Abstract
Activated hepatic stellate cells have been implicated in the fibrogenic process associated with iron overload, both in animal models and in human hemochromatosis. Previous studies have evaluated the role of ferritin/ferritin receptor interactions in the activation of stellate cells and subsequent fibrogenesis; however, the role of transferrin in hepatic stellate cell biology is unknown. This study was designed to identify and characterize the stellate cell transferrin receptor and to evaluate the influence of transferrin on stellate cell activation. Identification and characterization of the stellate cell transferrin receptor was determined by competitive displacement assays. The effect of transferrin on stellate cell activation was assessed using western blot analysis for α-smooth muscle actin expression, [3H]Thymidine incorporation, and real-time RT-PCR for procollagen α1(I) mRNA expression. A specific receptor for rat transferrin was observed on activated but not quiescent stellate cells. Transferrin significantly increased the expression of α-smooth muscle actin, but caused a decrease in proliferation. Transferrin induced a significant increase in procollagen α1(I) mRNA expression. In conclusion, this study has demonstrated for the first time a specific, high affinity receptor for rat transferrin on activated hepatic stellate cells, which via interaction with transferrin regulates stellate cell activation. This suggests that transferrin may be an important factor in the activation of hepatic stellate cells in conditions of iron overload.
Hemochromatosis is a common, genetic iron overload disorder characterized by iron accumulation within the parenchyma of various organs, in particular the liver. Without treatment hemochromatosis can progress to hepatic injury, fibrosis and cirrhosis. The activated hepatic stellate cell (HSC) has been shown to be responsible for fibrosis, which occurs in a variety of chronic liver diseases. 1-3 Previous studies by our group and others have demonstrated an association between increasing hepatic iron stores and the presence of the activated HSC phenotype in both C282Y hemochromatosis 4 and in animal models of iron overload. 5,6 However, the initiating events responsible for HSC activation in iron overload are not fully understood.
The pathways of iron metabolism in both hepatocytes and Kupffer cells have been extensively studied 7-9 ; however, there is a relative paucity of information on the pathways associated with HSC iron metabolism. HSC have been shown to possess a specific, high-affinity receptor for the iron-storage protein tissue ferritin 10 ; however, the existence of a receptor for the iron-transport protein transferrin and its role in HSC activation have not been previously examined. As the liver is the predominant site of iron accumulation in hemochromatosis and the site of often severe iron-induced fibrotic injury, an increased understanding of the pathways of iron metabolism in HSC is of great importance in elucidating the mechanisms of iron-induced liver injury.
Transferrin is a single-chain glycoprotein of molecular weight of approximately 80 kd. 11,12 The transferrin receptor (TfR) is a dimer of subunits of approximately 90 kd each, which are linked by a single disulphide bond. Uptake of iron by hepatic cells through the transferrin/transferrin receptor pathway is considered quantitatively the most important 13 ; however, this pathway has not previously been examined in HSC.
The aims of this study were to identify the existence of a HSC transferrin receptor, characterize the binding kinetics of this receptor, and examine the influence of transferrin on parameters of HSC activation. Iron metabolism in HSC is poorly understood and these studies are the first to investigate the kinetics of transferrin uptake and the role of transferrin in the HSC activation process. This study was designed to provide important new knowledge on the pathways associated with HSC iron metabolism and to elucidate the potential mechanisms associated with iron-induced HSC activation, which occurs in diseases of iron overload, such as hemochromatosis.
Materials and Methods
HSC Isolation
Rat HSC were isolated from male Sprague-Dawley rats by sequential pronase and collagenase digestion and separated on an arabinogalactan gradient as previously described. 10 HSC were maintained in M199 (supplemented with 12 mmol/L sodium bicarbonate, 12 mmol/L glucose, 4 U/L insulin, 100,000 U/L penicillin, 0.1 g/L streptomycin, 1 μmol/L corticosterone and 10 mmol/L HEPES) + 20% horse and calf serum. For uptake experiments this culture media was replaced with supplemented M199 + 1% calf serum. HSC purity at isolation was between 90 and 95% and viability was >95%. By 5 days in culture the purity and viability had reached 97% and 99%, respectively. HSC viability and purity were assessed as previously described by Ramm et al. 14 These studies were approved by the ethics committees of The Queensland Institute of Medical Research and The University of Queensland, Brisbane and complied with guidelines outlined in “Guide for the Care and Use of Laboratory Animals.”
Preparation of Proteins
Rat apo-transferrin was purchased from the Sigma Chemical Company (St. Louis, MO) and holo-transferrin was prepared as described previously. 15 To assess iron loading of transferrin the absorbance was measured, assuming that diferric transferrin has an A465/A280 ratio of 0.046 and an A465/A412 ratio of >1.4.
For all competitive displacement experiments, rat transferrin (RTf) was iodinated using 125I-labeled Bolton and Hunter reagent (NEN Research Products, Boston, MA) as described previously. 16
Optimization of 125I-RTf Concentration
To determine the optimal concentration of 125I-RTf for use in competitive binding experiments, HSC were incubated with increasing concentrations of 125I-RTf for 2 hours at 37°C. HSC were plated at 1 × 106 cells/ml and cultured for 5 days in media containing 20% serum. Before the optimization experiment the culture media was replaced by media containing 1% calf serum. HSC were exposed to increasing concentrations of 125I-RTf (0.1 to 5 μg/ml) ± 50-fold excess of unlabelled RTf for 2 hours at 37°C. Cells were washed three times in ice-cold PBS and lysed by addition of 0.2 N NaOH. Cell-bound 125I-RTf was determined using a gamma counter (Packard, Canberra, Australia).
Competitive Binding Assays
After isolation, HSC were plated onto 12-well tissue culture plates at 1 × 106 cells/ml. At days 1, 2, 3, 5, and 7 post-isolation, uptake of 125I-RTf was determined as described above with 0.5 μg/ml 125I-RTf ± 50-fold excess of unlabeled RTf.
To determine whether quiescent HSC express a TfR, freshly isolated HSC were also plated onto Teflon-coated inserts (Millipore Corporation, Bedford, MA) and cultured for 6 hours and 24 hours before incubation with 125I-RTf ± 50-fold excess of unlabeled RTf. A minimum 6-hour culture period post-enzyme digestion was used to ensure recovery of receptors post-digestion. Previously a 2-hour period postdigestion showed recovery of hepatocyte transferrin receptors. 17 Culture on Teflon wells ensures that HSC are maintained in a quiescent phenotype. To confirm that HSC were quiescent after culture on Teflon inserts, cell lysates were collected for α-SMA determination by Western blotting. Viability of HSC cultured on Teflon inserts was also examined by trypan blue exclusion and by incubating cells on plastic following seven days of culture on Teflon. By trypan blue exclusion, viability remained over 90%. Following 7 days of culture on Teflon wells and subsequent culture on plastic, cells remained viable and spontaneously activated as assessed by Western blotting for α-smooth muscle actin (α-SMA; see below)(results not shown).
Transferrin Receptor Specificity
After 5 days in culture, HSC were incubated with 0.5 μg/ml 125I-RTf in M199 + 1% calf serum. Cells were incubated with 125I-RTf ± 50-fold excess of unlabeled RTf, mouse transferrin, bovine serum albumin, rat liver ferritin and rat heart ferritin for 2 hours at 37°C. Cells were washed three times in ice-cold PBS and lysed by addition of 0.2 N NaOH. Cell-bound 125I-RTf was determined using a gamma counter.
Time Course Study
After 5 days in culture HSC were washed well in PBS (three times) and incubated with 0.5 μg/ml 125I-RTf ± 50-fold excess of unlabeled RTf in M199 + 1% calf serum for up to 180 minutes. Bound 125I-RTf was determined as described above.
Scatchard Analysis
After 5 days in culture, HSC were incubated with 0.5 μg/ml 125I-RTf ± increasing concentrations of unlabeled RTf (0.005–150 μg/ml) in M199 + 1% calf serum. Cells were incubated for 2 hours at 37°C. After this incubation cells were washed, lysed, and bound 125I-RTf determined. Scatchard analysis was performed using the LIGAND program as described by Munson and Rodbard. 18
Effect of Transferrin on HSC Activation
Preparation of Whole Cell Lysates and Western Blotting
Freshly isolated HSC were treated with holo-Tf (0.05−0.3 mg/ml) at days 0, 3, and 5 and cell extracts were harvested at day 7. HSC whole-cell lysates were prepared as described previously. 19 Protein concentration was determined using Peterson’s modification 20 of the micro-Lowry method (Protein Assay Kit; Sigma Diagnostics, St. Louis, MO). Determination of α-SMA protein expression was achieved by SDS-PAGE and Western blotting. Proteins were transferred to PVDF membrane (Bio-Rad, Regents Park, Australia) before immunodetection with the α-SMA antibody (α-SMA, Sigma Chemical Company; 1:2000). Membranes were then incubated in the secondary antibody (anti-mouse immunoglobulin, horseradish peroxidase [HRP] conjugated, Silenus, AMRAD, Richmond, Australia; 1:1000) before chemiluminescent detection with the ECL detection kit (Amersham, Little Chalfont, Buckinghamshire, UK).
[3H]Thymidine Incorporation Assay
Freshly isolated HSC were treated with holo-Tf (0.05−0.5 mg/ml) at days 0, 3, and 5 and cell extracts were harvested at day 7. After 6 days in culture 2 μCi/ml [methyl-3H]thymidine (5 Ci/mmol; 1 mCi/ml; Amersham) was added to each well as previously described 21 and cell lysates were collected at day 7. Lysates were counted in 4 ml of scintillation fluid (Emulsifier-Safe; Packard) in a LKB 1219 rackbeta liquid scintillation counter.
Determination of Procollagen α1(I) Gene Expression
For mRNA analysis, cells were either treated with RTf at day 0 and RNA collected at day 1, 2, and 3 post-isolation, or grown in culture until day 5, then treated with RTf and RNA collected at 6 and 48 hours post-treatment. HSC were treated with 0.2 mg/ml holo-RTf. Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA), as per manufacturer’s instructions. Concentration and purity of isolated RNA was assessed by spectrophotometry at A260/A280. Following RNA isolation, DNase 1 digestion and first-strand cDNA synthesis were performed using 2 μg of total RNA and oligo dT15 (Invitrogen). The resulting cDNA was then diluted 2.5-fold.
Real-time RT-PCR was performed to quantitate the expression of procollagen α1(I) mRNA and the housekeeping gene, β-actin, for each sample. A Rotorgene thermal cycler (Corbett Research, Brisbane, Australia) was used for real-time RT-PCR experiments. Reactions were performed in a 20 μl volume with 1 μmol/L forward primer, 1 μmol/L reverse primer, and 2 μl of cDNA. Nucleotides, MgCl2, Taq polymerase, and SYBR Green were included in the SYBR Green PCR master mix (Applied Biosystems, Warrington, UK). Additional SYBR Green (Fisher Biotech, Perth, Australia) was added at 1:1000 dilution. Each sample was amplified in triplicate. The PCR conditions included an incubation at 50°C for 5 minutes to activate uracil-N-glycosylase (UNGase), which removes any carry-over contamination from previous PCR products, followed by a denaturation step at 94°C for 10 minutes. Amplification was carried out for 35 cycles (denaturation at 94°C for 15 seconds, annealing at 60°C for 15 seconds and extension at 72°C for 30 seconds). Detection of the fluorescent product was measured at the end of the 72°C annealing step. Cycling was followed by a melt curve analysis (50–90°C with a heating rate of 1° per second) to confirm the amplification of specific PCR products (either procollagen α1(I) or β-actin), and the absence of non-specific amplification or primer dimer. Primer sequences used for analysis of β-actin and procollagen α1(I) were as follows: β-actin forward ACT ATC GCC AAT GAG CGG TTC; β-actin reverse ATG CCA CAG GAT TCC ATA CCC; procollagen α1(I) forward TCG ATT CAC CTA CAG CAC GC; procollagen α1(I) reverse GAC TGT CTT GCC CCA AGT TCC. Primers were designed using Primer Express software (PerkinElmer Life Sciences, Boston, MA). Results for each sample are calculated as a percentage of the β-actin concentration and compared to that of “control” untreated HSC and expressed as fold-increase of the “control” expression.
Statistical Analysis
Results for all experiments represent duplicate, triplicate, or quadruplicate determinations of between two and four experiments from separate HSC isolations. Statistical significance was calculated using Student’s t-test, and a P value of <0.05 was considered significant. Results are represented as means ± SE.
Results
Optimization of 125I-RTf Concentration and Time Course Study
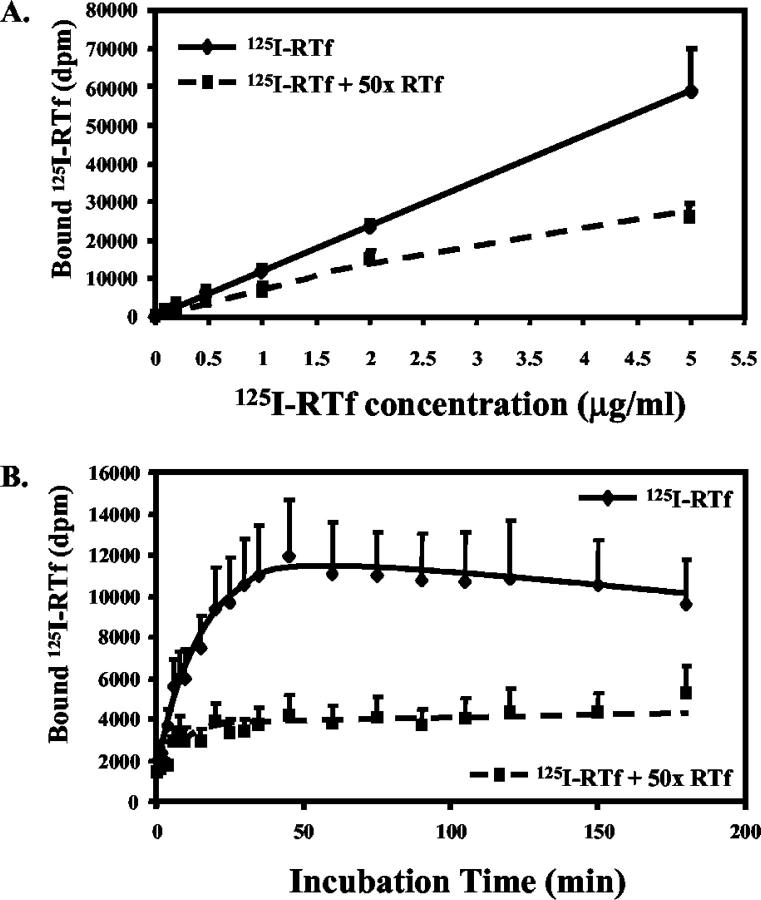
HSC were incubated with increasing concentrations of 125I-RTf for 120 minutes. There was an increase in the level of 125I-RTf binding by HSC. The level of specific binding (as determined by competitive displacement assays) increased to 59.9% ± 7.3% using 125I-RTf at a concentration of 0.5 μg/ml (Figure 1A) ▶ . The level of specific binding did not increase at higher 125I-RTf concentrations. 125I-RTf at 0.5 μg/ml was then used for all following uptake experiments. After incubation of HSC with 0.5 μg/ml 125I-RTf for increasing times from 1 to 180 minutes there was a rapid increase in 125I-RTf binding which reached a maximum at approximately 45 minutes (Figure 1B) ▶ .
Figure 1.
A: Optimization of 125I-RTf concentration. HSC were incubated with increasing concentrations of 125I-RTf ± 50-fold excess of unlabelled RTf at 37°C for 2 hours. Optimal specific binding was achieved using 0.5 μg/ml 125I-RTf (59.9 ± 7.3%). Mean ± SE, n = 2, DPM = disintegrations per minute. B: Time course of 125I-RTf uptake by HSC. HSC were incubated with 0.5 μg/ml 125I-RTf ± 50-fold excess of unlabeled RTf at 37°C for up to 180 minutes. Binding reached saturation at ∼45 minutes. Mean ± SE, n = 3. DPM, disintegrations per minute.
Competitive Binding Assay
Specificity for RTf
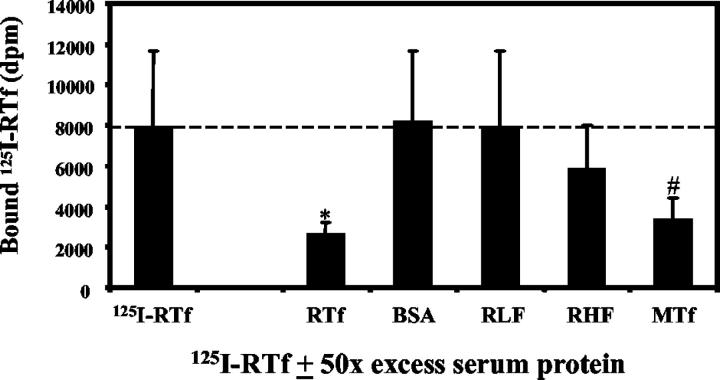
Transferrin receptor binding was demonstrated on HSC cultured for 5 days by competitive displacement of 125I-RTf by a 50-fold excess of unlabelled RTf. Specific binding of 125I-RTf to HSC was 67.4% ± 19.3, P = 0.01 (Figure 2) ▶ . There was no significant competitive displacement of 125I-RTf by bovine serum albumin, rat heart ferritin, or rat liver ferritin; however, there was significant displacement by mouse transferrin (specific binding 58.6% ± 14.4, P = 0.02).
Figure 2.
Identification of the HSC transferrin receptor. HSC were incubated with 0.5 μg/ml 125I-RTf ± 50-fold excess of either unlabelled RTf, BSA, RLF, RHF or MTf at 37°C for 2 hours. HSC demonstrated 67.4 ± 19.3% specific binding for RTf. Mean ± SE, n = 3. *P = 0.01, #P = 0.02 when compared to 125I-RTf. DPM, disintegrations per minute. MTf, mouse transferrin; BSA, bovine serum albumin; RLF, rat liver ferritin; RHF, rat heart ferritin.
Evidence for a TfR on Quiescent versus Activated HSC
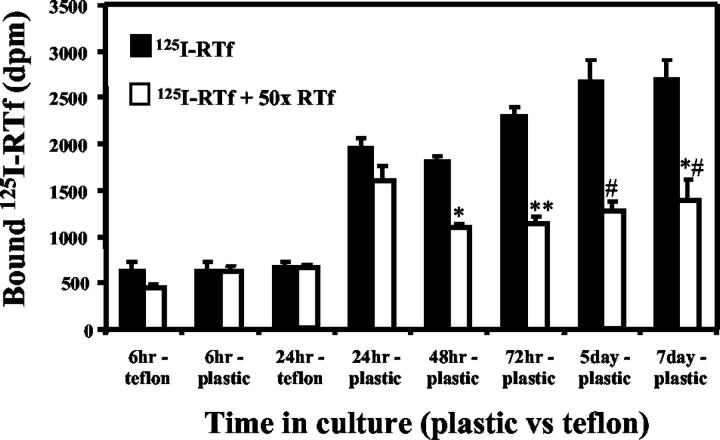
To determine whether binding for RTf by TfR was present on quiescent versus activated HSC, competitive displacement assays were performed on both cell populations. At 6 hours post-isolation, HSC did not demonstrate specific binding of RTf. There was no competitive displacement of 125I-RTf by a 50-fold excess of unlabeled RTf by HSC cultured on either Teflon inserts or cells plated directly onto tissue culture plastic. By 24 hours post-isolation, cells cultured on Teflon demonstrated no specific binding of 125I-RTf. HSC cultured on plastic for 24 hours post-isolation demonstrated specific binding of 18%. By 48 hours, 72 hours, 5 days, and 7 days there was significant displacement of 125I-RTf by a 50-fold excess of unlabelled RTf with specific binding of 38.8%, 50.3%, 52%, and 48.5%, respectively (P = 0.007, 0.003, 0.01, and 0.05, respectively; Figure 3 ▶ ).
Figure 3.
Competitive binding assay in quiescent and activated HSC. HSC were incubated at 37°C for 2 hours with 0.5 μg/ml 125I-RTf ± 50-fold excess of unlabelled RTf. Cells were cultured on Teflon inserts (to maintain HSC in a quiescent phenotype) and on tissue culture plastic (to activate HSC). 125I-RTf uptake was examined at 6, 24, 48, and 72 hours, and 5 and 7 days post-isolation. Quiescent HSC did not express Tf receptors; however, those cultured on plastic, and therefore culture-activated, did demonstrate competitive displacement of 125I-RTf by a 50-fold excess of unlabelled Tf. Mean ± SE, n = 3. *P = 0.007, **P = 0.003, #P = 0.01, ##P = 0.05, when compared to 125I-RTf. DPM, disintegrations per minute.
Scatchard Analysis
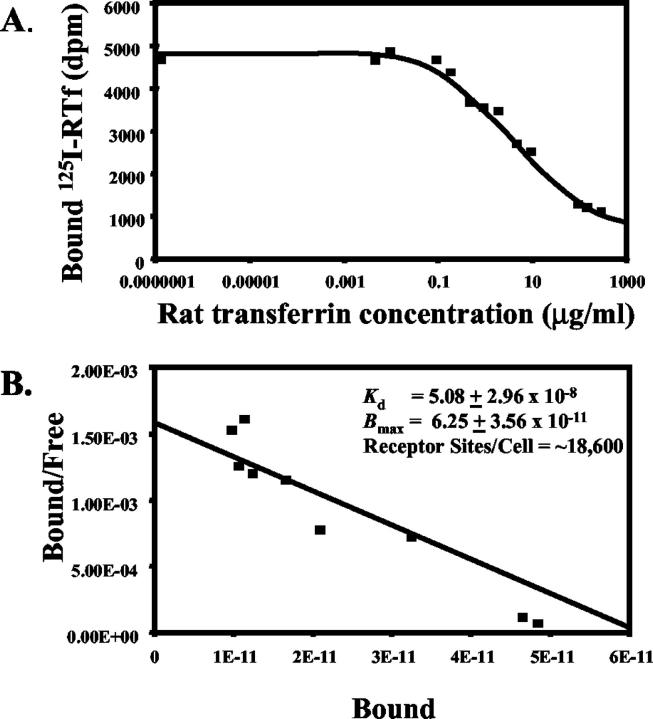
Scatchard analysis of the competitive displacement of 125I-RTf by unlabelled RTf (Figure 4A) ▶ suggested a single class of binding sites for RTf on HSC with an estimated Kd of 5.08 ± 2.96 × 10−8 mol/L and a maximum binding capacity (Bmax) of 6.25 ± 3.56 × 10−11 mol/L (Figure 4B) ▶ . Rat HSC express approximately 18,600 ± 7602 (mean ± SE; range, 11,200 – 26,400) receptor sites per cell.
Figure 4.
Scatchard analysis of the competitive displacement of 125I-RTf by unlabeled RTf by the HSC transferrin receptor. A: HSC were incubated with 0.5 μg/ml 125I-RTf ± increasing concentrations of unlabelled RTf at 37°C for 2 hours. B: Scatchard analysis indicated a single class of binding sites for RTf with an estimated Kd of 5.08 ± 2.96 × 10−8 and a Bmax of 6.25 ± 3.56 × 10−11, with approximately 18,600 receptor sites per cell. Results are expressed as means from duplicate determinations in each experiment from HSC isolated from two separate rats.
Effect of Transferrin on HSC Activation
α-SMA Expression
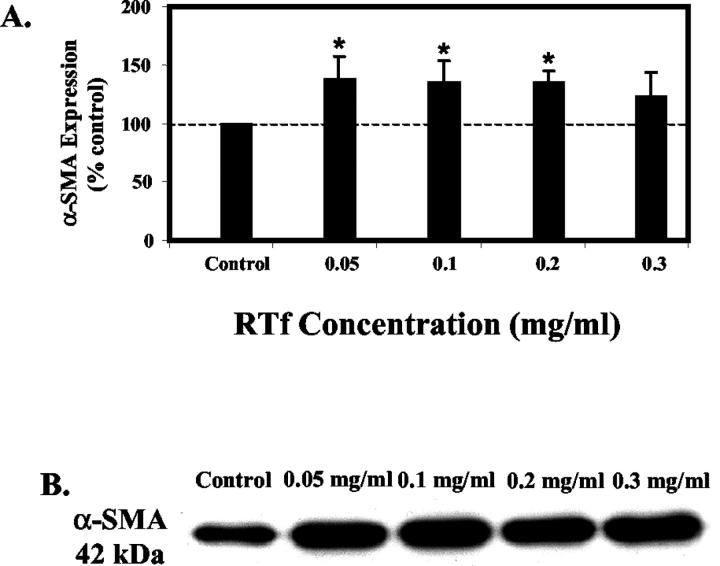
RTf caused a significant 35% to 40% increase in α-SMA expression (Figure 5) ▶ using 0.05, 0.1, and 0.2 mg/ml RTf. RTf used at a concentration of 0.3 mg/ml also caused an increase in α-SMA expression of approximately 20%; however, this did not reach statistical significance.
Figure 5.
Expression of α-SMA in HSC. A: HSC were treated with increasing concentrations of RTf and collected after 7 days in culture and quantitated using scanning laser densitometry following Western blotting. (B) Representative Western blot of HSC cell extracts (10 μg cell protein/lane) showing 42 kd band corresponding to α-SMA. Results are mean ± SE, n = 4. *P < 0.05 when compared to control.
HSC Proliferation
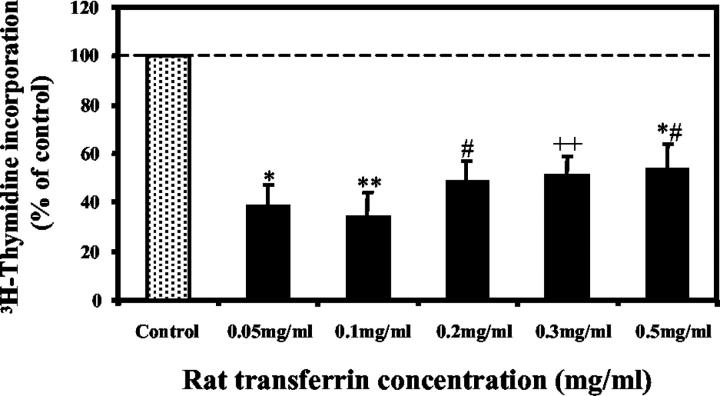
RTf significantly reduced HSC proliferation, as determined by [3H]thymidine incorporation. Proliferation decreased by approximately 45% to 66% at RTf concentrations ranging from 0.05 to 0.5 mg/ml (Figure 6) ▶ .
Figure 6.
Effect of RTf on HSC proliferation as determined by [3H]thymidine incorporation. Results are expressed as a percentage of control and calculated as disintegrations/min. Results are mean ± SE of quadruplicate determinations in each experiment from HSC isolated from two separate rats. *P = 0.002, **P = 0.001, #P = 0.009, ##P = 0.01, *#P = 0.03, when compared to controls.
Procollagen α1(I) Gene Expression
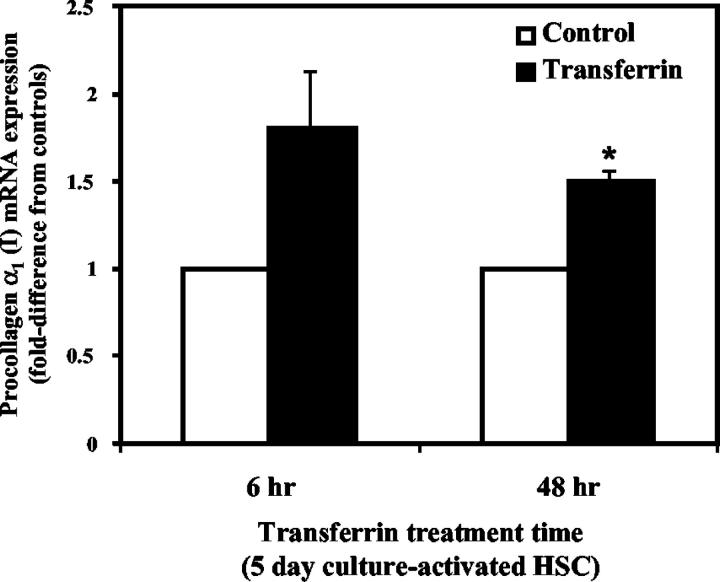
Freshly isolated HSC treated with RTf showed no significant difference in procollagen α1(I) gene expression compared to controls for up to 3 days post-isolation (results not shown). When 5-day culture-activated HSC were treated with RTf for 6 hours, there was an increase in procollagen α1(I) mRNA expression compared to untreated HSC (1.8-fold versus controls); however, this did not reach statistical significance (P = 0.17). After treatment for 48 hours there was a significant increase in procollagen expression (1.5-fold versus controls, P = 0.005) (Figure 7) ▶ .
Figure 7.
Effect of RTf on HSC procollagen α1 (I) mRNA expression as determined by real-time RT-PCR analysis. Day 5 HSC were treated with 0.2 mg/ml RTf. RNA was collected at 6 and 48 hours after treatment with RTf. Procollagen α1(I) mRNA expression in RTF-treated HSC are expressed as a fold-difference from controls (untreated HSC), after standardization against expression of the housekeeping gene β-actin. *P = 0.005. Mean ± SE, n = 3.
Discussion
These studies are the first to demonstrate the presence of a specific, high-affinity receptor for transferrin on culture-activated HSC. Scatchard analysis demonstrated a single class of binding sites with a Kd of 5.08 ± 2.96 × 10−8 mol/L, a maximum binding capacity of 6.25 ± 3.56 × 10−11 mol/L with approximately 18,600 receptor sites per cell. The binding of transferrin to its receptor reached a maximum at approximately 45 minutes with the greatest specific binding (67.4 ± 19.3%) observed using 0.5 μg/ml 125I-RTf. In addition, this study has provided important new information which shows that transferrin plays a role in regulating the activated phenotype of HSC, as demonstrated by the transferrin-induced increase in α-SMA and procollagen α1(I) mRNA expression, and decreased HSC proliferation.
This study has demonstrated that the HSC TfR is specific for transferrin, as a 50-fold excess of unlabeled rat transferrin was able to significantly inhibit binding of 125I-RTf. Mouse transferrin was also able to inhibit binding of 125I-RTf. This kind of cross-species specificity of TfR for transferrin has previously been demonstrated in other cell types by Lim et al, 22 who showed that uptake of heterologous human or rabbit transferrin by rat reticulocytes was actually in excess of that for rat transferrin (150% and 200% of control, respectively). Uptake of transferrin by rabbit reticulocytes has previously been shown to be a temperature-dependent process, which reaches a steady state by approximately 20 minutes of incubation. 23 We have assessed transferrin binding in HSC and have demonstrated maximal binding of transferrin after approximately 45 minutes of incubation at 37°C.
One of the aims of this study was to determine whether TfR were present on both quiescent and activated HSC. The fact that these receptors are not present on quiescent cells is of interest when attempting to understand the mechanisms of iron-induced HSC activation. This observation may be a reflection of the reduced iron requirements of the quiescent HSC phenotype. Most cells take up iron by predominantly receptor-mediated means, 12 but hepatocytes in culture also take up transferrin via a non-receptor-mediated pathway. 24 Quiescent HSC, which may have lower iron requirements than activated HSC, may also use alternative pathways of iron uptake, although further investigation is required to elucidate these pathways. A second transferrin receptor (TfR2) has been recently described, 25 which raises the possibility that this receptor may play a role in HSC iron uptake. However, we have examined TfR2 mRNA expression in HSC by RNase protection assay and have found no expression of this receptor in either quiescent or activated HSC (unpublished observations).
Transferrin receptors have been characterized on a number of cell types including hepatocytes, Kupffer cells, reticulocytes, fibroblasts, neuronal cells and various cell lines. 7,26-29 Previous reports have estimated that the TfR is a high affinity binding site for transferrin with a Kd ranging from 1.65 × 10−7 to 27 × 10−9 mol/L. We have provided the first evidence for a specific, high-affinity receptor for Tf on activated HSC with a Kd of 5.08 ± 2.96 × 10−8 mol/L.
While the identification and characterization of the HSC TfR are novel, the role of the TfR in HSC iron metabolism or in the transformation into myofibroblasts is unclear. It is postulated that the TfR may function to supply the activated HSC with its increasing requirement for iron. As HSC sequester little iron in comparison to hepatocytes and Kupffer cells in conditions of iron overload, it is unlikely that the HSC TfR plays a major role in the release of iron for storage within ferritin, as is the case in other cells. 30 Iron may rapidly transit intracellular iron pools before being used by the cell for the synthesis of iron-requiring enzymes and proteins. Ramm and colleagues 10 have previously demonstrated that activated HSC internalize iron-loaded tissue ferritin. It is postulated that iron derived from the phagocytosis of senescent red blood cells by Kupffer cells is released as ferritin, 31 which can then be taken up by hepatic cells possessing ferritin receptors, such as hepatocytes 32 and HSC, 10 in the paracellular movement of iron in the liver. 10,32,33 Thus, it is possible that HSC may play a role in the transit (via transferrin) and storage (via ferritin) of iron in liver iron metabolism in conjunction with other hepatic cells such as hepatocytes and Kupffer cells, although the precise physiological nature of these pathways remain unknown.
As activated rather than quiescent HSC possess receptors for transferrin it is also possible that the interaction of RTf with the HSC TfR plays a role in the regulation of iron-induced activation of HSC. This is the first study to demonstrate that RTf causes a significant increase in HSC activation, as determined by western blotting for α-SMA. In contrast, studies by Ramm et al 21 demonstrated that long-term exposure to rat liver ferritin caused a dose-dependent decrease in α-SMA expression by HSC over the same time course. While this group initially suggested that this effect of ferritin may be induced by iron, further studies suggest that long-term exposure to ferritin may act to inhibit protein kinase C, which may control α-SMA expression via cell signaling pathways. 34 While it would be of interest to examine the effects of iron-loaded transferrin versus iron-depleted transferrin (apo-transferrin), to delineate the role of iron delivery to HSC, inherent problems exist with this approach, because apo-transferrin is not recognized by the TfR. 35 Further studies are required to examine the mechanisms associated with the RTf-induced increase in α-SMA expression.
RTf caused a significant decrease in HSC proliferation as determined by [3H]thymidine incorporation. These observations appear to contradict the α-SMA results, as with an increased HSC activation the proliferative capacity of HSC might have been expected to increase. Transferrin has previously been shown to increase proliferation of a number of cell types, including pituitary tumor cells, 36 B-cells 37 and cultured blastemal cells. 38 In contrast, Hagiwara et al 39 have shown that iron-free transferrin is not growth-stimulating for a rat intestinal epithelial cell line, and Booth et al 40 failed to demonstrate a significant effect of transferrin on mouse colonic epithelium.
RTf did not significantly influence the expression of procollagen α1(I) mRNA in freshly isolated HSC for up to 3 days post-treatment. However, there was an increase in procollagen α1(I) gene expression at 6 and 48 hours post-transferrin treatment in 5-day culture-activated HSC. Interestingly, the effects of transferrin on both proliferation and type I collagen gene expression by HSC show similarities to the effects of transforming growth factor-β (TGF-β) on HSC activation in vitro. TGF-β has been shown to both inhibit HSC proliferation and increase procollagen α1(I) mRNA expression. 41,42 In addition to its role as an iron transport protein, transferrin may have cytokine-like actions on HSC, similar to those of TGF-β. Indeed it is possible that in HSC-signaling pathways elicited via the interaction of transferrin with its receptor may be similar to those involved in TGF-β signaling. However, further studies are required to fully elucidate the signaling pathways associated with transferrin-induced expression of the type I collagen gene in activated HSC.
In summary, these studies have identified a specific high-affinity TfR on activated HSC, which is not present in the quiescent HSC phenotype. In addition, this study is the first to demonstrate that transferrin plays a role in regulating HSC activation in vitro. Further studies are required to fully clarify the influence of transferrin on HSC activation, particularly in conditions of iron overload in vivo.
Acknowledgments
The rat holo-transferrin was a generous gift from Dr. Gregory Anderson, the Queensland Institute of Medical Research, Australia.
Footnotes
Address reprint requests to Grant A. Ramm, Ph.D., Hepatic Fibrosis Group, The Queensland Institute of Medical Research, Post Office, Royal Brisbane Hospital, Herston, QLD, 4029, Australia. E-mail: grantr@qimr.edu.au.
Supported by an Institute Block Grant from the National Health and Medical Research Council of Australia to The Queensland Institute of Medical Research (to G.A.R and D.H.G.C.).
References
- 1.Friedman SL: Hepatic stellate cells. Prog Liver Dis 1996, 14:101-130 [PubMed] [Google Scholar]
- 2.Ooi LP, Crawford DH, Gotley DC, Clouston AD, Strong RW, Gobe GC, Halliday JW, Bridle KR, Ramm GA: Evidence that “myofibroblast-like” cells are the cellular source of capsular collagen in hepatocellular carcinoma. J Hepatol 1997, 26:798-807 [DOI] [PubMed] [Google Scholar]
- 3.Ramm GA, Nair VG, Bridle KR, Shepherd RW, Crawford DH: Contribution of hepatic parenchymal and nonparenchymal cells to hepatic fibrogenesis in biliary atresia. Am J Pathol 1998, 153:527-535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramm GA, Crawford DH, Powell LW, Walker NI, Fletcher LM, Halliday JW: Hepatic stellate cell activation in genetic haemochromatosis. Lobular distribution, effect of increasing hepatic iron and response to phlebotomy. J Hepatol 1997, 26:584-592 [DOI] [PubMed] [Google Scholar]
- 5.Ramm GA, Li SC, Li L, Britton RS, O’Neill R, Kobayashi Y, Bacon BR: Chronic iron overload causes activation of rat lipocytes in vivo. Am J Physiol 1995, 268:G451-G458 [DOI] [PubMed] [Google Scholar]
- 6.Pietrangelo A, Gualdi R, Casalgrandi G, Montosi G, Ventura E: Molecular and cellular aspects of iron-induced hepatic cirrhosis in rodents. J Clin Invest 1995, 95:1824-1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumazawa M, Misaki M, Baba M, Shima T, Suzuki S: Transferrin receptor on rat Kupffer cells in primary culture. Liver 1986, 6:138-144 [DOI] [PubMed] [Google Scholar]
- 8.Osterloh K, Aisen P: Pathways in the binding and uptake of ferritin by hepatocytes. Biochim Biophys Acta 1989, 1011:40-45 [DOI] [PubMed] [Google Scholar]
- 9.Trinder D, Batey RG, Morgan EH, Baker E: Effect of cellular iron concentration on iron uptake by hepatocytes. Am J Physiol 1990, 259:G611-G617 [DOI] [PubMed] [Google Scholar]
- 10.Ramm GA, Britton RS, O’Neill R, Bacon BR: Identification and characterization of a receptor for tissue ferritin on activated rat lipocytes. J Clin Invest 1994, 94:9-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Worwood M: An overview of iron metabolism at a molecular level. J Intern Med 1989, 226:381-391 [DOI] [PubMed] [Google Scholar]
- 12.Aisen P: Transferrin, the transferrin receptor, and the uptake of iron by cells. Met Ions Biol Syst 1998, 35:585-631 [PubMed] [Google Scholar]
- 13.Page MA, Baker E, Morgan EH: Transferrin and iron uptake by rat hepatocytes in culture. Am J Physiol 1984, 246:G26-G33 [DOI] [PubMed] [Google Scholar]
- 14.Ramm GA, Britton RS, O’Neill R, Blaner WS, Bacon BR: Vitamin A-poor lipocytes: a novel desmin-negative lipocyte subpopulation, which can be activated to myofibroblasts. Am J Physiol 1995, 269:G532-G541 [DOI] [PubMed] [Google Scholar]
- 15.Halliday JW, Powell LW, Mack U: Iron absorption in the rat: the search for possible intestinal mucosal carriers. Br J Haematol 1976, 34:237-250 [DOI] [PubMed] [Google Scholar]
- 16.Bolton AE, Hunter WM: The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J 1973, 133:529-539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kishimoto T, Tavassoli M: Recovery of transferrin receptors on hepatocytes membrane after collagenase perfusion. Biochem Biophys Res Commun 1986, 134:711-715 [DOI] [PubMed] [Google Scholar]
- 18.Munson PJ, Rodbard D: Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem 1980, 107:220-239 [DOI] [PubMed] [Google Scholar]
- 19.Rockey DC, Boyles JK, Gabbiani G, Friedman SL: Rat hepatic lipocytes express smooth muscle actin upon activation in vivo and in culture. J Submicrosc Cytol Pathol 1992, 24:193-203 [PubMed] [Google Scholar]
- 20.Peterson GL: A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem 1977, 83:346-356 [DOI] [PubMed] [Google Scholar]
- 21.Ramm GA, Britton RS, O’Neill R, Kohn HD, Bacon BR: Rat liver ferritin selectively inhibits expression of α-smooth muscle actin in cultured rat lipocytes. Am J Physiol 1996, 270:G370-G375 [DOI] [PubMed] [Google Scholar]
- 22.Lim BC, McArdle HJ, Morgan EH: Transferrin-receptor interaction and iron uptake by reticulocytes of vertebrate animals-a comparative study. J Comp Physiol B 1987, 157:363-371 [DOI] [PubMed] [Google Scholar]
- 23.Iacopetta BJ, Morgan EH: The kinetics of transferrin endocytosis and iron uptake from transferrin in rabbit reticulocytes. J Biol Chem 1983, 258:9108-9115 [PubMed] [Google Scholar]
- 24.Trinder D, Morgan EH, Baker E: The effects of an antibody to the rat transferrin receptor and of rat serum albumin on the uptake of diferric transferrin by rat hepatocytes. Biochim Biophys Acta 1988, 943:440-446 [DOI] [PubMed] [Google Scholar]
- 25.Kawabata H, Yang R, Hirama T, Vuong PT, Kawano S, Gombart AF, Koeffler HP: Molecular cloning of transferrin receptor 2. A new member of the transferrin receptor-like family J Biol Chem 1999, 274:20826-20832 [DOI] [PubMed] [Google Scholar]
- 26.Ward JH, Kushner JP, Kaplan J: Regulation of HeLa cell transferrin receptors. J Biol Chem 1982, 257:10317-10323 [PubMed] [Google Scholar]
- 27.Grasso JA, Bruno M, Yates AA, Wei LT, Epstein PM: Calmodulin dependence of transferrin receptor recycling in rat reticulocytes. Biochem J 1990, 266:261-272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graziadei I, Kahler CM, Wiedermann CJ, Vogel W: The acute-phase protein α1-antitrypsin inhibits transferrin-receptor binding and proliferation of human skin fibroblasts. Biochim Biophys Acta 1998, 1401:170-176 [DOI] [PubMed] [Google Scholar]
- 29.Roskams AJ, Connor JR: Transferrin receptor expression in myelin deficient (md) rats. J Neurosci Res 1992, 31:421-427 [DOI] [PubMed] [Google Scholar]
- 30.Halliday JW, Ramm GA, Powell LW: Cellular iron processing and storage: The role of ferritin. Brock JH Halliday JW Pippard MJ Powell LW eds. Iron Metabolism in Health and Disease. 1994, :pp 97-122 Academic Press/United Kingdom, London [Google Scholar]
- 31.Kondo H, Saito K, Grasso JP, Aisen P: Iron metabolism in the erythrophagocytosing Kupffer cell. Hepatology 1988, 8:32-38 [DOI] [PubMed] [Google Scholar]
- 32.Sibille JC, Kondo H, Aisen P: Interactions between isolated hepatocytes and Kupffer cells in iron metabolism: a possible role for ferritin as an iron carrier protein. Hepatology 1988, 8:296-301 [DOI] [PubMed] [Google Scholar]
- 33.Ono T, Seno S: Transport of ferritin from Kupffer cells to liver parenchymal cells. Morphological and immunocytochemical observations Int J Hematol 1991, 54:93-102 [PubMed] [Google Scholar]
- 34.Ramm GA, Piva TJ, Nair VG, Garrone BM, Watters DJ, Crawford DHG: Role of tissue ferritin in regulation of α-smooth muscle actin expression in hepatic stellate cells via PKCζ and/or nitric oxide. Hepatology 1997, 26:451A(abstract)9252158 [Google Scholar]
- 35.Morgan EH: Effect of pH and iron content of transferrin on its binding to reticulocyte receptors. Biochim Biophys Acta 1983, 762:498-502 [DOI] [PubMed] [Google Scholar]
- 36.Tampanaru-Sarmesiu A, Stefaneanu L, Thapar K, Kontogeorgos G, Sumi T, Kovacs K: Transferrin and transferrin receptor in human hypophysis and pituitary adenomas. Am J Pathol 1998, 152:413-422 [PMC free article] [PubMed] [Google Scholar]
- 37.Bosseloir A, Bouzahzah F, Defrance TH, Heinen E, Simar LJ: The influence of follicular dendritic cells on B-cell proliferation depends on the activation of B cells and the mitogen used. Scand J Immunol 1996, 43:23-30 [DOI] [PubMed] [Google Scholar]
- 38.Mescher AL, Connell E, Hsu C, Patel C, Overton B: Transferrin is necessary and sufficient for the neural effect on growth in amphibian limb regeneration blastemas. Dev Growth Differ 1997, 39:677-684 [DOI] [PubMed] [Google Scholar]
- 39.Hagiwara T, Shinoda I, Fukuwatari Y, Shimamura S: Effects of lactoferrin and its peptides on proliferation of rat intestinal epithelial cell line, IEC-18, in the presence of epidermal growth factor Biosci Biotechnol Biochem 1995, 59:1875-1881 [DOI] [PubMed] [Google Scholar]
- 40.Booth C, Patel S, Bennion GR, Potten CS: The isolation and culture of adult mouse colonic epithelium. Epithelial Cell Biol 1995, 4:76-86 [PubMed] [Google Scholar]
- 41.Saile B, Matthes N, Knittel T, Ramadori G: Transforming growth factor β and tumor necrosis factor α inhibit both apoptosis and proliferation of activated rat hepatic stellate cells. Hepatology 1999, 30:196-202 [DOI] [PubMed] [Google Scholar]
- 42.Pinzani M: Novel insights into the biology and physiology of the Ito cell. Pharmacol Ther 1995, 66:387-412 [DOI] [PubMed] [Google Scholar]