Abstract
Phage display approaches are used increasingly in efforts to identify cancer-specific binding peptides and antibodies. Phage-derived reagents are likely to have broad applications in diagnostic and research pathology. A critical element in the identification of cell or tissue-type-specific phage is the ability to reproducibly quantify bound or eluted phage at various stages of panning and screening procedures. Traditional biological assays of phage numbers such as plaque counting are commonly applied but are time-consuming, labor-intensive, and poorly reproducible. Moreover, enzyme immunoassays only support a subset of target types. Here, we report on the use of real-time polymerase chain reaction (PCR) (M13qPCR) in developing methods for identification of cell- and tissue-type-specific binding peptides. With M13qPCR, we demonstrate a ≥5 log10 dynamic linear range with high reproducibility and significantly lower coefficients of variation (10 to 20%) than conventional methodology. Using M13qPCR in phage-panning experiments on live leukemia and prostate cancer cells, cancer-binding phage were identified. Similar results were obtained with conventional methodology such as flow cytometry. These results were extended to specific application of M13qPCR in panning phage libraries on tissue sections of prostate and breast cancer. With the PCR-based method, direct quantification of phage bound to tissue sections correlated well with staining intensity and yielded phage that bound to neoplastic and nonneoplastic epithelium. Thus, real-time PCR-based methodology significantly improves a number of aspects of conventional phage-panning protocols. Furthermore, identification of phage that bind specifically to diseased or cancerous tissue sections will likely be facilitated by this PCR-based approach.
Random peptide and antibody phage display libraries represent rich sources of high-diversity ligands from which selection procedures can yield specific, high-affinity binding reagents for a variety of targets. 1,2 A phage display library is a collection of infectious filamentous phage particles that incorporates uni- or multivalent display of either a random sequence oligopeptide or Fv fragments of antibodies fused to an outer coat protein. 1,3,4 These libraries can contain >10 9 unique sequences in a small volume (eg, 20 μl) and are thus becoming increasingly popular for many different applications. For example, random peptide phage display libraries have been used successfully to map antibody epitopes, 5,6 to characterize critical residues at interfaces between proteins, 7 to analyze protein structure, 8 and to discover receptor ligands. 9,10 Moreover, phage display approaches have been recently applied to identify cell- and tissue-type-specific binding ligands. 11-14 We have used random peptide phage display libraries to identify phage-bearing peptides that bind selectively to neutrophils and monocytes and that elicit functional responses from these cells. 15,16 Our laboratory is currently expanding the use of these libraries to identify breast cancer, prostate cancer, and leukemia selective-binding peptides.
Ligands of interest from a phage display library are identified through experiments that incorporate both selection and analysis phases. During the selection phase, a process also referred to as panning, 17 an aliquot of library that represents the library’s full diversity is applied to a target such as an antibody, a protein, or a cell. Nonbound phage are washed away and the bound subpopulation is eluted from the target. Eluted phage replicate in appropriate bacterial hosts and are secreted into the medium from which they may be purified and concentrated. 4 The amplified subpopulation is then reapplied to target for generally two to six rounds of panning and amplification, leading to an ever more refined subset of phage that bind target with higher avidity. 1 Individual phage clones bearing a specific peptide or Fv antibody may then be isolated for the analysis phase. The amino acid sequence of displayed peptides may be deduced from the DNA sequence of the appropriate region on the phage genome. Clones may be further screened and analyzed based on a desired property, for example, for their relative binding specificity for a target or the ability to elicit functional responses from a receptor of interest.
During both the panning and analysis phases, numerous quantifications of phage are generally required. Such quantitative data provide indication as to whether the panning method is yielding progressively higher avidity subpopulations or if it might require modification, and a basis for assigning relative binding avidities of individual phage clones during the analysis phase.
Our efforts have been directed at using phage display technology in the identification of cell- and tissue-type-specific binding phage for use in diagnostic and research pathology. Because the vast majority of material available for such studies is in the form of embedded tissue sections, development of methodology for producing phage particles that label tissue sections in a disease-specific manner have broad applications. In developing such methodology, it became apparent that conventional methods of phage quantification were cumbersome and not entirely reliable. We directed efforts at identifying techniques that show rapid turn-around time, high reproducibility, and facilitate high-throughput analysis for a variety of panning and screening target types. Here, we describe a new methodology for quantification of phage particles based on real-time polymerase chain reaction (PCR) using TaqMan chemistry to facilitate identification of cell- and tissue-type-selective binding phage. We analyze the performance parameters of the assay and demonstrate its utility in phage-panning and screening experiments on live cells and on formalin-fixed, paraffin-embedded (FFPE) tissue sections. The approach reported here for phage quantification may find broad applicability in experiments using phage display technology.
Materials and Methods
Reagents
Purchased reagents included bovine serum albumin (BSA) fraction V 96% (Sigma Chemical Co., St. Louis, MO), nonfat dry milk powder (Carnation, Nestle USA, Solon, OH), phycoerythrin-streptavidin (Jackson Laboratory, Bar Harbor, ME), and LB broth (Fisher Biotech, Fairlawn, NJ). The Emory University Microchemical Facility synthesized oligonucleotide primers and fluorochrome-labeled oligonucleotide probe.
Cells and Tissues
LNCaP prostate cancer cells 18 (a gift of Dr. John Petros, Emory University, Atlanta, GA) and the NB4 human acute myelogenous leukemia cell line 19 (a gift of Dr. P. Koeffler, UCLA, Los Angeles, CA) were cultured in RPMI 1640 and 10% fetal calf serum. Fresh blood leukocytes were isolated from acid citrate dextrose anti-coagulated blood obtained from healthy volunteers, using a gelatin sedimentation technique. 20 Cells were resuspended in modified Hanks’ balanced salt solution devoid of Ca2+ and Mg2+ [HBSS(−)] at a concentration of 5 × 10 7 cells/ml at 4°C until used. For some experiments, blood leukocytes were fixed in 1.8% paraformaldehyde in HBSS(−) for 10 minutes and blocked in HBSS(−) and 5% nonfat milk for 30 minutes, washed three times in HBSS(−), and resuspended in HBSS(−) to 1 × 107/ml.
FFPE human tissue sections were obtained from the archives of the Emory University Division of Surgical Pathology. Appropriate institutional approval for human tissue studies was obtained.
Phage Libraries
The linear nonapeptide (LL9) and constrained decapeptide (CL10) and hexapeptide (CL6) random peptide phage display libraries were prepared in our laboratory as previously described, 15 using the M13KBst vector. 1,21
Phage Clone Preparation
Selected phage clones for these experiments were propagated and concentrated to 1 × 1012 to 1 × 1013 plaque forming units (pfu)/ml as described. 1,4 Stock phage suspensions were stored in normal saline/HEPES buffer (NS/HEPES; 150 mmol/L NaCl, 10 mmol/L HEPES, pH 7.4). The amino acid sequences of the displayed peptides were deduced for a subset of phage preparations by sequencing the coding nucleotides in the viral DNA as described. 21
Plaque Counting Assay
This standard bioassay was performed using Escherichia coli strain K91 as described with some modification. 1,4 Briefly, K91 cells were grown from single colonies in standard LB broth overnight at 37°C and stored at 4°C for up to 1 week until used. Phage suspensions underwent serial dilutions of 1:100 in LB. A cocktail including 3 ml of top agar, 100 μl of the appropriate dilutions of phage, and 400 μl of K91 cells was poured onto 100 × 15-mm antibiotic-free LB agar plates for 37°C incubation overnight. Pfu counts were culled from plates for which pfu numbered from 5 to 200.
Quantitative PCR Assay (M13qPCR)
PE Biosystems Primer Express software (Applied Biosystems, Foster City, CA) was used to select the DNA template sequences for the primers and probe based on the sequence of the M13KBst phage vector. 1 In Table 1 ▶ , the sequences of primers and probe and oriented probe labels 22 including the 5′-end 6-carboxyfluorescein (FAM) fluorescent reporter and the 3′-end 6-carboxytetramethylrhodamine (TAMRA) quencher are presented. The primer sequences were chosen to yield an amplicon length of 100 bp, which was confirmed in a standard PCR reaction in which the amplicon product was visualized on an agarose gel. Primer 2 reverse was used for DNA sequencing of the pertinent region of the M13KBst genome using the USB Sequenase version 2.0 kit.
Table 1.
M13qPCR Reagents and Conditions
| Primer 1 forward | ||||||
| 5′ AAACTGGCAGATGCACGGTT 3′ | ||||||
| Probe | ||||||
| 6-FAM 5′ TGCGCCCATCTACACCAACGTAACCTAT 3′ TAMRA | ||||||
| Primer 2 reverse | ||||||
| 5′ AACCCGTCGGATTCTCCG 3′ | ||||||
| Thermal cycler conditions | ||||||
| Cycle | Temp (°C) | Time | Repeat | |||
| Hold | 50 | 2:00 | 1 | |||
| Hold | 95 | 10:00 | 1 | |||
| Cycle | 95 | 0:15 | 40 | |||
| 60 | 1:00 | |||||
The optimal primer and probe concentrations were determined to be 900 nmol/L for primers and 200 nmol/L for probe, using the Sequence Detection Systems Quantitative Assay Design and Optimization protocol (PE Biosystems, Foster City, CA) according to the manufacturer’s instructions. Phage templates were prepared in H2O or appropriate buffer, as specified in the Results, and were heated to 100°C for 5 minutes then cooled to room temperature before use. The 50-μl PCR reaction mixture included 10 μl of phage template, 25 μl of TaqMan Universal PCR Mastermix (Perkin Elmer, Emeryville, CA), and primers and probe in 15 μl of H2O. PCR was performed on the ABI Prism 7700 using thermal cycler conditions displayed in Table 1 ▶ . Data were acquired and analyzed using the Sequence Detector Software version 1.7 (PE Biosystems). A control devoid of template was included in each run.
Phage-Panning Experiments
Panning was performed with the CL6 library on NB4 cells for three rounds or the CL10 library on LNCaP cells for four rounds based on a previously described protocol. 15 Briefly, panning engendered application of 20 μl of phage library containing 2 × 1011 pfu to 1 × 107 cells in HBSS(−), containing 1 mmol/L CaCl2, 1 mmol/L MgCl2 [HBSS(+)], and 0.5% BSA for 1 hour at room temperature. Nonbound phage were removed through six washings with 5 ml of buffer. Phage elution was effected by addition of 0.6 ml of HBSS(+) and 0.05% Tween 20. Phage were amplified in K91 E. coli overnight and purified, concentrated, and resuspended in NS/HEPES 15 to generate sublibraries for use in the next round of panning.
For panning on FFPE tissues, 10 sections (∼1 × 1 cm) were cut to a thickness of 4 μm and processed as above for each assay. Tissues underwent standard deparaffinization and rehydration 23 in 1.5-ml microfuge tubes yielding innumerable tissue fragments of various sizes. Solutions were sequentially changed after centrifugation of tissues. After washing with H2O, tissues underwent heat-induced antigen unmasking in 1 ml of 10 mmol/L citrate buffer, pH 6.0, for 10 minutes at 15 psi and 120°C. Tissues were washed in H2O, blocked for 30 minutes in HBSS(−) and 5% powdered milk, and resuspended in 1 ml of the same buffer. A 20-μl aliquot of phage library containing 2 × 1011 pfu was incubated with tissue in 1 ml of buffer for 1 hour at room temperature with gentle mixing. Nonbound phage were removed by washing 20 times with 5 ml of buffer and centrifugation at 1500 × g. K91 E coli were added directly to tissue sections for amplification of bound phage in 25 ml of LB overnight at 37°C. Amplified phage populations were purified and concentrated as described. 15
Phage Screening Experiments
For cell-based screening using M13qPCR, 1 × 106 cells were washed and resuspended in 1 ml of HBSS(−) and 0.5% BSA. In one set of experiments, individual phage clones (1 × 108 pfu), selected randomly from the third round eluate of panning on NB4 cells, were added to live NB4 cells or blood leukocytes. In a second set of experiments, whole amplified phage populations (1 × 109 pfu) from each of four rounds of panning on LNCaP cells were added to live LNCaP cells. For both sets of experiments, samples were incubated for 30 minutes at room temperature and washed twice with buffer using a 2.5-minute centrifugation (0.6 × g) to completely pellet tissues. Samples were resuspended in 1 ml of HBSS(−), heated to 100°C for 5 minutes, and diluted 1:100 in H2O. A 10-μl aliquot of diluted supernatant served as template for M13qPCR.
For individual phage clones selected by panning on FFPE tissue, four sections at 4-μm thickness (∼1 × 1 cm) were cut and placed into microfuge tubes and processed as above. Tissues were finally suspended in 1 ml of HBSS(−) and 0.5% BSA to which 10 μl of a 1 × 1011 pfu/ml phage suspension was added (1 × 109 pfu total). Samples were incubated for 30 minutes at room temperature and washed twice with buffer by centrifugation (600 × g) for 2.5 minutes to pellet tissues. Samples were resuspended in 1 ml of H2O and heated to 100°C for 5 minutes. A 10-μl aliquot of a supernatant diluted 1:100 in H2O served as template for M13qPCR.
Flow Cytometry
Flow cytometric phenotyping with phage was performed as described. 16 Briefly, cells [1 to 5 × 106 cells in 100 μl in HBSS(−) and 0.5% BSA] were incubated for 45 minutes with 10 μl of phage suspension. After washing, cells were fixed with 1.8% paraformaldehyde for 10 minutes and washed once with buffer. Cell-bound phage were labeled with a biotinylated anti-phage M13 antibody (Serotec, Oxford, UK), washed, and stained with phycoerythrin-streptavidin. Cells were analyzed on a FACSort cytometer (Becton Dickinson, Mountain View, CA). At least 5000 events were measured per condition. Data acquisition and analysis were accomplished using Cellquest software, version 3.1 (Becton Dickinson).
Phage Histochemistry
Sections of 4-μm thickness on glass slides underwent standard deparaffinization, rehydration, and antigen unmasking as above. After blocking with phosphate-buffered saline and 5% milk, sections were stained with 2 × 1011 pfu/ml of phage for 1 hour. Bound phage were detected using an anti-M13 phage monoclonal antibody (Amersham Pharmacia Biotech, Piscataway, NJ) at a 1:400 dilution followed by the LSAB2 System (DAKO, Carpinteria, CA) for immunoperoxidase staining according to the manufacturer’s recommendations. After diaminobenzidine exposure, sections were counterstained with hematoxylin before mounting for microscopy.
Results
Design of the M13qPCR Assay
The set of primers and probes that were selected from the M13KBst template using Primer Express software is shown in Table 1 ▶ . The template region of M13KBst for PCR amplification encodes the α-complementing fragment of the LacZ gene, originally cloned into the M13mp18 vector 24,25 from which the M13KBst vector derives. 1,21 A search of GenBank with the amplicon sequence indicated no significant homology other than to the E. coli LacZ gene. This suggested that the likelihood of amplification of nonspecific target sequences would be minimized. In addition, we reasoned that probing the exogenous LacZ site would ensure that wild-type phage would not cause false-positive results.
Because the LacZ gene is not essential for phage survival, we speculated that this region might be subject to mutations that could render the assay less useful. To test this, we sequenced across a 120-bp stretch of template that included the amplified region for M13qPCR in 10 clones and 2 libraries. Selected clones were garnered from several different panning experiments involving three to five rounds of panning and amplification, conditions representative of the opportunity for mutation. Nonetheless, sequencing data showed no mutations in the tested specimens.
To minimize assay times, whole phage particles were used as template material for both test and standard curve samples, rather than purified phage genomes after a more time-consuming DNA purification step. Results reported below confirmed the feasibility of this approach.
M13qPCR Assay Reproducibly Quantifies Phage over a 5 Log10 Concentration Difference
Experiments were performed to establish the dynamic linear range for the assay. We used the M13KBst phage 1 as the arbitrarily defined standard. This phage displays no additional peptide on the pIII coat protein. The concentration of a stock was first quantified in pfu/ml and was used throughout these experiments as standard. Serial dilutions of stock material across 10 log10 underwent M13qPCR. A representative amplification plot shows relative fluorescence versus PCR cycle number in Figure 1A ▶ . As elaborated on in Figure 1 ▶ , this plot was used to determine each CT value, the cycle at which sample fluorescence exceeds an arbitrary threshold. 26 In Figure 1B ▶ , a plot of phage genome equivalents (PGEs), approximating the number of phage particles versus CT values is displayed. A representative result for the assay demonstrates linearity on a log scale across ≥5 log10 of concentration, from 1 × 102 to 1 × 107, with very narrow error bars. These data established a broad working range for quantification that is convenient for most phage experiments.
Figure 1.

Dynamic linear range and standard curve for M13qPCR. An amplification plot is shown in A as sequential curves from left to right for a subset of dilutions of M13KBst phage (1 × 107 to 1 × 101 pfu per reaction). The acquired ΔRn values are presented over PCR cycles 0 to 40, where ΔRn represents the difference between the reporter fluorescence and an unreacted sample each normalized to the fluorescence of a passive reference dye, ROX. The threshold (horizontal line) was set at 10 times the SD above the baseline average ΔRn throughout PCR cycles 3 to 12. The CT value represents the cycle number at which the threshold ΔRn is attained and occurs where amplification is still exponential. In B, the CT values obtained for all dilutions (1 ×10−1 to 1 × 109) are plotted against PGEs, which were set to be identical to the assigned pfu count. Lines connecting data points demarcate the linear range for which the correlation coefficient r 2 = 0.998, slope = −3.6, and y intercept = 40.8. Data points in B represent triplicate within-run measurements and error bars indicate standard deviations for measured CTs.
We next tested the within and between-run reproducibility of M13qPCR on individual phage clones. As shown in Figure 2, A and B ▶ , samples representing the low (sample 1), intermediate (sample 2), and high (sample 3) phage number across this linear range showed within-run percent coefficients of variation (%CV) that ranged from 1 to 16.5% and between-run %CVs from 7.8 to 16.3%. We found similar results regardless of whether the phage clone came from the LL9 or CL10 library (data not shown). These values are comparable with other viral assays that use TaqMan technology. 27-29 There was no trend for increasing %CV at either end of the range. In fact, these data suggest that as low as twofold relative differences in phage numbers between samples may be reproducibly identified by the assay.
Figure 2.

Within-run and between-run reproducibility of M13qPCR versus traditional plaque-counting assay. For reproducibility assessments of M13qPCR, three phage clone samples diluted in H2O were tested in triplicate in two (sample 1) or three (samples 2 and 3) separate runs representing 24 independent measurements. A displays the PGE values for within-run assessment of samples determined in runs a, b, and c. B shows PGE values for between-run reproducibility for each of the dilutions. For comparative purposes, reproducibility of the plaque assay was tested using two samples in triplicate in three separate runs totaling 18 measurements. For each replicate pfu determination, samples underwent serial 1:100 dilutions, from 10−2 to 10−10 in HBSS(−) from starting samples 4 and 5. Three dilutions, encompassing a 6 log10-fold range of concentration, were prepared for plating on LB plates as specified in the Material and Methods. After overnight incubations, pfu determinations were made for plates on which plaques numbered 10 to 200, usually only one of the three plates in the run. C displays pfu determinations for within-run assessment of samples 4 and 5 determined in separate runs labeled a, b, and c. D shows values for between-run reproducibility of the plaque assay. Standard deviations (SDs) and percent coefficients of variation (%CVs) are shown.
For comparison, we assessed the reproducibility of the plaque-counting assay using two phage clone samples. A separate dilution series for samples 4 and 5 was prepared for each run and is labeled a to c in Figure 2C ▶ . Results for intra-assay comparison, shown in Figure 2C ▶ , demonstrate %CVs that range from 13.5 to 48%. Figure 2D ▶ indicates interassay %CVs from 34 to 60%. These data contrast with the much lower %CVs in Figure 2, A and B ▶ , and confirm the significantly better reproducibility of M13qPCR.
Effects of Buffers, Exogenous Tissue, and Cellular Factors on M13qPCR Assay
We next tested whether commonly used buffers and/or target types affect assay measurements. Buffers included HBSS (−), HBSS (−) supplemented with 0.5% BSA, with 2% nonfat milk, or preneutralized elution buffer containing glycine and HEPES or phage treated with elution buffer (low pH glycine) that was neutralized with HEPES buffer after 5 minutes. The last of these conditions recapitulates a typical elution protocol. Pfu numbering 1 × 109 in 5 μl of H2O were added to 1 ml of each buffer, heated to 100°C for 5 minutes, and cooled to room temperature. For M13qPCR, 10 μl of samples were added separately as template for PGE determination. As shown in Figure 3 ▶ , phage template in HBSS(−) and HBSS(−) with BSA yielded results that did not statistically differ from phage control in H2O alone. Although undiluted elution buffers caused significant diminution in the detected PGE levels, dilution of these buffers at 1:100 in H2O afforded results consistent with phage control in H2O. Nonfat dry milk resulted in the most significant diminution in detected PGEs, almost 2 log10 if undiluted and 1 log10 when diluted 1:100. Therefore, these findings suggest that nonfat dry milk represents an incompatible carrier protein for use in this assay and that glycine/HEPES buffers should be diluted at least 1:100 before use in M13qPCR.
Figure 3.
Influence of common buffered solutions on M13qPCR. Abbreviations of buffered solutions displayed on the ordinate are the following: HBSS = HBSS(−); HBSS/BSA = HBSS(−), 0.5% BSA; HBSS/MILK = HBSS(+), 2% powdered nonfat milk; glycine/HEPES* = neutralized elution buffer (0.1 mol/L glycine, 0.2 mol/L HEPES, pH 7.5); glycine/HEPES# = phage added to elution buffer (0.1 mol/L glycine, pH 2.2) for 5 minutes, then neutralized to pH 7.5 with 1 mol/L of HEPES, pH 11). For M13qPCR, 10 μl of undiluted sample (checkered bars) or sample diluted 1:100 in H2O (solid bars) was added as template for PGE determination. To normalize for dilution, PGE values for diluted samples (solid bars) were multiplied by 100. Samples were run in triplicate. Error bars represent standard deviations.
Whole tissues and cells, whether fresh or fixed, represent complex targets that are increasingly used in phage display experiments. 15,30,31 To quantify the number of phage associated with these targets, it is preferable to release bound phage by either boiling samples directly or exposing samples to low pH elution buffers. Boiling of samples, however, would be the most efficient method for PCR-based assays and would likely release genomes from phage that show low pH-resistant binding to target. We tested whether direct boiling releases cellular or tissue factors into the supernatant that interfere with M13qPCR. A constant number of pfu was applied in 1 ml of HBSS(−) to representative substrate types: FFPE prostate tissue sections, paraformaldehyde-fixed blood leukocytes, fresh blood leukocytes, or buffer control. To determine false-positive background reactivity, a separate set of control reactions was run in parallel in which the above substrates included no added phage. Figure 4 ▶ shows that the average PGE values determined for samples that included phage (checkered bars) are all within threefold of the PGE value for control sample (labeled “none”) for which no cell or tissue substrate was added, regardless of the state of fixation of the sample. This variation is not notably different from the between-run variation for the M13qPCR assay displayed in Figure 2B ▶ . In addition, the background contribution from substrate-only samples (solid bars) in which no phage were added is below the lower limit of detection for the assay (≤1 × 102 PGEs). These results suggest that factors released from fresh and fixed cells and tissues in the quantities tested cause negligible interference with the M13qPCR assay. However, there was a slight decrease in recovered PGEs when live cells were used that, although acceptable for the cell-load used in Figure 4 ▶ (1 × 106 cells), might be significant for panning on larger quantities of fresh cells (eg, 1 × 107 or 108 cells). Panning and screening experiments were next performed to test the utility of the M13qPCR assay in prototype applications.
Figure 4.
Assessment of interference engendered by cell and tissue factors on M13qPCR. Phage particles numbering 1 × 109 pfu were added to each of three different substrates used in tissue and cell-based panning and screening experiments: 1) five 4-μm-thick, ∼1 × 1 cm, FFPE human prostate tissue sections that were previously deparaffinized and rehydrated (FFPE tissues); 2) 1 × 106 paraformaldehyde-fixed blood leukocytes (PF cells); and 3) 1 × 106 fresh blood leukocytes (fresh cells); buffer alone served as control (none). Samples were brought to 1 ml with HBSS(−) buffer, boiled for 5 minutes, and cooled to room temperature. A 10-μl aliquot of a 1:100 dilution in H2O was measured by M13qPCR for PGE determination. Checkered bars represent PGE values obtained for samples containing phage. Solid bars display background values obtained for substrate-only samples. PGE values shown on the log10 scale represent triplicate measurements for which error bars display standard deviations.
Application of M13qPCR in Phage Display-Panning Experiments on Live Cells
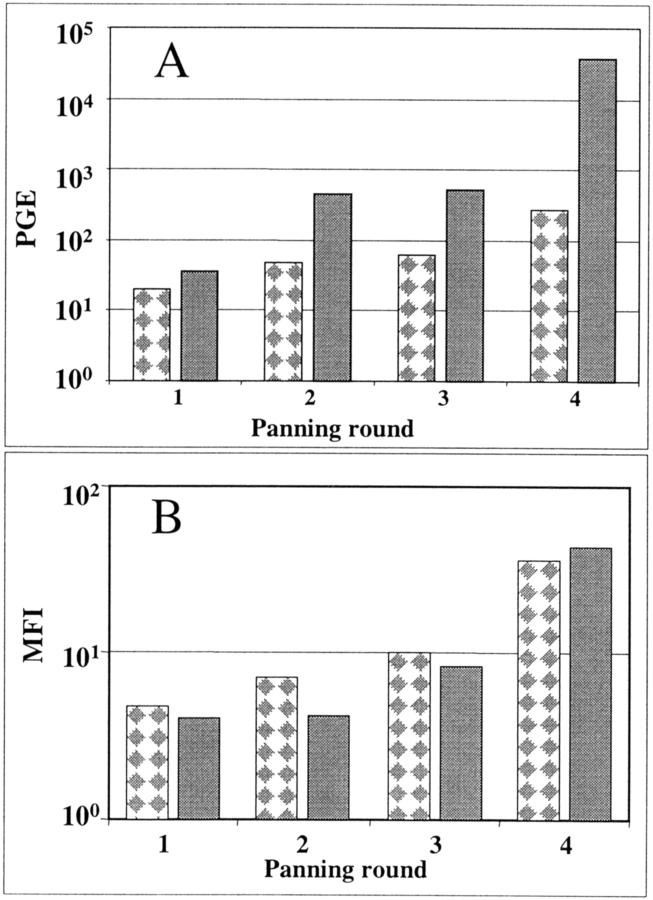
We and others have reported on panning phage libraries over suspensions of live cells to identify cell-type-selective binding phage. 12,15,30 We compared the M13qPCR assay versus flow cytometry to provide relative quantification of phage binding to cells in suspension. In the first set of experiments, LL9 and CL10 libraries were applied to live LNCaP cells for four rounds of panning using methods previously described. 15 After panning, 2 × 109 pfu of the amplified populations in 1 ml from rounds 1 to 4 for each library were incubated separately with 1 × 106 LNCaP cells. Nonbound phage were washed away and samples were either boiled before M13qPCR or prepared for flow cytometry. Results displayed in Figure 5A ▶ show that the amplified phage populations exhibit an increase in PGEs bound to LNCaP cells over rounds 1 to 4 of panning. Specifically, panning with the LL9 library (checkered bars) yields a >1 log10 increase in population binding to LNCaP cells whereas the CL10 library demonstrates a dramatic 3 log10 increase in binding. An alternative and proven methodology, flow cytometry, 15,16 was used in parallel and confirmed the validity of M13qPCR findings, as presented in Figure 5B ▶ . The mean fluorescent intensity (MFI), an indication of the relative number of bound phage per cell, increases steadily over rounds 1 to 4 of panning for both libraries, paralleling the increase demonstrated in Figure 5A ▶ . However, the relative increases in binding observed with flow cytometric methods were smaller than those observed with M13qPCR, demonstrating that M13qPCR is useful in the panning phase to monitor the relative changes in avidity of whole phage populations for live cells in suspension.
Figure 5.
M13qPCR indicates increases in phage population affinity for live cells during panning. In A, results of M13qPCR show PGE per reaction representing relative numbers of cell-bound phage for each amplified phage populations 1 to 4 using the LL9 library (checkered bars) or CL10 library (solid bars). In B, flow cytometry was performed as previously detailed in which cell-bound phage from the amplified phage populations from each round of panning were detected with a biotinylated anti-phage antibody and streptavidin-phycoerythrin. 16 The mean fluorescent intensities (MFI) are shown for each amplified phage population according to the specified round of panning (LL9 library, checkered bars; CL10 library, solid bars).
In a second set of experiments, the CL6 phage library was applied to the NB4 human leukemia cell line for three rounds of panning with the aim of obtaining phage clones that show leukemia-selective binding. After three rounds of panning as above, a 2 log10 increase in population binding was observed. We randomly selected several individual phage clones for analysis in binding assays on NB4 cells and nonneoplastic leukocytes. For each test and control clone, 1 × 108 pfu were incubated separately with 1 × 106 fresh cells. After washing to remove nonbound phage, samples were boiled for M13qPCR. In parallel experiments, flow cytometric analysis was performed as described 16 using the same phage clones and cells.
Relative binding profiles generated from both sets of experiments are shown in Figure 6 ▶ where data are displayed for nonneoplastic leukocytes (Figure 6, A and B) ▶ and NB4 leukemia cells (Figure 6, C and D) ▶ . All results have been normalized to binding measured for M13KBst-negative control and are expressed as normalized PGEs for M13qPCR (Figure 6, A and C) ▶ and normalized MFI for flow cytometry (Figure 6, B and D) ▶ . The most significant findings portrayed in these panels are the consistency of relative binding profiles comparing M13qPCR to flow cytometry within cell types and the detection of cell-type-selective differential binding for a subset of clones. In Figure 6A ▶ , the three positive controls, phage clones bearing peptides FGPNLTGRW (FGP), DLVTSKINI (DLV), and WDWLPW (WDW), display binding to leukocytes by M13qPCR, a finding paralleled by flow cytometry in Figure 6B ▶ and as previously described. 16 Although control clones FGP and DLV display binding only to leukocytes, clone WDW binds as well to NB4 cells (Figure 6C) ▶ . Of the test phage clones selected with target NB4 cells, most manifest notably lower binding to leukocytes compared to controls. By contrast, clones 2 and 3 show relatively prominent binding to NB4 cells by M13qPCR (Figure 6C) ▶ , a finding recapitulated in flow cytometric studies (Figure 6D) ▶ . In addition, clone 1 binds to both cell types, similar to the WDW control. Additional studies are underway to better characterize these phage clones, including studies with clinical leukemia samples and sequencing of the displayed peptides. In addition, we are adapting the screening method for high-throughput analysis of clones in 96-well plates. Together, the results indicate that M13qPCR is a reliable screening test for phage clones that show cell-type-selective binding.
Figure 6.
M13qPCR screening of phage clones selected for cell-type-selective binding. Six individual clones were randomly selected from the cell-bound eluates after the third round of panning on NB4 leukemia cells for these analyses. Clones were screened by M13qPCR to identify those that bind nonspecifically to nonneoplastic blood leukocytes (A) and to NB4 cells (C). M13qPCR quantification of cell-bound phage (PGE) is shown after test clones (labeled 1 to 6, solid bars) or positive controls (checkered bars) were applied to blood leukocytes. Results for PGEs are displayed normalized to PGEs bound for M13KBst-negative binding control phage (PGE/PGE for control). In parallel experiments, flow cytometry to detect cell-bound phage was performed as previously detailed. 16 Results are displayed for leukocytes (B) and NB4 cells (D). The mean fluorescent intensity (MFI) of each specific cell population is shown normalized to M13KBst-negative binding control phage (MFI/MFI for control). Standard forward versus side scatter plots allowed gating on granulocytes, monocytes, and lymphocytes combined (not shown).
Phage Display-Panning Experiments using M13qPCR on FFPE Tissue Sections
Although the aforementioned results demonstrate the utility of M13qPCR in phage display-panning experiments, it is clear, nonetheless, that flow cytometric-based assays provide reliable results although these assays are significantly more time consuming. Furthermore, flow cytometric analyses are not easily adaptable for use in panning experiments on tissue sections, whereas M13qPCR has immediate applications. We have begun incorporating M13qPCR into tissue section panning protocols. For these experiments, we developed a method for panning directly on FFPE sections of prostate and breast cancers. Human prostate tissue containing both adenocarcinoma and benign epithelium underwent deparaffinization, rehydration, antigen unmasking, and blocking for use in five rounds of panning with the CL10 library. K91 E. coli were added directly to tissue to effect amplification of bound phage. Tissue was apportioned so an equivalent, unused aliquot was available for each round.
After panning, M13qPCR was used to assess relative changes in the avidity for target of whole phage populations obtained from rounds 1, 3, and 5 of panning. Amplified phage populations were separately applied to a constant quantity of target tissue that had been deparaffinized, rehydrated, and blocked in a microfuge tube. After incubation with 2 × 1011 pfu in 1 ml and washing to remove nonbound phage, samples were boiled in 1 ml H2O to release bound phage. Samples underwent centrifugation to remove tissue fragments. Supernatants were diluted 1:100 in H2O before M13qPCR. Figure 7A ▶ displays the number of bound PGEs for each amplified population. The 1 log10 cumulative rise in the bound PGEs indicates an increase in the avidity of the population for target over the five rounds of panning. Furthermore, this finding suggested that some individual clones within the population manifest high-avidity binding for target.
Figure 7.

M13qPCR monitoring of phage population affinity for FFPE target tissues aids in identification of a phage histochemical staining positive control. In A, PGEs shown represent total phage liberated per reaction after correction for the 1:100 dilution. In B–D, tissue-bound phage were detected using an anti-phage monoclonal antibody and the LSA2 kit (DAKO) followed by a standard diaminobenzidine reaction. Representative results for prostate (B) and breast tissue (C) sections are displayed; staining with negative control phage M13KBst is shown for similar sections of prostate (D) and breast tissue (E). Single arrows indicate adenocarcinoma whereas double arrows designate benign epithelium. Original magnifications: ×200 (B, E); ×100 (C); ×400 (D).
Based on this promising result, we selected random individual clones from the fifth round avidity-enriched population for histochemical screening. Phage clones were applied to human prostate and breast tissue sections, much as primary antibodies, and detected as specified in the Materials and Methods. One selected clone, CL10-3-11, bears the cysteine-constrained 7-mer peptide delimited by parentheses, AECPWLKSQVCGPP, delimited by invariant AE at the N-terminus and spacer GPP that attaches to the pIII protein of phage. 15 A 7-mer represents a highly unusual sequence length from the CL10 library which is mostly 10-mers, suggesting strong selection during panning. Representative staining results for one clone, CL10-3-11, are displayed for prostate (Figures 7B) ▶ and breast (Figure 7D) ▶ . As shown, this clone strongly stains both benign and malignant epithelium. Variable staining was observed for endothelia, hematolymphoid cells, and stromal fibroblasts (data not shown). Surprisingly, no staining of acellular stromal connective tissue was identified, suggesting selectivity in binding. In contrast, negative control M13KBst phage manifests negligible staining overall as shown for prostate (Figures 7C) ▶ and breast (Figure 7E) ▶ tissue.
Discussion
Traditional methods for phage quantification rely on bioassays, in particular, the plaque-forming unit (pfu) assays and colony-forming unit [cfu, also called transducing unit (TU)] assays. 32 For pfu assays, a bacterial host is infected, plated on an agar plate, and phage plaques in the bacterial lawn are counted the following day. For phage vectors that encode for drug resistance markers, bacteria infected with phage form drug-resistant colonies that may be quantified as cfu after overnight incubation. However, these assays are time consuming and labor intensive. The assays engender multiple pipetting steps, plating of several dilutions onto agar plates, and manual counting of plaques or colonies, a subjective process. Moreover, phage reproduction in the bacterial host can vary depending on the host’s growth state, 33 and thus potentially affect the efficiency of plaque formation and therefore assay reproducibility. Although ELISA assays have been used, their applications are generally limited to targets that can readily be bound to a solid phase such as antibodies or proteins. 34 Moreover, the dynamic linear range of these assays is generally ∼2 logs 32 and therefore several titrations of phage suspensions may be required. Spectrophotometric methods are not readily extended to high-throughput formats and require purified phage particles. More cumbersome methods such as electron microscopy and high performance liquid chromatography have been used to accurately quantify viral particle numbers, 35,36 but these represent low throughput and expensive modalities.
In the current work, we present several novel approaches that incorporate quantitative PCR based on TaqMan chemistry to facilitate identification of phage clones that display cell- and tissue-type-selective binding. We use leukemia cells, and breast and prostate cancer cells and tissues as prototypical targets. These methods are applicable to both fresh and FFPE cells and tissues and represent the first step of an endeavor to identify phage clones bearing peptides that show cell- and tissue-type-selective binding in FFPE material. The M13qPCR assay demonstrates excellent performance characteristics with a reproducible ≥5 log10 dynamic linear range and %CVs of 10 to 20% that are similar to those of clinically used, quantitative RT-PCR assays for viruses, such as HIV. 37 In addition, M13qPCR overcomes many of the shortcomings of the aforementioned methods.
In addition, our findings provide proof-of-principle that M13qPCR screening identifies phage clones that manifest biochemically specific interactions between cells and phage-displayed peptides. In Figure 6 ▶ in which M13qPCR is compared to flow cytometric screening, data are displayed for control clones labeled FGP and DLV. We previously showed that these clones manifest leukocyte-selective binding by flow cytometry that is abrogated by the cognate synthetic peptide but not control peptide, consistent with peptide-mediated binding to specific cell receptor(s). 15,16 Here, screening by M13qPCR yields comparable findings to flow cytometry, suggesting that M13qPCR identifies phage interactions with cell and tissues that are mediated by displayed peptides. Nonetheless, additional studies would be essential to confirm molecular specificity. Such studies are currently underway for several clones identified from this work.
When we designed this methodology, we set several goals for performance characteristics for M13qPCR. In our opinion, the most important qualities were precision, a broad dynamic linear range, and high throughput for use with a variety of targets. Sensitivity and accuracy were less important attributes. With regard to accuracy, others have estimated that the pfu:phage particle ratio is ∼1:1 to 2. 32 If this estimate were correct, PGE values, which are tied to measured pfu values for the standard, might be expected to underestimate the actual phage particle number by up to twofold. Nonetheless, for most phage display experiments, inaccuracies of even greater magnitude, which are likely, would be considered insignificant. This is because quantifications of phage are performed typically for comparative purposes. For example, panning experiments generally include assessment of changes in binding of phage populations to target throughout the course of panning to deciding whether the method is yielding progressively higher avidity phage subpopulations. Likewise, postpanning analysis generally includes assessment of the relative binding avidities of selected individual clones, ie, which clones bind most tightly to target and not to control. These applications require reproducibility, such that measurements could be taken throughout time and reliably compared. Precise interlaboratory comparisons could be facilitated by a set of uniform standards of which material for hundreds of thousands of runs may be generated from a few milliliters of concentrated phage. In addition, because phage experiments rarely require quantitative measurements of <1 × 102 to 1 × 103, a lower limit of detection in this range, as displayed by our method, would be acceptable. Lastly, this method readily affords high throughput of samples because most quantitative PCR platforms can accommodate 96 wells or more.
Many phage display libraries are based on the Ff class of filamentous bacteriophage that include M13, fd, and fl, which have 98% homologous genomes. 38 Not all derivatives contain the LacZ gene and therefore the primer sets described in this assay would not be applicable. However, given the greater than 6-kb size of the phage genome, numerous combinations of useful primers and probe are feasible. In fact, the Primer Express software yielded dozens of primer and probe sets based on native sequences in phage genome. These or other alternative sets could be readily generated using this or similar software and rapidly tested as described in this article. Caveats would include the following: that target sequences should neither include the coding region with random oligonucleotides nor display homology to potential DNA contaminants from target tissues.
A major rationale for developing this methodology is to facilitate identification of cell- and tissue-type-selective binding peptides. Such peptide probes have the potential to aid in elucidating basic mechanisms of oncogenesis and cancer progression by fostering characterization of differentially expressed or modified molecules. Moreover, these peptides or subsequently developed mimetics will likely have broad applications in diagnostic pathology, as well as therapeutics and imaging. In addition, phage display-based approaches may have distinct advantages over competing technologies such as RNA/cDNA microarray technologies for profiling, although the information from both approaches will likely prove to be complementary. Advantages include the ability to directly address structural differences by detecting phenotypic variants in mixed populations of cells or tissues without previous selection of which molecules to assay. In fact, structural differences such as posttranslational modifications would not be encompassed by RNA/cDNA microarray techniques. Others 39 have already undertaken panning procedures using intact, glyceraldehyde-fixed tissue fragments to identify phage that bind selectively to thymic epithelial cells but not other thymic components. Therefore, like some antibodies, phage can manifest cell-type binding specificity despite possible structural modifications engendered by target fixation. Phage-bearing brain and kidney tissue-specific binding peptides have been isolated by panning directly on whole organs in vivo. 31 In another report of in vivo panning, tumor vessel-binding peptides were identified from phage display libraries. 14 These phage bound specifically to tumor vessels, although not normal vessels, in histochemical analyses using fresh tissue. However, by directly panning on tumor sections as in this report, there is the potential advantage of exposure to greater diversity of sites (eg, nuclear, cytoplasmic) that would not be exposed using the latter two approaches. Phage display technology, moreover, has not been adapted for histochemical studies of clinical specimens, particularly archived FFPE tissues. This material represents a rich source for investigating molecular differences between benign and malignant tissues and for identifying disease stage-specific characteristics. This report contributes toward that adaptation and will facilitate development of panning strategies on tissue sections.
Acknowledgments
We thank Yaohui Bai for expert technical assistance, Dr. James Burritt for critical comments and suggestions, and Dr. Sharon Weiss for her support of these studies.
Footnotes
Address reprint requests to David L. Jaye, Department of Pathology and Laboratory Medicine, 615 Michael St., Atlanta, GA 30322. E-mail: dljaye@emory.edu.
Supported by grants from the Avon Foundation, the National Institutes of Health (grants CA-91435, T32 DK07771, and DK60647), and the Swiss National Science Foundation (grant 31-66804.01).
References
- 1.Burritt JB, Bond CW, Doss KW, Jesaitis AJ: Filamentous phage display of oligopeptide libraries. Anal Biochem 1996, 238:1-13 [DOI] [PubMed] [Google Scholar]
- 2.McCafferty J, Griffiths AD, Winter G, Chiswell DJ: Phage antibodies: filamentous phage displaying antibody variable domains. Nature 1990, 348:552-554 [DOI] [PubMed] [Google Scholar]
- 3.Rader C, Barbas CF, III: Phage display of combinatorial antibody libraries Curr Opin Biotechnol 1997, 8:503-508 [DOI] [PubMed] [Google Scholar]
- 4.Smith GP, Scott JK: Libraries of peptides and proteins displayed on filamentous phage. Methods Enzymol 1993, 217:228-257 [DOI] [PubMed] [Google Scholar]
- 5.Burritt JB, Busse SC, Gizachew D, Siemsen DW, Quinn MT, Bond CW, Dratz EA, Jesaitis AJ: Antibody imprint of a membrane protein surface. Phagocyte flavocytochrome b. J Biol Chem 1998, 273:24847-24852 [DOI] [PubMed] [Google Scholar]
- 6.Burritt JB, DeLeo FR, Quinn MT, Siemsen DW, Bond CW, Jesaitis AJ: Identification of a discontinuous neutrophil cytochrome B epitope with a nonapeptide phage-display library. Mol Biol Cell 1995, 6:224A [Google Scholar]
- 7.Atwell S, Ultsch M, De Vos AM, Wells JA: Structural plasticity in a remodeled protein-protein interface. Science 1997, 278:1125-1128 [DOI] [PubMed] [Google Scholar]
- 8.Jesaitis AJ, Gizachew D, Dratz EA, Siemsen DW, Stone KC, Burritt JB: Actin surface structure revealed by antibody imprints: evaluation of phage-display analysis of anti-actin antibodies. Protein Sci 1999, 8:760-770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norris JD, Paige LA, Christensen DJ, Chang CY, Huacani MR, Fan D, Hamilton PT, Fowlkes DM, McDonnell DP: Peptide antagonists of the human estrogen receptor. Science 1999, 285:744-746 [DOI] [PubMed] [Google Scholar]
- 10.Lowman HB: Bacteriophage display and discovery of peptide leads for drug development. Annu Rev Biophys Biomol Struct 1997, 26:401-424 [DOI] [PubMed] [Google Scholar]
- 11.Arap W, Haedicke W, Bernasconi M, Kain R, Rajotte D, Krajewski S, Ellerby HM, Bredesen DE, Pasqualini R, Ruoslahti E: Targeting the prostate for destruction through a vascular address. Proc Natl Acad Sci USA 2002, 99:1527-1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lekkerkerker A, Logtenberg T: Phage antibodies against human dendritic cell subpopulations obtained by flow cytometry-based selection on freshly isolated cells. J Immunol Methods 1999, 231:53-63 [DOI] [PubMed] [Google Scholar]
- 13.Huls GA Heijnen IA, Cuomo ME Koningsberger JC, Wiegman L Boel E, van der Vuurst de Vries AR, Loyson SA, Helfrich W, van Berge Henegouwen GP, van Meijer M, de Kruif J, Logtenberg T: A recombinant, fully human monoclonal antibody with antitumor activity constructed from phage-displayed antibody fragments Nat Biotechnol 1999, 17:276-281 [DOI] [PubMed] [Google Scholar]
- 14.Arap W, Pasqualini R, Ruoslahti E: Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science 1998, 279:377-380 [DOI] [PubMed] [Google Scholar]
- 15.Mazzucchelli L, Burritt JB, Jesaitis AJ, Nusrat A, Liang TW, Gewirtz AT, Schnell FJ, Parkos CA: Cell-specific peptide binding by human neutrophils. Blood 1999, 93:1738-1748 [PubMed] [Google Scholar]
- 16.Jaye DL, Edens HA, Mazzucchelli L, Parkos CA: Novel G protein-coupled responses in leukocytes elicited by a chemotactic bacteriophage displaying a cell type-selective binding peptide. J Immunol 2001, 166:7250-7259 [DOI] [PubMed] [Google Scholar]
- 17.Parmley SF, Smith GP: Antibody-selectable filamentous fd phage vectors: affinity purification of target genes. Gene 1988, 73:305-318 [DOI] [PubMed] [Google Scholar]
- 18.Wu GJ, Varma VA, Wu MW, Wang SW, Qu P, Yang H, Petros JA, Lim SD, Amin MB: Expression of a human cell adhesion molecule, MUC18, in prostate cancer cell lines and tissues. Prostate 2001, 48:305-315 [DOI] [PubMed] [Google Scholar]
- 19.Lanotte M, Martin-Thouvenin V, Najman S, Balerini P, Valensi F, Berger R: NB4, a maturation inducible cell line with t(15;17) marker isolated from a human acute promyelocytic leukemia (M3). Blood 1991, 77:1080-1086 [PubMed] [Google Scholar]
- 20.Henson PM, Oades ZG: Stimulation of human neutrophils by soluble and insoluble immunoglobulin aggregates: secretion of granule constituents and increased oxidation of glucose. J Clin Invest 1975, 56:1053-1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burritt JB, Quinn MT, Jutila MA, Bond CW, Jesaitis AJ: Topological mapping of neutrophil cytochrome b epitopes with phage-display libraries. J Biol Chem 1995, 270:16974-16980 [DOI] [PubMed] [Google Scholar]
- 22.Livak KJ, Flood SJ, Marmaro J, Giusti W, Deetz K: Oligonucleotides with fluorescent dyes at opposite ends provide a quenched probe system useful for detecting PCR product and nucleic acid hybridization. PCR Methods Appl 1995, 4:357-362 [DOI] [PubMed] [Google Scholar]
- 23.Prophet EB: Tissue processing: dehydration, clearing, and infiltration. Prophet EB Mills B Arrington JB Sobin LH eds. Laboratory Methods in Histotechnology. 1994:pp 29-32 American Registry of Pathology, Washington D.C.
- 24.Yanisch-Perron C, Vieira J, Messing J: Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 1985, 33:103-119 [DOI] [PubMed] [Google Scholar]
- 25.Ebright R, Dong Q, Messing J: Corrected nucleotide sequence of M13mp18 gene III. Gene 1992, 114:81-83 [DOI] [PubMed] [Google Scholar]
- 26.Heid CA, Stevens J, Livak KJ, Williams PM: Real time quantitative PCR. Genome Res 1996, 6:986-994 [DOI] [PubMed] [Google Scholar]
- 27.Martell M, Gomez J, Esteban JI, Sauleda S, Quer J, Cabot B, Esteban R, Guardia J: High-throughput real-time reverse transcription-PCR quantitation of hepatitis C virus RNA. J Clin Microbiol 1999, 37:327-332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miley WJ, Suryanarayana K, Manns A, Kubota R, Jacobson S, Lifson JD, Waters D: Real-time polymerase chain reaction assay for cell-associated HTLV type I DNA viral load. AIDS Res Hum Retroviruses 2000, 16:665-675 [DOI] [PubMed] [Google Scholar]
- 29.Niesters HG, van Esser J, Fries E, Wolthers KC, Cornelissen J, Osterhaus AD: Development of a real-time quantitative assay for detection of Epstein-Barr virus. J Clin Microbiol 2000, 38:712-715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giordano RJ, Cardo-Vila M, Lahdenranta J, Pasqualini R, Arap W: Biopanning and rapid analysis of selective interactive ligands. Nat Med 2001, 7:1249-1253 [DOI] [PubMed] [Google Scholar]
- 31.Pasqualini R, Ruoslahti E: Organ targeting in vivo using phage display peptide libraries. Nature 1996, 380:364-366 [DOI] [PubMed] [Google Scholar]
- 32.Rider JE, Sparks AB, Adey NB, Kay BK: Microbiological methods. Kay BK Winter J McCafferty J eds. Phage Display of Peptides and Proteins: A Laboratory Manual. 1996, :pp 55-65 Academic Press, San Diego [Google Scholar]
- 33.Kadavy DR, Shaffer JJ, Lott SE, Wolf TA, Bolton CE, Gallimore WH, Martin EL, Nickerson KW, Kokjohn TA: Influence of infected cell growth state on bacteriophage reactivation levels. Appl Environ Microbiol 2000, 66:5206-5212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valadon P, Scharff MD: Enhancement of ELISAs for screening peptides in epitope phage display libraries. J Immunol Methods 1996, 197:171-179 [DOI] [PubMed] [Google Scholar]
- 35.Mittereder N, March KL, Trapnell BC: Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol 1996, 70:7498-7509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clark KR, Liu X, McGrath JP, Johnson PR: Highly purified recombinant adeno-associated virus vectors are biologically active and free of detectable helper and wild-type viruses. Hum Gene Ther 1999, 10:1031-1039 [DOI] [PubMed] [Google Scholar]
- 37.Nolte FS, Boysza J, Thurmond C, Clark WS, Lennox JL: Clinical comparison of an enhanced-sensitivity branched-DNA assay and reverse transcription-PCR for quantitation of human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol 1998, 36:716-720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Webster R: Filamentous phage biology. Barbas CF, III Burton DR Scott JK Silverman GJ eds. Phage Display: A Laboratory Manual. 2001, :pp 1.1-1.37 Cold Spring Harbor Press, Cold Spring Harbor [Google Scholar]
- 39.Van Ewijk W, de Kruif J, Germeraad WT, Berendes P, Ropke C, Platenburg PP, Logtenberg T: Subtractive isolation of phage-displayed single-chain antibodies to thymic stromal cells by using intact thymic fragments. Proc Natl Acad Sci USA 1997, 94:3903-3908 [DOI] [PMC free article] [PubMed] [Google Scholar]