Abstract
Ischemic and solid tumor tissues are less well perfused than normal tissue, leading to metabolic changes and chronic hypoxia, which in turn promotes angiogenesis. We identified human angiopoietin-like 4 (angptl4) as a gene with hypoxia-induced expression in endothelial cells. We showed that the levels of both mRNA and protein for ANGPTL4 increased in response to hypoxia. When tested in the chicken chorioallantoic membrane assay, ANGPTL4 induced a strong proangiogenic response, independently of vascular endothelial growth factor. In human pathology, ANGPTL4 mRNA is produced in ischemic tissues, in conditions such as critical leg ischemia. In tumors, ANGPTL4 is produced in the hypoxic areas surrounding necrotic regions. We observed particularly high levels of ANGPTL4 mRNA in tumor cells of conventional renal cell carcinoma. Other benign and malignant renal tumor cells do not produce ANGPTL4 mRNA. This molecule therefore seems to be a marker of conventional renal cell carcinoma. ANGPTL4, originally identified as a peroxisome proliferator-activated receptor α and γ target gene, has potential for use as a new diagnostic tool and a potential therapeutic target, modulating angiogenesis both in tumors and in ischemic tissues. This study also suggests that ANGPTL4 may provide a link between metabolic disorders and hypoxia-induced angiogenesis.
There is growing evidence that changes in oxygen partial pressure (ΔpO2) play a key role in the regulation of many cellular functions. A ΔpO2 is observed during ischemia that involves a decrease in blood supply resulting from a fall in arterial flow or venous drainage. Tumor blood flow may be chaotic, resulting in hypoxia. 1 A complex collection of biochemical and physiological interactions, including imbalances in levels of angiogenic regulators, occurs during hypoxia. 2-4 These interactions are not fully understood, but recent studies have demonstrated the essential role of hypoxia-inducible factor (HIF)-1α in hypoxia-induced angiogenesis. 5-7 Indeed, HIF-1α regulates many genes, including those for erythropoietin, vascular endothelial growth factor (VEGF), tyrosine hydroxylase, inducible NO synthase (iNOS), and angiopoietin-2. 8-10
Endothelial cells (ECs) are the prime targets of hypoxia-induced growth factors. 11,12 In this study, we used the human microvascular endothelial cell line HMEC-1, the first immortalized microvascular EC line to retain the characteristics of microvascular ECs. 13 HMEC-1 cells were subjected to chemical hypoxia, by exposing them to 100 μmol/L of desferrioxamine (DFO) 14 for 20 hours. We then used representational difference analysis (RDA) 15 to identify 350 hypoxia-induced genes (data not shown). Two of the cDNA fragments identified corresponded to the human angptl4 gene (nucleotides 498 to 904 and nucleotides 1147 to 1344) that encodes a secreted growth factor, ANGPTL4, also known as hepatic fibrinogen/angiopoietin-related protein (HFARP) 16 and peroxisome proliferator-activated receptor-γ (PPARγ) angiopoietin-related gene (PGAR) 17 or fasting-induced adipose factor (FIAF). 18 ANGPTL4 has been reported to induce an anti-apoptotic activity in ECs 16 and its gene has been shown to be a target of PPARγ or PPARα. 17,18
In this study, we demonstrated the induction of ANGPTL4 production by hypoxia at the mRNA and protein levels. We suggest that this protein may be involved in the mechanisms that compensate for ischemia by angiogenesis and/or arteriogenesis. 19,20 ANGPTL4 induced a strong in ovo angiogenic response in the chicken chorioallantoic membrane (CAM) assay, independently of VEGF. We also found that the angptl4 gene was expressed in ischemic tissues in humans, in conditions such as critical leg ischemia. In tumors, we found that ANGPTL4 was produced in liposarcomas and hepatocellular carcinomas, in amounts similar to those previously observed in adipose tissue and liver. 16-18 It was also produced in perinecrotic hypoxic regions in a wide variety of cancers. In addition, ANGPTL4 mRNA was present in very large amounts in tumoral cells from conventional renal cell carcinomas (RCCs), but not in other kidney tumors (benign or malignant). It thus seems to be a bona fide marker of conventional RCC. Both as a protein encoded by a target gene of PPARα and PPARγ, which have been shown to be associated with the regulation of lipid metabolism and/or glucose homeostasis and as a hypoxia-inducible secreted protein, ANGPTL4 has potential for use as a new diagnostic tool and potential therapeutic target, modulating angiogenesis in tumors and ischemic tissues.
Materials and Methods
Cell Culture
We used the HMEC-1 cell line of human dermal microvascular endothelial cells (a gift from Thomas J. Lawley, Emory University, School of Medicine, Atlanta, GA). HMEC-1 cells were cultured in MCDB-131 medium supplemented with 20% heat-inactivated fetal calf serum, 2 mmol/L of l-glutamine, 100 U/ml of penicillin, 100 μg/ml of streptomycin, 10 ng/ml of human recombinant epidermal growth factor, and 1 μg/ml of hydrocortisone. For hypoxic treatments, cells were grown in an atmosphere containing 3% O2, in an IG750 incubator (Jouan, France), or in the presence of 100 μmol/L of DFO. For growth factor treatments, cells were incubated for 15 hours in fresh serum-free medium supplemented with 1 ng/ml of transforming growth factor-β1, 10 nmol/L of insulin-like growth factor-1, 30 ng/ml of VEGF, 100 nmol/L of angiotensin II, 0.1 ng/ml of basic fibroblast growth factor, or 100 nmol/L of epidermal growth factor.
Recombinant Chinese hamster ovary (CHO)/ANGPTL4 cells were established by transfecting CHO cells with the full-length human ANGPLT4 cDNA in pCDNA3.1mycHis (Invitrogen) and selecting stably transfected cells with geneticin. Cells were maintained in Ham’s F12 medium (Life Technologies) supplemented with 7% fetal calf serum, 1% glutamine, 100 U/ml of penicillin, and 100 μg/ml of streptomycin.
RNA Isolation and cDNA Synthesis
RNA was extracted with the RNeasy midiprep kit (Qiagen) from HMEC-1 cells grown to 80% confluence, untreated or treated with 100 μmol/L of DFO for 20 hours. RNA was digested with RNase-free DNase RQ1 (Promega) for 30 minutes and PolyA+ RNA was isolated by performing the Oligotex mRNA kit (Qiagen) procedure twice. cDNAs were synthesized from 10 μg of polyA+ RNA by oligodT priming, using Superscript II.
Representational Difference Analysis (RDA)
The RDA procedure used was similar to previously published methods, 15 with several important modifications. 21 The ratios for rounds 1, 2, and 3 were 1:10, 1:500, and 1:1000, respectively. Subtracted RDA products were inserted into the pGEM.T cloning vector (Promega) and analyzed by sequencing.
Gene Expression Studies
Northern blots were performed using the NorthernMax and Strip-EZ RNA kits from Ambion. Normal kidney and conventional RCC RNAs were obtained from Stratagene. Paraffin section preparation, probe labeling by in vitro transcription, and in situ hybridization, were performed as previously described. 22 Real-time quantitative polymerase chain reaction was used to evaluate ANGPTL4 levels by means of a SYBR Green assay (Applied Biosystems), with 18S as a control, using an Iq Cycler (BioRad). ANGPTL4 was amplified with the following primers: 5′-GCTGCATGCGTTGCCTC-3′ (forward) and 5′-CCCTTGGTCCACGCCTCTA-3′ (reverse). 18S ribosomal RNA was amplified with the following primers: 5′-CGCCGCTAGAGGTGAAAT TC-3′ (forward) and 5′-TCTTGGCAAATGCTTTCGC-3′ (reverse). Thermocycling conditions were as follows: 2 minutes at 50°C followed by an initial denaturation for 10 minutes at 95°C, followed by 40 cycles of two-step polymerase chain reaction consisting of 15 seconds at 95°C, and 1 minute at 60°C. Duplicate experiments were performed three times to compare each target cDNA using the ΔΔCt method.
CAM Assay
Approximately one million wild-type CHO cells, used as a negative control, or CHO cells producing ANGPTL4 were placed on the surface of the CAM of 9-day-old chick embryos, following the shell-less protocol. 23 Cells were allowed to grow for 3 to 5 days, as previously described. 24 The VEGF inhibitor PTK787/ZK 222584 [1-(4-chloroanilinol)-4-(4-pyridylmethyl) phthalazine succinate, dihydrochloride salt; a gift from Dr. J Wood, Novartis Pharmaceuticals, Basel, Switzerland] 25,26 was dissolved in dimethyl sulfoxide at 100 μmol/L. This solution was then diluted 100 × in water and applied twice per day (20 μl of a 1 μmol/L solution) to the CAM, not to the CHO/ANGPTL4 cells, starting 48 hours after grafting. We injected fluorescein isothiocyanate-dextran into the CAM to make the vasculature more visible, and CAMs were then examined by two observers blind to the cells used. CHO nodules and the surrounding CAMs were then excised, fixed in 4% paraformaldehyde, and processed for immunohistochemical analysis.
Tissue Collection
All tumor specimens were obtained from the Pathological Anatomy Department of Tenon Hospital (Paris, France). They were originally obtained from patients undergoing surgery. Tissues were fixed in 20% formaldehyde and embedded in paraffin. The sections were stained with hematoxylin, eosin, and safran and conventional histological diagnosis was made by standard light microscopy. Human tumors were classified according to the revised World Health Organization criteria for tumors. Small leg tissue samples were obtained from patients undergoing amputation for critical leg ischemia at Saint Joseph Hospital or Hopital Europeen Georges Pompidou (Paris, France). For each patient, normal tissue, from a nonischemic part of the leg, was taken as a control. Samples were fixed in 4% paraformaldehyde and processed for histology.
Immunohistochemistry
Immunostaining with anti-α-smooth muscle actin (1:2000, clone 1A4; DAKO) or anti-endothelial CD34 (1:200, clone QBEND10; Immunotech) antibodies was performed by routine methods using a biotinylated secondary antibody and the ABC-peroxidase complex (Vector Laboratories) with diaminobenzidine-H2O2 used as the chromogen for detection.
Antibody Production
An antibody directed against human ANGPTL4 was generated by conjugating a 15-amino acid peptide from the coiled-coil domain (amino acids 149 to 165) with ovalbumin and injecting the conjugate into rabbits (Neosystem, France). This antibody made it possible to detect endogenous human ANGPTL4 protein on immunoblots, but not by immunohistochemistry on paraffin sections.
Western Blotting
HMEC-1 cells were rinsed and fresh serum-free medium was added. The media of untreated cells and of cells treated with 100 μmol/L of DFO for 24 hours were concentrated by a factor of 60, by centrifugation (Ultrafree-4 Biomax-15-kd membrane; Millipore). Western blots were probed with antibodies directed against human ANGPTL4 (dilution, 1:1000) and antibody binding was detected with a biotin-streptavidin system.
Results
ANGPTL4 Is Induced in Hypoxic Conditions in HMEC-1 Cells
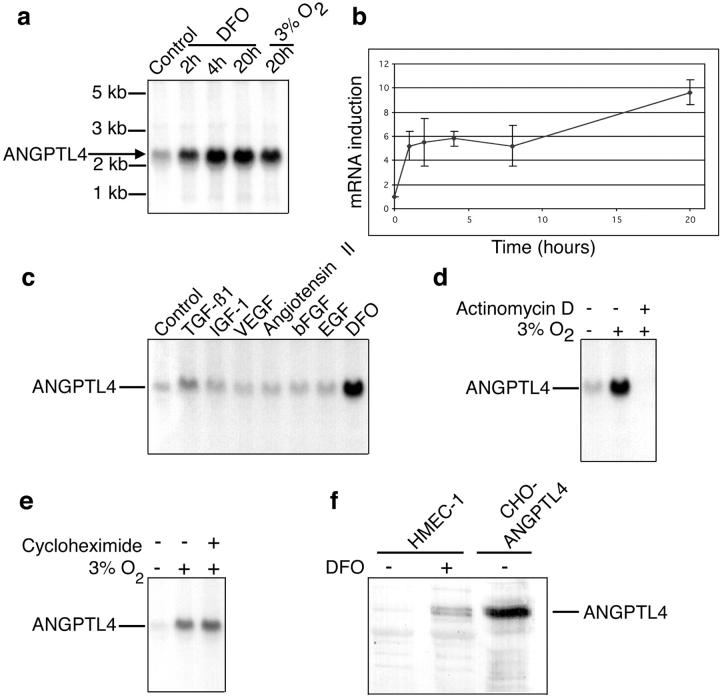
ANGPTL4 was identified by cDNA RDA as 1 of the 350 hypoxia-induced mRNAs isolated from ECs (data not shown). The hypoxic stimulation of ANGPTL4 mRNA expression in these cells was confirmed by Northern blotting with RNA isolated from HMEC-1 cells cultured under chemical hypoxia (100 μmol/L DFO) or gaseous hypoxia (3% O2) and comparison with RNA from HMEC-1 cells cultured under normoxia. Both chemical and gaseous hypoxia induced robust induction of ANGPTL4 mRNA (Figure 1a) ▶ . Induction by DFO was quantified by real-time quantitative polymerase chain reaction, using the 18S RNA gene as a reference gene (Figure 1b) ▶ . ANGPTL4 mRNA levels had increased by a factor of six at 2 hours after the start of hypoxia. ANGPTL4 mRNA levels remained high thereafter, reaching a maximum of eight times basal levels after 20 hours of hypoxia (Figure 1, a and b) ▶ .
Figure 1.
ANGPTL4 is a direct target of hypoxia in HMEC-1 cells. a: Northern blot analysis of ANGPTL4 mRNA induction by chemical hypoxia (100 μmol/L DFO) and gaseous hypoxia (3% O2). b: Kinetic analysis by real-time quantitative reverse transcriptase-polymerase chain reaction of ANGPTL4 mRNA induction by 100 μmol/L of DFO. c: Northern blot analysis of ANGPTL4 mRNA levels after 15 hours of treatment with various growth factors and 100 μmol/L of DFO, demonstrating the specificity of the effects of hypoxia. d: Northern blot analysis of ANGPTL4 mRNA levels after incubation for 15 hours in an atmosphere containing 3% O2, with or without 1 μg/ml of actinomycin D. e: Northern blot analysis of ANGPTL4 mRNA levels after incubation for 4 hours in an atmosphere containing 3% O2, with or without 10 μg/ml of cycloheximide. f: Western blot analysis of ANGPTL4 protein, secreted into the culture medium in response to hypoxia. Recombinant ANGPTL4 protein secreted by stably transfected CHO cells was used as a control (right lane).
We investigated whether the induction of ANGPTL4 production by hypoxia was specific, by incubating HMEC-1 cells for 15 hours with various growth factors: transforming growth factor-β1, insulin-like growth factor-1, VEGF, angiotensin II, basic fibroblast growth factor, epidermal growth factor, and DFO. Only DFO caused a marked increase in ANGPTL4 mRNA levels, the other growth factors having little (transforming growth factor-β1) or no (all of the other growth factors) effect. We investigated the mechanisms responsible for ANGPTL4 mRNA accumulation in response to hypoxia by incubating HMEC-1 cells for 15 hours in an atmosphere containing 3% O2, in the presence of 1 μg/ml of actinomycin D. Actinomycin D totally abolished the induction of ANGPTL4 mRNA (Figure 1d) ▶ , indicating that hypoxia exerts its effects on angptl4 gene expression at the transcriptional level. We then investigated whether hypoxia directly affected angptl4 gene expression. The induction of ANGPTL4 mRNA persisted in the presence of cycloheximide, which inhibits protein synthesis (Figure 1e) ▶ , consistent with angptl4 being a direct target gene of hypoxia. Finally, we showed, by Western blotting, that ANGPTL4 is secreted into the culture medium of HMEC-1 cells in response to hypoxia (Figure 1f) ▶ .
ANGPTL4 Promotes Angiogenesis in Ovo
Because the production of ANGPTL4 by ECs is induced by hypoxia and because this protein displays similarity to ANGPTL3, which was recently described as an angiogenic factor, 27 we investigated the potential angiogenic effects of ANGPTL4 in ovo. We used an original experimental model recently developed by our group that involves grafting CHO cells producing the protein of interest onto the chick chorioallantoic membrane (CAM). 24 Any effect on the development of CAM vascularization can then be observed throughout the next few days. CHO cells stably secreting ANGPTL4 (Figure 1f ▶ , right lane) were grafted onto the outer surface of the CAM on day 9 of embryonic development. These cells (CHO/ANGPTL4) implanted efficiently in the CAM (34 of 36 grafts performed), resulted in the development of a three-dimensional structure resembling a nodule. ANGPTL4 production led to a major reorganization of mesodermal vessels in the CAM, with the formation of a spoke-wheel pattern of vascularization toward the nodule, a feature typical of active neoangiogenesis (Figure 2a ▶ , left). In contrast, wild-type CHO cells, used as a control, implanted very rarely (2 of 38 grafts performed) and had no angiogenic effect (Figure 2a ▶ , right).
Figure 2.
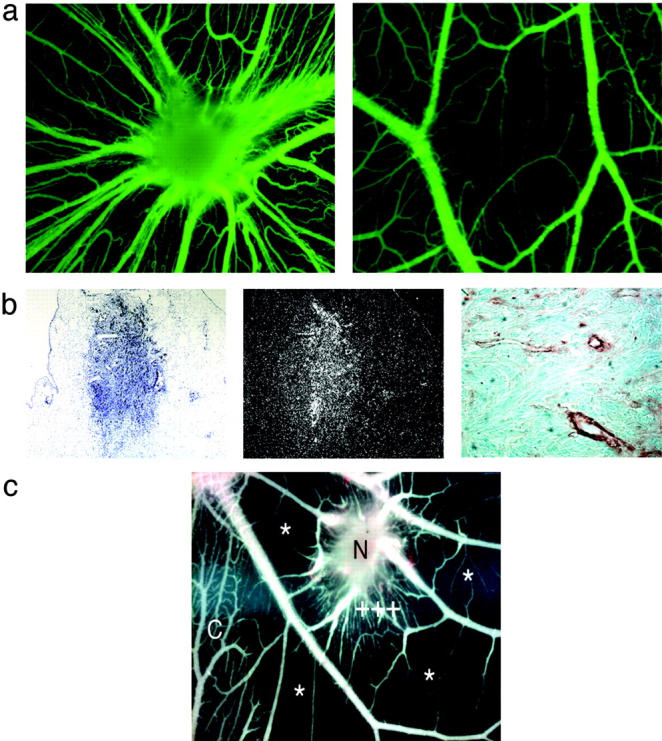
Angiogenic activity of ANGPTL4 in the chicken CAM assay. a: Left: CHO-ANGPTL4 nodule 5 days after grafting, displaying a spoke-wheel pattern of vascularization. Highly vascularized nodule after in vivo fluorescein isothiocyanate-dextran injection. Right: Cell aggregates from CHO control cells remained on the ectodermal surface and displayed no induced angiogenic response. b: CHO/ANGPTL4 nodule counterstained with toluidine blue (left). Dark-field view of ANGPTL4 mRNA in the implanted nodule 5 days after grafting (middle). Invasion of the nodule by newly formed vessels from the CAM is shown by staining with an anti-smooth muscle cell actin antibody (right). c: Inhibition of the protein tyrosine kinase activity of the VEGF receptor by PTK787/ZK 222584. PTK787/ZK 222584 applied close to the cell aggregates (N) 2 days after grafting does not inhibit CHO-ANGPTL4-mediated angiogenesis, as shown by the nonregression of blood vessels surrounding the CHO-ANGPTL4 cells (+++). In contrast, PTK787/ZK 222584 inhibits CAM blood vessel formation away from the CHO-ANGPTL4-producing cells (*), but only in the treated area in which growth of the microvasculature is inhibited. As a control, an untreated area of the CAM is shown on the left of C. Scale bars: 2 mm (a), 150 μm (b, left and middle); and 20 μm in (b, right).
Histological analysis of the nodule showed that CHO/ANGPTL4 cells crossed the ectoderm and formed spherical nodules in the mesoderm (Figure 2b ▶ , left). In situ hybridization demonstrated that the CHO/ANGPTL4 cells stably produced ANGPTL4 mRNA within the nodule 5 days after grafting (Figure 2b ▶ , left and middle). Vascular chemotaxis, induced by the ANGPTL4 produced by the grafted CHO/ANGPTL4 cells, resulted in the invasion of newly formed vessels from the CAM into the growing nodule, as shown by staining with anti-smooth muscle cell-α-actin antibody (Figure 2b ▶ , right). The robust induction of new blood vessel formation and the reorganization of the vascular system provoked by ANGPTL4 demonstrate that ANGPTL4 is an angiogenic factor.
We characterized the angiogenic effect of ANGPTL4 further by repeating the CAM experiment in the presence of PTK787/ZK 222584 [1-(4-chloroanilino)-4-(4-pyridylmethyl) phthalazine succinate], a potent inhibitor of VEGF receptor tyrosine kinases. 25 Interestingly, PTK787/ZK 222584 inhibited the formation of CAM microvessels in areas far from the grafted ANGPTL4-producing cells, but did not efficiently inhibit the neovascularization induced in the vicinity of the ANGPTL4-secreting cells (Figure 2c) ▶ , strongly suggesting that VEGF, despite being involved in most angiogenic processes, is not involved in ANGPTL4-induced angiogenesis.
ANGPTL4 Is Produced in Human Hypoxic/Ischemic Tissue
We investigated the physiological relevance of ANGPTL4 in hypoxic situations in humans by evaluating ANGPTL4 mRNA levels in critical leg ischemia by in situ hybridization. Human leg specimens were obtained from patients who had undergone amputation. The distal (most diseased) and proximal (most healthy) parts of the leg were compared for each patient. ANGPTL4 mRNA levels were below the threshold of detection by in situ hybridization in the normal part of the leg (Figure 3, a through e ▶ ; left). In contrast, ANGPTL4 mRNA levels were repeatedly found to be higher in the ischemic tissues of several patients suffering from critical leg ischemia (Figure 3, a and b ▶ ; compare left and right panels). We tried to identify the types of cell producing ANGPTL4 mRNA by performing in situ hybridization without (Figure 3c ▶ , right) or with double-immunohistochemical labeling, using anti-smooth muscle actin (Figure 3d ▶ , right) and anti-CD34 antibodies (Figure 3e ▶ , right). We found that ANGPTL4 mRNA was produced in the wall of the blood vessel in both vascular smooth muscle cells and ECs.
Figure 3.
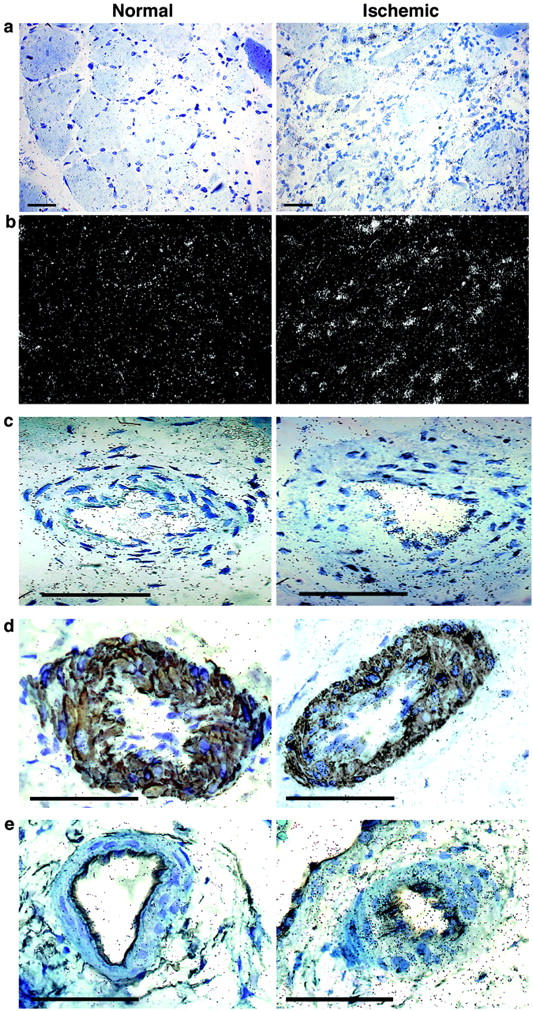
In situ hybridization analysis of ANGPTL4 mRNA levels in critical leg ischemia. Slides were counterstained with toluidine blue. The normal part of the leg is shown on the left and the ischemic part of the leg on the right. a: Bright-field view showing ANGPTL4 mRNA in tissues. b: Dark-field view showing ANGPTL4 mRNA. c: ANGPTL4 mRNA production in large vessels showing restricted labeling in the ECs and smooth muscle cells in the ischemic part. d: ANGPTL4 mRNA in SMCs in the ischemic part (right) of the leg, compared with the normal part (left), by double-labeling with an anti-smooth muscle cell actin antibody. e: ANGPTL4 mRNA in ECs of a large vessel of the ischemic part of the leg (right), compared with the normal part of the leg (left), by double-labeling with an anti-CD34 antibody. Scale bars, 100 μm.
ANGPTL4 Produced in Large Amounts in Conventional RCC Cells
Solid tumor tissues are subject to chronic or intermittent low partial pressures of oxygen. We therefore investigated whether ANGPTL4 mRNA was produced by various types of tumor (Table 1) ▶ in addition to glioblastomas, as demonstrated by Lal and colleagues. 28 As ANGPTL4 was first detected in the placenta, liver, and adipose tissue, 17,18 we began by assessing angptl4 expression in hepatocellular carcinoma and liposarcoma. ANGPTL4 levels in in situ hybridization experiments were not higher in hepatocellular carcinoma than in normal hepatocytes (data not shown). ANGPTL4 levels were slightly higher in well-differentiated liposarcoma than in normal adipocytes, and tumor adipocytes displayed a heterogeneous pattern of expression (data not shown). Interestingly, ANGPTL4 was also detected in the perinecrotic region of several types of tumor in which hypoxia has been reported to up-regulate angiogenic factors. 29 For example, angptl4 expression was observed in the perinecrotic areas of pulmonary carcinomas (Figure 4a) ▶ , renal oncocytomas (Figure 4b) ▶ , and chromophobic RCCs (Figure 4c) ▶ .
Table 1.
In Vivo ANGPTL4 mRNA Expression in Tumoral Tissues
| Organ | Tumor type | Histological type | Number | Normal tissue | Perinecrotic areas | Tumoral cells |
|---|---|---|---|---|---|---|
| Kidney | Malignant primary lesion | Conventional carcinoma | 65 | − | +++ | +++ |
| Tubulo-papillary carcinoma | 5 | − | + | − | ||
| Chromophobe cell carcinoma | 4 | − | + | − | ||
| Composite tumor | 3 | − | + | − | ||
| Benign tumor | Oncocytoma | 4 | − | + | − | |
| Angiomyolipoma | 1 | − | NA | − | ||
| Preneoplasic lesion | Tubulo-papillary adenoma | 2 | − | NA | − | |
| Breast | Malignant primary lesion | Canalar invasive carcinoma | 7 | − | − | − |
| Lobular invasive carcinoma | 2 | − | NA | − | ||
| Metastatic lesion | Adenocarcinoma ganglionar metastasis | 1 | − | NA | − | |
| Lung | Malignant primary lesion | Adenocarcinoma | 4 | − | + | − |
| Squamous cell carcinoma | 8 | − | − | |||
| Small cell carcinoma | 1 | − | − | |||
| Colon | Malignant primary lesion | Adenocarcinoma | 6 | − | * | − |
| Preneoplasic lesion | Tubulo-villous adenoma | 3 | − | NA | − | |
| Metastatic lesion | Adenocarcinoma ganglionar metastasis | 4 | − | NA | − | |
| Adenocarcinoma hepatic metastasis | 2 | − | * | − | ||
| Adenocarcinoma pelvian metastasis | 2 | − | * | − | ||
| Brain | Malignant primary lesion | Glioblastoma | 2 | − | + | − |
| Oligodendroglioma | 2 | − | + | − | ||
| Prostate | Malignant primary lesion | Adenocarcinoma | 3 | − | * | − |
| Benign tumor | Adenomyoma | 3 | − | NA | − | |
| Preneoplasic lesion | High grade prostatic intra-epithelial neoplasia | 1 | − | NA | − | |
| Soft tissues | Malignant primary lesion | Angiosarcoma | 4 | − | * | − |
| Liposarcoma | 6 | ++ | ++ | ++ | ||
| Liver | Malignant primary lesion | Hepatocellular carcinoma | 2 | + | * | + |
| Miscellaneous | Malignant primary lesion | Carcinoid tumors | 3 | − | NA | NA |
Tumor specimens used in this study. Expression levels are indicated by + signs. NA, not applicable, in cases in which no necrotic areas were found;
* , the presence only of very small regions of necrosis, in which ANGPTL4 mRNA was barely detected.
Figure 4.
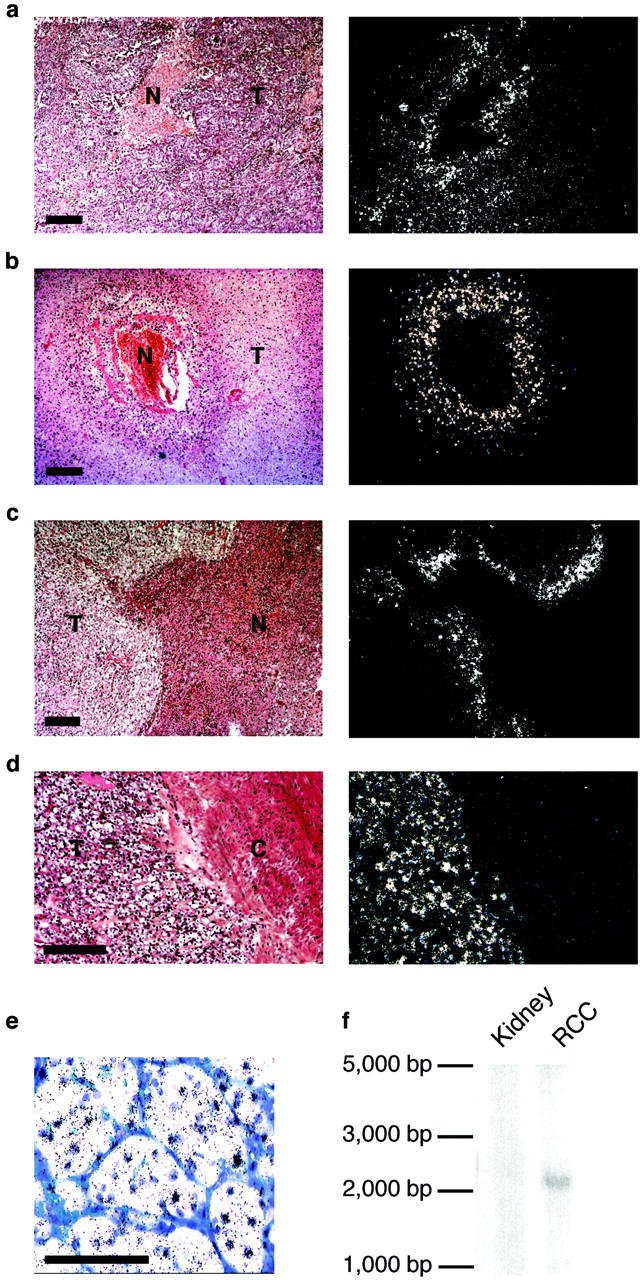
Analysis of ANGPTL4 mRNA production in various types of tumor. a–d: Bright-field (left) and dark-field (right) views of ANGPTL4 mRNA production in necrotic areas of a pulmonary carcinoma (a), renal oncocytoma (b), chromophobic RCC (c), and in conventional RCC (d). e: Production of ANGPTL4 mRNA by tumor cells of conventional RCC. f: Northern blot analysis of ANGPTL4 mRNA levels in normal kidney and conventional RCC. T, tumor tissue; N, necrotic area; C, kidney capsule. Scale bars, 100 μm.
One of the major findings of this study was the much higher levels of ANGPTL4 mRNA in tumoral epithelial cells from conventional RCC than in the adjacent nontumoral part of the kidney (Figure 4, d and e) ▶ . Northern blot analysis clearly showed the 2.3-kb ANGPTL4 mRNA to be present in conventional RCC, whereas it was not detected in the adjacent normal renal tissue (Figure 4f) ▶ . We examined other renal tumors to determine whether ANGPTL4 was specific to this tumor type. We found that tumoral cells from tubulopapillary carcinomas and chromophobe carcinomas did not produce ANGPTL4 mRNA (data not shown). Benign tumors (oncocytomas or angiomyolipomas) and preneoplasic lesions (tubulopapillary adenomas) also lacked ANGPTL4 mRNA (data not shown). This result contrasts strongly with the high levels of ANGPTL4 mRNA that we found in all 65 conventional RCCs (Table 1) ▶ . Thus, there seem to be two different patterns of ANGPTL4 production in tumor tissues: ANGPTL4 is produced in a limited number of cells around the necrotic and hypoxic areas of most tumors, and specifically in the tumoral cells of conventional RCC.
Discussion
ANGPTL4 has been identified by several groups: Kim and colleagues 16 identified it as a novel fibrinogen/angiopoietin-related protein by homology cloning, Kersten and colleagues 18 reported the corresponding gene to be a novel peroxisome proliferator-activated receptor-α target gene (PPARα), and Yoon and colleagues 17 identified the angtpl4 gene as a PPARγ target gene. ANGPTL4 has been detected in the liver, adipose tissue, and placenta, and it has been suggested that this protein is involved in lipid metabolism, adaptation to fasting, and adipose tissue differentiation. 17,18
We identified angptl4 as a hypoxia-induced gene while performing a systematic search for genes expressed by ECs under chemical and/or gaseous hypoxia. ANGPTL4 mRNA levels rapidly (2 hours) and markedly (by a factor of up to eight) increased under these conditions and the amount of the corresponding protein in the medium also increased. Hypoxic induction of ANGPTL4 mRNA production has also been reported in glioblastomas 28 and in cultured cardiomyocytes. 30 In this study, using our anti-human ANGPTL4 antibody, we showed for the first time the induction by hypoxia of ANGPTL4 protein production.
ANGPTL4 belongs to a family of angiopoietin-like proteins, including ANGPTL3, that display angiogenic activity. 27 It is also involved in the regulation of plasma lipid levels. 31 In this study, we have clearly demonstrated that ANGPTL4 has a proangiogenic effect. We used the production of the recombinant protein by CHO cells grafted onto the chick CAM as an experimental model. ANGPTL4 is a secreted protein. 16 It is therefore steadily released by CHO/ANGPTL4 cells and induces significant neoangiogenesis and the rearrangement of the blood vessels. ANGPTL4-induced neovascularization was not inhibited by the VEGF inhibitor PTK787/ZK 222584. In control CAMs, or away from the ANGPTL4-producing cells, the effect of PTK787/ZK 222584 was similar to that reported by Cruz and colleagues, 24 who described the inhibition of CAM vessel growth, which was restricted to the microvasculature. As previously reported, 26 PTK787/ZK 222584 did not inhibit kinases from other enzyme families at the concentrations used in this study. We therefore suggest that ANGPTL4 is a proangiogenic factor that acts independently of VEGF. Nevertheless, more work is needed to better characterize vascular proliferation in response to ANGPTL4, the mechanism of action of remains unknown as Kim and colleagues 16 showed that ANGPTL4 binds neither Tie-2 nor Tie-1.
We confirmed that ANGPTL4 production was induced by hypoxia/ischemia by means of in situ hybridization experiments comparing healthy and ischemic tissue samples from patients suffering from leg ischemia. In the chronically ischemic part of the leg, VEGF and VEGF-R2 have been shown to be restricted to atrophic and regenerating muscle cells. 32 We show here that ANGPTL4 is present in larger amounts in the ischemic tissue than in the healthy part of the leg. ANGPTL4 was produced principally by ECs, but also, to a lesser extent, by vascular smooth muscle cells. ANGPTL4 was also produced in the area surrounding the necrotic portions of various benign or malignant tumors. A similar pattern has been observed for endothelial Per-ARNT-Sim domain protein-1(EPAS-1)/hypoxia-inducible factor-2, the production of which in hypoxic perinecrotic regions is correlated with tumor progression and angiogenesis. 29
ANGPTL4 production was also clearly up-regulated in tumoral cells from conventional RCCs. In addition, ANGPTL4 was produced only in conventional RCCs, and not in other benign or malignant renal lesions. ANGPTL4 therefore seems to be a marker for conventional RCC and the determination of plasma ANGPTL4 levels may serve as an indicator of the recurrence of this cancer or the likelihood of metastasis. ANGPTL4 may also be of value as a therapeutic target in conventional RCC, which is resistant to radiotherapy and conventional chemotherapy, and prone to metastasis via the bloodstream.
Finally, human conventional RCCs have been shown to display altered carbohydrate metabolism, a feature not shared by other renal tumors. 33 In fact, conventional RCC arises from proximal tubular cells, which have a very high metabolic demand as they ensure ion transport. The role of ANGPTL4 in the metabolic pathways of these cells and in conventional RCCs requires further investigation, but it seems likely that survival in hypoxia and the growth of conventional RCCs may require PPARγ target genes such as angptl4. Interestingly, PPARγ has been detected in conventional RCC specimens and both the synthetic PPAR-γ agonists (pioglitazone and troglitazone) and the endogenous PPAR-γ ligand, 15-deoxy-delta12,14-prostaglandin J(2) [15dPGJ(2)] inhibit the growth of conventional RCC cells. 34 These findings suggest that there is a complex, as yet unknown interplay between the regulation of PPARγ-regulated genes and the hypoxic pathway in some pathological conditions such as cancer. 35
Acknowledgments
We thank Dr. Joseph Emmerich (Hôpital Européen Georges Pompidou) for providing ischemic tissues; Dr. Jeanette M. Wood (Novartis Research, Basel, Switzerland) for the gift of PTK787/ZK 222584 (the inhibitor of the protein tyrosine kinase activity of the VEGF receptor); Marie-Thérèse Morin and Francoise Mongiat for technical assistance; and Dr. Luc Pardanaud, Dr. Xavier Jeunemaitre, and Dr. Kristen Frenzel for helpful discussions and critical reading of the manuscript.
Footnotes
Address reprint requests to Stephane Germain, INSERM U36, Collège de France, 11, place M. Berthelot, 75005 Paris, France. E-mail: stephane.germain@college-de-france.fr.
Supported in part by a Fondation de France grant (to S. G.), the Ministère de la Recherche et de l’Enseignement Supérieur (to S. L. J.), the Association Claude Bernard (to C. A.), the Académie Nationale de Médecine (to A. C.), and the Ligue Contre le Cancer (to J. F.).
C. A. and A. C. contributed equally to this work.
References
- 1.Helmlinger G, Yuan F, Dellian M, Jain RK: Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat Med 1997, 3:177-182 [DOI] [PubMed] [Google Scholar]
- 2.Folkman J: Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995, 1:27-31 [DOI] [PubMed] [Google Scholar]
- 3.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD: Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 1997, 277:55-60 [DOI] [PubMed] [Google Scholar]
- 4.Ferrara N, Alitalo K: Clinical applications of angiogenic growth factors and their inhibitors. Nat Med 1999, 5:1359-1364 [DOI] [PubMed] [Google Scholar]
- 5.Ryan HE, Poloni M, McNulty W, Elson D, Gassmann M, Arbeit JM, Johnson RS: Hypoxia-inducible factor-1alpha is a positive factor in solid tumor growth. Cancer Res 2000, 60:4010-4015 [PubMed] [Google Scholar]
- 6.Kung AL, Wang S, Klco JM, Kaelin WG, Livingston DM: Suppression of tumor growth through disruption of hypoxia-inducible transcription. Nat Med 2000, 6:1335-1340 [DOI] [PubMed] [Google Scholar]
- 7.Hockel M, Vaupel P: Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst 2001, 93:266-276 [DOI] [PubMed] [Google Scholar]
- 8.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL: Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev 1998, 12:149-162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryan HE, Lo J, Johnson RS: HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J 1998, 17:3005-3015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mandriota SJ, Pepper MS: Regulation of angiopoietin-2 mRNA levels in bovine microvascular endothelial cells by cytokines and hypoxia. Circ Res 1998, 83:852-859 [DOI] [PubMed] [Google Scholar]
- 11.Hanahan D: Signaling vascular morphogenesis and maintenance. Science 1997, 277:48-50 [DOI] [PubMed] [Google Scholar]
- 12.Zhu H, Bunn HF: Signal transduction. How do cells sense oxygen? Science 2001, 292:449-451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ades EW, Candal FJ, Swerlick RA, George VG, Summers S, Bosse DC, Lawley TJ: HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol 1992, 99:683-690 [DOI] [PubMed] [Google Scholar]
- 14.Melillo G, Taylor LS, Brooks A, Musso T, Cox GW, Varesio L: Functional requirement of the hypoxia-responsive element in the activation of the inducible nitric oxide synthase promoter by the iron chelator desferrioxamine. J Biol Chem 1997, 272:12236-12243 [DOI] [PubMed] [Google Scholar]
- 15.Hubank M, Schatz DG: cDNA representational difference analysis: a sensitive and flexible method for identification of differentially expressed genes. Methods Enzymol 1999, 303:325-349 [DOI] [PubMed] [Google Scholar]
- 16.Kim I, Kim HG, Kim H, Kim HH, Park SK, Uhm CS, Lee ZH, Koh GY: Hepatic expression, synthesis and secretion of a novel fibrinogen/angiopoietin-related protein that prevents endothelial-cell apoptosis. Biochem J 2000, 346:603-610 [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon JC, Chickering TW, Rosen ED, Dussault B, Qin Y, Soukas A, Friedman JM, Holmes WE, Spiegelman BM: Peroxisome proliferator-activated receptor gamma target gene encoding a novel angiopoietin-related protein associated with adipose differentiation. Mol Cell Biol 2000, 20:5343-5349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kersten S, Mandard S, Tan NS, Escher P, Metzger D, Chambon P, Gonzalez FJ, Desvergne B, Wahli W: Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J Biol Chem 2000, 275:28488-28493 [DOI] [PubMed] [Google Scholar]
- 19.Carmeliet P: Mechanisms of angiogenesis and arteriogenesis. Nat Med 2000, 6:389-395 [DOI] [PubMed] [Google Scholar]
- 20.Deindl E, Buschmann I, Hoefer IE, Podzuweit T, Boengler K, Vogel S, van Royen N, Fernandez B, Schaper W: Role of ischemia and of hypoxia-inducible genes in arteriogenesis after femoral artery occlusion in the rabbit. Circ Res 2001, 89:779-786 [DOI] [PubMed] [Google Scholar]
- 21.Pastorian K, Hawel L, III, Byus CV: Optimization of cDNA representational difference analysis for the identification of differentially expressed mRNAs. Anal Biochem 2000, 283:89-98 [DOI] [PubMed] [Google Scholar]
- 22.Sibony M, Commo F, Callard P, Gasc JM: Enhancement of mRNA in situ hybridization signal by microwave heating. Lab Invest 1995, 73:586-591 [PubMed] [Google Scholar]
- 23.Auerbach R, Kubai L, Knighton D, Folkman J: A simple procedure for the long-term cultivation of chicken embryos. Dev Biol 1974, 41:391-394 [DOI] [PubMed] [Google Scholar]
- 24.Cruz A, Parnot C, Ribatti D, Corvol P, Gasc JM: Endothelin-1, a regulator of angiogenesis in the chick chorioallantoic membrane. J Vasc Res 2001, 38:536-545 [DOI] [PubMed] [Google Scholar]
- 25.Ozaki H, Seo MS, Ozaki K, Yamada H, Yamada E, Okamoto N, Hofmann F, Wood JM, Campochiaro PA: Blockade of vascular endothelial cell growth factor receptor signaling is sufficient to completely prevent retinal neovascularization. Am J Pathol 2000, 156:697-707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wood JM, Bold G, Buchdunger E, Cozens R, Ferrari S, Frei J, Hofmann F, Mestan J, Mett H, O’Reilly T, Persohn E, Rosel J, Schnell C, Stover D, Theuer A, Towbin H, Wenger F, Woods-Cook K, Menrad A, Siemeister G, Schirner M, Thierauch KH, Schneider MR, Drevs J, Martiny-Baron G, Totzke F: PTK787/ZK 222584, a novel and potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, impairs vascular endothelial growth factor-induced responses and tumor growth after oral administration. Cancer Res 2000, 60:2178-2189 [PubMed] [Google Scholar]
- 27.Camenisch G, Pisabarro MT, Sherman D, Kowalski J, Nagel M, Hass P, Xie MH, Gurney A, Bodary S, Liang XH, Clark K, Beresini M, Ferrara N, Gerber HP: ANGPTL3 stimulates endothelial cell adhesion and migration via integrin alpha vbeta 3 and induces blood vessel formation in vivo. J Biol Chem 2002, 277:17281-17290 [DOI] [PubMed] [Google Scholar]
- 28.Lal A, Peters H, St Croix B, Haroon ZA, Dewhirst MW, Strausberg RL, Kaanders JH, van der Kogel AJ, Riggins GJ: Transcriptional response to hypoxia in human tumors. J Natl Cancer Inst 2001, 93:1337-1343 [DOI] [PubMed] [Google Scholar]
- 29.Onita T, Ji PG, Xuan JW, Sakai H, Kanetake H, Maxwell PH, Fong GH, Gabril MY, Moussa M, Chin JL: Hypoxia-induced, perinecrotic expression of endothelial Per-ARNT-Sim domain protein-1/hypoxia-inducible factor-2alpha correlates with tumor progression, vascularization, and focal macrophage infiltration in bladder cancer. Clin Cancer Res 2002, 8:471-480 [PubMed] [Google Scholar]
- 30.Belanger AJ, Lu H, Date T, Liu LX, Vincent KA, Akita GY, Cheng SH, Gregory RJ, Jiang C: Hypoxia up-regulates expression of peroxisome proliferator-activated receptor gamma angiopoietin-related gene (PGAR) in cardiomyocytes: role of hypoxia inducible factor 1alpha. J Mol Cell Cardiol 2002, 34:765-774 [DOI] [PubMed] [Google Scholar]
- 31.Koishi R, Ando Y, Ono M, Shimamura M, Yasumo H, Fujiwara T, Horikoshi H, Furukawa H: Angptl3 regulates lipid metabolism in mice. Nat Genet 2002, 30:151-157 [DOI] [PubMed] [Google Scholar]
- 32.Rissanen TT, Vajanto I, Hiltunen MO, Rutanen J, Kettunen MI, Niemi M, Leppanen P, Turunen MP, Markkanen JE, Arve K, Alhava E, Kauppinen RA, Yla-Herttuala S: Expression of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 (KDR/Flk-1) in ischemic skeletal muscle and its regeneration. Am J Pathol 2002, 160:1393-1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steinberg P, Storkel S, Oesch F, Thoenes W: Carbohydrate metabolism in human renal clear cell carcinomas. Lab Invest 1992, 67:506-511 [PubMed] [Google Scholar]
- 34.Inoue K, Kawahito Y, Tsubouchi Y, Kohno M, Yoshimura R, Yoshikawa T, Sano H: Expression of peroxisome proliferator-activated receptor gamma in renal cell carcinoma and growth inhibition by its agonists. Biochem Biophys Res Commun 2001, 287:727-732 [DOI] [PubMed] [Google Scholar]
- 35.Panigrahy D, Singer S, Shen LQ, Butterfield CE, Freedman DA, Chen EJ, Moses MA, Kilroy S, Duensing S, Fletcher C, Fletcher JA, Hlatky L, Hahnfeldt P, Folkman J, Kaipainen A: PPARgamma ligands inhibit primary tumor growth and metastasis by inhibiting angiogenesis. J Clin Invest 2002, 110:923-932 [DOI] [PMC free article] [PubMed] [Google Scholar]