagues 6 looked for β-catenin mutations in IPF patients but did not find any. This may not be surprising because it is likely that the aberrant activation of the Wnt pathway is a response and not a cause of IPF. However, it will be important in the future to look for both β-catenin mutations and mutations in other Wnt pathway members such as APC in lung tumors and other hyperproliferative lung diseases. Because of the complexity of the Wnt pathway and the fact that it is capable of signaling through at least three distinct pathways, it will be a challenge to determine what if any genetic mutations are responsible for IPF or other lung diseases. The recent advances in deciphering both the human and mouse genomes should help in this endeavor.
Lung Development and Wnt Signaling
In the mouse, the lung arises from the primitive foregut endoderm starting at approximately E9.5 during mouse development. 17 This primitive epithelium is surrounded by mesodermally derived multipotent mesenchymal cells, which in time will differentiate into several cell lineages including bronchial and vascular smooth muscle, pulmonary fibroblasts, and endothelial cells of the vasculature. During gestation, the airway epithelium evolves and grows through a process termed branching morphogenesis. This process results in the three-dimensional arborized network of airways required to generate sufficient surface area for postnatal respiration. Mouse embryonic lung development can be divided into at least four stages: embryonic (E9.5 to E12.5), pseudoglandular (E12.5 to E16.0), canalicular (E16.0 to E17.5), and saccular/alveolar (E17.5 to postnatal). During development, epithelial-mesenchymal signaling plays an important role in the regulation of both epithelial and mesenchymal cell differentiation and development. Several important signaling molecules are expressed in the airway epithelium and signal to the adjacent mesenchyme including members of the bone morphogenetic family (BMP-4), transforming growth factor family (TGF-β1, -2), and sonic hedgehog (SHH). In turn, the mesenchyme expresses several signaling molecules such as FGF-7, -9, and -10, important for lung epithelial development and proliferation. Gain of function and loss of function experiments in mice have demonstrated an important role for each of these factors in regulating lung epithelial and mesenchymal proliferation and differentiation. 18-25
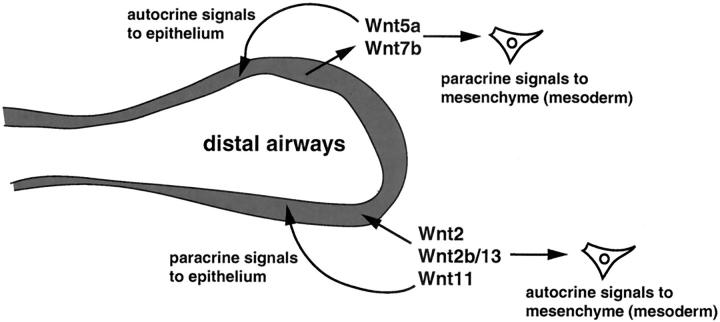
Wnt signaling has been shown to play an essential role in brain, limb, mammary, skin, and most recently cardiovascular development. 26-35 However, until recently, little was known of what role Wnt signaling played during lung development. Several Wnt genes are expressed in the developing and adult lung including Wnt2, Wnt2b/13, Wnt7b, Wnt5a, and Wnt11. 30,31,36-38 Of these, Wnt5a and Wnt7b are expressed at high levels exclusively in the developing airway epithelium during lung development. Wnt2, Wnt5a, and Wnt7b have been inactivated through homologous recombination in mice. Wnt2-null mice do not display an overt lung phenotype and Wnt5a null mice have late-stage lung maturation defects, corresponding to expression of Wnt5a later in lung development. 36,39 Two reports show that inactivation of Wnt7b results in either early embryo demise because of defects in extra-embryonic tissues or perinatal demise because of defects in lung development. 40,41 These lung defects include decreased mesenchymal proliferation, lung hypoplasia caused by reduced branching, and pulmonary vascular smooth muscle defects leading to blood vessel hemorrhage in the lung. 41 Thus, Wnt signaling regulates important aspects of both epithelial and mesenchymal development during gestation, likely through both autocrine and paracrine signaling mechanisms (Figure 1) ▶ .
Figure 1.
Autocrine and paracrine Wnt signaling in the lung. Several Wnt ligands are expressed in either the epithelium or mesenchyme during development and in the adult. β-catenin is expressed in both alveolar epithelium as well as the adjacent mesenchyme. Epithelial-mesenchymal signaling via Wnts may regulate diverse aspects of lung development and injury repair.
Chilosi and colleagues 6 also observed accumulation of nuclear β-catenin in both epithelial and mesenchymal (myofibroblasts) cell lineages in adult human lung. Other reports support these observations during mouse lung development. 42 Type 2 pneumocytes appear to express high levels of β-catenin both in the embryo and in the adult (current study). 42 Type 2 cells are precursors of type 1 cells, which form the thin diffusible stratum important for gas exchange in the lung. Type 2 cells have been shown to re-enter the cell cycle, grow, and differentiate into type 1 cells in some models of lung re-epithelialization. 43,44 Importantly, type 2 cells proliferate excessively during IPF and other proliferative lung diseases and increased nuclear β-catenin in these cells suggests that Wnt signaling regulates this proliferation. 45,46 Increased proliferation of type 2 cells in IPF may also inhibit their differentiation into type 1 cells because excessive proliferation is often antagonistic to cellular differentiation. In this context, it is important to note that expression of certain important transcriptional and signaling regulators in the lung decreases with gestational age. Forced overexpression of some of these such as BMP-4, GATA6, and Foxa2 results in aberrant lung development that exhibits many aspects of arrested lung epithelial maturity. 25,47,48 Thus, a careful balance of the correct spatial and temporal expression of certain regulatory genes is required for normal lung development and improper activation of these pathways can result in severe defects in epithelial differentiation.
The finding by Chilosi and colleagues 6 that nuclear β-catenin is found in the mesenchyme adjacent to the airway epithelium is significant especially because these cells appear to be myofibroblastic in nature and may contribute to bronchial and vascular smooth muscle in the lung. Although the authors suggest that Wnt signals in these mesenchymal cells could be autocrine in nature, it is just as likely that the mesenchymal cells are responding to a paracrine signal from the airway epithelium where Wnts such as Wnt5a and Wnt7b are expressed. In this way, the epithelium may be responsible for causing the aberrant activation of Wnt signaling in adjacent mesenchyme, leading to increased fibrosis and damage to the lung. This is particularly relevant because of the increase in the number of type 2 cells in the airways of IPF patients. This may also be reflective of a switch to an embryonic phenotype in the alveolus, where type 1 cells are rare. In turn, this would result in an increase in expression of several genes, including Wnts such as Wnt7b, whose expression is dramatically down-regulated in postnatal development. 38,41 The increased level of Wnts may inhibit the proper differentiation of more mature alveolar cells such as type 1 cells, impairing the repair process.
Because nuclear translocation of β-catenin is a result of Wnt signaling activity, its presence in cells such as distal airway epithelium and in mesenchyme adjacent to airway epithelium suggests that epithelial-mesenchymal Wnt signaling is active and likely plays an important role during both lung development and disease states such as IPF. It will be important in the future to use LEF/TCF-lacZ reporter mice that reveal active canonical Wnt signaling through the nuclear translocation of β-catenin and co-activation of LEF/TCF transcription factors, in mouse models of IPF and other lung injury models. 49 These studies may reveal a more complete, and possibly more complex, picture of Wnt signaling during the epithelial and mesenchymal repair process in the lung as well as normal lung development.
Regulation of Cell-Matrix Interactions by Wnt Signaling
Several recent reports have demonstrated a link between Wnt signaling and regulation of cell-matrix interactions including cell adhesion and migration. In particular, Wnt signaling has been shown to affect cell motility and invasiveness of melanoma cells. 50 In this system, melanoma cells overexpressing Wnt5a displayed increased adhesiveness, which correlated to a reorganized actin cytoskeleton. 50 These data suggest that Wnt5a expression correlates directly with the metastatic ability of melanoma tumors and as such may provide a novel target for new therapies. Wnt5a has also been shown to induce cardiac myocyte aggregation via a β-catenin-dependent pathway, suggesting a role in cell-cell adhesion. 51 Together with the emerging role of both canonical and noncanonical Wnt signaling playing an essential role in cardiac myocyte specification, 32,33 Wnt5a regulation of cardiac myocyte cell aggregation may implicate this Wnt in the regulation of both specification and morphogenesis of the heart.
In IPF lung tissue, Chilosi and colleagues 6 found that the important extracellular matrix metalloproteinase matrilysin was overexpressed in some of the cells containing high levels of nuclear β-catenin. This is supported by previous studies showing that matrilysin is a molecular target of Wnt signaling. 52 Matrilysin has been linked to a role in carcinogenesis both in intestinal and endometrial tumors. Increased matrilysin expression strongly correlates with increased nuclear β-catenin expression and inhibition of this nuclear translocation results in decreased matrilysin expression. 52 So, what does increased expression of matrilysin do in IPF? One hypothesis is that increased degradation of the extracellular matrix from increased matrilysin expression leads to decreased cell adhesion and increased cell motility. In IPF, this might reduce the ability of both epithelial and mesenchymal cells to properly restructure the alveolar architecture after injury. In addition, extracellular matrix integrity may be required for type 1 cell differentiation, which might be predicted because of their flattened morphology and the very large surface area that they cover in the alveolus. This process may contribute to an increase in type 2 cell proliferation, which in turn could decrease type 1 cell differentiation.
Wnt Signaling and IPF
Several models could explain the finding that Wnt signaling is aberrantly activated in IPF. First, unregulated activation of the Wnt signaling pathway could be a physiological response to either lung injury or the repair process. This may be because of the requirement of the Wnt pathway for proliferation in cells such as type 2 alveolar epithelium and adjoining myofibroblasts. In this model, Wnt signaling should deactivate once the repair process is complete, leading to a return to normal proliferation. In the second model, aberrant Wnt signaling is the initiating event leading to increased cell proliferation in type 2 cells, which may inhibit their ability to differentiate into type 1 cells and restructure the alveolar architecture properly. Either injury-induced or spontaneous mutations in certain components of the canonical Wnt pathway or in regulatory molecules that regulate this pathway may result in this dysregulation of cell proliferation. The fact that nuclear β-catenin is up-regulated in other lung proliferative diseases suggests that the data presented by Chilosi and colleagues 6 may be a response and not a primary causative event in IPF. Moreover, the unregulated proliferation in type 2 cells and mesenchymal fibroblasts along with the increased presence of nuclear β-catenin suggests that the Wnt pathway is continuously stimulated in lung diseases such as IPF and that inhibitors of Wnt signaling may provide a means to control this proliferation.
It is intriguing to note that the Chilosi and colleagues 6 detected increased nuclear β-catenin in the mesenchyme adjacent to the airway epithelium, what the authors describe as myofibroblasts. Several lines of evidence suggest that these myofibroblasts can induce apoptosis in neighboring epithelial cells in vitro and in vivo, probably through degradation of the extracellular matrix. 53-55 In addition, in IPF there appears to be either a lack of re-epithelialization or an increase in type 2 cells with little if any maturation of type 1 cells, leading to injured areas with exposed mesodermal components or re-epithelialized with immature type 2 cells. Since it has been demonstrated that type 2 cells express high levels of TGF-β1, which is a profibrotic cytokine, in IPF either scenario would inhibit the proper re-epithelialization of these injured areas, causing more fibrosis. 4,5 This process could go unchecked and eventually lead to massive changes in tissue architecture, eventual tissue destruction, and loss of lung function.
Although it is unclear from the studies by Chilosi and colleagues 6 whether activation of the canonical Wnt pathway is reflective of IPF or possibly a causative mechanism underlying the phenotypic characteristics of IPF, it is becoming increasingly clear that Wnt signaling plays an important role both in lung development and differentiation. As the authors in the present study state, inhibitory molecules for components of the Wnt pathway are currently under intensive investigation and will hopefully lead to possible treatments of diseases as varied as gastrointestinal and mammary tumors as well as IPF.
Footnotes
Address reprint requests to Edward E. Morrisey, Ph.D., University of Pennsylvania, 956 BRB II/III, 421 Curie Blvd., Philadelphia, PA 19104. E-mail: emorrise@mail.med.upenn.edu.
References
- 1.Murphy A, O’Sullivan BJ: Familial fibrosing alveolitis. Ir J Med Sci 1981, 150:204-209 [DOI] [PubMed] [Google Scholar]
- 2.Bitterman PB, Rennard SI, Keogh BA, Wewers MD, Adelberg S, Crystal RG: Familial idiopathic pulmonary fibrosis. Evidence of lung inflammation in unaffected family members. N Engl J Med 1986, 314:1343-1347 [DOI] [PubMed] [Google Scholar]
- 3.Mageto YN, Raghu G: Genetic predisposition of idiopathic pulmonary fibrosis. Curr Opin Pulm Med 1997, 3:336-340 [DOI] [PubMed] [Google Scholar]
- 4.Kapanci Y, Desmouliere A, Pache JC, Redard M, Gabbiani G: Cytoskeletal protein modulation in pulmonary alveolar myofibroblasts during idiopathic pulmonary fibrosis. Possible role of transforming growth factor beta and tumor necrosis factor alpha. Am J Respir Crit Care Med 1995, 152:2163-2169 [DOI] [PubMed] [Google Scholar]
- 5.Khalil N, O’Connor RN, Unruh HW, Warren PW, Flanders KC, Kemp A, Bereznay OH, Greenberg AH: Increased production and immunohistochemical localization of transforming growth factor-beta in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol 1991, 5:155-162 [DOI] [PubMed] [Google Scholar]
- 6.Chilosi M, Poletti V, Zamo A, Lestani M, Montagna L, Piccoli P, Pedron S, Bertaso M, Scarpa A, Murer B, Cancellieri A, Maestro R, Semenzato G, Doglioni C: Aberrant Wnt/beta-catenin pathway activation in idiopathic pulmonary fibrosis. Am J Pathol 2003, 162:1497-1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cadigan KM, Nusse R: Wnt signaling: a common theme in animal development. Genes Dev 1997, 11:3286-3305 [DOI] [PubMed] [Google Scholar]
- 8.Parr BA, McMahon AP: Wnt genes and vertebrate development. Curr Opin Genet Dev 1994, 4:523-528 [DOI] [PubMed] [Google Scholar]
- 9.Smalley MJ, Dale TC: Wnt signalling in mammalian development and cancer. Cancer Metastasis Rev 1999, 18:215-230 [DOI] [PubMed] [Google Scholar]
- 10.Willert K, Nusse R: Beta-catenin: a key mediator of Wnt signaling. Curr Opin Genet Dev 1998, 8:95-102 [DOI] [PubMed] [Google Scholar]
- 11.Tsukamoto AS, Grosschedl R, Guzman RC, Parslow T, Varmus HE: Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell 1988, 55:619-625 [DOI] [PubMed] [Google Scholar]
- 12.Jue SF, Bradley RS, Rudnicki JA, Varmus HE, Brown AM: The mouse Wnt-1 gene can act via a paracrine mechanism in transformation of mammary epithelial cells. Mol Cell Biol 1992, 12:321-328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landesman-Bollag E, Romieu-Mourez R, Song DH, Sonenshein GE, Cardiff RD, Seldin DC: Protein kinase CK2 in mammary gland tumorigenesis. Oncogene 2001, 20:3247-3257 [DOI] [PubMed] [Google Scholar]
- 14.Song DH, Sussman DJ, Seldin DC: Endogenous protein kinase CK2 participates in Wnt signaling in mammary epithelial cells. J Biol Chem 2000, 275:23790-23797 [DOI] [PubMed] [Google Scholar]
- 15.Polakis P, Hart M, Rubinfeld B: Defects in the regulation of beta-catenin in colorectal cancer. Adv Exp Med Biol 1999, 470:23-32 [DOI] [PubMed] [Google Scholar]
- 16.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW: Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science 1997, 275:1787-1790 [DOI] [PubMed] [Google Scholar]
- 17.Warburton D, Schwarz M, Tefft D, Flores-Delgado G, Anderson KD, Cardoso WV: The molecular basis of lung morphogenesis. Mech Dev 2000, 92:55-81 [DOI] [PubMed] [Google Scholar]
- 18.Bellusci S, Grindley J, Emoto H, Itoh N, Hogan BL: Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development 1997, 124:4867-4878 [DOI] [PubMed] [Google Scholar]
- 19.Simonet WS, DeRose ML, Bucay N, Nguyen HQ, Wert SE, Zhou L, Ulich TR, Thomason A, Danilenko DM, Whitsett JA: Pulmonary malformation in transgenic mice expressing human keratinocyte growth factor in the lung. Proc Natl Acad Sci USA 1995, 92:12461-12465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark JC, Tichelaar JW, Wert SE, Itoh N, Perl AK, Stahlman MT, Whitsett JA: FGF-10 disrupts lung morphogenesis and causes pulmonary adenomas in vivo. Am J Physiol 2001, 280:L705-L715 [DOI] [PubMed] [Google Scholar]
- 21.Min H, Danilenko DM, Scully SA, Bolon B, Ring BD, Tarpley JE, DeRose M, Simonet WS: Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev 1998, 12:3156-3161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Motoyama J, Liu J, Mo R, Ding Q, Post M, Hui CC: Essential function of Gli2 and Gli3 in the formation of lung, trachea and oesophagus. Nat Genet 1998, 20:54-57 [DOI] [PubMed] [Google Scholar]
- 23.Litingtung Y, Lei L, Westphal H, Chiang C: Sonic hedgehog is essential to foregut development. Nat Genet 1998, 20:58-61 [DOI] [PubMed] [Google Scholar]
- 24.Pepicelli CV, Lewis PM, McMahon AP: Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr Biol 1998, 8:1083-1086 [DOI] [PubMed] [Google Scholar]
- 25.Weaver M, Yingling JM, Dunn NR, Bellusci S, Hogan BL: Bmp signaling regulates proximal-distal differentiation of endoderm in mouse lung development. Development 1999, 126:4005-4015 [DOI] [PubMed] [Google Scholar]
- 26.Baker JC, Beddington RS, Harland RM: Wnt signaling in Xenopus embryos inhibits bmp4 expression and activates neural development. Genes Dev 1999, 13:3149-3159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradley RS, Brown AM: A soluble form of Wnt-1 protein with mitogenic activity on mammary epithelial cells. Mol Cell Biol 1995, 15:4616-4622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang C, Hemmati-Brivanlou A: Neural crest induction by Xwnt7B in Xenopus. Dev Biol 1998, 194:129-134 [DOI] [PubMed] [Google Scholar]
- 29.Kispert A, Vainio S, McMahon AP: Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development 1998, 125:4225-4234 [DOI] [PubMed] [Google Scholar]
- 30.Kispert A, Vainio S, Shen L, Rowitch DH, McMahon AP: Proteoglycans are required for maintenance of Wnt-11 expression in the ureter tips. Development 1996, 122:3627-3637 [DOI] [PubMed] [Google Scholar]
- 31.Lin Y, Liu A, Zhang S, Ruusunen T, Kreidberg JA, Peltoketo H, Drummond I, Vainio S: Induction of ureter branching as a response to Wnt-2b signaling during early kidney organogenesis. Dev Dyn 2001, 222:26-39 [DOI] [PubMed] [Google Scholar]
- 32.Marvin MJ, Di Rocco G, Gardiner A, Bush SM, Lassar AB: Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev 2001, 15:316-327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pandur P, Lasche M, Eisenberg LM, Kuhl M: Wnt-11 activation of a non-canonical Wnt signalling pathway is required for cardiogenesis. Nature 2002, 418:636-641 [DOI] [PubMed] [Google Scholar]
- 34.Reya T, O’Riordan M, Okamura R, Devaney E, Willert K, Nusse R, Grosschedl R: Wnt signaling regulates B lymphocyte proliferation through a LEF-1 dependent mechanism. Immunity 2000, 13:15-24 [DOI] [PubMed] [Google Scholar]
- 35.Stark K, Vainio S, Vassileva G, McMahon AP: Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 1994, 372:679-683 [DOI] [PubMed] [Google Scholar]
- 36.Monkley SJ, Delaney SJ, Pennisi DJ, Christiansen JH, Wainwright BJ: Targeted disruption of the Wnt2 gene results in placentation defects. Development 1996, 122:3343-3353 [DOI] [PubMed] [Google Scholar]
- 37.Yamaguchi TP, Bradley A, McMahon AP, Jones S: A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development 1999, 126:1211-1223 [DOI] [PubMed] [Google Scholar]
- 38.Weidenfeld J, Shu W, Zhang L, Millar SE, Morrisey EE: The WNT7B promoter is regulated by TTF-1, GATA6, and Foxa2 in lung epithelium. J Biol Chem 2002, 277:21061-21070 [DOI] [PubMed] [Google Scholar]
- 39.Li C, Xiao J, Hormi K, Borok Z, Minoo P: Wnt5a participates in distal lung morphogenesis. Dev Biol 2002, 248:68-81 [DOI] [PubMed] [Google Scholar]
- 40.Parr BA, Cornish VA, Cybulsky MI, McMahon AP: Wnt7b regulates placental development in mice. Dev Biol 2001, 237:324-332 [DOI] [PubMed] [Google Scholar]
- 41.Shu W, Jiang YQ, Lu MM, Morrisey EE: Wnt7b regulates mesenchymal proliferation and vascular development in the lung. Development 2002, 129:4831-4842 [DOI] [PubMed] [Google Scholar]
- 42.Tebar M, Destree O, de Vree WJ, Ten Have-Opbroek AA: Expression of Tcf/Lef and sFrp and localization of beta-catenin in the developing mouse lung. Mech Dev 2001, 109:437-440 [DOI] [PubMed] [Google Scholar]
- 43.Borok Z, Hami A, Danto SI, Zabski SM, Crandall ED: Rat serum inhibits progression of alveolar epithelial cells toward the type I cell phenotype in vitro. Am J Respir Cell Mol Biol 1995, 12:50-55 [DOI] [PubMed] [Google Scholar]
- 44.Danto SI, Shannon JM, Borok Z, Zabski SM, Crandall ED: Reversible transdifferentiation of alveolar epithelial cells. Am J Respir Cell Mol Biol 1995, 12:497-502 [DOI] [PubMed] [Google Scholar]
- 45.Kawanami O, Ferrans VJ, Crystal RG: Structure of alveolar epithelial cells in patients with fibrotic lung disorders. Lab Invest 1982, 46:39-53 [PubMed] [Google Scholar]
- 46.Kasper M, Haroske G: Alterations in the alveolar epithelium after injury leading to pulmonary fibrosis. Histol Histopathol 1996, 11:463-483 [PubMed] [Google Scholar]
- 47.Koutsourakis M, Keijzer R, Visser P, Post M, Tibboel D, Grosveld F: Branching and differentiation defects in pulmonary epithelium with elevated Gata6 expression. Mech Dev 2001, 105:105-114 [DOI] [PubMed] [Google Scholar]
- 48.Zhou L, Dey CR, Wert SE, Yan C, Costa RH, Whitsett JA: Hepatocyte nuclear factor-3beta limits cellular diversity in the developing respiratory epithelium and alters lung morphogenesis in vivo. Dev Dyn 1997, 210:305-314 [DOI] [PubMed] [Google Scholar]
- 49.DasGupta R, Fuchs E: Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 1999, 126:4557-4568 [DOI] [PubMed] [Google Scholar]
- 50.Weeraratna AT, Jiang Y, Hostetter G, Rosenblatt K, Duray P, Bittner M, Trent JM: Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell 2002, 1:279-288 [DOI] [PubMed] [Google Scholar]
- 51.Toyofuku T, Hong Z, Kuzuya T, Tada M, Hori M: Wnt/frizzled-2 signaling induces aggregation and adhesion among cardiac myocytes by increased cadherin-beta-catenin complex. J Cell Biol 2000, 150:225-241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crawford HC, Fingleton BM, Rudolph-Owen LA, Goss KJ, Rubinfeld B, Polakis P, Matrisian LM: The metalloproteinase matrilysin is a target of beta-catenin transactivation in intestinal tumors. Oncogene 1999, 18:2883-2891 [DOI] [PubMed] [Google Scholar]
- 53.Uhal BD, Joshi I, Hughes WF, Ramos C, Pardo A, Selman M: Alveolar epithelial cell death adjacent to underlying myofibroblasts in advanced fibrotic human lung. Am J Physiol 1998, 275:L1192-L1199 [DOI] [PubMed] [Google Scholar]
- 54.Uhal BD, Joshi I, True AL, Mundle S, Raza A, Pardo A, Selman M: Fibroblasts isolated after fibrotic lung injury induce apoptosis of alveolar epithelial cells in vitro. Am J Physiol 1995, 269:L819-L828 [DOI] [PubMed] [Google Scholar]
- 55.Selman M, Ruiz V, Cabrera S, Segura L, Ramirez R, Barrios R, Pardo A: TIMP-1, -2, -3, and -4 in idiopathic pulmonary fibrosis. A prevailing nondegradative lung microenvironment? Am J Physiol 2000, 279:L562-L574 [DOI] [PubMed] [Google Scholar]