Abstract
Chemokines and adhesion molecules play a critical role in the recruitment of leukocytes into specific organ sites. Little is known, however, regarding the repertoire of chemokines and adhesion molecules expressed within different vascular beds. In this study, we compare adhesion molecule expression, chemokine induction, and T-cell subset-endothelial interactions under defined flow conditions on resting and tumor necrosis factor (TNF)-α-activated murine lung endothelial cells (MLECs) and heart endothelial cells (MHECs). Our study revealed that only MHECs exhibited high constitutive VCAM-1 expression. Exposure to TNF-α up-regulated adhesion molecule expression and chemokine production in both MLECs and MHECs. However, high levels of Regulated on Activation Normal T cell Expressed And Secreted (RANTES) expression were detected only in TNF-α-activated MHECs. TNF-α-stimulated MLECs and MHECs both supported T-helper cell interactions under defined flow conditions. Most T cells instantaneously arrested on MHECs but exhibited a rolling phenotype on MLECs. Blocking studies revealed that T-cell arrest on MHECs was mediated by constitutive VCAM-1 and TNF-α-induced RANTES. These findings are consistent with the hypothesis that functional heterogeneity of endothelial cells from different sites exists and some of it is retained in vitro. Furthermore, these results provide an insight into the molecular mechanisms that may mediate T-helper cell recruitment to these organs.
Proinflammatory cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL)-1β, as well as certain gram-negative bacterial endotoxins (lipopolysaccharide), activate the endothelium and trigger the induction of adhesion molecules as well as production of numerous chemokines. The interactions of these adhesion molecules with their cognate ligands on the leukocyte surface and the binding of chemokines to their respective leukocyte receptors regulate the recruitment of leukocytes to the inflammatory site. The recruitment of T-cell subsets is thought to contribute significantly to a variety of cardiovascular diseases including cardiomyopathies, autoimmune myocarditis, atherosclerosis, as well as cardiac transplant rejection.
Chemokines such as RANTES (regulated on activation, normal T cell-expressed and secreted), MIP-1β (macrophage inflammatory protein-1β), and MIP-2 (the mouse equivalent to human IL-8) have been implicated in various pathological conditions. 1-3 In murine models, as well as in rejected human heart transplants, expression of the RANTES gene and protein has been localized to the graft-infiltrating cells (T cells and macrophages), within the intimal lesions, and in the vessel walls of intramyocardial arterioles and capillaries. 3,4 Similarly, chemokines such as MIP-1β and MIP-2 produced in the lungs are believed to be major chemotactic factors responsible for the recruitment of neutrophils into the alveolar spaces during infection and inflammation. 1,5,6 In most cases, the cellular source of these chemokines remained undefined and it was unclear whether endothelial cells were significant contributors. Furthermore, it is not known whether endothelial cells from different organs produce the same repertoire of chemokines or in response to a proinflammatory stimulus exhibit different chemokine production profiles.
Mouse strains deficient in specific genes have been widely used to understand the roles of those genes in developmental, physiological, and disease situations. Studies of in vivo models of inflammation using these genetically modified animals and intravital microscopy have provided major insights into the role of specific adhesion molecules in leukocyte trafficking. 7-9 Because high optical resolution is necessary to visualize each individual step of leukocyte-endothelial interactions, intravital microscopy can be applied only to a few tissues, such as the cremaster muscle, 8,10 bone marrow, 7 lymph nodes, 11,12 and dermal microvessels in the ear. 13,14 Most other organs are considered too opaque for this technique. Because there is functional heterogeneity between endothelial cells from different organ sites, 15 the availability of an in vitro model of cultured endothelial cells derived from different organs of these genetically modified animals would be a useful tool for the study of organ-specific endothelial cell-leukocyte interactions. Here we described the isolation and culture of murine lung and heart endothelial cells. This endothelial cell culture model allows us the opportunity to test which chemokines are produced by the heart and lung endothelium and what effects these chemokines have on specific T-cell subset adhesion and transmigration under flow conditions that mimic postcapillary venules in vivo.
We report here the different responses of cultured endothelial cells from the lung (MLECs) and the heart (MHECs) to stimulation with inflammatory cytokine TNF-α. Cultured MHECs but not MLECs exhibited high constitutive expression of VCAM-1. Although MLECs and MHECs exhibited similar temporal profiles for the up-regulation of P-selectin, E-selectin (after 6 hours), and ICAM-1 after 24 hours of TNF-α stimulation, the expression of VCAM-1 was not significantly up-regulated on either cell type. Of importance, MLECs and MHECs exhibited different expression patterns and temporal profiles of various chemokines as assessed by mRNA analysis. MLECs would rapidly up-regulate and then down-regulate the production of chemokines. In contrast, the high induction of chemokines in MHECs was sustained throughout a longer time period. TNF-α-stimulated MLECs and MHECs both supported interactions of T-cell subsets under defined flow conditions. Blocking studies revealed that these interactions were mediated by constitutive VCAM-1 and TNF-α-induced RANTES found in MHECs. The results are consistent with the notion that endothelial cells isolated from different organs and cultured in vitro do retain certain of their functional differences. Furthermore, our findings also suggest that the VCAM-1 VLA-4 adhesion pathway in conjunction with production of the RANTES chemokine may have a role in the recruitment of T-cell subsets to the heart.
Materials and Methods
Materials
All culture media and supplements [Dulbecco’s modified Eagle’s medium (DMEM), RPMI 1640, l-glutamine, nonessential amino acids, and sodium pyruvate] were obtained from Invitrogen Corporation (Grand Island, NY). Endothelial cell growth stimulant (catalog no. BT-203) was purchased from Biomedical Technologies (Stoughton, MA), and porcine heparin was from Sigma Chemical Co. (St. Louis, MO). Fetal calf serum (FCS) was purchased either from Life Technologies, Inc., Grand Island, NY (lot no.1010713) or Sigma (catalog no. F-2442). Reagents used for the endothelial cell isolation procedure were from the following sources: type I collagenase was from Worthington Biochemical Corporation (Lakewood, NJ); Dynabeads M-450 sheep anti-rat IgG (catalog no. 110.07) was from Dynal (Great Neck, NY); and purified rat anti-mouse CD102 (ICAM-2) antibody (catalog no. 01800D) and anti-mouse CD31 (PECAM-1, clone MEC13.3) antibody (catalog no. 01951D) were from Pharmingen (San Diego, CA). Antibodies used in the characterization and blocking studies, anti-mouse VE-cadherin (catalog no. 555289), biotinylated anti-mouse VCAM-1 (catalog no. 553331), anti-mouse CD49d (clone 9C10, catalog no. 553314 and clone R1-2, catalog no. 53154), and anti-mouse CCR5 (catalog no. 559921), were purchased from Pharmingen. Purified rat anti-mouse ICAM-1 (YN1.1) and anti-mouse VCAM-1 (MK 2) were acquired from the American Type Culture Collection (Rockville, MD). Purified mouse anti-mouse E-selectin (RME-1) 16 and mouse anti-mouse P-selectin (RMP-1) 17 were kind gifts from Dr. Andrew Issekutz, Dalhousie University (Halifax, Canada). Unless otherwise stated, all other reagents were obtained from M.A. BioWhittaker (Walkersville, MD).
Mice
Mice from either the C57BL/6 or the 129S6 background were purchased from Taconics Farm (Germantown, NY). Animals were housed in a pathogen- and virus-free facility at the Longwood Medical Research Center. The animals (8 to 10 weeks of age) were sacrificed by carbon dioxide asphyxiation as approved by the panel on euthanasia at the American Veterinary Association.
Th1 and Th2 Cell Preparation
In vitro differentiated CD4+ T cells were prepared as previously described. 18-20 Briefly, DO.11 T cells express a transgenic Ag receptor specific for OVA peptide (323 to 339) plus I-Ad. Lymph nodes and spleens were removed from DO.11 mice after euthanasia and cell suspensions were made by passing the tissues through wire mesh. CD4+ T cells were purified by positive selection using CD4+-coated Dynal beads and Detachabead reagent (Dynal, Lake Success, NY) according to the manufacturer’s instructions. Naive T cells were suspended in RPMI 1640 medium supplemented with 10% FCS, 2 mmol/L l-glutamine, 10 mmol/L HEPES, 100 U/ml penicillin, 100 U/ml streptomycin, 100 μmol/L nonessential amino acids, 1 mmol/L sodium pyruvate, and 55 μmol/L mercaptoethanol. The cells plated out in 2-ml polystyrene culture wells at a cell density of 2.5 × 105/well. Antigen presenting cells (APCs) (2.5 × 106/well) and OVA peptide at a final concentration of 1 μg/ml were added to each well. For Th1 differentiation, recombinant murine IL-12 (10 ng/ml final concentration) plus neutralizing anti-IL-4 monoclonal antibody (mAb) (11B11, 500 ng/ml) were added to individual wells. For Th2 differentiation, murine recombinant IL-4 (300 U/ml) was added to individual wells. After 3 days, the cultures were fed fresh culture medium containing recombinant murine IL-2 (10 U/ml final concentration). Cells were harvested 2 days later and centrifuged through a Ficoll density gradient to remove dead APCs and cell debris. Cells were tested immediately in flow assays. Th1 cells express robust levels of ligands for E- and P-selectin. T-cell PSGL-1 is the principal ligand for both selectins. During Th1 differentiation and exposure to IL12, posttranslational modification by glycosylation (Core-2 and fucosyltransferase IV and VII) enzymes generates functional PSGL-1. In contrast, Th2 differentiation by IL-4 limits posttranslational glycosylation of PSGL-1 and leads to minimal binding to E- and P-selectins, which we and others have noted previously. 19-21
Isolation and Culture of Murine Lung and Heart Endothelial Cells
Sheep anti-rat-IgG Dynal beads were coated with either anti-PECAM-1 or anti-ICAM-2 monoclonal antibody (2.5 μg antibody for 2 × 107 beads) according to the manufacturer’s instructions. The beads were precoated and stored at 4°C (4 × 108 beads/ml of Dulbecco’s phosphate-buffered saline (DPBS) with 0.1% FCS without sodium azide) for up to 2 weeks.
The method for isolation and purification of endothelial cells was modified from published protocols. 22-24 In brief, the hearts and lungs were harvested from two mice. The lung lobes were carefully dissected out from any visible bronchi and mediastinal connective tissue. Organs were washed in 50 ml of DMEM containing 20% FCS (DMEM-20%) to remove erythrocytes, minced finely with scissors, and digested in 15 ml of collagenase (180 to 200 U/ml) at 37°C for 45 minutes. 22,24 The digested tissue was then mechanically dissociated by titurating, filtered through a 70-μm disposable cell strainer (Becton Dickinson Labware, Bedford, MA) and centrifuged at 400 × g for 10 minutes at 4°C. The cell pellet was resuspended in cold DPBS (∼2 ml for the lung preparation and 1 ml for the heart preparation) and incubated with PECAM-1-coated beads (15 μl/ml of cells) at room temperature for 10 minutes with end-over-end rotation. A magnetic separator was used to recover the bead-bound cells. The recovered cells were washed with DMEM-20%, suspended in 10 ml of complete culture medium (DMEM containing 20% FCS, supplemented with 100 μg/ml porcine heparin, 100 μg/ml endothelial cell growth stimulant, nonessential amino acids, sodium pyruvate, l-glutamine, and antibiotics, at standard concentrations), and then plated in a single gelatin-coated 75-cm2 tissue culture flask. 24 After overnight incubation, the nonadherent cells were removed, the adherent cells washed with Hanks’ balanced salt solution, and 10 ml of fresh complete media was added. Cultures were fed routinely on alternate days with fresh complete culture medium. When the cells reached 70 to 80% confluence, they were detached with warm trypsin-ethylenediaminetetraacetic acid to generate a single cell suspension. The cells were pelleted and then resuspended in 2 ml of DPBS and sorted for a second time using ICAM-2-coated beads (15 μl/ml of cells). The bead-bound cells were washed and plated in complete culture medium and passaged further at a 1:2 ratio. Confluent monolayers of multiple preparations (10 to 12 different) MLEC and MHEC isolates were used at passages 1 to 3 for this study. Because both PECAM-1 and ICAM-2 are cell surface markers expressed on endothelial cells from different vascular beds, 25-28 these positively selected cells are a mixture of endothelial cells of capillary, arterial, or venous origin.
For the longitudinal studies using real-time polymerase chain reaction, MLECs or MHECs from passage 1 were plated at an initial density of 75,000 cells/cm2 onto 0.1% gelatin-coated 9.62-cm2 culture dishes. Cells were allowed to reach confluency (∼48 hours) and subcultured until passage 9. Cell samples were obtained from subcultures 1 to 9 (derived from a single isolate) and processed for RNA isolation as described below. This was performed for three different isolates of MLECs and MHECs.
Immunohistochemistry
Mouse hearts were processed, embedded in OCT, and snap-frozen. Immunohistochemistry on cryostat sections was performed as described. 22,29
Immunostaining by Fluorescence-Activated Cell Sorting (FACS) and Fluorescence Immunoassay for Constitutive and Inducible Expression of Adhesion Molecules
MLECs and MHECs were grown in either 100-mm culture dishes or 96-multiwell plates. At confluence, the endothelial cells were incubated with murine TNF-α (Genzyme, Boston, MA) at a final concentration of 120 ng/ml for 6 and 24 hours. MLECs or MHECs cultured in complete media alone served as controls (designated as 0 hours). For FACS analysis, the endothelial cells were detached from the culture dish by either a brief trypsinization (no longer than 2 minutes at 37°C) or by nonenzymatic cell dissociation buffer (Invitrogen Corporation). Proteolysis was arrested by the addition of ice-cold RPMI-20% FCS. The pelleted cells were titurated in RPMI-5% FCS to create a single cell suspension. For the fluorescence immunoassay, the unfixed endothelial cells were washed twice with DPBS. Analysis of the expression of cell-surface adhesion molecules was performed with two-step immunofluorescence staining. The cells first were incubated with purified monoclonal antibodies (rat anti-PECAM-1, rat anti-ICAM-2, rat anti-ICAM-1, rat anti-VCAM-1, mouse anti-E-selectin, and mouse anti-P-selectin) for 45 minutes on ice. After the cells were washed with DPBS- 0.5% bovine serum albumin, they were incubated with fluorescein isothiocyanate-conjugated anti-rat IgG or fluorescein isothiocyanate-conjugated anti-mouse IgG (Caltag, Burlingame, CA) for 30 minutes on ice. For FACS analysis, the stained endothelial cells were washed twice, fixed in 1% formaldehyde in phosphate-buffered saline (PBS), and analyzed with the FACS Calibur (BD Biosciences, San Jose, CA). For fluorescence immunoassay, the washed stained cells in each well were lysed in 150 μl of Tris-sodium dodecyl sulfate lysis buffer and fluorescence was quantified with a microtiter plate fluorometer at 485 nm, as previously described for human umbilical vein endothelial cells. 30,31
RNase Protection Assay for Induction of Chemokine mRNA
TNF-α-stimulated or control MLECs and MHECs were washed twice with DPBS and then lysed with 3 ml of TRIzol reagent (Life Technologies, Inc., Grand Island, NY) per 100-mm dish. The lysate was stored as 1-ml aliquots at −80°C until the day of the assay. On the day of the assay, total RNA was prepared from the TRIzol lysate according to the manufacturer’s instructions. A commercially available RPA kit that contains templates that probe for eight different chemokine mRNAs and two housekeeping genes (MCK-5) was purchased from BD Pharmingen. 32P-labeling of the RNA-probes, RNA hybridization, and the subsequent RNase protection assay were performed according to the manufacturer’s instructions. The protected RNA fragments were separated on a 5% denaturing polyacrylamide gel, and the density of the separate RNA bands was determined with a Phosphor-Imager and ImageQuant software. For quantification, the density of each band was corrected for background levels and normalized to the mean levels of the housekeeping genes GAPDH and L32. 20
Real-Time Polymerase Chain Reaction
Confluent endothelial cell monolayers were washed twice with ice-cold PBS and scraped into TRIzol reagent. Total RNA was isolated by ethanol precipitation, treated with DNase, and purified on a RNeasy mini column (Qiagen, Valencia, CA). The RNA integrity was assessed by a microfluidics RNA 6000 Nano-Assay chip with a 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). RNA was transcribed with a MultiScribe-based reverse transcriptase reaction. Polymerase chain reaction conditions were 2 minutes at 50°C, 10 minutes at 95°C, and 40 cycles of 15 seconds at 95°C and 60 seconds at 60°C. Reactions were performed in a GeneAmp 5700 sequence detection system (Applied Biosystem, Foster City, CA). Relative RNA equivalents for each sample were obtained by standardizing to β2-microglobulin levels. The primer/probe pairs used for reverse transcriptase analysis were mouse Tie-2, forward primer 5′-AAGCCAGACAGCTCTAAATTTGACTT-3′ and reverse primer 5′-GGTTCCCAGGCACTTTGATG-3′ and probe, 5′(FAM)-AGAGGCGATCCCTGCAAACAACAAGTG-(TAMRA)p3′; mouse VE-cadherin, forward primer 5′-ACCGCTGATCGGCACTGT-3′, reverse primer 5′-GATGGAGTACCCGATGCTGC-3′, probe 5′(FAM)-TGGCCAAAGACCCTGACAAGGCTC-(TAMRA)p3′; mouse PECAM-1, forward primer 5′-CTGCAGGCATCGGCAAA-3′, reverse primer 5′-ATACTGGGCTTCGAGAGCATTT-3′, probe 5′(FAM)-CACCTGGATCGGTACCAGGCCG-(TAMRA)p3′; mouse β2-microglobulin, forward primer 5′-TTCAGTCGCGGTCGCTTC-3′, reverse primer 5′-AGTGAGACAAGCACCAGAAAGACTAG-3′, probe 5′(FAM)-TCACCGAGCGAGCCATGCTGA-(TAMRA)p3′ (FAM, 6-carboxyfluorescein; TAMRA, 6-carboxytetramethylrhodamine).
In Vitro Flow Assay
Th1 and Th2 cells were prepared as described above. MLECs or MHECs were plated at confluence on fibronectin-coated 25-mm glass coverslips and stimulated with murine TNF-α (120 ng/ml) for 6 hours. The interactions of in vitro differentiated T-cell subsets with activated MLECs or MHECs under defined flow conditions (1.0, 0.7, and 0.5 dynes/cm2) were assessed with the use of a parallel plate flow chamber as described previously. 32,33 For function-blocking studies, the binding of RANTES to CCR5 on the T cells was neutralized by preincubating the T cells with an anti-CCR5 monoclonal antibody (20 μg/ml final concentration) for 15 minutes on ice. VCAM-1-VLA-4 interactions were blocked by either incubating the endothelial monolayer with anti-VCAM-1 (MK-2, 20 μg/ml) for 30 minutes at 37°C or the T cells with the combination of anti-CD49d antibodies (clone 9C10 and R1-2 at 10 μg/ml for each antibody) for 15 minutes at 37°C. Total accumulation was determined after 1 minute of flow for each flow rate by counting the number of cells in four fields as detailed previously. 34 T-cell transmigration across the endothelial monolayer was determined using a ×60 magnification objective lens. Cell rolling velocities were determined with the Visual Lab Imaging software program (Ed Marcus Lab, Boston, MA).
Results
Characterization of MLECs and MHECs
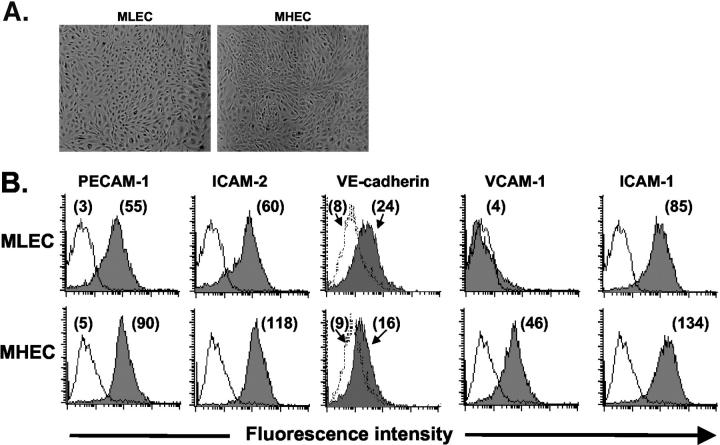
Both MLECs and MHECs isolated by this double-selection method exhibited classical cobblestone morphology typical of vascular endothelium (Figure 1A) ▶ . The endothelial cell lineage was confirmed by staining MLECs and MHECs (at subculture 1 or 2) and analyzing them for cell surface expression of PECAM-1 and ICAM-2, the two endothelial-specific markers that were used for the positive selection of these cells. The percentage of PECAM-1- and ICAM-2-positive cells in these cultures generally ranged from 85 to 99% (Figure 1B) ▶ . Furthermore, three-color staining revealed that MLECs and MHECs positive for both PECAM-1 and ICAM-2 also expressed VE-cadherin (Figure 1B) ▶ . These cells, however, were not positive for expression of VEGF-R3 (Flt-4) thus suggesting cultures were not contaminated with lymphatic endothelial cells (data not shown). It was previously reported that murine PECAM-1 is sensitive to trypsin treatment. 35 However, we found that the PECAM-1 expression remained robust by using a short trypsin treatment (2 minutes at 37°C), which was sufficient to detach >95% of cells from the flasks.
Figure 1.
Endothelial cells isolated from MLECs and MHECs grow as monolayers and express endothelial-specific markers. A: Cultures of MLEC and MHEC monolayers exhibited the classical cobblestone morphology. B: Single-cell suspensions of MLECs and MHECs (at sc-1) were stained with antibodies to PECAM-1, ICAM-2, VE-cadherin, ICAM-1, and VCAM-1 as detailed in Materials and Methods. FACS analyses revealed that >90% of MLECs and MHECs expressed PECAM-1 and ICAM-2 as compared with the negative control (thin line). Cells positive for PECAM-1 and ICAM-2 were also positive for VE-cadherin. The mean fluorescence intensity (MFI) for each test mAb is shown in parentheses.
The number of passages that MLECs and MHECs retain endothelial cell markers in culture was determined by performing a longitudinal study to examine the expression of the mRNAs for three endothelial markers, PECAM-1, VE-cadherin, and Tie-2, over a total of nine subcultures (n = 3). MHECs retained high levels of expression of PECAM-1, VE-cadherin, and Tie-2 mRNA up to subculture 4, after which the expression of these mRNAs declined gradually by passage 9 to 83%, 67%, and 76% of their original expression, respectively (Figure 2) ▶ . It is interesting that whereas the profile of expression of PECAM-1 mRNA in MLECs was similar to that in MHECs, the expression of VE-cadherin and Tie-2 in MLECs was sustained only for two passages (Figure 2) ▶ . By passage 3, the expression of VE-cadherin mRNA declined rapidly to 62%, and by passage 9, it fell to 32%. Expression of Tie-2 mRNA showed a profile similar to that of VE-cadherin although the rate of decline was not as rapid.
Figure 2.
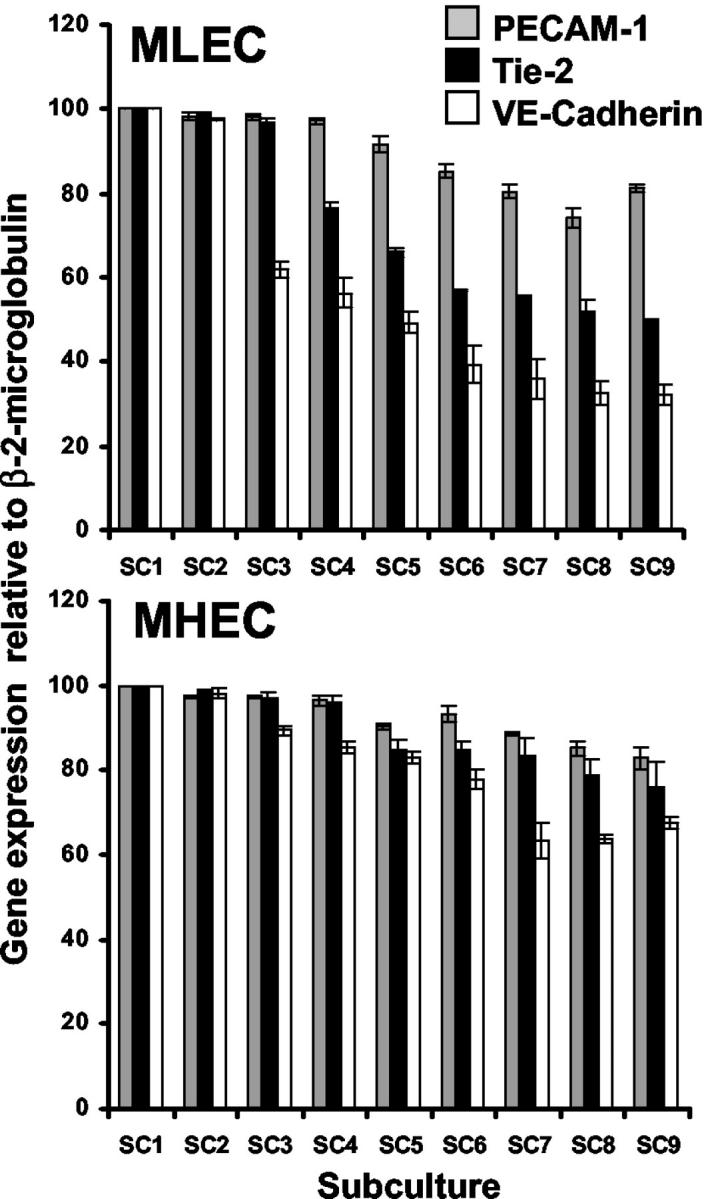
Expression of PECAM-1, Tie-2, and VE-cadherin mRNA in MHECs and MLECs throughout nine passages. The expression of PECAM-1, Tie-2, and VE-cadherin mRNA in MLECs and MHECs was determined by real-time polymerase chain reaction from passages 1 to 9 as described in Materials and Methods. The level of mRNA of these endothelial cell-specific markers decreased more rapidly in MLECs than in MHECs with continuous subcultures. Data shown is mean ± SEM of three different experiments.
MHECs but Not MLECs Exhibit High Constitutive Expression of VCAM-1 in Culture
The recruitment of leukocytes to an inflammatory site is mediated in part by the interactions of leukocyte adhesion molecules with their cognate ligands expressed on the endothelium. Thus, we first examined the induction of adhesion molecules on these murine endothelial cells in response to stimulation by inflammatory cytokines. MLECs and MHECs were incubated with TNF-α for 6 and 24 hours and the expression of adhesion molecules was detected by fluorescence immunoassay.
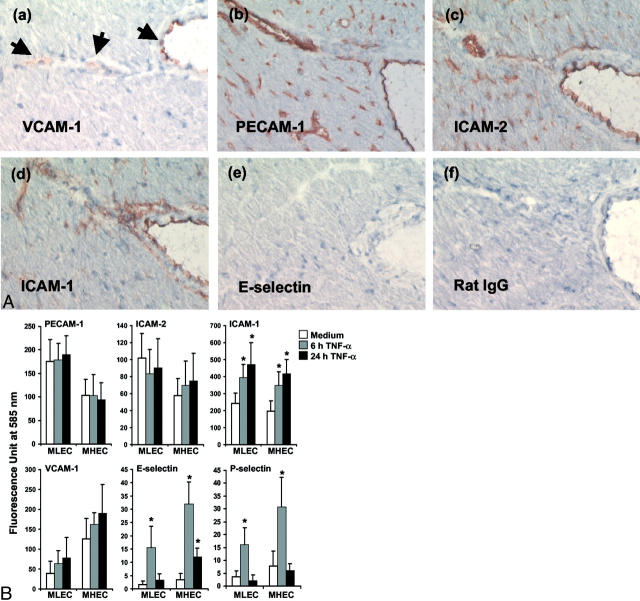
A high level of VCAM-1 expression was detected consistently on the cell surface of MHECs as early as subculture 1, but expression was either low or negligible on MLECs in early subcultures (Figure 1B) ▶ . The constitutive expression of VCAM-1 on freshly isolated MLECs and MHECs was examined by staining collagenase-digested heart and lung tissues for expression of VCAM-1. Endothelial cells were identified by four-color FACS analysis as PECAM-1/ICAM-2/VE-cadherin triple-positive cells. Data from two different experiments consistently suggested that constitutive expression of VCAM-1 on MHECs was twofold higher than that on MLECs (Table 1) ▶ . Constitutive VCAM-1 expression but not E-selectin expression in the mouse heart was confirmed by immunohistochemistry (Figure 3A) ▶ . These results suggest that endothelial cells from distinct vascular beds exhibit significant differences in their constitutive expression of VCAM-1 in vivo. Surprisingly, TNF-α stimulation did not induce a significant increase in VCAM-1 expression on either MLECs or MHECs beyond the constitutive levels seen in medium control endothelium (Figure 3B) ▶ .
Table 1.
Expression of Constitutive VCAM-1 on Freshly Digested Heart and Lung Tissue
| Corrected mean channel fluorescence | ||
|---|---|---|
| Lung tissue | Heart tissue | |
| Experiment 1 | 57.74 | 169.99 |
| Experiment 2 | 40.79 | 81.98 |
Single-cell suspensions were prepared from freshly collagenase-digested murine heart and lung tissues and were stained for expression of PECAM-1, ICAM-2, VE-cadherin, and VCAM-1. Endothelial cells were identified as PECAM-1/ICAM-2/VE-cadherin triple-positive cells. The fluorescence from 104 cells was analyzed and the nonspecific fluorescence was corrected by subtracting the mean channel fluorescence of biotin-conjugated rat IgG2a from the mean channel fluorescence of the test monoclonal antibody, biotin-conjugated VCAM-1.
Figure 3.
MLECs and MHECs up-regulate their cell-surface expression of ICAM-1, E-selectin, and P-selectin after stimulation with TNF-α. A: Cryostat heart sections were stained with various mAbs to detect VCAM-1, ICAM-1, ICAM-2, PECAM-1, and E-selectin and as a control purified rat IgG as described. 22,29 Photomicrographs were taken with the ×20 objective. B: Confluent monolayers of MLECs and MHECs in 96-multiwell plates were stimulated with TNF-α (120 ng/ml final concentration) for 6 and 24 hours. Triplicate wells were stained with optimally diluted primary antibodies to PECAM-1 (10 μg/ml final concentration), ICAM-2 (10 μg/ml), ICAM-1 (1:200 dilution), VCAM-1 (1:50), E-selectin (10 μg/ml), and P-selectin (10 μg/ml), followed by their respective fluorescein isothiocyanate-conjugated secondary polyclonal antibodies (1:80) as indicated in Materials and Methods. Data are the mean ± SEM from three different experiments.
TNF-α treatment did not influence the expression of PECAM-1 and ICAM-2 on either MLECs or MHECs (Figure 3B) ▶ . Both MLECs and MHECs exhibited high constitutive ICAM-1 expression, which was increased with 6 hours of TNF-α stimulation and further up-regulated after 24 hours. Unstimulated MLECs and MHECs did not express E-selectin or P-selectin, but both E-selectin and P-selectin were induced after 6 hours of TNF-α stimulation (Figure 3B) ▶ . The elevated levels of E-selectin and P-selectin returned to baseline levels by 24 hours.
Differential Expression of Chemokine mRNAs throughout Time by MLECs and MHECs in Response to TNF-α
The binding of chemokines to their receptors on the leukocyte is thought to trigger activation of the leukocyte β1 and β2 integrins, leading to leukocyte arrest. 36 Because chemokines usually are produced locally at sites of inflammation (ie, by the inflamed endothelium, activated tissue macrophages, smooth muscle cells, or fibroblasts), we next examined the ability of our cultured MLECs and MHECs to up-regulate their expression of chemokine mRNA in response to TNF-α stimulation.
Although we probed for the expression of eight different chemokine mRNAs, only four of these were detected in the murine endothelial cells in response to stimulation with TNF-α. Both unactivated MLECs and MHECs exhibited high constitutive expression of MCP-1, which did not increase further after TNF-α stimulation (Figure 4, A and B) ▶ . The low levels of MIP-2 expression detected in unactivated MLECs and MHECs was rapidly up-regulated by TNF-α treatment (Figure 4B) ▶ . Furthermore, a higher expression of MIP-2 was detected in TNF-α-stimulated MHECs than MLECs. Negligible levels of MIP-1β were detected in MHECs (Figure 4B) ▶ . In contrast, the low but detectable constitutive expression of MIP-1β in unactivated MLECs was rapidly up-regulated at 5 hours after TNF-α stimulation and then declined toward basal level by 24 hours (Figure 4B) ▶ . Interestingly, TNF-α treatment induced high levels of RANTES mRNA expression in MHECs but extremely low levels in MLECs (Figure 4, A and B) ▶ .
Figure 4.
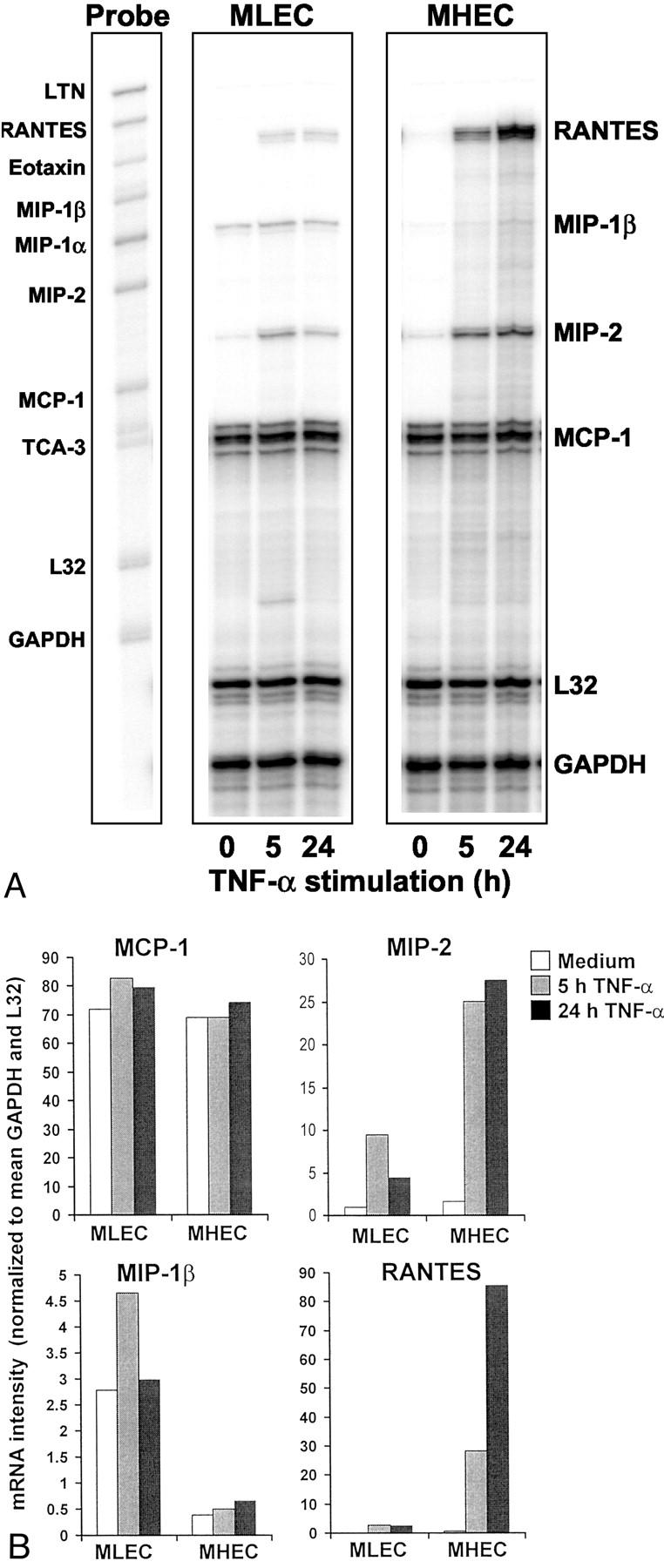
On TNF-α stimulation, MLECs and MHECs up-regulate their expression of chemokine mRNA. Total mRNA was purified from MLECs and MHECs after 5 and 24 hours of TNF-α stimulation. The presence of various chemokine mRNAs was detected by RNase protection assay as described in Material and Methods. A: The protected fragments were separated on a 5% polyacrylamide gel and visualized by phosphorimaging. B: Quantification of the bands was performed using the ImageQuant software and normalized to the mean RNA levels of housekeeping genes L32 and GAPDH. The gel and graph shown are a representative experiment from three separate cultures.
TNF-α-Activated MHECs Mediate T-cell Interactions under Defined Flow Conditions via the VCAM-1-VLA-4 and RANTES-CCR5 Pathways
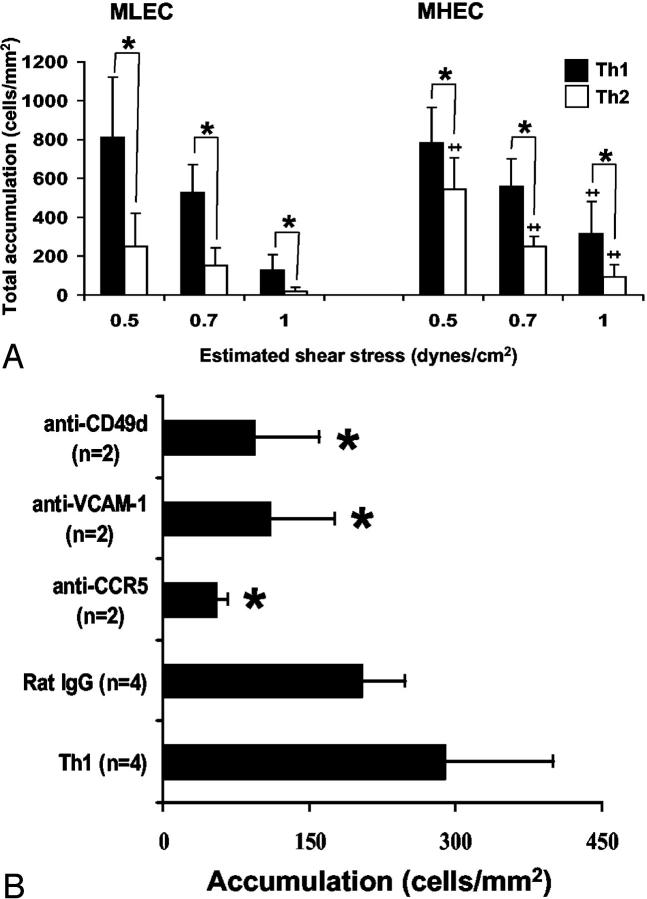
Th1 and Th2 accumulation on unactivated MLEC and MHEC monolayers under flow conditions was quantified (Table 2) ▶ and revealed baseline adhesion of both Th1 and Th2 cells to both MLECs and MHECs. The dominant phenotype of Th1 and Th2 adhesive interactions was arrest without spreading on the apical surface of the endothelium. No T cells were observed to transmigrate either MLEC or MHEC monolayers. We next examined the interactions between T-helper cell subsets and 6 hour TNF-α-activated MLECs or MHECs using the same conditions. Figure 5A ▶ shows the total number of Th1 and Th2 cells that interacted with the MLECs (left) and MHECs (right) under various levels of defined laminar shear stress. TNF-α-activated MHECs or MLECs consistently supported interactions with more Th1 cells than Th2 cells across the range of shear stresses studied (*, P < 0.05 for both MLECs and MHECs; n = 3 experiments). Interestingly, more Th2 cells interacted with MHECs than MLECs (**, P < 0.05). Focusing on the Th1 to MHEC adhesive interactions, the majority (40 of 47 total, or 87%) of Th1 cells arrested on the apical surface, spread rapidly and many ultimately transmigrated. After 10 to 15 minutes of flow, 35.7 ± 8.0% of Th1 cells and 35.1 ± 6.4% of Th2 cells had transmigrated the MHEC monolayer (n = 3 separate experiments). In contrast to MHECs, only 27% of Th1 cells arrested on the MLECs (9 of 33 total, 27%). The Th1 rolling velocity on MLECs was approximately twofold higher than MHECs but did not reach statistical significance (MHECs, 5.6 ± 4.0 μm/sec versus MLECs, 10.6 ± 9.0 μm/sec; P ≥ 0.5, NS). After 10 to 15 minutes of flow, 23.5 ± 4.4% of Th1 cells had transmigrated the MLEC monolayer. TNF-α-activated MLECs were not as efficient in supporting Th2 cell interactions (Figure 5A ▶ , left), however, ∼19.7 ± 5.6% of the interacting cells were able to transmigrate the monolayer after 15 minutes of perfusion.
Table 2.
Accumulation of T-Helper Cell Subsets on Unstimulated MLEC and MHEC Monolayers under Defined Flow in Vitro
| Shear stress (dynes/cm2) | Th1 (cells/mm2) | Th2 (cells/mm2) | |
|---|---|---|---|
| MLEC (n = 3) | 0.5 | 301 ± 181 | 241 ± 140 |
| 0.7 | 230 ± 141 | 136 ± 39 | |
| 1.0 | 26 ± 6 | 10 ± 6 | |
| MHEC (n = 5) | 0.5 | 501 ± 103 | 263 ± 40 |
| 0.7 | 307 ± 81 | 129 ± 35 | |
| 1.0 | 85 ± 48 | 41 ± 13 |
Th1 and Th2 cells at 1.0 × 106/ml were drawn across unstimulated MLECs or MHECs at the indicated shear stress. The total number of interacting cells (ie, rolling and arrested) from four different fields was determined after a minute of perfusion at each level of shear stress. No Th1 or Th2 transmigrations of MLEC or MHEC monolayers were observed.
Figure 5.
MLECs and MHECs stimulated with TNF-α for 6 hours can support T-cell interactions under defined flow conditions. MLECs and MHECs were plated on fibronectin-coated coverslips at confluence and stimulated with TNF-α (120 ng/ml) for 6 hours. T-helper cell subsets (Th1 and Th2) were differentiated in vitro from splenocytes as previously reported. 18,19 A: Th1 and Th2 cells at 1.0 × 106/ml were perfused over TNF-α-stimulated MLECs and MHECs at the indicated shear stress. The total number of interacting cells (ie, rolling, arrested, or transmigrated) from four different fields was determined after a minute of perfusion at each level of shear stress. *, Statistical significance (P < 0.05) between Th1 versus Th2 for both MLECs and MHECs; #, statistical significance (P < 0.05) between MHECs versus MLECs. B: Either Th1 cells were pretreated with anti-CCR5 mAb, a combination of two anti-CD49d mAb (or with rat IgG) or the MHECs were preincubated with anti-VCAM-1 mAb as detailed in Materials and Methods. The total number of interacting cells was determined at 1.0 dynes/cm2. *, P < 0.05 for comparison with untreated Th1 cells. Data are mean ± SEM from two to four different experiments.
To further examine the molecular mechanisms that play a role in these T-cell interactions, we performed a series of function-blocking studies. Either the MHEC monolayer was pretreated with an anti-VCAM-1 antibody (MK2) or Th1 cells were incubated with an anti-CCR5 antibody or a combination of anti-CD49d antibodies before perfusion. Blocking either the VCAM-1-VLA-4 pathway, or the binding of RANTES to the CCR5 on Th1 cells significantly reduced MHEC to T-cell interactions (Figure 5B) ▶ . However, antibody-treated T cells that retained the ability to interact with MHECs were equally efficient in transmigrating the MHEC monolayer (data not shown), suggesting that other adhesive and chemokine pathways such as E-selectin-E-selectin ligand, LFA-1-ICAM-1, or MCP-1 to CCR2, may also be important mediators of such interactions.
Discussion
In this study, we have successfully isolated, cultured, and propagated endothelial cells from murine lung and heart in vitro. Their endothelial origin was confirmed by the expression by these cells of the endothelial-specific markers: PECAM-1, ICAM-2, and VE-cadherin. Analyses showed that MHECs cultured in vitro exhibit high constitutive expression of VCAM-1. On exposure to the inflammatory cytokine, TNF-α, MHECs up-regulated their cell surface expression of E-selectin, P-selectin, and ICAM-1 as well as produced an abundance of RANTES. T-helper cell subsets Th1 and Th2 were able to arrest and transmigrate the MHEC monolayer under defined flow conditions. Blocking studies revealed that these interactions were mediated predominantly by VCAM-1-VLA-4 adhesion interactions and RANTES-induced integrin activation. In contrast, TNF-α-activated MLECs did not produce RANTES and a smaller percentage of the rolling Th1 cells arrested and ultimately transmigrated.
Our group previously published a method for the isolation and propagation of murine pulmonary microvascular endothelium. 22 In that report, Gerritsen and colleagues 22 isolated a subpopulation of the pulmonary endothelium from lipopolysaccharide-stimulated mice by sterile sorting for VCAM-1-expressing cells by FACS. Immunohistochemical analysis showed that the cells were primarily from small venules. In this study, we made use of magnetic beads to positively sort for PECAM-1- and ICAM-2-expressing cells. The endothelial cell lineage of these cells was further confirmed by their expression of VE-cadherin mRNA or protein. These MLECs and MHECs are essentially of mixed vascular bed origins, however, this protocol has significant advantages over the previous method. Primarily, the endothelial cells are not systemically activated in situ, the yield is dramatically increased and the tedious nature of FACS sorting has been eliminated.
The phenotype of endothelial cells is greatly influenced by their environmental signals. Recent studies have shown that biomechanical stimuli such as laminar shear stress can differentially modulate the expression of various genes in endothelial cells. 37-39 A report by Aird and colleagues 40 showed that induction of the vWR gene in cardiac microvascular endothelial cells was influenced by signals from cardiac myocytes. Interestingly, in the longitudinal study, it was MLECs that underwent phenotypic modification first. Because both MLECs and MHECs were cultured under similar conditions, we are uncertain at this juncture what signal(s) was lacking that resulted in the early phenotypic changes in MLECs.
Few published studies have examined expression of adhesion molecules on cultured endothelial cells from different organs. Various recent studies using a variety of strategies, 41-43 however, have examined the in vivo constitutive and cytokine-induced expression of various adhesion molecules in different organs in mice. Using immunohistochemical techniques, Fries and colleagues 41 reported focal (ie, patchy) VCAM-1 expression in multiple organs. Similarly, Henniger and colleagues 42 reported significant differences in constitutive expression of ICAM-1 and VCAM-1 within different tissues as accessed by the dual radiolabel technique. The normalized data by Henniger and colleagues 42 indicated that the highest level of VCAM-1 expression was in the brain, followed by expression in the heart, and that the lowest level of expression was in the intestine. In addition, they showed that TNF-α treatment could induce only a twofold increase in VCAM-1 expression in the heart. In a separate study, Langley and colleagues 44 reported the detection of basal VCAM-1 expression in pulmonary vessels. Our immunohistochemical analysis of normal heart also detected VCAM-1 expression (Figure 3A) ▶ . Consistent with this finding, FACS analysis on freshly dissociated MHECs showed that constitutive VCAM-1 expression was twofold higher than that on MLECs. Although our data cannot be directly compared with the results from these studies, together these data strongly suggest that constitutive VCAM-1 expression could be maximally up-regulated in our cultured cells in vitro and thus were no longer responsive to TNF-α stimulation. Alternatively, we cannot rule out the possibility that disruption of the tissues for immunoisolation of endothelium have triggered the differences observed in VCAM-1 expression during subsequent culture in vitro.
On TNF-α activation cultured MLECs and MHECs exhibited a differential expression of chemokine mRNAs. Most interesting was the finding that TNF-α-activated MHECs express high levels of RANTES mRNA. This finding is consistent with previous studies that have immunolocalized expression of RANTES protein to vessel walls of intramyocardial arterioles and capillaries in rejected heart allografts, 3,4 suggesting that our in vitro-expanded MHECs have retained their ability to produce RANTES on stimulation with inflammatory cytokines. By comparison, MLECs cultured and stimulated with TNF-α under similar conditions were unable to up-regulate RANTES mRNA. This finding strongly suggests that endothelial cells from different vascular beds may respond differently to a common stimulus. This hypothesis is supported further by a recent study from Shukaliak and colleagues 45 who reported the secretion of RANTES by primary cultures of human brain microvessel endothelial cells but not by human umbilical vein endothelial cells after stimulation with TNF-α.
Both the induction of RANTES and infiltration of CCR5-positive lymphocytes have been implicated to play a major role in cardiac allograft rejections. 3,4,46,47 Our present findings that TNF-α-induced RANTES in MHECs and the VCAM-1-VLA-4 pathway are important for the arrest and subsequent transmigration of Th1 cells across the MHEC monolayer in vitro provides insight to the molecular mechanisms that mediate the recruitment of T cells into the heart during the development of graft rejection. A better understanding of the roles of these molecules and the signaling pathways involved will provide the knowledge necessary for the design of novel immunomodulatory interventions that may prevent chronic graft rejections.
High levels of MIP-2 and MIP-1β detected in bronchoalveolar lavage fluid from patients of respiratory distress syndrome 5,6 or from animal models of lung inflammation 1 have suggested critical roles for these chemokines in the recruitment of neutrophils into the alveolar spaces. However, our data (low levels of MIP-1β or MIP-2 expression by TNF-α-stimulated MLECs) does not support a role for endothelial cells as a major source of MIP-1β or MIP-2 in lung inflammation. Other cell types present in the lung environment, such as the alveolar macrophages, are likely major producers of these chemokines. This idea is consistent with various in vivo studies that have reported on the role of alveolar macrophages in the recruitment of neutrophils to the inflamed lungs and as the initiator of early proinflammatory signals. 48,49
Our data showed TNF-α triggered rapid up-regulation and sustained production of chemokines in MHECs throughout time. In contrast, TNF-α-induced chemokine expression in MLECs was rapidly down-regulated to basal or near basal levels within 24 hours. This difference in temporal profile of chemokine expression may be one of several adaptations by endothelial cells that help them meet specific requirements of their local environment. During inflammation, the endothelium is the site of initial contact with blood leukocytes. In the absence of resident macrophages, the endothelium in the heart (and the brain 45 ) may be functionally adapted to serve as an early and sustained source of chemokines that would recruit specific leukocytes such as lymphocyte subsets and macrophages to the site of antigenic challenge. In contrast, in the lung, that responsibility may be shared between the endothelium and resident alveolar macrophages, or in some instances, taken over by macrophages and other infiltrating cells.
In conclusion, we have successfully isolated, cultured, and expanded endothelial cells from murine heart and lungs. Our data suggest that these endothelial cells can retain at least some of their functional differences for several subcultures when they are expanded in vitro. In addition, our study has also provided evidence that T-cell interactions with lung and heart endothelium may be mediated by different adhesion pathways. The availability of a reliable method to isolate endothelial cells from different organs and ability of the endothelial cells to retain some of their organ-specific functions in vitro will be a useful tool to elucidate the genetic contributions and molecular mechanisms that mediate endothelial cell-leukocyte interactions and their roles toward the pathogenesis and progression of various fatal conditions such as graft rejection, atherosclerosis, or even tumor metastasis.
Acknowledgments
We thank the late William J. Atkinson for his invaluable early contributions to this project; Sonali Bose and Jason Comander for help in the development of the primer/probes sets; Dr. Andrew Issekutz for his kind gifts of reagents; George Stavrakis, David Milstone, and the Morphology Core for immunohistochemical staining of cardiac tissues; and Brandy N. Perkins and Deanna Lamont for technical assistance.
Footnotes
Address reprint requests to F. William Luscinskas, Ph.D., Vascular Research Division, Department of Pathology, 221 Longwood Ave., LMRC 423A, Boston MA 02115. E-mail: fluscinskas@rics.bwh.harvard.edu.
Supported by National Institutes of Health grants HL36028 (to A. J. C., M. A. G. Jr., F. W. L.), HL56985 (to M.A.G.), HL65090 (to F.W.L.) and HL53993 (to Y.-C. L., F. W. L.).
References
- 1.Zhang P, Nelson S, Holmes MC, Summer WR, Bagby GJ: Compartmentalization of macrophage inflammatory protein-2, but not cytokine-induced neutrophil chemoattractant, in rats challenged with intratracheal endotoxin. Shock 2002, 17:104-108 [DOI] [PubMed] [Google Scholar]
- 2.Mercer-Jones MA, Shrotri MS, Peyton JC, Remick DG, Cheadle WG: Neutrophil sequestration in liver and lung is differently regulated by C-X-C chemokines during experimental peritonitis. Inflammation 1999, 23:305-319 [DOI] [PubMed] [Google Scholar]
- 3.Yun JJ, Fischbein MP, Laks H, Irie Y, Espejo ML, Fishbein MC, Berliner JA, Ardehali A: RANTES production during development of cardiac allograft vasculopathy. Transplantation 2001, 71:1649-1656 [DOI] [PubMed] [Google Scholar]
- 4.Pattison JM, Nelson PJ, Huie P, Sibley RK, Krensky AM: RANTES chemokine expression in transplant-associated accelerated atherosclerosis. J Heart Lung Transplant 1996, 15:1194-1199 [PubMed] [Google Scholar]
- 5.Hirani N, Antonicelli F, Strieter RM, Weisener MS, Ratcliffe PJ, Haslett C, Donnelly SC: The regulation of IL-8 by hypoxia in human macrophages—a potential role in the pathogenesis of the acute respiratory distress syndrome. Mol Med 2001, 7:685-697 [PMC free article] [PubMed] [Google Scholar]
- 6.Aggarwal A, Baker CS, Evans TW, Haslam PC: G-CSF and IL-8 but not GM-CSF correlate with severity of pulmonary neutrophilia in acute respiratory distress syndrome. Eur Respir J 2002, 15:895-901 [DOI] [PubMed] [Google Scholar]
- 7.Mazo IB, Gutierrez-Ramos J-C, Frenette PS, Hynes RO, Wagner DD, von Andrian UH: Hemotopoietic progenitor cell rolling in bone marrow microvessels: parallel contributions by endothelial selectins and vascular cell adhesion molecule 1. J Exp Med 1998, 188:465-474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forlow SB, White EJ, Barlow SC, Feldman SH, Lu H, Bagby GJ, Beaudet AL, Bullard DC, Ley K: Severe inflammatory defect and reduced viability in CD18 and E-selectin double-mutant mice. J Clin Invest 2000, 106:1457-1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andre P, Denis CV, Ware J, Saffaripour S, Hynes RO, Ruggeri ZM, Wagner DD: Platelets adhere to and translocate on von Willebrand factor presented by endothelium in stimulated veins. Blood 2000, 96:3322-3328 [PubMed] [Google Scholar]
- 10.Sperandio M, Thatte A, Foy D, Ellies LG, Marth JD, Ley K: Severe impairment of leukocyte rolling in venules of core 2 glucosaminyltransferase-deficient mice. Blood 2001, 97:3812-3819 [DOI] [PubMed] [Google Scholar]
- 11.von Andrian UH: Intravital microscopy of the peripheral lymph node microcirculation in mice. Microcirculation 1996, 3:287-300 [DOI] [PubMed] [Google Scholar]
- 12.Stein JV, Cheng G, Stockton BM, Fors BP, Butcher EC, von Andrian UH: L-selectin-mediated leukocyte adhesion in vivo: microvillous distribution determines tethering efficiency, but not rolling velocity. J Exp Med 1999, 189:37-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Proske S, Vollmar B, Menger MD: Microvascular consequences of thrombosis in small venules: an in vivo microscopic study using a novel model in the ear of the hairless mouse. Thromb Res 2000, 98:491-498 [DOI] [PubMed] [Google Scholar]
- 14.West CA, He C, Su M, Secomb TW, Konerding MA, Young AJ, Mentzer SJ: Focal topographic changes in inflammatory microcirculation associated with lymphocyte slowing and transmigration. Am J Physiol 2001, 281:H1742-H1750 [DOI] [PubMed] [Google Scholar]
- 15.Stevens T, Rosenberg R, Aird W, Quertermous T, Johnson FL, Garcia JGN, Hebbel RP, Tuder RM, Garfinkel S: NHLBI workshop report: endothelial cell phenotypes in heart, lung, and blood diseases. Am J Physiol 2001, 281:C1422-C1433 [DOI] [PubMed] [Google Scholar]
- 16.Walter UM, Ayer LM, Manning AM, Frenette PS, Wagner DD, Hynes RO, Wolitzky BA, Issekutz AC: Generation and characterization of a novel adhesion function blocking monoclonal antibody recognizing both rat and mouse E-selectin. Hybridoma 1997, 16:355-361 [DOI] [PubMed] [Google Scholar]
- 17.Walter UM, Ayer LM, Wolitzky BA, Wagner DD, Hynes RO, Manning AM, Issekutz AC: Characterization of a novel adhesion function blocking monoclonal antibody to rat/mouse P-selectin generated in the P-selectin-deficient mouse. Hybridoma 1997, 16:249-257 [DOI] [PubMed] [Google Scholar]
- 18.Xie H, Lim Y-C, Luscinskas FW, Lichtman AH: Acquisition of selectin binding and peripheral homing properties by CD4+ and CD8+ T cells. J Exp Med 1999, 189:1765-1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim Y-C, Henault L, Wagers AJ, Kansas GS, Luscinskas FW, Lichtman AH: Expression of functional selectin ligands on Th cells is differentially regulated by IL-12 and IL-4. J Immunol 1999, 162:3193-3201 [PubMed] [Google Scholar]
- 20.Lim Y-C, Xie H, Come CE, Alexander SI, Grusby MJ, Lichtman AH, Luscinskas FW: IL-12, STAT4-dependent up-regulation of CD4+ T cell core2 β-1,6-n-acetylglucosaminyltransferase, an enzyme essential for biosynthesis of P-selectin ligands. J Immunol 2001, 167:4476-4484 [DOI] [PubMed] [Google Scholar]
- 21.Wagers AJ, Waters CM, Stoolman LM, Kansas GS: IL-12 and IL-4 control T cell adhesion to endothelial selectins through opposite effects on FucT-VII gene expression. J Exp Med 1998, 188:2225-2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerritsen ME, Shen CP, Mchugh MC, Atkinson WJ, Kiely JM, Milstone DS, Luscinskas FW, Gimbrone MA, Jr: Activation-dependent isolation and culture of murine pulmonary microvascular endothelium. Microcirculation 1995, 2:151-163 [DOI] [PubMed] [Google Scholar]
- 23.Bowden RA, Entman ML, Smith CW, Burns AR: Neutrophil transendothelial migration on cultured murine endothelial cells. EMBO J 2000, 14:A704 [Google Scholar]
- 24.Dong QG, Bernasconi S, Lostaglio S, De Calmanovici RW, Martin-Padura I, Breviario F, Garlanda C, Ramponi S, Mantovani A, Vecchi A: A general strategy for isolation of endothelial cells from murine tissues—characterization of two endothelial cell lines from the murine lung and subcutaneous sponge implants. Arterioscler Thromb Vasc Biol 1997, 17:1599-1604 [DOI] [PubMed] [Google Scholar]
- 25.de Fougerolles AR, Stacker SA, Schwarting R, Springer TA: Characterization of ICAM-2 and evidence for a third counter-receptor for LFA-1. J Exp Med 1991, 174:253-267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu H, Tong IL, de Fougerolles AR, Springer TA: Isolation, characterization, and expression of mouse ICAM-2 complementary and genomic DNA. J Immunol 1992, 149:2650-2655 [PubMed] [Google Scholar]
- 27.Albelda SM, Oliver PD, Romer LH, Buck CA: EndoCAM: a novel endothelial cell-cell adhesion molecule. J Cell Biol 1990, 110:1227-1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newman PJ, Berndt MC, Gorski J, White GCI, Lyman S, Paddock C, Muller WA: PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science 1990, 247:1219-1222 [DOI] [PubMed] [Google Scholar]
- 29.Milstone D, Fukumura D, Padgett RC, O’Donnell P, Davis VM, Benavidez OJ, Monsky WL, Melder RJ, Jain RK, Gimbrone MA, Jr: Mice lacking E-selectin show normal numbers of rolling leukocytes but reduced leukocyte stable arrest on cytokine-activated microvascular endothelium. Microcirculation 1998, 5:153-171 [PubMed] [Google Scholar]
- 30.Luscinskas FW, Cybulsky MI, Kiely J-M, Peckins CS, Davis VM, Gimbrone MA, Jr: Cytokine-activated human endothelial monolayers support enhanced neutrophil transmigration via a mechanism involving both endothelial-leukocyte adhesion molecule-1 and intercellular adhesion molecule-1. J Immunol 1991, 146:1617-1625 [PubMed] [Google Scholar]
- 31.Bochner BS, Luscinskas FW, Gimbrone MA, Jr, Newman W, Sterbinsky SA, Derse-Anthony CP, Klunk D, Schleimer RP: Adhesion of human basophils, eosinophils and neutrophils to IL-1-activated human endothelial cells: contributions of endothelial cell adhesion molecules. J Exp Med 1991, 173:1553-1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lichtman AH, Ding H, Henault L, Vachino G, Camphausen R, Cumming D, Luscinskas FW: CD45RA−RO+ (memory) but not CD45RA+RO− (naive) T cells roll efficiently on E- and P-selectin and vascular cell adhesion molecule-1 under flow. J Immunol 1997, 158:3640-3650 [PubMed] [Google Scholar]
- 33.Luscinskas FW, Ding H, Lichtman AH: P-selectin and vascular cell adhesion molecule 1 mediate rolling and arrest, respectively, of CD4+ T lymphocytes on tumor necrosis factor alpha-activated vascular endothelium under flow. J Exp Med 1995, 181:1179-1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim Y-C, Wakelin MW, Henault L, Goetz DJ, Yednock T, Cabanas C, Sanchez-Madrid F, Lichtman AH, Luscinskas FW: α4β1-integrin activation is necessary for high-efficiency T-cell subset interactions with VCAM-1 under flow. Microcirculation 2000, 7:201-214 [PubMed] [Google Scholar]
- 35.Marelli-Berg F, Peek E, Lidington EA, Stauss HJ, Lechler RI: Isolation of endothelial cells from murine tissue. J Immunol Methods 2000, 244:205-215 [DOI] [PubMed] [Google Scholar]
- 36.Lawrence MB, Springer TA: Leukocytes roll on a selectin at physiologic flow rates: distinct from and prerequisite for adhesion through integrins. Cell 1991, 65:1-20 [DOI] [PubMed] [Google Scholar]
- 37.Topper JN, Cai J, Falb D, Gimbrone MA, Jr: Identification of vascular endothelial genes differentially responsive to fluid mechanical stimuli: cyclooxygenase-2, manganese superoxide dismutase, and endothelial cell nitric oxide synthase are selectively up-regulated by steady laminar shear stress. Proc Natl Acad Sci USA 1996, 93:10417-10422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Topper JN, Cai J, Qiu Y, Anderson KR, Xu Y, Deeds JD, Feeley R, Gimeno CJ, Woolf EA, Tayber O, Mays GG, Sampson BA, Schoen FJ, Gimbrone MA, Jr, Falb D: Vascular MADs: two novel MAD-related genes selectively inducible by flow in human vascular endothelium. Proc Natl Acad Sci USA 1997, 94:9314-9319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garcia-Cardena G, Comander J, Anderson KR, Blackman BR, Gimbrone MA, Jr: Biomechanical activation of vascular endothelium as a determinant of its functional phenotype. Proc Natl Acad Sci USA 2001, 98:4478-4485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aird WC, Edelberg JM, Weiler-Guettler H, Simmons WW, Smith TW, Rosenberg RD: Vascular bed-specific expression of an endothelial cell gene is programmed by the tissue microenvironment. J Cell Biol 1997, 138:1117-1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fries JWU, Williams AJ, Atkins RC, Newman W, Lipscomb MF, Collins T: Expression of VCAM-1 and E-selectin in an in vivo model of endothelial activation. Am J Pathol 1993, 143:725-737 [PMC free article] [PubMed] [Google Scholar]
- 42.Henninger DD, Panes J, Eppihimer MJ, Russell J, Gerritsen ME, Anderson DC, Granger DN: Cytokine-induced VCAM-1 and ICAM-1 expression in different organs of the mouse. J Immunol 1997, 158:1825-1832 [PubMed] [Google Scholar]
- 43.Eppihimer MJ, Wolitzky BA, Anderson AO, Labow MA, Granger DN: Heterogeneity of expression of E- and P-selectin in vivo. Circ Res 1996, 79:560-569 [DOI] [PubMed] [Google Scholar]
- 44.Langley RR, Russell J, Eppihimer MJ, Alexander SJ, Gerritsen ME, Specian RD, Granger DN: Quantification of murine endothelial cell adhesion molecules in solid tumors. Am J Physiol 1999, 277:H1156-H1166 [DOI] [PubMed] [Google Scholar]
- 45.Shukaliak JA, Dorovini-Zis K: Expression of the β-chemokines RANTES and MIP-1β by human brain microvessel endothelial cells in primary culture. J Neuropathol Exp Neurol 2000, 59:339-352 [DOI] [PubMed] [Google Scholar]
- 46.Mulligan MS, McDuffie JE, Shanley TP, Guo R-F, Sarma JV, Warner RL, Ward PA: Role of RANTES in experimental cardiac allograft rejection. Exp Mol Pathol 2000, 69:167-174 [DOI] [PubMed] [Google Scholar]
- 47.Gao W, Faia KL, Csizmadia V, Smiley ST, Soler D, King JA, Danoff TM, Hancock WW: Beneficial effects of targeting CCR5 in allograft recipients. Transplantation 2001, 72:1199-1205 [DOI] [PubMed] [Google Scholar]
- 48.Hashimoto S, Pittet JF, Hong K, Folkesson HG, Bagby GJ, Kobzik L, Frevert C, Watanabe K, Tsurufuji S, Wiener-Kronish J: Depletion of alveolar macrophage decreases neutrophil chemotaxis to Pseudomonas airspace infections. Am J Physiol 1996, 270:L819-L828 [DOI] [PubMed] [Google Scholar]
- 49.Zsengeller Z, Otake K, Hossain S-A, Berclaz P-Y, Trapnell BC: Internalization of adenovirus by alveolar macrophages initiates early proinflammatory signaling during acute respiratory tract infection. J Virol 2000, 74:9655-9667 [DOI] [PMC free article] [PubMed] [Google Scholar]