Abstract
T cell factor-1 (TCF-1) and lymphoid enhancer factor-1 (LEF-1), members of the TCF/LEF family of transcription factors, play a significant role in T cell development and are expressed in thymocytes and peripheral CD3+ T cells. Previously, precursor T lymphoblastic leukemia/lymphoblastic lymphoma (T-ALL/LyL) was found to express TCF-1, and we find that 9 of 10 cases of T-ALL/LyL express LEF-1 as well as TCF-1, exhibiting uniform nuclear immunostaining for both transcription factors. In addition, a significant subset of cases of peripheral T cell lymphoma (PTCL), 39 of 81 cases (48%), are immunoreactive for LEF-1 and/or TCF-1, with 36 of 38 cases immunoreactive for both, indicating that these transcription factors are coordinately expressed in PTCL. The vast majority of LEF-1+ and/or TCF-1+ PTCL (34 of 39 or 87%) exhibit a composite Th1 T-cell-like immunophenotype, based on expression of Th1 T cell-associated, but not Th2 T cell-associated, chemokine receptors and activation markers. Of the Th1-like PTCL studied, 33 of 42 (79%) were immunoreactive for LEF-1 and 32 of 42 (76%) were immunoreactive for TCF-1, including most cases of angioimmunoblastic lymphoma and all cases of lymphoepithelioid lymphoma. Surprisingly, none of the 21 cases of Th2-like PTCL studied, all cases of anaplastic large cell lymphoma, were immunoreactive for LEF-1 or TCF-1 (P < 0.0001), suggesting that LEF-1 and TCF-1 transcription factor expression may be lost in Th2 T cells or Th2-like PTCL. LEF-1 and TCF-1 immunostaining can serve to identify specific subtypes of PTCL, and lends support to a bipartite model of PTCL development, based on expression of activation markers.
T cell factor-1 (TCF-1) and lymphoid enhancer factor-1 (LEF-1), members of the TCF/LEF family of transcription factors, are components of the WNT/β-catenin signal transduction cascade that is important in early lymphocyte development. 1 Wnt pathway signaling stabilizes β-catenin, which can then associate with TCF/LEF family proteins to form transcriptional activation complexes that regulate target genes involved in lymphocyte development. 1
TCF-1 and LEF-1 are expressed in thymocytes and mature T cells in mice and humans; LEF-1 is also expressed in B cell precursors, but not in mature B cells. 1-6 TCF-1 is an early marker of T cell development in the fetal thymus, 7 and mouse knockout experiments have shown that it is essential for maintenance of early thymocyte progenitor cells, but not for proliferation and function of mature T cells. 8,9 Double knockout of the TCF-1 and LEF-1 genes results in a complete block of thymocyte development. 10
Mutations that result in constitutive activation of the Wnt pathway, particularly through loss of APC function, are a common cause of colorectal carcinoma. 11 Wnt pathway mutations are present in a number of other malignant neoplasms, including malignant melanoma and gastric and hepatocellular carcinoma. 11-13 The role of the Wnt pathway in B cell and T cell oncogenesis has not been extensively investigated. Castrop and co-workers 14 found that TCF-1 is expressed in thymocytes and peripheral CD3+ T cells, as well as being expressed in 20 cases of T-cell acute lymphoblastic leukemia (ALL), 1 case of Tγ lymphocytosis (T-cell large granular lymphocyte leukemia), and 1 case of peripheral T cell lymphoma (PTCL) studied, but was not expressed in B-cell lymphoproliferative disorders, including ALL, chronic lymphocytic leukemia, and non-Hodgkin’s lymphoma, or in myeloid neoplasms, including acute myelogenous leukemia of chronic myelogenous leukemia.
Because TCF-1 and LEF-1 are expressed in postthymic CD3+ T cells, we examined a variety of cases of PTCL for TCF-1 and LEF-1 expression. We found that a significant subset of PTCL expresses TCF-1 and LEF-1 in a coordinate manner, and that this expression correlates with that of markers of Th1 T-cell activation. Surprisingly, cases of PTCL expressing markers of Th2 T-cell activation did not express TCF-1 or LEF-1.
Materials and Methods
Case material was obtained from the Brigham and Women’s Hospital, Boston, MA, in accordance with institutional policies. All diagnoses were based on the features described in the World Health Organization Lymphoma Classification system. 15 Cases were characterized immunophenotypically with antibodies directed against the B cell marker CD20 (L26) and the T cell markers CD3, CD45RO, CD43 (Leu22), CD8, CD30, ALK-1, CXCR3, CD134/OX40, and CD69, using formalin-fixed, paraffin-embedded tissue sections, as previously described. 16-18 In cases in which frozen tissue was available, immunophenotypic analysis was also performed using antibodies directed against CD4 and CCR4, as previously described. 17 Cases of PTCL were included if they demonstrated CD4 immunoreactivity by frozen section immunohistochemical staining and/or absence of CD8 immunoreactivity by paraffin section immunohistochemical staining.
Immunostaining for LEF-1 and TCF-1 was performed on formalin-fixed paraffin-embedded tissue sections after microwave antigen retrieval in 1 mmol/L of ethylenediaminetetraacetic acid, pH 8.0, with an anti-human LEF-1 monoclonal antibody (monoclonal antibody REMB1; Exalpha Biologicals, Boston, MA) and anti-human TCF-1 (monoclonal antibody 7H3, Exalpha Biologicals) using a standard indirect avidin-biotin horseradish peroxidase method and diaminobenzidine color development, as previously described. 16-18 In cases that were immunoreactive for LEF-1 and/or TCF-1, typically greater than 75% of neoplastic cells exhibited positive staining. Fewer than 5% of neoplastic cells exhibited staining for LEF-1 and/or TCF-1 in nonimmunoreactive cases.
Statistical analysis of LEF-1 and TCF-1 staining between various PTCL subtypes was performed using Fisher’s exact test.
Results
Monoclonal antibodies for LEF-1 and TCF-1 were used to stain formalin-fixed, paraffin-embedded specimens of reactive lymphoid tissue, thymus, and a range of cases of T-cell lymphoproliferative disorders. In specimens of reactive lymphoid tissue with follicular hyperplasia, extensive nuclear staining for LEF-1 and TCF-1 was seen in the interfollicular T cell zone (Figure 1; A to C) ▶ . Scattered, small LEF-1- and TCF-1-positive cells were also noted within germinal centers, consistent with trafficking T cells. B cell centroblasts and centrocytes within germinal centers as well as mantle zone B cells were nonreactive for LEF-1 and TCF-1. Similar results were seen in histological sections of reactive lymph node and spleen (data not shown).
Figure 1.
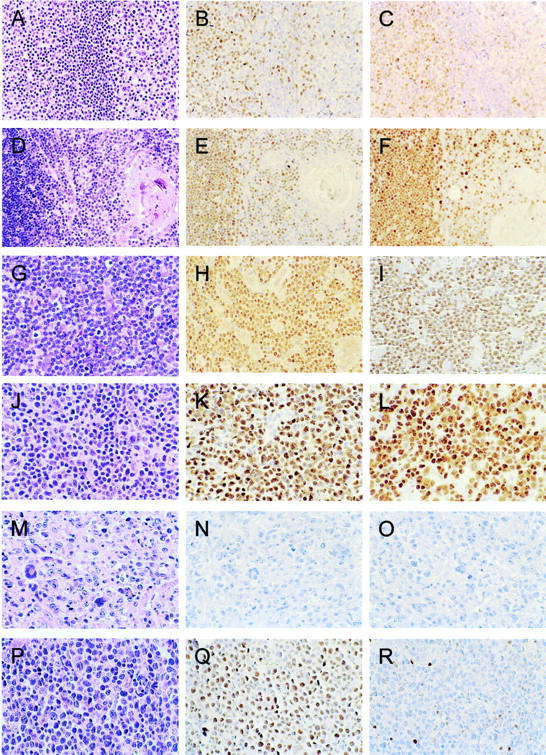
LEF-1 and TCF-1 immunostaining in reactive lymphoid tissue, thymus, and T-cell lymphoproliferative disorders. Tonsil with follicular hyperplasia (A), in which there are numerous LEF-1-positive (B) and TCF-1-positive (C) cells in the interfollicular T cell zone, as well as scattered LEF-1- and TCF-1-positive cells within germinal centers, consistent with trafficking T cells. Mantle zone and germinal center B cells are negative for LEF-1 and TCF-1. Adult thymus (D) with numerous LEF-1-positive (E) and TCF-1-positive (F) cells in the thymic cortex (left), with fewer LEF-1- and TCF-1-positive cells in the thymic medulla (right). Thymic epithelial cells, including Hassall’s corpuscles, are negative for LEF-1 and TCF-1. Precursor T lymphoblastic leukemia/lymphoblastic lymphoma (G) with uniform staining of neoplastic cells for LEF-1 (H) and TCF-1 (I). Peripheral T cell lymphoma, unspecified (J) with uniform staining of neoplastic cells for LEF-1 (K) and TCF-1 (L). Anaplastic large-cell lymphoma (M), in which neoplastic cells are uniformly negative for LEF-1 (N) and TCF-1 (O). A case of peripheral T cell lymphoma, unspecified (P) with uniform staining of neoplastic cells for LEF-1 (Q) and absence of staining of large neoplastic cells for TCF-1 (R). Scattered, small reactive T cells are immunoreactive for TCF-1 (R).
In adult thymus, virtually all cortical thymocytes were immunoreactive for LEF-1 and TCF-1 (Figure 1, E and F) ▶ . A subset of medullary thymocytes was reactive for LEF-1 and TCF-1, but thymic epithelial cells, including Hassall’s corpuscles located in the medullary thymus, did not stain for LEF-1 and TCF-1 (Figure 1, E and F) ▶ . Similar results were seen in histological sections of fetal thymus (data not shown).
Castorp and co-workers 14 reported previously that B-cell lymphoproliferative disorders do not exhibit TCF-1 expression. We studied a small series of cases of B-cell lymphoproliferative disorders, including three cases of chronic lymphocytic leukemia/small lymphocytic lymphoma, three cases of marginal zone lymphoma, two cases of mantle cell lymphoma, and five cases of follicular lymphoma (Table 1) ▶ . All were negative for LEF-1 and TCF-1 immunostaining except for the cases of chronic lymphocytic leukemia/small lymphocytic lymphoma, which exhibited faint nuclear staining for LEF-1 (data not shown). In all cases studied, intermixed, reactive, CD3+ T cells were present and exhibited strong nuclear staining for both LEF-1 and TCF-1 (not shown).
Table 1.
LEF-1 and TCF-1 Immunostaining in T-Cell and B-Cell Lymphoproliferative Disorders
| LEF-1 immunostaining | TCF-1 immunostaining | |
|---|---|---|
| B NHL | 0/13* | 0/13* |
| T-LyL/ALL | 9/10 | 9/10 |
| Peripheral T cell NHL | 38/81 | 37/81 |
| Th-1 T cell-like NHL | 33/42† | 32/42† |
| Th-2 T cell-like NHL | 0/21† | 0/21† |
| Unable to subtype | 5/18‡ | 5/18‡ |
*Three cases of chronic lymphocytic leukemia/small lymphocytic lymphoma, three cases of marginal zone lymphoma, two cases of mantle cell lymphoma, and five cases of follicular lymphoma.
†P < 0.0001; the Th-1 T cell-like NHL group of tumors includes 5 cases of lympho-epithelioid (Lennert’s) lymphoma, all of which were immunoreactive for LEF-1 and TCF-1, and 13 cases of angioimmunoblastic lymphoma, 11 of which were immunoreactive for LEF-1 and TCF-1.
‡Nine cases of PTCL, unspecified, five of which were immunoreactive for LEF-1 and TCF-1, and nine cases of ALCL, all of which were negative for LEF-1 and TCF-1.
Nine of 10 cases of T-ALL/LyL exhibited uniform nuclear staining of neoplastic cells for LEF-1 and TCF-1 (Figure 1; G to I) ▶ . One case was negative for both LEF-1 and TCF-1 (data not shown). These results are consistent with those of Castorp and co-workers, 14 who reported that 20 of 20 cases of T cell ALL were immunoreactive for TCF-1.
We then studied a series of PTCL cases that were previously characterized for the expression of a number of T-cell activation markers and other markers that correlate with Th1 and Th2 T cell activation. 16-18 Thirty-nine of 81 cases (48%) were immunoreactive for LEF-1 and/or TCF-1, with 36 cases immunoreactive for both, 2 cases immunoreactive for LEF-1 and not TCF-1, and 1 case immunoreactive for TCF-1 and not LEF-1, indicating that these transcription factors are coordinately expressed in T cell lymphomas. The results are summarized in Table 1 ▶ . In cases that were immunoreactive for LEF-1 and/or TCF-1, typically the vast majority of neoplastic cells exhibited positive nuclear staining, as shown in Figure 1 ▶ . Of note, the vast majority of PTCL cases (34 of 39 or 87%) that were immunoreactive for LEF-1 and/or TCF-1 exhibited a composite Th1 T-cell-like immunophenotype, based on previous immunostaining for chemokine receptor CXCR3, activation markers CD69 and CD134, and absence of immunostaining for chemokine receptor CXCR4 and activation marker CD30. 16-18 This included 13 cases of angioimmunoblastic lymphoma, 11 of which were immunoreactive for LEF-1 and TCF-1, and five cases of lymphoepithelioid (Lennert’s) lymphoma, all of which were immunoreactive for LEF-1 and TCF-1. A representative case of LEF-1-positive, TCF-1-positive PTCL is shown in Figure 1, J-L ▶ . Nuclear staining for LEF-1 and TCF-1 is seen in virtually all of the intermediate to large neoplastic cells.
Thirty-three of 42 cases (79%) of PTCL with a composite Th1-like T cell immunophenotype were immunoreactive for LEF-1, and 32 of 42 cases (76%) were immunoreactive for TCF-1. Two cases of PTCL, unspecified, showed strong nuclear immunostaining for LEF-1 but not for TCF-1. One of the cases is shown in Figure 1, P to R ▶ , with scattered small reactive T cells exhibiting TCF-1 immunostaining (Figure 1R) ▶ . One case of PTCL, unspecified, showed nuclear staining for TCF-1 but not LEF-1. The three cases of PTCL that failed to exhibit coordinate expression of LEF-1 and TCF-1 did not have any unique or unusual characteristics when compared with other cases of PTCL, unspecified.
Twenty-one cases of peripheral T cell lymphoma that were studied exhibited a composite Th2 T-cell-like immunophenotype, based on previous immunostaining for activation marker CD30, chemokine receptors CXCR4, and, in some cases, CCR4, and absence of staining for chemokine receptor CXCR3 and activation markers CD69 and CD134. 16-18 All of these cases fulfilled diagnostic criteria for anaplastic large-cell lymphoma (ALCL), and 13 of 24 cases were immunoreactive for ALK kinase, characteristic of a subset of cases of ALCL. Surprisingly, all of the peripheral T cell lymphomas with a composite Th2 T-cell immunophenotype failed to exhibit staining for LEF-1 or TCF-1. A representative case of ALCL is shown in Figure 1, M to O ▶ . The association of LEF-1 and TCF-1 staining with expression of Th1-type, but not Th-2-type T-cell activation markers was statistically significant (P < 0.0001).
Eighteen additional cases of PTCL were studied that could not be classified as Th1 T cell-like or Th2 T cell-like based on their composite immunophenotypes. This group included nine cases of ALCL, all of which were negative for LEF-1 and TCF-1 by immunostaining, and nine cases of PTCL, unspecified, five of which were immunoreactive for LEF-1 and TCF-1 (Table 1) ▶ . When all cases of ALCL are combined (21 with a Th2 T cell-type and 9 that could not be otherwise classified), none of the 30 cases was immunoreactive for LEF-1 or TCF-1, indicating that absence of expression of these transcription factors is a characteristic of ALCL in general.
Discussion
Here we report that transcription factors LEF-1 and TCF-1 are coordinately expressed in PTCL as well as in T-ALL/LyL, but are not expressed to a significant extent in B-cell lymphoproliferative disorders. We found that slightly less than half of PTCL cases express LEF-1 and TCF-1, and that in the vast majority of cases this expression correlates with that of markers of Th1 T-cell differentiation. Previously we reported that a number of T-cell activation markers and chemokine receptors that are specific for Th1 or Th2 T cells are expressed in generally nonoverlapping subsets of PTCL. 16-18 This included chemokine receptors CXCR3 and CCR4, which are expressed in Th1 and Th2 T cells, respectively. Cases of ALCL, which express Th2 T-cell-associated activation marker CD30, 19-21 were generally also immunoreactive for CCR4 and not CXCR3. 17 In support of these findings, the murine anaplastic lymphoid cell line TS1G6, an animal model for ALCL, has been shown to exhibit a Th2 T-cell cytokine profile, 22 and L82, a human ALCL cell line, exhibits Th2 T-cell-type cytokine expression. 23 In contrast, a number of cases of PTCL, unspecified, including cases of lymphoepithelioid (Lennert’s) lymphoma, as well as most cases of angioimmunoblastic lymphoma, were immunoreactive for Th1 T-cell-associated CXCR3 and not Th2 T-cell-associated CCR4 or CD30. 16,17 A subsequent study of Th1 T-cell-associated activation marker CD69 in PTCL found that CD69 expression correlated with that of other Th1 T cell markers and was not present in PTCL expressing Th2 T cell markers. 18 These results suggest a bipartite model of PTCL development, in which at least a subset of cases of PTCL may arise from neoplastic transformation of peripheral T cells activated to a Th1 or Th2 state. The correlation of LEF-1 and TCF-1 expression with Th1 T-cell markers in PTCL lends support to a bipartite model of T cell lymphomagenesis.
The association of LEF-1 and TCF-1 expression with markers of Th1 T-cell activation in PTCL raises the possibility that these transcription factors are specifically expressed in or required for the development of normal Th1 T cells but not Th2 T cells. In support of this association, protein complexes regulating T cell-specific transcription, including TCF/LEF transcription factor components, are known to bind to the proximal promoter of the IL-2 gene, 24-26 which is expressed in T helper precursor and Th1 T cells, but not Th2 cells. 27,28 Further studies are needed to determine whether the loss of LEF-1 and TCF-1 expression is a general feature of Th2 T-cell development or is specific to Th2 T-cell-like lymphomas such as ALCL. An approach to address this question is to examine the expression of LEF-1 and TCF-1 in reactive states such as acute hypersensitivity reaction and chronic graft-versus-host disease, in which Th2 T cells predominate. 20,29 These studies are in progress.
Wnt pathway mutations, a common cause of colorectal carcinoma, and present in other neoplasms, 11-13 usually result in constitutive pathway activation. Typically the cytoplasmic pool of β-catenin is elevated, resulting in increased binding to TCF/LEF family members, entry into the nucleus, and enhanced transcriptional activation leading to carcinogenesis. 11 Increased nuclear immunostaining for β-catenin has been observed in a number of malignant neoplasms as a result of these mutations. 11-13,30,31 Preliminary immunostaining of cases of LEF-1+/TCF-1+ PTCL identified in the current study did not reveal increased nuclear staining for β-catenin (D.M.D. and A.S., unpublished data). If confirmed, these findings would suggest that the WNT/β-catenin signaling pathway is not abnormally activated in Th1 T-cell-like lymphomas. However, additional studies are needed to determine whether such Th1 T-cell-like lymphomas may still require signaling through the WNT/β-catenin pathway for their proliferation or survival.
In the current classification of B-cell lymphoproliferative disorders, various subtypes of B cell lymphoma are thought to arise from, and thus to resemble different stages of normal B cell development, from their origin as pro-B/pre-B cells in the marrow, to resting (naïve and memory) B cells and germinal center B cells in peripheral lymphoid organs, and finally to terminally differentiated plasma cells. 15 For example, the expression profiles of follicular lymphomas and diffuse large B cell lymphomas resemble those of germinal center B cells, although differences between individual cases and subsets of these lymphomas may reflect differences in either oncogenic mechanism or precise cell of origin.
The current classification of T cell leukemias and lymphomas, however, is primarily descriptive, without a clear correspondence between specific tumor subtypes and specific stages in T cell development. 15 The majority of T-cell non-Hodgkin’s lymphomas, which are difficult to subclassify morphologically and immunophenotypically, are postulated to arise from peripheral T cells in various, but as yet undefined, stages of development and/or transformation. 15 Recently, Ferrando and co-workers 32 identified several gene expression profiles in T-cell acute lymphoblastic leukemia indicative of leukemic arrest at specific stages of normal thymocyte development. A similar approach may permit a developmental analysis of the postthymic T-cell lymphoproliferative disorders, and may reveal whether subtypes of PTCL have gene expression profiles that correspond to that of Th1 and Th2 T cells, or other, distinct T-cell activation states.
Transcription factors expressed in the B cell lineage and in B cell lymphomas, such as Oct1, Oct2, B-cell-specific activator protein (BSAP), and PU.1 appear to be widely expressed in all subtypes of B cell lymphoma. 33-35 In contrast, LEF-1 and TCF-1, transcription factors that are expressed in mature T cells, are only expressed in a subset of T-cell lymphoproliferative disorders that seem to correspond to a specific subtype of activated T cell. This result lends support to a model of T cell lymphomagenesis from specific subtypes of activated T cells. Recently, additional T-cell transcription factors, differentially expressed in activated T cell subtypes, have been identified, including T-bet, c-Maf, and GATA-3. 36 Preliminary results reveal that T cell lymphomas with a Th1 T-cell-type immunophenotypic signature appear to be immunoreactive for T-bet, a Th1 T-cell-specific transcription factor, 36 whereas T cell lymphomas with a Th2 T-cell-type immunophenotype appear to be negative for T-bet immunostaining (D.M.D. and A.S., unpublished data). Additional studies to examine the expression of other T-cell subtype-specific transcription factors in PTCL, including c-Maf and GATA-3, Th2-type T-cell-specific transcription factors, 36 are in progress.
Footnotes
Address reprint requests to David M. Dorfman, M.D., Ph.D., Department of Pathology, Brigham and Women’s Hospital, 75 Francis St., Boston, MA 02115. E-mail: ddorfman@partners.org.
References
- 1.van de Wetering M, de Lau W, Clevers H: WNT signaling and lymphocyte development. Cell 2002, 109:S13-S119 [DOI] [PubMed] [Google Scholar]
- 2.Oosterwegel M, van de Wetering M, Dooijes D, Klomp L, Winoto A, Georgopoulos K, Meijlink F, Clevers H: Cloning of murine TCF-1, a T cell-specific transcription factor interacting with functional motifs in the CD3-epsilon and T cell receptor alpha enhancers. J Exp Med 1991, 173:1133-1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van de Wetering M, Oosterwegel M, Dooijes D, Clevers H: Identification and cloning of TCF-1, a T lymphocyte-specific transcription factor containing a sequence-specific HMG box. EMBO J 1991, 10:123-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Travis A, Amsterdam A, Belanger C, Grosschedl R: LEF-1, a gene encoding a lymphoid-specific protein with an HMG domain, regulates T-cell receptor alpha enhancer function. Genes Dev 1991, 5:880-894 [DOI] [PubMed] [Google Scholar]
- 5.Waterman ML, Fischer WH, Jones KA: A thymus-specific member of the HMG protein family regulates the human T cell receptor C alpha enhancer. Genes Dev 1991, 5:656-669 [DOI] [PubMed] [Google Scholar]
- 6.Korinek V, Barker N, Willert K, Molenaar M, Roose J, Wagenaar G, Markman M, Lamers W, Destree O, Clevers H: Two members of the Tcf family implicated in Wnt/beta-catenin signaling during embryogenesis in the mouse. Mol Cell Biol 1998, 18:1248-1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hattori N, Kawamoto H, Fujimoto S, Kuno K, Katsura Y: Involvement of transcription factors TCF-1 and GATA-3 in the initiation of the earliest step of T cell development in the thymus. J Exp Med 1996, 184:1137-1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verbeek S, Izon D, Hofhuis F, Robanus-Maandag E, te Riele H, van de Wetering M, Oosterwegel M, Wilson A, MacDonald HR, Clevers H: An HMG-box-containing T-cell factor required for thymocyte differentiation. Nature 1995, 374:70-74 [DOI] [PubMed] [Google Scholar]
- 9.Schilham MW, Wilson A, Moerer P, Benaissa-Trouw BJ, Cumano A, Clevers HC: Critical involvement of Tcf-1 in expansion of thymocytes. J Immunol 1998, 161:3984-3991 [PubMed] [Google Scholar]
- 10.Okamura RM, Sigvardsson M, Galceran J, Verbeek S, Clevers H, Grosschedl R: Redundant regulation of T cell differentiation and TCRalpha gene expression by the transcription factors LEF-1 and TCF-1. Immunity 1998, 8:11-20 [DOI] [PubMed] [Google Scholar]
- 11.Bienz M, Clevers H: Linking colorectal cancer to Wnt signaling. Cell 2000, 103:311-320 [DOI] [PubMed] [Google Scholar]
- 12.Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P: Stabilization of β-catenin by genetic defects in melanoma cells lines. Science 1997, 275:1790-1792 [DOI] [PubMed] [Google Scholar]
- 13.Polakis P: Wnt signaling and cancer. Genes Dev 2000, 14:1837-1851 [PubMed] [Google Scholar]
- 14.Castrop J, van Wichen D, Koomans-Bitter M, van de Wetering M, De Weger R, van Dongen J, Clevers H: The human TCF-1 gene encodes a nuclear DNA-binding protein uniquely expressed in normal and neoplastic T-lineage lymphocytes. Blood 1995, 86:3050-3059 [PubMed] [Google Scholar]
- 15. Jaffe ES Harris NL Stein H Vardima JW eds. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. 2001:pp 189-235 IARC Press Lyon
- 16.Jones D, Fletcher CDM, Pulford K, Shahsafaei A, Dorfman M: The T-cell activation markers CD30 and OX40/CD134 are expressed in nonoverlapping subsets of peripheral T-cell lymphoma. Blood 1999, 93:3487-3493 [PubMed] [Google Scholar]
- 17.Jones D, O’Hara C, Kraus MD, Perez-Atayde AR, Shahsafaei A, Wu L, Dorfman DM: Expression pattern of T-cell-associated chemokine receptors and their chemokines correlates with specific subtypes of T-cell non-Hodgkin lymphoma. Blood 2000, 96:685-690 [PubMed] [Google Scholar]
- 18.Dorfman DM, Shahsafaei A: CD69 expression correlates with expression of other markers of Th1 T cell differentiation in peripheral T cell lymphomas. Hum Pathol 2002, 33:330-334 [DOI] [PubMed] [Google Scholar]
- 19.Caligaris-Cappio F, Bertero MT, Converso M, Stacchini A, Vinante F, Romagnani S, Pizzolo G: Circulating levels of soluble CD30, a marker of cells producing Th2-like cytokines, are increased in patients with systemic lupus erythematosus and correlate with disease activity. Clin Exp Rheumatol 1995, 13:339-343 [PubMed] [Google Scholar]
- 20.D’Elios MM, Romagnani P, Scaletti C, Annunziato F, Marghetti M, Mavilia C, Parronchi P, Pupilli C, Pizzolo G, Maggi E, Del Prete GF, Romagnani S: In vivo CD30 expression in human disease with predominant activation of Th2-like T cells. J Leukoc Biol 1997, 61:539-544 [PubMed] [Google Scholar]
- 21.Annunziato F, Manetti R, Cosmi L, Galli G, Heusser CH, Romagnani S, Maggi E: Opposite role for interleukin-4 and interferon-gamma on CD30 and lymphocyte activation gene-3 (LAG-3) expression by activated naïve T cells. Eur J Immunol 1997, 27:2239-2244 [DOI] [PubMed] [Google Scholar]
- 22.Bittner C, Feller AC, Renauld JC, Lange K, Pietrzik R, Jenetzky C, Briese J, Gaiser T, Muller A, Wiedemann GJ, Van Snick J, Merz H: An animal model for anaplastic large cell lymphoma in the immunocompetent syngeneic C57BL/6 mouse. Lab Invest 2000, 80:1523-1531 [DOI] [PubMed] [Google Scholar]
- 23.Merz H, Lange K, Gaiser T, Muller A, Kapp U, Bittner C, Harder S, Siebert R, Bentz M, Binder T, Diehl V, Feller AC: Characterization of a novel human anaplastic large cell lymphoma cell line tumorigenic in SCID mice. Leuk Lymphoma 2002, 43:165-172 [DOI] [PubMed] [Google Scholar]
- 24.Radler-Pohl A, Pfeuffer I, Karin M, Serfling E: A novel T-cell trans-activator that recognizes a phorbol ester-inducible element of the interleukin-2 promoter. New Biol 1990, 2:566-573 [PubMed] [Google Scholar]
- 25.Briegel K, Hentsch B, Pfeuffer I, Serfling E: One base pair change abolishes the T cell-restricted activity of a kB-like proto-enhancer element from the interleukin 2 promoter. Nucl Acids Res 1991, 19:5929-5936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ward SB, Hernandez-Hoyos G, Chen F, Waterman M, Reeves R, Rothenberg EV: Chromatin remodeling of the interleukin-2 gene: distinct alterations in the proximal versus distal enhancer regions. Nucl Acids Res 1998, 26:2923-2934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brombacher F, Schafer T, Weissenstein U, Tschopp C, Andersen E, Burki K, Baumann G: IL-2 promoter-driven lacZ expression as a monitoring tool for IL-2 expression in primary T cells of transgenic mice. Int Immunol 1994, 6:189-197 [DOI] [PubMed] [Google Scholar]
- 28.Lederer JA, Liou JS, Todd MD, Glimcher LH, Lichtman AH: Regulation of cytokine gene expression in T helper cell subsets. J Immunol 1994, 152:77-86 [PubMed] [Google Scholar]
- 29.Abbas AK, Murphy KM, Sher A: Functional diversity of helper T lymphocytes. Nature 1996, 383:787-793 [DOI] [PubMed] [Google Scholar]
- 30.Clements WM, Wang J, Sarnaik A, Kim OJ, MacDonald J, Fenoglio-Preiser C, Groden J, Lowy AM: β-catenin mutation is a frequent cause of Wnt pathway activation in gastric cancer. Cancer Res 2002, 62:3503-3506 [PubMed] [Google Scholar]
- 31.Nakatani Y, Masudo K, Miyagi Y, Inayama Y, Kawano N, Tanaka Y, Kato K, Ito T, Kitamura H, Nagashima Y, Yamanaka S, Nakamura N, Sano J, Ogawa N, Ishiwa N, Notohara K, Resl M, Mark EJ: Aberrant nuclear localization and gene mutation of β-catenin in low-grade adenocarcinoma of fetal lung type: up-regulation of the Wnt signaling pathway may be a common denominator for the development of tumors that form morules. Mod Pathol 2002, 15:617-624 [DOI] [PubMed] [Google Scholar]
- 32.Ferrando AA, Neuberg DS, Staunton J, Loh ML, Huard C, Raimondi SC, Behm FG, Pui C-H, Downing JR, Gilliland DG, Lander ES, Golub TR, Look AT: Gene expression signatures define novel oncogenic pathways in T cell acute lymphoblastic leukemia. Cancer Cell 2002, 1:75-87 [DOI] [PubMed] [Google Scholar]
- 33.Saez A-I, Artiga M-J, Sanchez-Beato M, Sanchez-Verde L, Garcia J-F, Camacho F-I, Franco R, Piris MA: Analysis of octamer-bonding transcription factors Oct2 and Oct1 and their coactivators BOB.1/OBF.1 in lymphomas. Mod Pathol 2002, 15:211-220 [DOI] [PubMed] [Google Scholar]
- 34.Foss H-D, Reusch R, Demel G, Lenz G, Anagnostopoulos I, Hummel M, Stein H: Frequent expression of the B-cell specific activator protein in Reed-Sternberg cells of classical Hodgkin’s disease provides further evidence for its B-cell origin. Blood 1999, 94:3108-3113 [PubMed] [Google Scholar]
- 35.Torlakovic E, Tierens A, Dang HD, Delabie J: The transcription factor PU.1, necessary for B-cell development is expressed in lymphocyte predominance, but not classical Hodgkin’s disease. Am J Pathol 2001, 159:1807-1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ho I-C, Glimcher LH: Transcription: tantalizing times for T cells. Cell 2002, 109:S109-S120 [DOI] [PubMed] [Google Scholar]


